CDX Screen Shots - TSCA Section 4 ECAs
CDX_Screen Shots_Section 4 ECAs_2012-01-25.doc
Electronic Reporting of TSCA Section 4, Section 5 NOC and Supporting Documents, 8(a) Preliminary Assessment Information Rule (PAIR), and 8(d) Submissions
CDX Screen Shots - TSCA Section 4 ECAs
OMB: 2070-0183
![]()
Screen mock-ups are approximations and may change. Each optional field is outlined by a red text box.
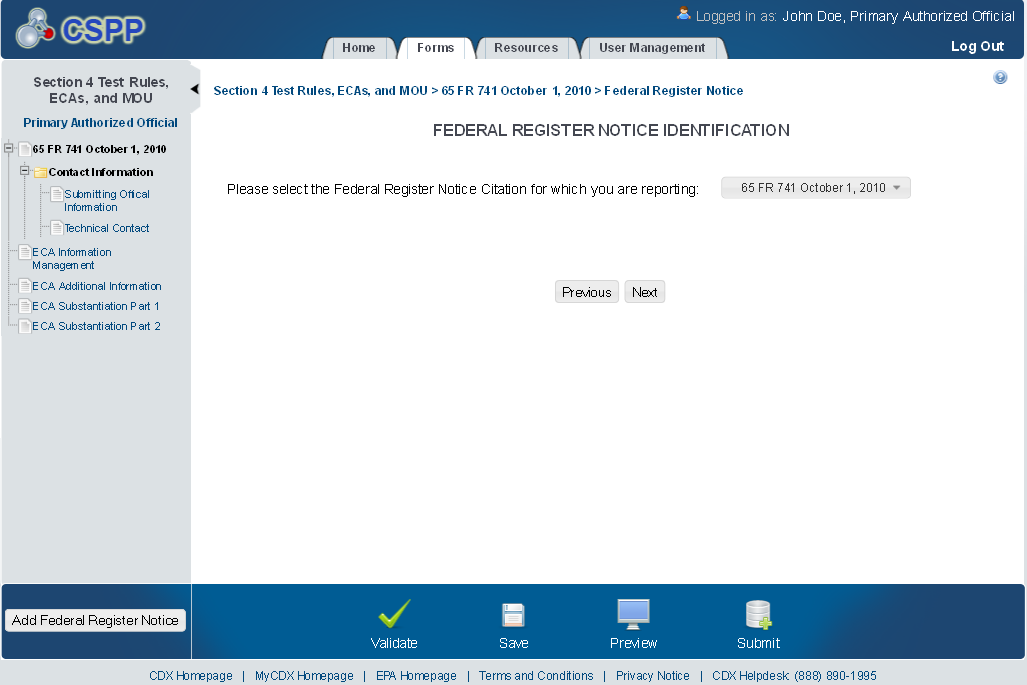
Federal Register Notice Identification - ECA
The ‘Federal Register Notice Identification - ECA’ screen provides a list of Federal Register Notice Citations for the user to choose. Based on the selection, the system directs the user to the appropriate subsequent screen.
The following exhibit shows the screen capture for the ‘Federal Register Notice Identification - ECA’ screen for ECA submissions:
Exhibit 1‑1 Federal Register Notice Identification - ECA
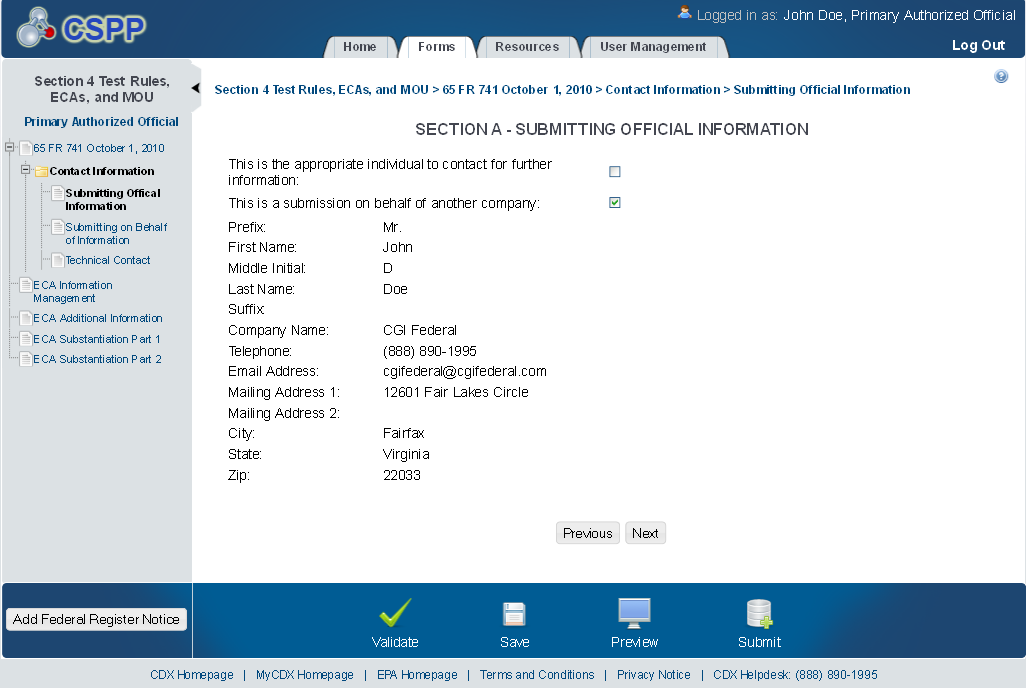
Submitting Official Information
The ‘Section A – Submitting Official Information’ screen allows the user to confirm their contact information. The screen is pre-populated with the information previously entered by the user during CDX registration.
The following exhibit shows the screen capture for the ‘Submitting Official Information’ screen for ECA submissions:
Exhibit 1‑2 Submitting Official Information Screen


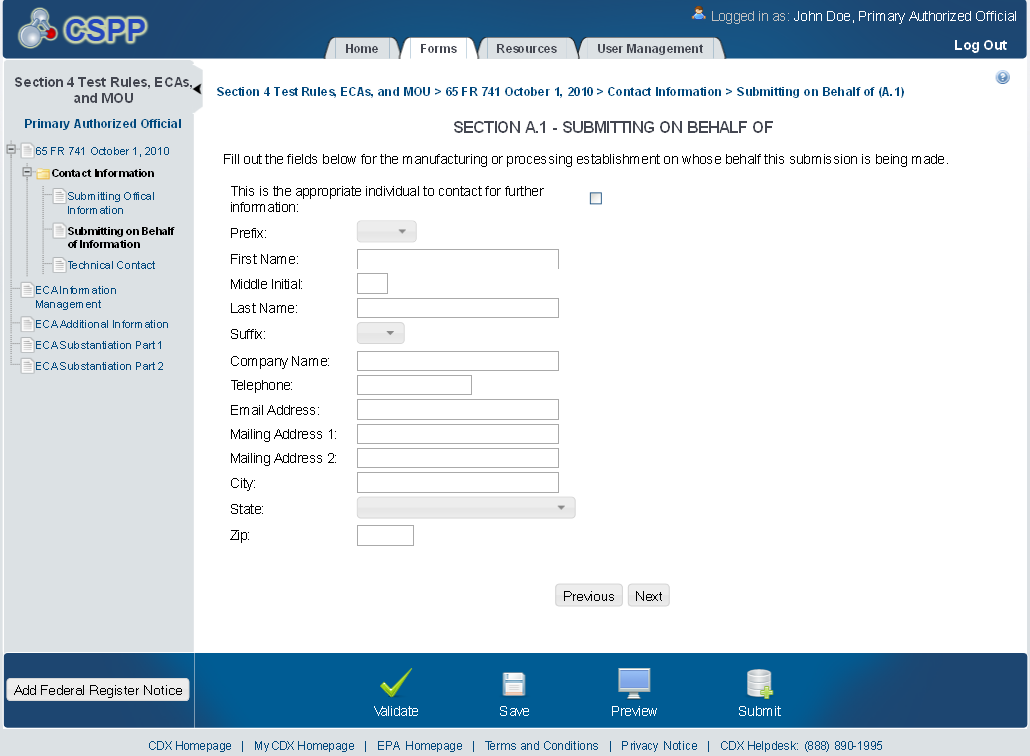
Submitting on Behalf of
The ‘Section A.1 – Submitting on Behalf of’ screen allows the user to submit forms on behalf of another company covered under the reporting requirement. The screen displays a list of text fields that allows the user to input contact information of the manufacturing or processing establishment on whose behalf the submission is made.
The following exhibit shows the screen capture for the ‘Submitting on Behalf of’ screen for ECA submissions:
Exhibit 1‑3 Submitting on Behalf of Screen





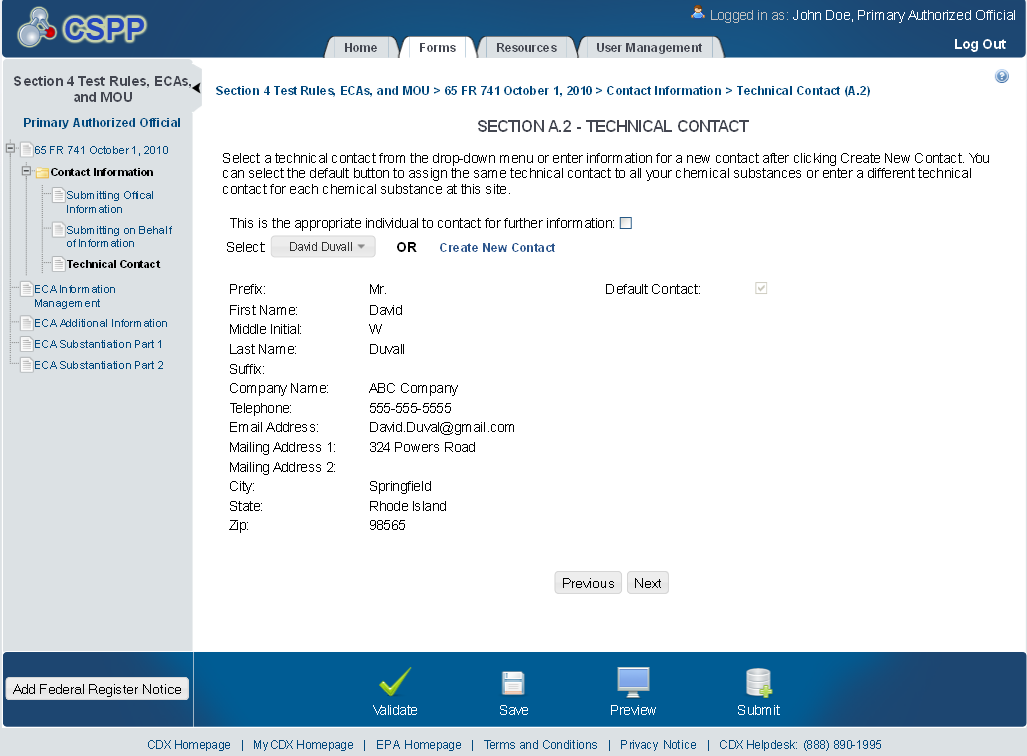
Technical Contact
The ‘Technical Contact’ screen allows the user to add multiple technical contacts, if needed. The system will provide a virtual rolodex address book dropdown menu listing all added technical contacts.
The following exhibit shows the screen capture for the ‘Technical Contact’ screen for ECA submissions:
Exhibit 1‑4 Technical Contact Screen

ECA Information Management
The ‘ECA Information Management’ screen allows the user to add information regarding their study plans, conduct of testing, and reports. The user may upload documents to supply information regarding reports, but must manually enter information for study plans and conduct of testing.
The following exhibits show the screen captures for the ‘Technical Contact’ screen for ECA submissions:
Exhibit 1‑5 ECA Information Management Screen
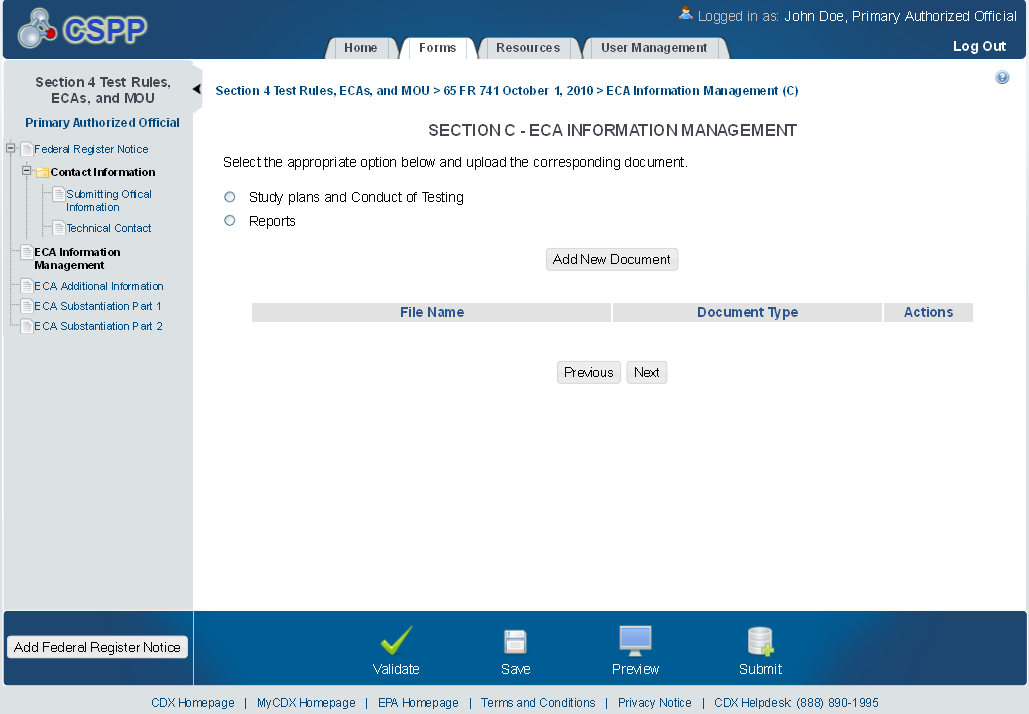
Exhibit 1‑6 Study Plans and Conduct of Testing – Scroll 1
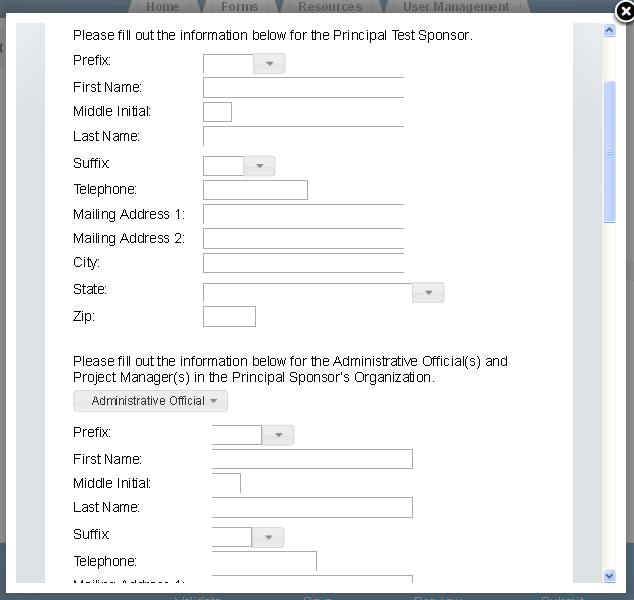







Exhibit 1‑7 Study Plans and Conduct of Testing – Scroll 2
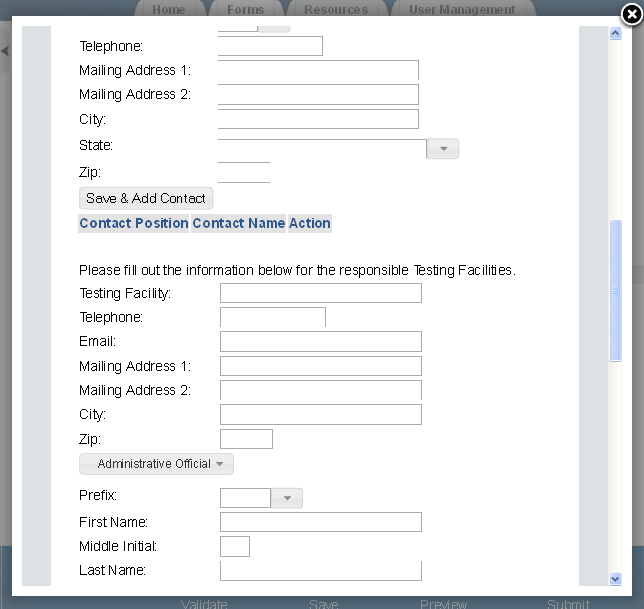




Exhibit 1‑8 Study Plans and Conduct of Testing – Scroll 3
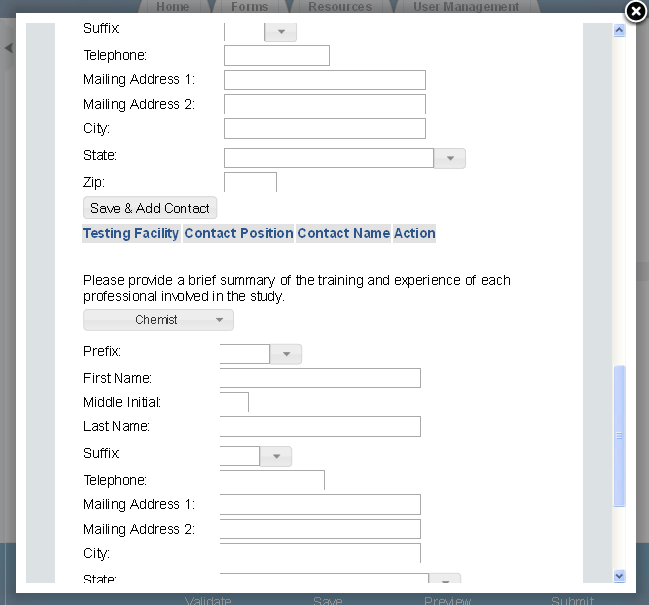






Exhibit 1‑9 Study Plans and Conduct of Testing – Scroll 4
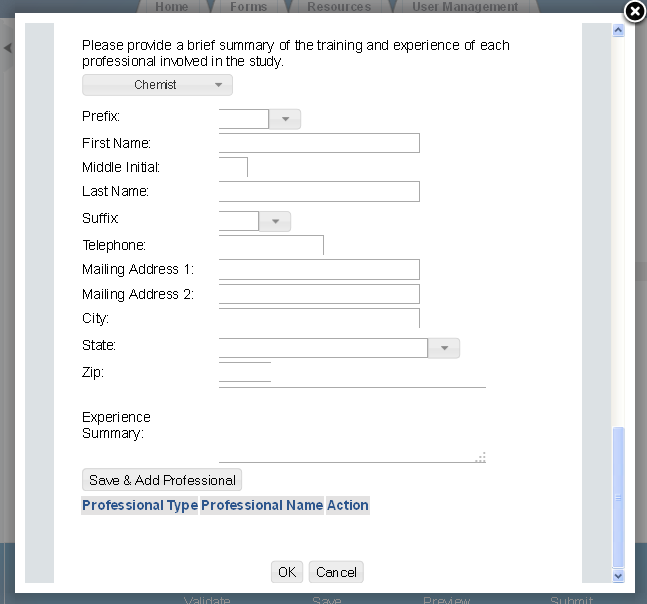




Exhibit 1‑10 ECA Information Management
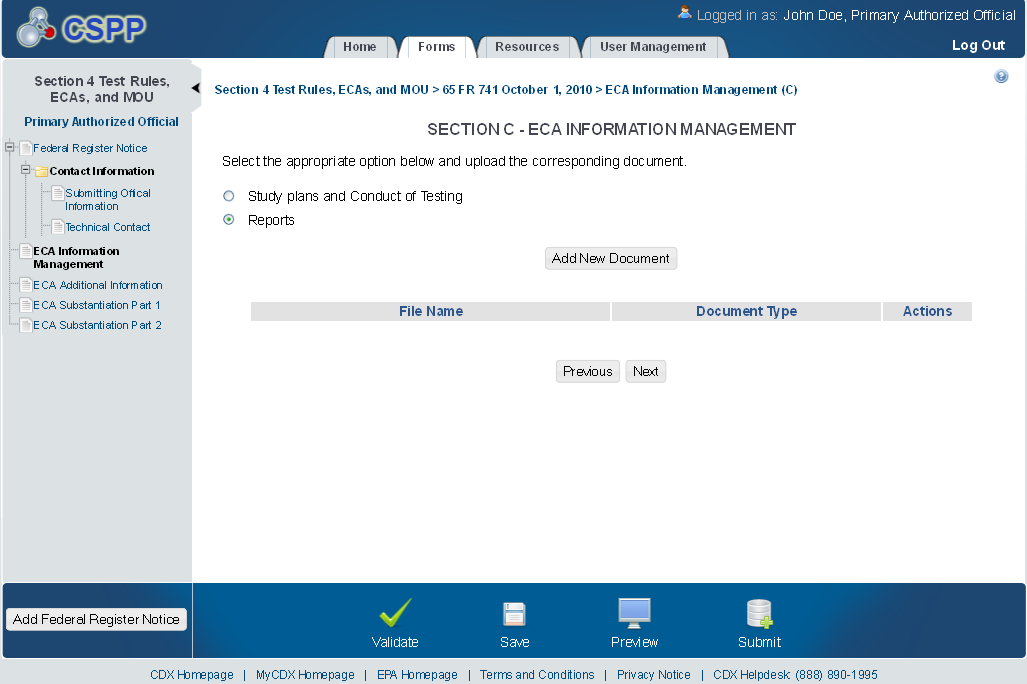
Exhibit 1‑11 ECA Reports Pop-Up
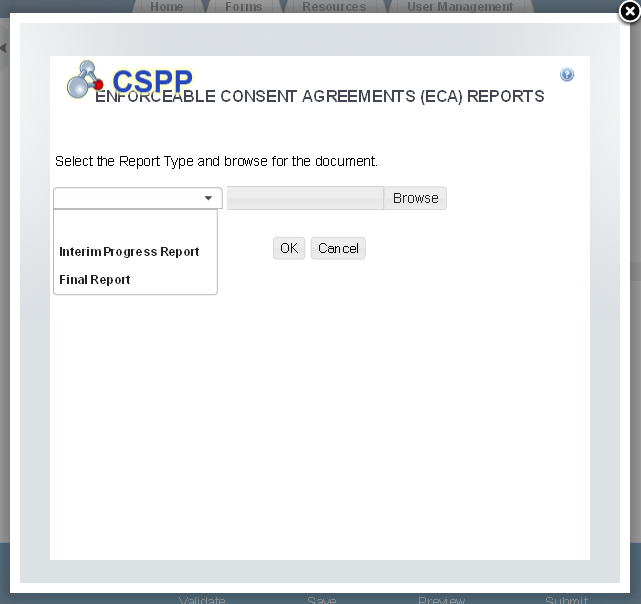
Exhibit 1‑12 ECA Information Management Screen
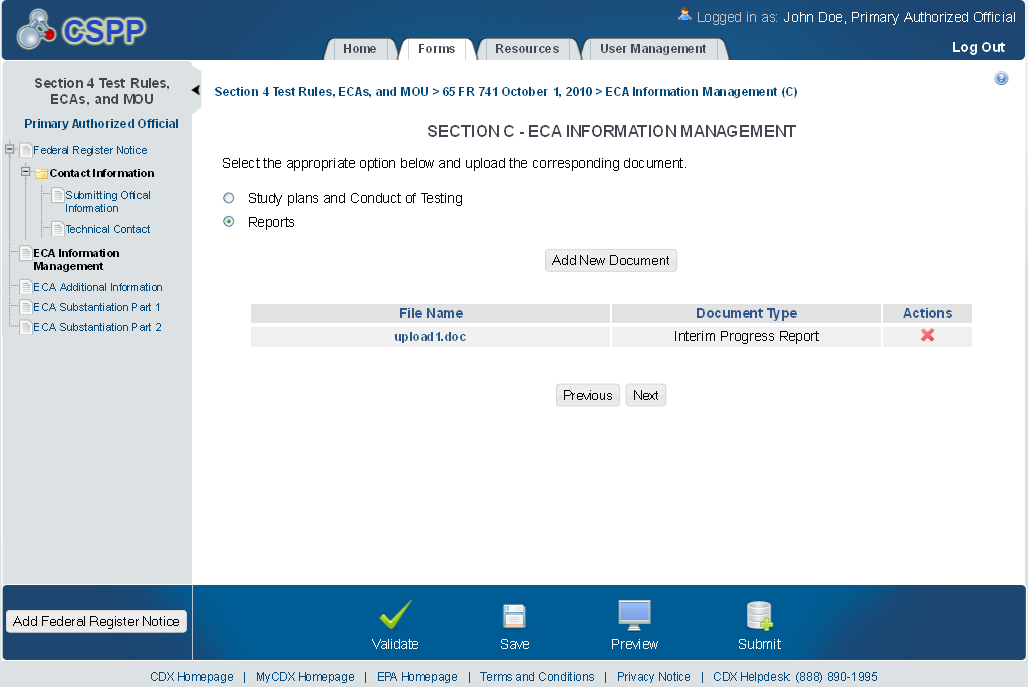
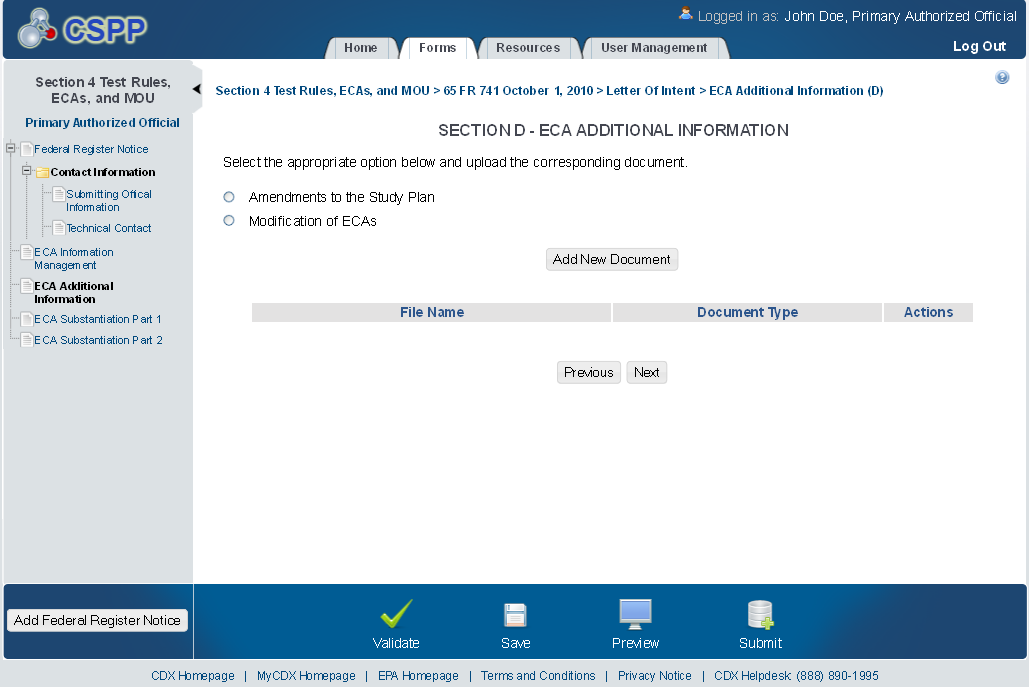
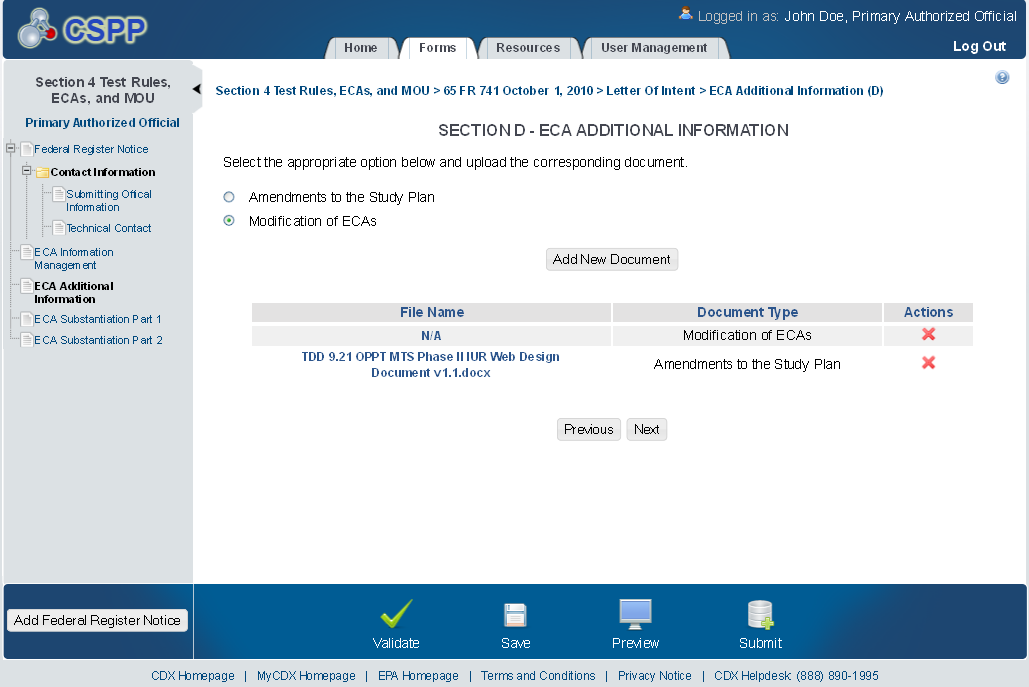
ECA Additional Information
The ‘ECA Additional Information Management’ screen allows the user to submit amendments to their study plans and modifications of ECAs. The user may upload documents to supply information regarding amendments to a study plan, but must manually enter information for modifications of ECAs.
The following exhibits show the screen captures for the ‘ECA Additional Information Management’ screen for ECA submissions:
Exhibit 1‑13 ECA Additional Information Screen
Exhibit
1‑14 Amendments to the
Study Plan Pop-Up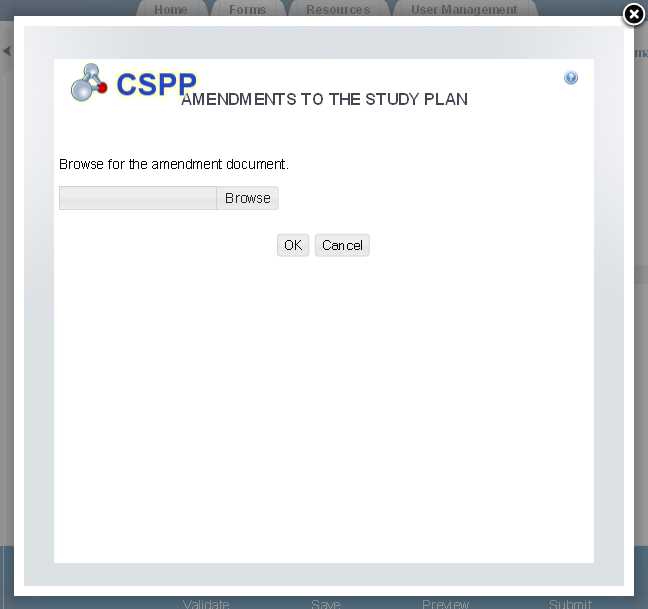
Exhibit 1‑15 ECA Additional Information Screen
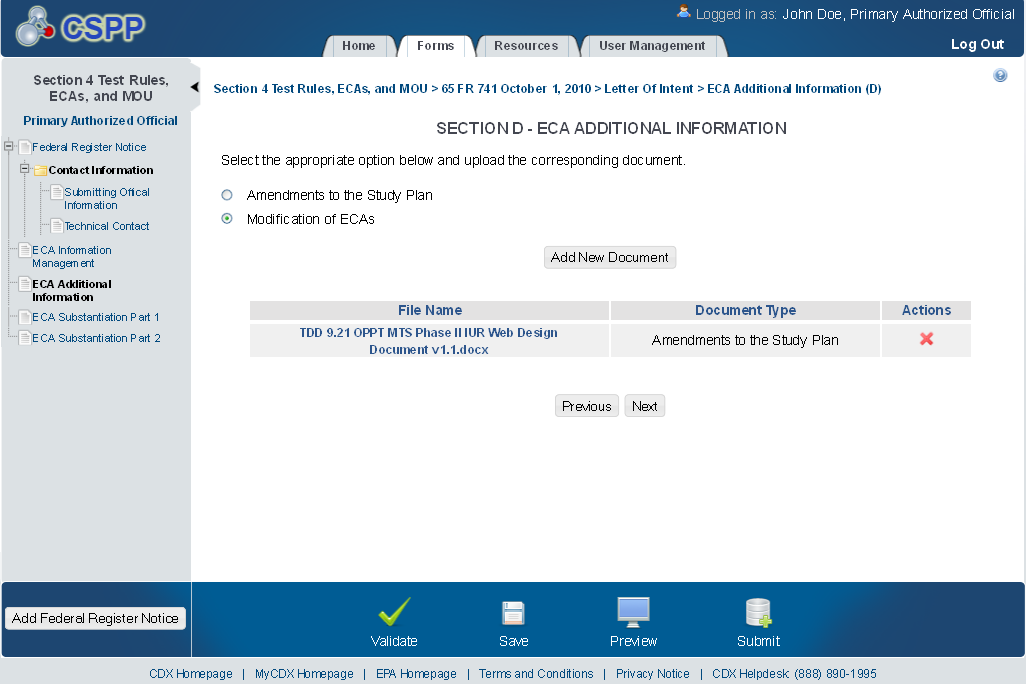
Exhibit 1‑16 Modification of ECAs Pop-Up
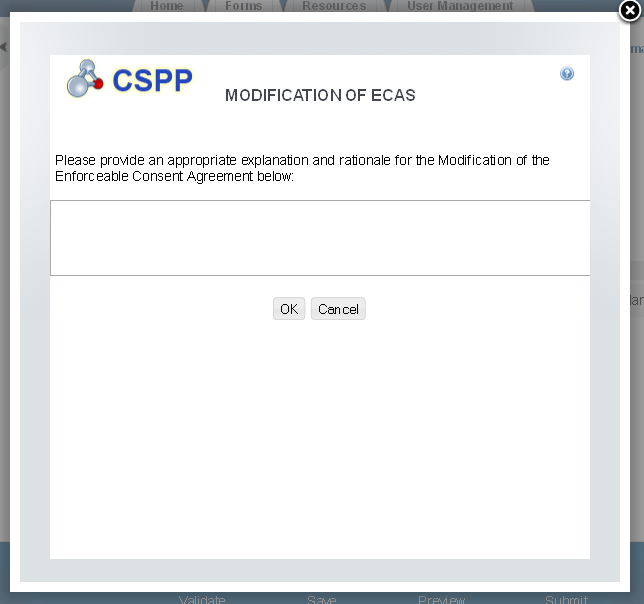
Exhibit 1‑17 Additional Information
| File Type | application/msword |
| File Title | CDX Memo Template v1.0 |
| Author | Stacy |
| Last Modified By | EPA |
| File Modified | 2012-04-12 |
| File Created | 2012-04-12 |
© 2026 OMB.report | Privacy Policy