Provider-Based Sampling Arm Supporting Statement B FINAL 20120817
Provider-Based Sampling Arm Supporting Statement B FINAL 20120817.docx
Provider-Based Sampling Feasibility Study for the Vanguard (Pilot) Study and Data Collection Updates for the National Children's Study (NICHD)
OMB: 0925-0593
REQUEST FOR OMB CLEARANCE
Provider-Based Sampling Feasibility Study for the Vanguard (Pilot) Study and Data Collection Updates for the National Children’s Study (NICHD)
Part B only
CDR Colleen O. Lee, MS, CRNP
National Children's Study
Eunice Kennedy Shriver National Institute for Child Health and Human Development
6100 Executive Boulevard
Room 3A01
Bethesda, MD 20892-7510
Phone 301-451-2135
E-mail: [email protected]
General e-mail inquiries: [email protected]
www.nationalchildrensstudy.gov
B. Collection of Information Employing Statistical Methods
B.1 Respondent Universe and Sampling Methods
Inclusion and Exclusion Criteria
Alternate Recruitment Substudy (ARS)
The fifteen study locations will participate in this further expansion of the sampling associated with the ARS. These are the same 15 study locations that participated in the already approved Phase 2 expanded data collection (see list in Supporting Statement Part A). These fifteen locations were initially selected from among all ARS study locations based on several criteria including readiness to collect environmental samples and biospecimens, physical measures, and the quality of prior data submissions, and number of participants. Within these fifteen locations, the following groups are eligible for inclusion as participants for the data collection updates to the ARS:
Pregnant women of the age of majority (typically, age 18) or older residing in a selected NCS geographic segment at the time of enrollment
Men of the age of majority (typically, age 18) named by enrolled women during the pre- to neonatal period as their baby’s father
Children born to enrolled women
Adult caregivers for enrolled children who have legal responsibility to authorize needed care for an enrolled child
Among ARS participants, we will ask enrolled women if we may contact the father of their baby to invite these fathers to participate in the study. We will then invite fathers of the age of majority to enroll in the study; these fathers will be enrolled and will complete the Father Interview.
Enrolled mothers will be asked to consent to enrolling their child in the study upon birth through the age of majority. Anyone unable to understand NCS participation and grant informed consent will be considered ineligible to participate. .
Provider-Based Sampling Feasibility Study
The target population is all pregnant women of the age of majority residing in one of the three selected PSUs with a first prenatal care visit or birth visit during the recruitment period. This includes the birth visit for women who had no prenatal care.
The following groups are eligible for inclusion as participants in the Provider-Based Sampling Feasibility Substudy:
Pregnant women of the age of majority (typically, age 18) or older residing in a selected NCS primary sampling unit (PSU; each PSU corresponds to a county) at the time of enrollment
Children born to enrolled women
Adult caregivers for enrolled children who have legal responsibility to authorize needed care for an enrolled child
The sampling frame will include two stratum (one for hospitals and birthing centers and one for prenatal care providers, in order to both a) test the approach to recuiting a birth cohort and b) ensure women without pre-natal care are included in the study. .A pregnant woman who is seen on her first prenatal visit for her current pregnancy at a selected prenatal care provider, or who presents at a selected hospital or birthing center will be eligible for recruitment. In this pilot, a Provider can be an individual (physician, midwife, nurse practitioner, or physician assistant), an office-based practice, or a facility, including hospitals and birthing centers. This will inform the Main Study in two ways: recruitment yields of women, and provider sampling frame coverage.
Women who received no prenatal care
Any sampling that is taking place at the prenatal care provider location would miss women who do not seek prenatal care until delivery. We suspect that these women have unique exposures and would like to include them in the Main Study. Recruiting women at delivery who indicate that they have not sought prenatal care gives the NCS the opportunity to determine the willingness of these women to participate in the NCS. We intend to sample these women by using a separate stratum to the sampling frame for hospitals, selecting the hospital locations as in the rest of the protocol (please see pages 7-8 of Supporting Statement A and pages 5-12 of Supporting Statement B), by measure of size, then using a targeted approach to identify women who indicate that they have not received prenatal care. The same instruments would be used on this cohort of women as the rest of the women enrolled, beginning with the Birth Visit.
Sampling Women Not on the Prenatal Care Provider Sampling Frame
We propose to use the same sampling method as above for the prenatal care cohort, and again, use the hospitals to perform a targeted screen for women with prenatal care from providers not on the prenatal care provider list frame. This should not be confused with the list of selected providers; rather, this is an evaluation of the entire list frame of providers, as a measure of frame coverage. This would serve to inform the Main Study to ensure that we enumerate, determine a measure of size, and determine stratification characteristics of all providers including hospitals and birthing centers. The same instruments would be used on this cohort of women as the rest of the women enrolled, beginning with the Birth Visit.
Hospital engagement and participant recruitment processes at the birth visit are explained fully on pages 14 –17 in Supporting Statement B.
Overview of Provider-Based Sampling Feasibility Study as an Arm of the Vanguard (Pilot) Study
The purpose of Provider-Based Sampling is to determine the acceptability, feasibility and cost of a Provider-Based Sampling strategy. We anticipate that Provider-Based Sampling, and associated recruitment methods will be less costly and more efficient than the other sampling and recruitment methods piloted in the Alternate Recruitment Substudy (ARS).
We will sample women in three stages. At the first stage, we will use geographically defined Primary Sampling Units (PSUs) with each PSU corresponding to one county. The second stage of sampling will select prenatal care and birth providers who treat women living in the selected PSU. To do so, field contractors in each PSU will compile an exhaustive list of provider locations that provide health care services to women residing in that PSU (for additional information on the development of the Sampling Frame, see pages 4-9) as one stratum and a list of hospitals and birthing centers that serve women residing in the PSU as a second stratum. The strata will be about equal in size. The resulting Sampling Frames will be provided to the NCS sampling and statistical support contractor who will, for each PSU, select a probability sample of provider or hospital locations, with probabilities proportionate to the estimated numbers of women seen for first prenatal care visits or births by the particular provider location. The sample will also be stratified by the type of provider, geographic location, and other information available for the provider locations. In the third stage of sampling, using a systematic sampling method, we will identify women at their first prenatal visit who reside in the sampled PSU and are eligible for enrollment in the NCS. We describe each stage in further detail below:
First Stage: Geographic-Based Primary Sampling Units
Second Stage: Provider-Based Secondary Sampling Units (SSU)
Third Stage: Sampling of women within a selected provider location
First Stage of Provider-Based Sampling: Primary Sampling Units
Three existing NCS counties (PSUs) were selected for implementation of the Provider-Based Sampling Feasibility Study. These counties, while geographically and demographically diverse, do not, and were not intended to, support generalizations to regions or to the broader U.S. target population. The two factors that drove the decision to include three field contractors were budget limitations and refining efficiency in operations. We anticipate that improvements in operational efficiency and adjustment of sampling criteria we can enroll the same number of women, about 1,200, in the same time frame from three locations as we previously did with ten who comprised the Provider-Based Recruitment arm of the ARS. The three field contractors and corresponding county locations are listed below in Table B.1.
Table B.1. Field Contractors and Locations for Provider-Based Sampling |
|
Field Contractor |
County Location |
Baylor College of Medicine |
Harris County, TX |
University of Louisville University of Massachusetts |
Jefferson County, KY Worcester County, MA |
|
|
Second Stage of Provider-Based Sampling: Prenatal Care and Birth Providers
The NCS ARS tested three recruitment strategies: Provider-Based Recruitment, Enhanced Household-Based Recruitment, and Two-Tier High-Intensity / Low-Intensity Recruitment. The underlying geographic multi-stage, sampling design was the same across the three recruitment strategies. Within each PSU, we selected a stratified random sample of geographic segments, called Secondary Sampling Units (SSUs), each designed to yield the same number of annual births. Eligibility for enrollment in the NCS was restricted to women who resided within the geographic boundaries of the SSUs.
During the recruitment phase of the ARS, we identified operational limitations with overlaying Provider-Based Recruitment on top of the SSUs. These limitations included the need to approach nearly all providers to seek their cooperation in allowing their patients’ addresses to be screened for geographic eligibility. Within provider offices, field contractors screened thousands of addresses to identify women living in households located in the relatively small SSUs (for additional information, see page 5 of Supporting Statement A). In PSUs with large numbers of health care providers, many of the providers had few patients that actually resided within one of the SSUs.
Provider-Based Sampling differs from this earlier approach in that geographic eligibility for women is based on residence in the PSU and not a SSU. This feasibility study informs the following questions:
Can a comprehensive list of prenatal care and birth providers who serve women residing in a selected county – inclusive of providers located both within and adjacent to the selected county – be constructed?
Can we associate a measure of size (MOS) with each of the providers on the list that accurately reflects the number of women who reside in the selected county receiving first prenatal care or birth services?
What percentage of providers selected from the list will agree to participate in the Study?
Can substitute providers be selected with similar characteristics (e.g., MOS, race/ethnic makeup, Medicaid usage) as original sample provider locations be identified as replacements for providers who decline participation?
Do the methods developed for sampling women at the selected provider locations work as operationally intended?
Do the three stages of sampling and subsequent recruitment methods yield the expected numbers of eligible women?
Does pre-screening of patients’ geographic eligibility (county residence) by provider office staff affect expected enrollment yields?
Are there differences in the efficiencies in recruitment between prenatal care providers and birth providers?
Are there differences in the quality and quantity of prenatal exposure data collected retrospectively versus prospectively?
Are there differences in the demographics of women enrolled at prenatal care provider locations versus birth providers?
Are there differences in the feasibility of collecting perinatal samples retrospectively versus prospectively?
Potential evaluation and assessment methods and plans to address these and other questions are discussed in Section B.4 below.
Construction of the Sampling Frame
In each selected PSU, we will construct a list frame of locations of prenatal care providers that will be known as the Sampling Frame. Hospitals and birthing centers will be included in the sampling frame in order to provide sample coverage for those women who do not receive prenatal care through a first visit with a selected provider. The resulting frame will have two strata - one for prenatal care providers and one for hospitals and birthing centers. We aim to have about the same number of women recruited from the two strata.
We considered various approaches to construction of this sampling frame and decided to include all provider locations that serve women residing in the PSU. As a result, some provider locations will be located outside of the PSU, but only women who reside in the PSU will be eligible for enrollment.
The three key steps in the construction of a PSU-specific sampling frame are:
Generating a list of all provider practice locations that provide prenatal and birthing services to women who reside in the sampled PSU.
Collecting information about the characteristics of each provider location guided by the use of sources such as publicly-available information, sources containing previously collected data, and the NCS Provider-Based Sampling Frame Questionnaire.
Compute the MOS based on data from the Provider-Based Sampling Frame Questionnaire and other data sources.
Variability in population size, numbers of providers, and state and local institutions across the three PSUs necessitated tailored strategies for creating the sampling frames. Differing population sizes result in vastly different numbers of providers, and each PSU has variable availability of standardized data sources, such as birth certificate data, to construct the frame. The methods of sampling frame construction employed by each PSU are described in detail below, and will allow the NCS to assess the feasibility, cost and sample coverage of the different approaches, which constitute a likely mosaic of circumstances that we could expect in the Main Study. The sections below describe the efforts planned or underway at each of the three PBS locations to accomplish step 1—-generating a list of provider locations.
Provider Location and Hospital or Birthing Center Frame
Each Study Center generates a list of provider locations that provide prenatal care services to women who reside in the sample county. The measure of size (MOS) and stratification data are collected from birth certificate data, from a provider location questionnaire, or from other data sources for each provider location on the list. To define the final provider location sampling frame, provider locations with MOS below a threshold that is determined for each location are dropped.
Two populations are not covered by the provider sampling frame: Women with no prenatal care and women with prenatal care only at locations not in the final provider location frame (the small MOS provider locations and provider locations missing from the original list). In order to give these women a chance for inclusion, a frame that includes the associated births will be constructed. To do so, a list of hospitals and birthing centers will be compiled. The list will include all hospitals and birthing centers where women who reside in the sample county go to have births. An MOS for each of these hospitals and birthing centers will need to be determined. Potentially, it could be based on an allocation of the estimated total number of no prenatal care births, the total MOS from all the small provider locations and an estimate of total MOS associated with provider locations not on the original list. In order to support a more stable understanding of the sampling and operational processes associated with recruiting hospitals and birthing centers, reviewing and sampling from lists of births, and contacting and recruiting women sampled from birth records into the NCS, the stratum of hospitals and birthing centers will likely need to be oversampled.
The two strata, prenatal care providers and hospitals or birthing centers, can be compared in many ways including efficiency of recruitment, the feasibility to collect perinatal samples, quantity and quality of prenatal exposure data, demographics of enrolled populations and retention rate.
Harris County, TX
Harris County, TX was able to gain access to electronic birth records and these records served as the first step in developing of the sampling frame. The contractor has an approved IRB protocol (IRB# 08‐22) with the Texas Department of State Health Services (TDSHS) for the access to and receipt of electronic birth record data for Harris County, including key birth attendant variables such as name and address of the attendant. Electronic records of all births to women residing in Harris County (HC) for the three most recent available years of data - 2008, 2009 and 2010 were accessed. HC has an average of 68,000 births per year and we expected to obtain a total of 204,000 birth certificates for the three years.
For each year, a list of birth attendants was compiled and collated into provider groups based on the practice address that was available from other data sources. Using the birth certificate data, the following was performed:
The percentage of birth attendants who consistently appear on birth certificates over the three years was evaluated to estimate the turnover of prenatal care providers in HC. This statistic will inform the extent to which the list fluctuates yearly and how frequently we will need to review new birth certificates for changes to the provider list.
The name and address of each birth attendant was examined for errors in spelling of the names and incorrect street locations. Note that we anticipate approximately 19,400 birth records will require cross-checking of information with alternate data sources (assuming an average of 68,000 births per year, and an anticipated rate of 10% error). See item 3 below for additional sources.
The list of birth attendants from birth certificates was augmented with additional lists of providers from multiple sources. Specifically, lists of providers and their office locations were derived from the telephone directory and internet listings, listings by insurance plan, hospital lists of providers with delivery privileges, the Texas Medical Board, academic departments including obstetrics, and medical societies. The complexity of this activity is demonstrated by one example where we identified 7,000 obstetric providers (and their addresses) who are members of Blue Cross Blue Shield insurance plan in HC, where the same provider is mentioned more than once when he/she has more than one location of her/his obstetric practice. We mapped the various locations of the different practices of each obstetrician and quickly recognized the vast distances of the offices, the proximity of one office among a number of offices of a provider to the Texas Medical Center in downtown Houston, and the diversity of patients based on previous analysis of geocoded birth certificate data that might be provided care at each office location. This example illustrates information from a data source for provider addresses that can be linked to the birth certificate and that can link each provider with other members of his/her practice location. Note that this process can be used for different types of providers including family practice, midwives, and others providers of prenatal care to women in HC. Furthermore, we might consider utilizing the medical societies in Harris County to compile additional information as for example on the age of the obstetrician, a potential indicator that the provider might switch from obstetrics to gynecology only and therefore no longer included in the pool of providers for the pilot.
With multiple data sources such as the internet and phone book, the providers were clustered by office address into providers who share the same practice at the same office location.
Lists were then cross‐referenced to determine the percentages of provider offices in each list and the extent of overlap of listings of providers and their offices within each sampling frame.
Each provider office address was geocoded and the provider groups were mapped onto a HC grid.
Using data from the electronic birth records, estimates of provider office characteristics were calculated, including: births per year, maternal characteristics, infant characteristics, geographic area within HC. These characteristics will be helpful to identify and establish variables for stratification and estimate the numbers of births per provider office per year within each sampling frame of provider offices if a multi‐frame approach is taken. We recognize that we want to have an ample number of providers per stratum for replacement of refusals by other provider offices of similar characteristics and numbers of births.
The hospitals that these practices deliver in were enumerated. It is common for practices to deliver almost entirely in one hospital.
Further investigation of various scenarios that exist in HC regarding birth attendants was necessary. It is common in HC teaching hospitals for residents/fellows to sign off on the birth certificate rather than the obstetrician/prenatal care provider. To understand the possible coding errors associated with the birth attendant portion of the birth certificate, we met with appropriate representatives of these delivery hospitals to informally discuss their practices and approaches to link the resident/fellow to the supervising obstetrician/prenatal care provider office. Several practices also provide cross‐coverage. Following OMB review and approval of the Provider-Based Sampling Feasibility Study, we will identify these practices via a telephone survey to office managers and formulate a plan for how best to account for this.
There is a subset of women who receive prenatal care outside of HC and deliver in HC. We identified those providers in the surrounding counties from the electronic birth certificates in HC and included them in the list.
Jefferson County, KY
To construct the provider list frame, all OB/GYN practices, individual physicians, and other providers of prenatal care (midwifes, nurse practitioners, and physician assistants) were enumerated utilizing known data sources, including state boards of licensure, local medical association membership lists, telephone listings, and other public records. Our preliminary review indicates that there are fewer than 20 separate practices located in Jefferson County. Some of those practices have 2-4 locations. The largest practice is University Health Care. Most of the private practice groups have 6-12 MDs per group. There are only a few that appear to be practicing alone. In addition to the University and private practice groups, five Family Health Centers managed by the Metro Louisville government, also provide prenatal care.
In order to collect information about provider practices to allow sampling proportional to the number of deliveries as well as for the formation of strata, each practice will be interviewed by Jefferson County NCS staff to learn the number of Jefferson County women currently receiving prenatal care and the total number of deliveries in the previous 12-month period. In addition, data on the geographic locations of provider offices and demographic information on the geographic areas served will be assembled for the purpose of informing the sampling contractor who will select the practice locations.
Worcester County, MA
The contractor for Worcester County will use a three-pronged approach to identifying provider practices. The initial stage will be the identification of prenatal care providers through hospitals. Currently, nearly 86% of Worcester County women receive prenatal care that is almost exclusively provided by board-certified OB/GYNs and family practitioners affiliated with Worcester County hospitals (http://www.mass.gov/eohhs/researcher/community-health/masschip/custom-reports.html. Accordingly, our primary strategy for listing all prenatal care providers in our county is to engage these birthing hospitals to collect information about the prenatal care providers of women who deliver in their hospitals. We can anticipate a high level of success from this strategy because: 1) 41% of county deliveries occur at UMass Memorial Health Care (UMMHC) where the NCS Principal Investigator is Chair of the Department of Pediatrics and Physician-in-Chief of the UMass Memorial Children’s Medical Center (the only tertiary care facility for children in Central New England) and we have access to complete internal data; 2) 43% of county deliveries occur in five other area hospitals with maternity services where we have existing contacts and relationships, including CEO membership on our NCS Community Advisory Board; and 3) UMMHC operates the only Level III Neonatal Intensive Care Unit in Central New England and provides quarterly outreach education and chart review at every hospital that has maternity services in the county. Specific activities for hospital-based identification of prenatal care providers will include:
Accessing internal data from UMass Memorial Health Care (UMMHC) to list affiliated providers.
Accessing data from other Worcester County birthing hospitals with a high percentage of county deliveries: St. Vincent's Hospital at the Worcester Medical Center (17%); Health Alliance Hospital (11%); Milford Regional Medical Center (6%); Heywood Hospital (5%); and Harrington Memorial Hospital (4%), by:
Involving UMMHC neonatologists and High Risk OB physicians and their contacts at every birthing hospital in the county to help identify prenatal care providers
Contacting leadership at each hospital by phone, email, and in-person meetings to acquire information about affiliated prenatal practices and providers
Utilizing current hospital-affiliated NCS Community Advisory Board (CAB) members to access and provide information (senior leadership from St. Vincent's Hospital at the Worcester Medical Center and its affiliated practices; Health Alliance Hospital CEO; and Milford Regional Medical Center CEO)
Utilizing Community Outreach contacts and partnerships with Health Alliance, Heywood and Harrington Hospitals to access local provider information
Accessing hospital birth certificate data as appropriate to identify Worcester County prenatal care providers
Utilizing existing hospital-based venues like leadership groups, executive committee meetings, grand rounds and departmental meetings. Announcements will be made to providers notifying them and their associates of the NCS and about potential future contact from NCS staff regarding provider-based recruitment and associated needed information.
The second tier of provider identification relies on community-level information. To list additional prenatal care providers that may not be readily identified by local hospitals or who provide prenatal care for women that do not deliver in Worcester County birthing hospitals (e.g. lay midwife practices, individual family physicians, practices in Worcester County that are affiliated with hospitals outside of Worcester County), we will:
Form a subcommittee of our Community Advisory Board to focus on Provider-Based Recruitment strategies and contacts.
Seek information about prenatal care providers from individuals and groups currently engaged via our NCS community outreach activities
Gather information via phone, email and in-person contacts with community individuals and groups, including:
Membership of local Community Health Networks
Community leaders and opinion leaders
Professional organizations and networks including Worcester District Medical Society (WDMS), MA-ACOG and other groups
Community-based organizations and groups
Parenting–focused businesses and organizations (e.g. Mothers & Company; Childbirth Education-Holistic Practitioner Group/midwives; WIC; etc.)
The third approach to developing the list frame is access public information to identify any other providers of prenatal care that were not already listed. To do so, we will also access any information that is publicly available about prenatal health care providers in Worcester County, for example, from Internet sites (e.g., WebMD; Mass. Department of Public Health; MassCHIP; massresources.org, MassACOG, websites related to home birth and midwifery care etc.), phone book listings, health insurance provider lists, local parent resource guides and local or state medical society listings.
Finally, information from the recent acquisition of a birth certificates record file from Massachusetts Vital Statistics did not allow for the review of the provider location list in terms of coverage, estimated measure of size, and estimated characteristics of provider locations. Most of information will be determined through centralized hospital databases that have counts of first prenatal visits, prenatal provider name, and street addresses. The remainder of the information will be obtained through responses to the frame questionnaire.
Stratified Probability Sampling of the Provider Locations
Once we construct the Sampling Frame for each PSU, we will construct two strata- one for prenatal care providers and one for hospitals and birthing centers. We will select a subset of provider locations using a stratified Probability Proportional to Size (PPS) sampling design. The variables used for stratifying and sorting the provider practice locations will come from the Provider-Based Sampling Frame Questionnaire (e.g., method of payment, race, ethnicity, language spoken, age) and geocoding information. As described above, this information will also be available from birth records for Harris County, TX and possibly, Worcester County, MA. The MOS for providers will be the estimated number of first prenatal care visits from women who reside in the sampled PSU. To avoid highly inefficient operational situations, we will likely determine a lower bound threshold for the MOS for a provider practice location, below which the location would not be eligible for selection. The small sample of hospitals and birthing centers will be selected purposively to give a range of types (e.g., by size). A higher threshold for the prenatal provider practice locations will be used to identify women eligible for the hospital and birthing cohort in order to enroll a sufficient number of women without prenatal care and women who received prenatal care from a provider location not on the sampling frame or that falls below the higher threshold. This activity will enable the NCS to collect a sufficient number of informative events to advise data collection in the design of the Main Study.
In some cases, provider practice location-level information for a multiple-location practice may be unavailable and only practice-level information will be reflected on a single record on the frame. If such a practice is selected, we will employ an additional stage of sample selection to randomly select one (or more) of the practice locations.
The number of provider practice locations that will be included in the development of the subset determined by the stratification procedure will be selected depending on several factors, such as the total sample size, the expected number of women to be selected from each sample location, the number of locations on the frame and the distribution of the MOS across all the locations on the frame. Since the number of locations and the distribution of the MOS across the locations will likely vary among the PSUs, the number of provider practice locations that will be included in the development of the subset determined by the stratification procedure will also vary among the PSUs. That said, we estimate the selection of approximately 60 provider locations over the 3 PSUs.
Provider Location Recruitment
The field contractors will contact each provider location that was selected after the stratification procedure is completed to gain its cooperation in participating in Provider-Based Sampling Feasibility Study. In order to maintain the desired sample size yield, a provider location that declines to participate in the study will be replaced by a substitute provider location that matches the declining location on various characteristics such as, type of practice (public clinic, private provider), size of practice, and geographic location. We will then activate participant recruitment in the substitute location.
Sampled provider and hospital or birthing center locations will be approached to participate using a variety of methods. Recruitment will primarily be done via phone and in-person visits to discuss their participation in the Study. It may take several contacts to secure participation, as field contractors will need to secure cooperation from the medical staff and administrative staff. In the event of a refusal, field contractors may engage NCS staff to assist with refusal conversion. In the event that the field contractor cannot engage the provider location after a reasonable number of attempts determined in consultation with the NCS Program Office, or the provider gives a final hard refusal, a substitute provider location will be selected. Only one substitution will be made for each originally sampled provider location. Substitute provider locations will be approached in the same manner as an originally sampled provider location.
Field contractors will be provided with the tools and knowledge necessary in order to engage each provider location and to successfully negotiate the provider location’s role in the recruitment of women into the NCS. Provider engagement experts consulted by the NCS have put together a battery of questions and information that may be useful when engaging providers. These were put together into a form for the field contractors to utilize during their provider engagement (please see the attached Checklist for Negotiating Logistics with Providers for the NCS.docx). For each selected provider location, the sampling and statistical support contractor will provide the field contractor with a table similar to the ones found in the attached Appendix 1 – Methods for Sampling Women within Selected Providers.xlsx. These tables will be used to aid the study locations in determining the best method(s) for selecting women in a particular provider location. These tables are not intended to be shown to staff at the provider locations; rather, they are intended for NCS internal use only. In an effort to better demonstrate to the study locations how the information in these tables would be used, a memo was issued by the NCS Field Support Office (please see the attached PBS-FSM 2012-04 Patient Recruitment Methods and Scenarios.docx) that gave several example scenarios of how to engage different types of provider locations. Because we anticipate nonresponse from some provider locations, a protocol to invite a substitute sampled provider location to participate was introduced (please see the attached PBS-SDM 2012-05 Provider Substitution Guidelines.docx). These substitute provider locations will have similar characteristics as the nonresponding provider location they are replacing. A form was created for use by field contractor staff to keep track of all relevant information regarding the replacement provider location and the provider location being replaced (please see the attached NCS-FS_PBS Provider Substitution Form_2012-07-06.pdf). Study locations will be required to submit one of these forms for each provider location substitution. Each case will be analyzed to ensure that the substitution is warranted.
The Provider-Based Sampling Frame Questionnaire is a brief instrument that will be completed by staff at each of the prenatal care provider or hospital and birthing center locations. Data collection for the Provider-Based Sampling Frame Questionnaire can be done over the telephone, in person, or as a self-administered questionnaire returned via mail, fax, or email. The Provider-Based Sampling Frame Questionnaire will be used in two different ways. Some study locations will use the Provider-Based Sampling Frame Questionnaire to collect data from all providers serving the county to determine the number of pregnant patients they see. Other locations have used local birth certificate data to estimate the number of births at each location, and will use the Provider-Based Sampling Frame Questionnaire with selected providers to validate the estimates derived from birth certificates. For more information on the role of the Provider-Based Sampling Frame Questionnaire in establishing MOS and constructing the sampling frame, please see pages 7-10 of Supporting Statement A.
In most cases, data collection for the Provider-Based Sampling Frame Questionnaire will begin with a mailing of the questionnaire, followed up by a phone call if the provider location does not return the completed questionnaire. In-person visits will be made to non-responding providers. However, data collection procedures for the Provider-Based Sampling Frame Questionnaire can be tailored based on the purpose of the data collection. Study locations with a smaller number of providers may opt to do all data collection in-person. Also, Study locations may complete the Provider-Based Sampling Frame Questionnaire in-person with provider locations sampled for the Study as part of the process of recruiting the provider location.
Additionally, if a selected provider location is closed, efforts will be made to determine if the practice has re-located to a new location previously not on the frame. If so, the provider at the new location will be recruited to participate.
Third Stage of Provider Based Sampling: Sampling of Women within a Selected Provider or Hospital and Birthing Center Location
Design Parameters
To avoid multiple chances of selection, we will sample women at their first prenatal visit to the provider locations on the sampling frame. In this context, we define the first prenatal visit as the first visit to a provider location listed in the sampling frame. Visits to provider locations not on the sampling frame are not classified as “first prenatal visits.”
For planning purposes the target is 250 births in each PSU. We expect that about 1 in 8 women screened will meet the eligibility criteria as stated in Inclusion and Exclusion Criteria on page 2. Based on our experience with Provider-Based Recruitment, we can expect that 80% of consented/enrolled women will be retained through to the birth and that 80% of sampled eligible women will agree to consent/enroll into the NCS. So, this means about 315 (250/0.8) women will need to be consented/enrolled in each PSU and that about 400 (315/0.8) eligible women will need to be identified through the application of the eligibility screening process in each PSU. The last figure of 400 eligible women is used in the calculation to determine the overall sampling rate needed in each PSU.
In each PSU, we expect that that between 15 and 25 provider locations will be selected. The actual number sampled in each PSU will be dependent on the distribution of the provider location MOS in each PSU’s frame. And in a complementary sense, the average number of eligible women sampled from each of the selected provider offices will roughly be between 15 and 25.
For example, if 20 provider locations are to be randomly sampled, then the aim would be to sample about 20 eligible women from each location (in practice, the number selected from a location will differ from 20 because of imprecision in the MOS). In reality, some very large provider locations may be included with certainty and more than 20 eligible women would be expected to be selected from these locations. Current plans are to enroll the sample of women up to a four-month period. The sampling rates for sampling women within the selected provider locations will be computed based on this assumption.
We considered
several statistically valid methods for selection of eligible women
within a selected provider location. Operational considerations at
the provider location level and by the field contractor staff will
need to be taken into account in determining plausible procedures.
Our general approach is to aim for an equal probability sample of
eligible women over the two stages of sampling within the PSU. The
selection probability of a woman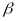 in provider location
in provider location
![]() is
given by:
is
given by:

where
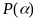 is
the PPS selection probability for location
is
the PPS selection probability for location and
and
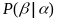 is
the rate for sampling women at that location. For an equal
probability of selection for each woman,
is
the rate for sampling women at that location. For an equal
probability of selection for each woman,
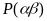 is a
constant, say
is a
constant, say Thus, the sampling rate for that location is given by
Thus, the sampling rate for that location is given by
 Application of this rate will yield an equal probability sample, but
the sample size will vary by location depending on accuracy of the
measures of size used in selecting the locations.
Application of this rate will yield an equal probability sample, but
the sample size will vary by location depending on accuracy of the
measures of size used in selecting the locations.
The procedures for the identification, screening and enrollment of pregnant women can be accomplished several ways. Some possible methods are listed:
One in every 4 women on a continuously updated list of women kept by the provider location staff. Possibly, where every 4th line is identified as the sampled women;
One in every 4th day is selected for the provider location staff to maintain a list of all women coming on the selected days;
Every other week is selected for the provider location staff to maintain a list of all women coming in the selected weeks. Every other woman on the list is selected;
One in every 4th week is selected for the provider location staff to maintain a list of all women coming in the selected weeks;
One of the 4 months is selected for the provider location staff to maintain a list of all women coming in that month.
Note that for methods 1-4, the general expectation is that on average about 5 eligible women would be sampled each month over the 4-month period. For method 5, all eligible women would be sampled in the randomly selected month, with an expectation of 20 women sampled in that month.
Different methods can be employed across the sampled providers within each location. The choice may be influenced by the particular provider location’s logistics, operations, and staff willingness to support the study. However, there can be some benefits derived from coordinating the sampling methods employed across the sampled provider locations in terms of making the overall process more efficient for the staff at the provider location. For example, if some of the provider locations employed a method based on time periods, the sampled time periods could be coordinated to selected non-overlapping time periods for the different locations to the extent possible, thus spreading the workload for the field contract staff as smoothly as possible over the enrollment period.
Hospital Engagement and Participant Recruitment Processes at the Birth Visit
Summary of prior experience from ARS informing PBS
One of the major challenges in the Provider-Based Recruitment (PBR) is engaging the provider locations themselves to participate. The PBR method in the ARS revealed that it takes significant time and labor to engage the provider locations, averaging approximately 6 months. While Study Centers were able to successfully engage providers (to allow for the collection of birth specimens), these efforts were time-consuming and complicated by the original NCS sample design, where any provider or hospital may only serve a handful of NCS-eligible women.
To gain provider cooperation, PBR study locations first needed to identify the key personnel, decision makers and gatekeepers in each provider office. Once determined, staff introduced the NCS, negotiated cooperation, and sought all necessary local approvals (IRB, etc.). Though time- and resource-intensive, Study locations were successfully able to engage providers to recruit women and hospitals/birthing centers to allow for the collection of birth specimens.
The PBS effort will build on what was learned during the PBR arm of the ARS, while increasing efficiency of recruitment through changes in the sample design. Field contractors who participated in the PBR sought to enroll women who met specific geographic eligibility criteria, specifically residence within the secondary sampling unit or segment. This constraint caused difficulty in non-rural areas with large numbers of providers, as the likelihood of a given provider treating a NCS-eligible woman was low. Similar difficulties were experienced while engaging hospital and birthing centers, as the location of birth was not known until each woman was recruited, leading to substantial effort by staff and hospital personnel to support NCS participants, whose numbers were initially unknown. The PBS effort, in contrast, is intended by design to be more efficient, as each selected provider location or hospital will support a known number of NCS participants.
Approaching Birth Centers and Hospitals
The current PBS design is intended to select providers based on their rates of service to eligible women. Hospital and birthing centers are represented to ensure coverage of women who receive no prenatal care or received care from a provider not included in the overall list frame. Therefore, selection of hospitals will occur to ensure adequate coverage of the sampled population. However, women who receive no prenatal care are likely to be quite different than those who are treated earlier in pregnancy. In addition to any demographic differences between these groups, women first identified at or immediately after giving birth will require a different approach for enrollment than those identified earlier in pregnancy. Given these factors, the process of recruiting women at hospitals or birthing centers will be distinct from those recruited at other provider locations.
One critical difference is the timing of enrollment. The clinic/provider model assumes recruitment, enrollment, administration of informed consent and the conduct of initial study assessments during pregnancy. The hospital/birthing center approach does not allow for prenatal assessments, and informed consent generally cannot be anticipated and administered until post-delivery. Some women have several visits to the hospital during the perinatal period and it is possible that such women may be offered enrollment prior to delivery. Once enrolled, however, there is no variation in the experience of participants enrolled in clinics and office practices with those enrolled at hospitals and birthing centers.
Ensuring comprehensive identification of eligible women at hospitals will also require a different approach. To avoid a pregnant woman having more than one chance at enrollment, women who give birth at a facility will be screened through admission records or asked if they receive care from selected listed providers from the other stratum. Only women who do not receive care from the listed providers will be offered enrollment during the hospital visit. If the selection of each woman relies on hospital admission information and initial contact with her cannot occur until after delivery, we risk missing women who are unavailable at the hospital as the result of early discharge or birth complications. NCS must take such situations into account to limit possible bias in the sample.
Recruitment of participants at the birth visit: how and when and by whom is it conducted?
The NCS anticipates some variation in how participants are identified and recruited at hospitals and birthing centers, based on institutional characteristics – such as size or affiliation, or demographic characteristics such as urbanicity. Variation by field contractor is anticipated as resulting from differential staffing models, resources and levels of effort assigned to the task.
Field contract staff will contact selected hospitals to understand their local procedures and determine how to best conduct study activities within these constraints. Some examples are provided below.
Selection of eligible women: Potential participants who meet eligibility criteria (described earlier in B.1) will be identified based on a systematic selection process, such as every nth delivery on a specific day of the week. Ongoing contact between field contractor and hospital staff will be required to review admission logs to identify recent deliveries (e.g., within the past 24 hours). Some hospitals or birthing centers may have pre-admission records that could be accessed to identify eligible women prior to delivery.
Possible specimen collection: If implemented during the PBS, field contractors will need to negotiate an agreement with selected hospitals and birthing centers to preserve critical birth samples for some set period of time after delivery.
Contacting eligible women: Based on the determined selection routine, eligible women will be identified and contacted by field contractor staff prior to discharge from the hospital or birthing center. At this time, NCS staff will introduce the Study to the woman and her family (as applicable) and invite her to enroll.
Initial Administration of Informed Consent: Women will generally be enrolled/consented post-delivery. After answering any questions, a NCS staff member will administer the informed consent document and obtain the participant’s written documentation of consent. The consent form will include all activities completed at the hospital and birthing center, including the collection of birth specimens (saved by hospital staff); chart abstraction; and initial birth interview; and physical assessments and specimens from the infant. Any woman who is not able to provide consent due to birth complications will be contacted once her health improves. The NCS will never attempt to administer consent to women who are cognitively unable to provide an informed response. Participation in specimen collection activities is not required for enrollment in the NCS. Note that the NCS is not planning for sample collection at this time but this type of collection is described in the consent form to facilitate later collection if we request and receive appropriate regulatory approval. Detailed contact information on the participant will be collected to facilitate follow-up visits.
Administration of Consent for Continued Participation: At the beginning of each Study visit, all participants are read a visit information script describing that voluntary nature of the Study and reminding participants that they can skip any data collection activity. At visits involving specimen collection, participants are provided with a hard copy visit information sheet (VIS) describing the collections offered and provided a hard copy visit information sheet describing procedures for collection of specimens and any associated risks. The VIS reminds participants that participation in Study data collections is voluntary and they can choose not to participate (or not have their child participate) in any data collection. Participants provide their verbal consent or dissent for the data collection activities at each Study visit. Regardless of their previous consent preferences, participants can choose to decline participation on their own behalf (or for their child) for any specimen collection or data collection at any time during the visit and are asked if they choose to participate in the sample collection prior to the start of the sample collection activity. Participants who previously declined to provide consent for biospecimen and/or environmental sample collection, as well as the use of biospecimens for genetic testing, will have the opportunity to reconsider their original decision (on their own behalf and on their child’s behalf if they are the legally authorized representative) if a given sample collection is re-introduced at a later date in the study visit sequence.
Process evaluation specific to hospital engagement: how are we evaluating this?
Field contractors are asked to collect information that will allow the NCS to understand the process of and resources required to engage hospitals and birthing centers. Each metric will be examined by available stratification variables, such as urbanicity, size, academic affiliation and others. Specifically, we seek to understand the level of effort and steps required to identify the key decision makers and gatekeepers; and the various types of negotiations and agreements required by hospitals. We are also interested in what is required to engage nursing staff, admissions, or other critical hospital personnel who may restrict access to potential participants. A key metric of interest is the participation rates of hospitals and birthing centers. If the NCS submits and receives approval to collect birth specimens we will also track availability of enrolled women’s birth specimens by hospitals. Data to conduct these analyses are being collected by field contractors and recorded in the NCS informatics systems. Detailed documentation and training on how to correctly implement code frames will be provided to all field contractors. These data represent operational and logistical processes and do not require systematic information collection from the public.
Process evaluation specific to participant recruitment via hospitals: how are we evaluating this?
Participant recruitment at hospitals will be evaluated on multiple levels. For example, the issue of sample coverage will be examined. The PBS study locations are taking two different approaches to provider frame construction. One location (Harris County, TX) is using birth certificate data to identify all prenatal care providers and estimate the number of deliveries (and therefore, potential pregnant patients) from each provider location. The other two study locations are building a list of prenatal care providers in the area, and then administering a questionnaire to determine the measure of size (number of prenatal patients per year) and potential stratification variables. The selection of hospitals and birthing centers may potentially vary by method.
Participant enrollment will be further evaluated by these metrics. These include but are not limited to (1) participation rates of women contacted for enrollment peri-natally; (2) ease of access to potential participants to describe the Study and attempt enrollment; (3) the percent of women unavailable due to birth complications; and (4) the percent of women unavailable due to early discharge from hospital. These data represent operational and logistical processes and do not require systematic information collection from the public.
Link to Main Study
The NCS is proposing a Main Study design that relies on identification of eligible women at multiple locations, including hospitals or birthing centers; and prenatal care provider offices. The original PBS design heavily focused on the latter, building on the lessons learned during the Provider-Based Recruitment arm of the ARS, while extending that protocol to a probability sample of providers. To better inform the methods for the Main Study, the NCS seeks a broader understanding of both sampling and operational issues related to working with the full range of provider institutions to identify and enroll participants. To best leverage the PBS experience to inform the Main Study, we need to understand the operational and logistical variations across types of providers and provider institutions, and any associated costs. To do so, we are documenting the amount of staff labor, other resources, and required length of time to recruit provider locations – including hospitals and birthing centers – and complete operational negotiations. Having accurate projections of these processes and their associated timelines will be critical in planning for the Main Study. Additionally, we will track provider, institution, and participant cooperation rates, to help us understand what is required to engage hospitals and providers to recruit women into a cohort study, and what effort is required to recruit the women themselves. Understanding these cooperation rates will allow us to more accurately project the necessary resources and implementation approach for the Main Study.
B.2 Procedures for the Collection of Information
Provider-Based Sampling will feature two additional instruments: 1) The Provider-Based Sampling Frame Questionnaire, which will be interviewer-administered in-person or by phone, or as an SAQ completed by the providers. Mode of administration will depend on the preferences of the individual provider; and 2) The Provider-Based Sampling Eligibility Screener, which will be interviewer-administered in-person or by phone. The purpose of these instruments is to collect information about the specific provider practice location to determine its MOS and patient characteristics, and to determine the geographic and age eligibility of sampled women; respectively.
The three field contractors participating in Provider-Based Sampling will use data collection instruments that are based on the current Alternate Recruitment Substudy (Phase 2, please see A.2 for further information and exceptions).
Additionally, alternate acceptable modes, such as telephone administration or secure Web-based administration are proposed in cases where participants are not available for in-person interviews. We have included additional modes of administration for several data collection instruments to accommodate the transition of the seven Study locations that participated in the Initial Vanguard Study (noted in A.1) to a single contract research organization (Table B.2.b).
New Data Collections and Updates for the National Children’s Study
We also seek approval for use of the 14-, 16-, 20-, 22-, 27-, and 30-Month Ages and Stages Questionnaires (ASQ) Self-Administered Questionnaire in addition to the currently approved 18 and 24 month ASQs in order to broaden the window of administration for the 18- and 24-Month visits. Currently, the approved 18-and 24-Month ASQs have a very limited window of administration compared to their corresponding 18-and 24-Month visits. In practice, if the 18- or 24-Month visit is scheduled outside of the 18- or 24-Month ASQ’s window of administration, then the ASQ would not be administered during the visit. With the addition of use of the 14-, 16-, 20-, and 22-Month ASQs, the window is broadened so that an age-appropriate ASQ may be administered during the 18-Month visit. With the addition of the 27- and 30-Month ASQs the window is broadened so that an age-appropriate ASQ may be administered during the 24-Month visit.
The NCS implemented a phased rollout of the ARS. This phased launch featured minimal study instruments initially to allow field contractors to gain familiarity with data collection operations and logistics. Subsequently, all ARS strategies employed additional and more robust instruments (in length and complexity) commensurate with those used in the Initial Vanguard Study. In this Information Collection Request, we include additional biospecimen collections in the ARS, including collection of biospecimens and physical measures at six and twelve months and physical measures at 24 months. Additionally, we include the introduction of a Core Questionnaire and 30-Month Interview Module. For a discussion on the contents of the 30-Month Interview, please see pages 11-12 of Supporting Statement A.
Data collection activities are described fully in A.2. A list of study locations that will collect samples and physical measures is located on page 5 of A.1.
In Table B.2.#, we indicate changes to the postnatal study visit structure, mention the individuals to whom the changes apply, and state the expected number of participants from whom we will collect data until the Vanguard Study clearance expires on July 31, 2013.
Table B.2.#. Postnatal Study Visit Changes
Visit |
Visit Type |
Changes to the Visit |
Individuals to whom the changes apply |
Expected number of participants we will collect data from until clearance expiration 7/31/2013 |
1mo |
New |
Re-introduction of the breast milk collection, SAQ |
Mother |
*100 sample collections |
3 mo |
New |
Re-introduction of the breast milk collection, SAQ |
Mother |
*100 sample collections |
6 mo |
Established |
Addition of child anthropometric measure and urine collections |
Child |
*850 sample collections |
12 mo |
Established |
Addition of child BP, and anthropometric measures and child urine/saliva/blood |
Child |
*2,700 sample collections |
18mo |
Established |
Addition of validation questionnaire |
Mother/ Father/ Guardian |
2,468 |
24 mo |
Established |
Addition of M-CHAT interview, child BP and anthropometric measures, validation questionnaire |
Mother/ Father/ Guardian/ Child |
1,279 |
30 mo |
New |
30-Month interview, BITSEA SAQ, BSI-R SAQ, Infant / Toddler Sensory Profile SAQ, Core questionnaire, tracing module, validation questionnaire |
Mother/ Father/ Guardian |
587 |
Tracing Module: Tracing questions were removed from all study visits, and a tracing module was developed for administration at all study visits for all recruitment methods.
Low-Intensity Visits: Addition of 3-, 9-,18-, and 24- month interviews to Low-Intensity participants; 30-month interview and core questionnaire
*The participant burden associated with postnatal biospecimen collection at 1, 3, 6, and 12 months is explained at the bottom of table on page 27 of A.12.A.
Table B.2.a. Study Instruments by Recruitment Strategy and Phase |
|||||||||||
Initial Vanguard Study Protocol |
Phase 1 |
Phase 2 |
|||||||||
Initial House-hold |
Provider-Based |
Enhanced House-hold |
Two-Tier High-Low Intensity |
Initial Household |
Provider-Based |
Enhanced Household |
Two-Tier High-Low Intensity |
Provider-Based Sampling |
|||
Low |
High |
Low |
High |
||||||||
Household Enumeration |
Household Enumeration |
Not Applicable |
Household Enumeration |
Not Applicable |
Not Applicable |
Household Enumeration |
Not Applicable |
Household Enumeration |
Not Applicable |
Not Applicable |
Not Applicable |
Provider-Based Sampling Frame Questionnaire |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Frame Questionnaire |
Provider-Based Sampling Eligibility Screener |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Eligibility Screener |
Pregnancy Screener |
Pregnancy Screener |
Provider-Based Pregnancy Screener |
Pregnancy Screener |
Low Intensity CATI Pregnancy Screener |
Pregnancy Screener |
Pregnancy Screener |
Provider-Based Pregnancy Screener |
Pregnancy Screener |
Low Intensity CATI Pregnancy Screener |
Pregnancy Screener |
Not Applicable |
General Study Informed Consent Form** |
Women’s Informed Consent Form |
Women’s Informed Consent Form |
Women’s Informed Consent Form |
Low Intensity Informed Consent Script |
Women’s Informed Consent Form |
Women’s Informed Consent Form |
Women’s Informed Consent Form |
Women’s Informed Consent Form |
Low Intensity Informed Consent Script |
Women’s Informed Consent Form |
Women’s Informed Consent Form |
Preconception (PI) and Visit Information Sheet (VIS) |
Pre-Pregnancy Interview and VIS |
Pre-Pregnancy Interview and VIS |
Pre-Pregnancy Interview and VIS |
Low Intensity Questionnaire |
Pre-Pregnancy Interview and VIS |
Pre-Pregnancy Interview and VIS |
Pre-Pregnancy Interview and VIS |
Pre-Pregnancy Interview and VIS |
Low Intensity Questionnaire |
Pre-Pregnancy Interview and VIS |
Not Applicable |
Multiple Biospecimen Collections |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Maternal Blood and Urine Collection^ |
Maternal Blood and Urine Collection^ |
Maternal Blood and Urine Collection^ |
Not Applicable |
Maternal Blood and Urine Collection^ |
Not Applicable |
Pregnancy Probability Group Follow-up Script |
Pregnancy Probability Group Follow-up Script |
Pregnancy Probability Group Follow-up Script |
Pregnancy Probability Group Follow-up Script |
Pregnancy Probability Group Follow-up Script |
Pregnancy Probability Group Follow-up Script |
Pregnancy Probability Group Follow-up Script |
Pregnancy Probability Group Follow-up Script |
Pregnancy Probability Group Follow-up Script |
Pregnancy Probability Group Follow-up Script |
Pregnancy Probability Group Follow-up Script |
Not Applicable |
First Trimester (T1) and Visit Information Sheet (VIS) |
Pregnancy Visit 1 Interview, SAQ, and VIS |
Pregnancy Visit 1 Interview, SAQ, and VIS |
Pregnancy Visit 1 Interview, SAQ, and VIS |
Low Intensity CATI Questionnaire |
Pregnancy Visit 1 Interview, SAQ, and VIS |
Pregnancy Visit 1 Interview, SAQ, Multi-Mode Visit Introductory Script (VISCR), and Tracing Module |
Pregnancy Visit 1 Interview, SAQ, VISCR, and Tracing Module |
Pregnancy Visit 1 Interview, SAQ, VISCR, and Tracing Module |
Low Intensity CATI Question-naire, VISCR, and Tracing Module |
Pregnancy Visit 1 Interview, SAQ, VISCR, and Tracing Module |
Pregnancy Visit 1 Interview, SAQ, VISCR, and Tracing Module |
Pregnancy Medical Care Log |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Pregnancy Health Care Log |
Pregnancy Health Care Log |
Pregnancy Health Care Log |
Not Applicable |
Pregnancy Health Care Log |
Pregnancy Health Care Log |
Multiple Biospecimen Collections |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Sample Collection VIS, Reconsideration Script, and Maternal Blood and Urine Collection^ |
Sample Collection VIS, Reconsideration Script, and Maternal Blood and Urine Collection^ |
Sample Collection VIS, Reconsideration Script, and Maternal Blood and Urine Collection^ |
Not Applicable |
Sample Collection VIS, Reconsideration Script, and Maternal Blood and Urine Collection^ |
Not Applicable |
|
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Sample Collection VIS, Reconsideration Script, and Household Dust and Tap Water Collection+ |
Sample Collection VIS, Household Dust and Tap Water Collection+ |
Sample Collection VIS, Reconsideration Script, and Household Dust and Tap Water Collection+ |
Not Applicable |
Sample Collection VIS, Reconsideration Script, and Household Dust and Tap Water Collection+ |
Not Applicable |
Multiple Environmental Sample Collections |
|||||||||||
Father Informed Consent |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Father Informed Consent |
Father Informed Consent |
Father Informed Consent |
Not Applicable |
Father Informed Consent |
Not Applicable |
First Trimester (T1) Father Interview and VIS |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Father Interview , VISCR, and Tracing Module |
Father Interview, VISCR, and Tracing Module |
Father Interview, VISCR, and Tracing Module |
Not Applicable |
Father Interview, VISCR, and Tracing Module |
Not Applicable |
Second Trimester (T2) and Visit Information Sheet |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Third Trimester (T3) and Visit Information Sheet |
Pregnancy Visit 2 Interview and VIS |
Pregnancy Visit 2 Interview and VIS |
Pregnancy Visit 2 Interview and VIS |
Not Applicable |
Pregnancy Visit 2 Interview and VIS |
Pregnancy Visit 2 Interview, VISCR, and Tracing Module |
Pregnancy Visit 2 Interview, VISCR, and Tracing Module |
Pregnancy Visit 2 Interview, VISCR, and Tracing Module |
Not Applicable |
Pregnancy Visit 2 Interview, VISCR, and Tracing Module |
Pregnancy Visit 2 Interview, VISCR, and Tracing Module |
Multiple Biospecimen Collections |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Sample Collection VIS, Reconsideration Script, and Maternal Blood and Urine Collection^ |
Sample Collection VIS, Reconsideration Script, and Maternal Blood and Urine Collection^ |
Sample Collection VIS, Reconsideration Script, and Maternal Blood and Urine Collection^ |
Not Applicable |
Sample Collection VIS, Reconsideration Script, and Maternal Blood and Urine Collection^ |
Not Applicable |
Multiple |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Sample Collection VIS, Reconsideration Script, and Household Dust and Tap Water Collection+ |
Sample Collection VIS, Reconsideration Script, and Household Dust and Tap Water Collection+ |
Sample Collection VIS, Reconsideration Script, and Household Dust and Tap Water Collection+ |
Not Applicable |
Sample Collection VIS, Reconsideration Script, and Household Dust and Tap Water Collection+ |
Not Applicable |
Environmental Sample Collections |
|||||||||||
Birth Visit (B1) and Visit Information Sheet (VIS) |
Birth Instrument and VIS |
Birth Instrument and VIS |
Birth Instrument and VIS |
Not Applicable |
Birth Instrument and VIS |
Birth Instrument, VISCR, and Tracing Module |
Birth Instrument, VISCR, and Tracing Module |
Birth Instrument, VISCR, and Tracing Module |
Low Intensity Birth Interview, VISCR, and Tracing Module |
Birth Instrument, VISCR, and tracing Module |
Birth Instrument, VISCR, and Tracing Module |
Infant Medical Care Log and Instructions |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Infant and Child Health Care Log |
Infant and Child Health Care Log |
Infant and Child Health Care Log |
Not Applicable |
Infant and Child Health Care Log |
Infant and Child Health Care Log |
Birth Visit Specimen Collections |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Sample Collection VIS and Cord Blood Specimen Collection |
Sample Collection VIS and Cord Blood Specimen Collection |
Sample Collection VIS and Cord Blood Specimen Collection |
Not Applicable |
Sample Collection VIS and Cord Blood Specimen Collection |
Not Applicable |
Biospecimen Collection |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
1-Month Breast Milk Collection, Breast Milk Collection SAQ, and Sample Collection VIS** |
1-Month Breast Milk Collection ,Breast Milk Collection SAQ, and Sample Collection VIS** |
1-Month Breast Milk Collection ,Breast Milk Collection SAQ, and Sample Collection VIS** |
Not Applicable |
1-Month Breast Milk Collection ,Breast Milk Collection SAQ, and Sample Collection VIS** |
Not Applicable |
3-Month Interview |
3-Month Interview (Minimal) |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
3-Month Interview (Minimal), VISCR, and Tracing Module |
3-Month Interview (Minimal), VISCR, and Tracing Module |
3-Month Interview (Minimal), VISCR, and Tracing Module |
3-Month Interview (Minimal), VISCR, and Tracing Module |
3-Month Interview (Minimal), VISCR, and Tracing Module |
3-Month Interview (Minimal), VISCR, and Tracing Module |
Biospecimen Collection |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
3-Month Breast Milk Collection ,Breast Milk Collection SAQ, and Sample Collection VIS ** |
3-Month Breast Milk Collection , Breast Milk Collection SAQ, and Sample Collection VIS ** |
3-Month Breast Milk Collection , Breast Milk Collection SAQ, and Sample Collection VIS ** |
Not Applicable |
3-Month Breast Milk Collection , Breast Milk Collection SAQ, and Sample Collection VIS ** |
Not Applicable |
6-Month Visit Interview, SAQ, VIS |
6-Month Interview (Minimal) |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
6-Month Interview (Minimal), SAQ, ,VISCR, Parental Permission for Child Participation (6 MO through age of majority), and Tracing Module |
6-Month Interview (Minimal), SAQ, VISCR, Parental Permission for Child Participation (6 MO through age of majority), and Tracing Module |
6-Month Interview (Minimal), SAQ, VISCR, Parental Permission for Child Participation (6 MO through age of majority), and Tracing Module |
Not Applicable |
6-Month Interview (Minimal), SAQ, VISCR, Parental Permission for Child Participation (6 MO through age of majority), and Tracing Module |
6-Month Interview (Minimal), SAQ, VISCR, Parental Permission for Child Participation (6 MO through age of majority), and Tracing Module |
6-Month Child Urine Collection |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Sample Collection VIS, Reconsideration Script, Child Urine Collection, and Child Urine Collection Instrument++ |
Sample Collection VIS, Reconsideration Script, Child Urine Collection, and Child Urine Collection Instrument++ |
Sample Collection VIS, Reconsideration Script, Child Urine Collection, and Child Urine Collection Instrument++ |
Not Applicable |
Sample Collection VIS, Reconsideration Script, Child Urine Collection, and Child Urine Collection Instrument++ |
Not Applicable |
Multiple Child Physical Measures |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Sample Collection VIS and Child Anthropometry Measures† |
Sample Collection VIS and Child Anthropometry Measures† |
Sample Collection VIS and Child Anthropometry Measures† |
Not Applicable |
Sample Collection VIS and Child Anthropometry Measures† |
Not Applicable |
9-Month Interview |
9-Month Interview (Minimal) |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
9-Month Interview (Minimal), VISCR, and Tracing Module |
9-Month Interview (Minimal), VISCR, and Tracing Module |
9-Month Interview (Minimal), VISCR, and Tracing Module |
9-Month Interview (Minimal), VISCR, and Tracing Module |
9-Month Interview (Minimal), VISCR, and Tracing Module |
9-Month Interview (Minimal), VISCR, and Tracing Module |
12-Month Visit Interview, SAQ, and VIS |
12-Month Interview (Minimal) |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
12-Month Interview (Minimal), SAQ, VISCR, and Tracing Module |
12-Month Interview (Minimal), SAQ, VISCR, and Tracing Module |
12-Month Interview (Minimal), SAQ, and VISCR |
Not Applicable |
12-Month Interview (Minimal), SAQ, and VISCR |
12-Month Interview (Minimal), SAQ, and VISCR |
Multiple Biospecimen Collections |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Sample Collection VIS, Reconsideration Script, Child Blood Collection &Instrument; Child Saliva Collection, Instrument, and SAQ; Child Urine Collection & Instrument++ |
Sample Collection VIS, Reconsideration Script, Child Blood Collection &Instrument; Child Saliva Collection, Instrument, and SAQ; Child Urine Collection & Instrument++ |
Sample Collection VIS, Reconsideration Script, Child Blood Collection &Instrument; Child Saliva Collection, Instrument, and SAQ; Child Urine Collection & Instrument++ |
Not Applicable |
Sample Collection VIS, Reconsideration Script, Child Blood Collection & Instrument; Child Saliva Collection, Instrument, and SAQ; Child Urine Collection & Instrument++ |
Not Applicable |
Multiple Child Physical Measures |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Sample Collection VIS and Child Blood Pressure and Anthropometry Measures† |
Sample Collection VIS and Child Blood Pressure and Anthropometry Measures† |
Sample Collection VIS and Child Blood Pressure and Anthropometry Measures† |
Not Applicable |
Sample Collection VIS and Child Blood Pressure and Anthropometry Measures† |
Not Applicable |
18-Month Interview and Child Food Questionnaire |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
18-Month Interview (Minimal), VISCR, and Tracing Module |
18-Month Interview (Minimal), VISCR, and Tracing Module |
18-Month Interview (Minimal), VISCR, and Tracing Module |
18-Month Interview (Minimal), VISCR, and Tracing Module |
18-Month Interview (Minimal), VISCR, and Tracing Module |
18-Month Interview (Minimal), VISCR, and Tracing Module |
ASQ Accompanying 18-Month Interview |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
14, 16-, 18- 20-, or 22-Month ASQ |
14, 16-, 18- 20-, or 22-Month ASQ |
14, 16-,18- 20-, or 22-Month ASQ |
14, 16-, 18- 20-, or 22-Month ASQ |
14, 16-,18, 20-, or 22-Month ASQ |
14, 16-,18- 20-, or 22-Month ASQ |
24-Month Interview |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
24-Month Interview (Minimal) and VISCR |
24-Month Interview (Minimal) and VISCR |
24-Month Interview (Minimal) and VISCR |
24-Month Interview (Minimal) and VISCR |
24-Month Interview (Minimal) and VISCR |
24-Month Interview (Minimal) and VISCR |
Modified Checklist for Autism in Toddlers |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
M-CHAT |
M-CHAT |
M-CHAT |
M-CHAT |
M-CHAT |
M-CHAT |
ASQ Accompanying 24-Month-Interview |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
24. 27- or 30-Month ASQ |
24, 27- or 30-Month ASQ |
24, 27- or 30-Month ASQ |
24, 27- or 30-Month ASQ |
24, 27- or 30-Month ASQ |
24, 27- or 30-Month ASQ |
Multiple Child Physical Measures |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Sample Collection VIS and Child Blood Pressure and Anthropometry Measures† |
Sample Collection VIS and Child Blood Pressure and Anthropometry Measures† |
Sample Collection VIS and Child Blood Pressure and Anthropometry Measures† |
Not Applicable |
Sample Collection VIS and Child Blood Pressure and Anthropometry Measures† |
Not Applicable |
30-Month Visit Interview |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Core Questionnaire, 30-Month Interview, and VISCR |
Core Questionnaire, 30-Month Interview, and VISCR |
Core Questionnaire, 30-Month Interview, and VISCR |
Core Questionnaire, and VISCR |
Core Questionnaire, 30-Month Interview, and VISCR |
Core Questionnaire, 30-Month Interview, and VISCR |
Validation Interview1 |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Validation Interview |
Validation Interview |
Validation Interview |
Validation Interview |
Validation Interview |
Validation Interview |
Non-respondent Questionnaire2 |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Non-Respondent Questionnaire |
Non- Respondent Questionnaire |
Non- Respondent Questionnaire |
Non-Respondent Questionnaire |
Non-Respondent Questionnaire |
Non-Respondent Questionnaire |
NOTE: Study Instruments that are no longer being used following the discontinuation of ARS recruitment procedures are greyed.
* A subset of items from the Household Enumeration Instrument may be
administered to the Provider-Based Recruitment and the Two-Tier
Recruitment strategies as a mechanism for determining eligibility of
a dwelling unit and identifying women that may be age-eligible to
participate in the study.
^ Maternal blood and urine will be
collected at Pregnancy Visits 1 and 2. If an enrolled woman declines
to provide specimens at Pregnancy Visit 1, we will ask her to
reconsider and request to obtain specimens up to and including
Pregnancy Visit 2.
+ Household dust and tap water collection
will be collected at the Pregnancy Visits 1 and 2.
** Breast
milk will be collected at 1 month and at the time of the 3 month
interview. If the initial scheduled pick-up call is unsuccessful,
there can be 1 reminder call to re-schedule within 4 weeks of the
visit.
++ Child urine will be collected at the 6 month and 12
month interview visits. We will make up to 2 attempts to return to
the participant's home to collect the sample as long as it is within
the window for the event. No attempt will be made to collect the
sample at the next visit because it will be out of the window for the
event.
† Child physical measures (blood pressure,
height, weight, and anthropometry measures) will be taken at 6, 12,
and 24 month visits. Further attempts can be made to collect child's
physical measures only in conjunction with collection of samples or
specimens from at least one other domain.
1The Validation
Interview will be randomly administered to some, but not all,
Vanguard (Pilot) Study participants.
2The Validation Interview
is administered to all participants who decline enrollment into the
study.
Table B.2.b. Mode of Instrument Administration by Recruitment Strategy and Phase |
|||||||||
Data Collection Event |
Phase 1 |
Phase 2 |
|||||||
Initial Household |
Provider-Based and High Intensity |
Enhanced Household |
Low Intensity |
Initial Household |
Provider-Based and High Intensity |
Enhanced Household |
Low Intensity |
Provider-Based Sampling |
|
Household Enumeration |
In person |
Not Applicable |
In person |
Not Applicable |
In person |
Not Applicable |
In person |
Not Applicable |
Not Applicable |
Provider-Based Pregnancy Screener |
Not Applicable |
Telephone (Provider Only) |
Not Applicable |
Not Applicable |
Not Applicable |
Telephone (Provider Only) |
Not Applicable |
Not Applicable |
Not Applicable |
Pregnancy Screener |
In person |
In person |
In person |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
Not Applicable |
(High Intensity Only) |
(High Intensity Only) |
||||||||
Provider-Based Sampling Frame Questionnaire |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Telephone, in-person, mail |
Provider-Based Sampling Eligibility Screener |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Not applicable |
Not applicable |
Not applicable |
Not applicable |
Telephone, in-person |
Low Intensity CATI Pregnancy Screener |
Not Applicable |
Not Applicable |
Not Applicable |
Telephone |
Not Applicable |
Not Applicable |
Not Applicable |
Telephone, mail, web |
Not Applicable |
Low Intensity CATI Questionnaire |
Not Applicable |
Not Applicable |
Not Applicable |
Telephone, mail, web |
Not Applicable |
Not Applicable |
Not Applicable |
Telephone, mail, web |
Not Applicable |
Low Intensity Informed Consent Script |
Not Applicable |
Not Applicable |
Not Applicable |
Telephone with direct mail information sheet |
Not Applicable |
Not Applicable |
Not Applicable |
Telephone with direct mail information sheet |
Not Applicable |
Women’s Informed Consent Form |
In person |
In person |
In person |
Not Applicable |
In person, telephone, mail |
In person, telephone, mail |
In person, telephone, mail |
Not Applicable |
In person, telephone, mail |
Pre-Pregnancy Interview |
In person |
In person |
In person |
Direct mail, telephone, web |
In person |
In person |
In person |
Direct mail, telephone, web |
Not Applicable |
Maternal Blood and Urine Collection^ |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
Not Applicable |
Pregnancy Probability Group Script |
Telephone |
Telephone |
Telephone |
Direct mail, telephone, web |
Telephone |
Telephone |
Telephone |
Direct mail, telephone, web |
Not Applicable |
Pregnancy Visit 1 Interview, SAQ, and Visit Information Sheet |
In person |
In person |
In person |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
In person |
Pregnancy Health Care Log |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
In person, mail, web |
Maternal Blood and Urine Collection^ |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
Not Applicable |
Household Dust and Tap Water Collection+ |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Participant Collect or Technician Collect~ |
Participant Collect or Technician Collect~ |
Participant Collect or Technician Collect~ |
Not Applicable |
Not Applicable |
Father Informed Consent |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone, mail, web |
In person, telephone, mail, web |
In person, telephone, mail, web |
Not Applicable |
Not Applicable |
Father Interview and VIS |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
Not Applicable |
Not Applicable |
Pregnancy Visit 2 Interview, SAQ, and Visit Information Sheet |
In person |
In person |
In person |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
In person |
Maternal Blood and Urine Collection^ |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
Not Applicable |
Household Dust and Tap Water Collection+ |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
Participant Collect or Technician Collect~ |
Participant Collect or Technician Collect~ |
Participant Collect or Technician Collect~ |
Not Applicable |
Participant Collect or Technician Collect~ |
Birth Visit Instrument and Visit Information Sheet |
In person |
In person |
In person |
Not Applicable |
In person, telephone |
In person, telephone |
In person, telephone |
Not Applicable |
In person, telephone |
Infant and Child Health Care Log |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
In person, mail, web |
Cord Blood Specimen Collection |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
In person |
1-Month Breast Milk Collection** |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
In person |
3-Month Interview (Minimal) |
Telephone |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone, mail, web |
3-Month Breast Milk Collection** |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
In person |
6-Month Interview |
In Person |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone, mail, web |
In person, telephone, mail, web |
In person, telephone, mail, web |
Not Applicable |
In person, telephone, mail, web |
Parental Permission for Child’s Participation (6 MO through age of majority) |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone, mail |
In person, telephone, mail |
In person, telephone, mail |
Not Applicable |
In person, telephone, mail |
6-Month SAQ |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, mail |
In person, mail |
In person, mail |
Not Applicable |
In person, mail |
6-Month Child Urine Collection++ |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
Not Applicable |
6-Month Child Blood Pressure & Anthropometry Measurements† |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
Not Applicable |
9-Month Interview |
Telephone |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone, mail, web |
12-Month Interview |
In Person |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone, mail, web |
In person, telephone, mail, web |
In person, telephone, mail, web |
In person, telephone, mail, web |
In person, telephone, mail, web |
12-Month SAQ |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, mail |
In person, mail |
In person, mail |
Not Applicable |
In person, mail |
12-Month Child Blood Collection |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
Not Applicable |
12-Month Child Saliva Collection |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
Not Applicable |
12-Month Child Urine Collection++ |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
Not Applicable |
12-Month Child Blood Pressure & Anthropometry Measurements† |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
Not Applicable |
18-Month Interview |
Telephone |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone, mail, web |
18-Month ASQ |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
24-Month Interview |
Telephone |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone, mail, web |
M-CHAT |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
24-Month ASQ |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
24-Month Child Blood Pressure & Anthropometry Measurements† |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person |
In person |
In person |
Not Applicable |
Not Applicable |
30-Month Interview Module |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone , mail, web |
In person, telephone, mail, web |
Core Questionnaire (up to 60 Months) |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone, web |
In person, telephone , web |
In person, telephone , web |
Not Applicable |
In person, telephone, web |
Tracing Module |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In-person, telephone |
In-person, telephone |
In-person, telephone |
In-person, telephone |
In-person, telephone |
Validation Interview (up to 30 Months) |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone , web, mail |
In person, telephone , web, mail |
In person, telephone , web, mail |
In person, telephone , web, mail |
In person, telephone, web, mail |
Nonrespondent Questionnaire |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone , web, mail |
In person, telephone , web, mail |
In person, telephone , web, mail |
In person, telephone , web, mail |
In person, telephone, web, mail |
Multi-Mode Visit Introductory Script (VISCR) |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone , web |
In person, telephone , web |
In person, telephone , web |
In person, telephone , web |
In person, telephone, web |
Sample Collection VIS |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone , web |
In person, telephone , web |
In person, telephone , web |
Not Applicable |
Not Applicable |
Re-Consideration Script |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone, web |
In person, telephone, web |
In person, telephone, web |
Not Applicable |
Not Applicable |
Participant Verification Questionnaire |
Not Applicable |
Not Applicable |
Not Applicable |
Not Applicable |
In person, telephone , web |
In person, telephone , web |
In person, telephone , web |
In person, telephone, web |
In person, telephone, web |
NOTE: Study Instruments that are no longer being used following the discontinuation of ARS recruitment procedures are greyed.
~ Random assignment of participant-collect or technician-collect
modality.
^ Maternal blood and urine will be collected at
Pregnancy Visits 1 and 2. If an enrolled woman declines to provide
specimens at Pregnancy Visit 1, we will ask her to reconsider and
request to obtain specimens up to and including Pregnancy Visit 2.
+
Household dust and tap water collection will be collected at the
Pregnancy Visit 1 and 2 visits.
** Breast milk will be
collected at 1 month and at the time of the 3 month interview. If the
initial scheduled pick-up call is unsuccessful, there can be 1
reminder call to re-schedule within 4 weeks of the visit.
++
Child urine will be collected at the 6- and 12-month visits. We will
make up to 2 attempts to return to the participant's home to collect
the sample, as long as it is within the window for the event. No
attempt will be made to collect the sample at the next visit because
it will be out of the window for the event.
† Child
physical measures (blood pressure, height, weight, and anthropometry
measures) will be taken at the 6- and, 12-, and 24 month visits.
Further attempts can be made to collect child's physical measures
only in conjunction with collection of samples or specimens from at
least one other domain.
B.2.1 Informed Consent
As noted earlier in A.1, the Alternate Recruitment Substudy locations will no longer consent participants as recruitment was halted. As referenced in A.2, the Provider-Based Sampling locations will consent pregnant women prior to Pregnancy Visit 1.
Participants provide consent for their own involvement and permission for their children’s participation in the NCS in stages. Participating mothers provide written consent for their own participation when they join the Study. Mothers (or other legally authorized representative (LAR)) provide their written permission for children’s participation at two separate time points. First, the mother or LAR is asked to provide written permission for a child’s participation from birth through 6 months (using either the Birth Visit Information Sheet (with samples) or the Birth Visit Information Sheet (no sample collection)) parental permission forms. At the time of the administration of the 6-month data collection, the NCS administers the Parental Permission for Child’s Participation (6 months through Age of Majority) to the mother or the child’s legally authorized representative. This form requests written permission for the NCS to collect information and samples involving enrolled children from six months through the child’s age of majority. The parental permission mentions that parents and guardians will be provided with descriptions of the data collection activities to be conducted during a particular visit at the start of that visit. Please see pages 17-18 for complete explanation of Initial Administration of Informed Consent and Administration of Consent for Continued Participation.
To date, the NCS has used a series of visit-specific Visit Information Sheets (VISs) – for administration during in-person visits that involve questionnaire administration and/or sample collection. The VISs supplement the initial consent forms and process throughout the in-person Study visit schedule by informing the participants of the activities to be completed at the particular visit and reminding them that participation is voluntary and that they may skip questions and samples as they choose. Similarly, instruments that are administered by telephone include a very brief informational script with less detail than the written VIS, which reminds participants that their participation is voluntary and that they may skip questions as they choose.
We have merged the existing written VIS and short informational script into a single multi-mode introductory Visit Information Script (VISCR) that can be read to participants during both in-person study visits and for all visits conducted via telephone. For visits that do not involve data collection activities other than questionnaire administration, this script will represent the entirety of the VIS process. The use of a single script will benefit both participants and Study operations by providing: (1) consistent language for all participants for a given visit that includes only questionnaires and/or self-administered questionnaires (regardless of mode of administration); and (2) flexibility to the Study and participants by supporting administration of questionnaires both in-person or by telephone. For visits that include other data collection activities, such as biological and environmental sample collection and physical measurements, we have drafted VIS language specific to each data collection activity. For postnatal visits that include non-questionnaire based other data collection activities, such as biological and environmental sample collection and physical measurements, we have created VIS language describing the administration and possible risk specific to each data collection activity. Therefore, administration of postnatal visits involving sample collection and collection of anthropometric or physical measures will include a reading of the multi-mode introductory VISCR followed by reading and distribution of a hard copy sample collection visit information sheet describing the specific sample collection and other procedures that will take place during the visit (Sample Collection VIS Specifications). When applicable, a Reconsideration Script for data collections that can be captured at one of multiple visits, are administered as part of the VIS administration to recognize that participants may choose not to provide specimens during a particular specimen at a given visit, but may choose to provide the specimen at a subsequent visit. The language of the script can be administered to caregivers of enrolled children as well as adult participants.
From an operational perspective, instead of providing a template VIS for each postnatal visit, we are providing a VIS Specifications document which includes introductory and closing language and modular language describing each collection procedure and any associated risks (see attached Sample VIS Specifications document). Field contractors collecting samples and/or physical measures can develop visit-specific VISs by drawing from the language in the specifications document for collection activities corresponding to the visit. The VIS process will appear unchanged to Study participants, as they will continue to receive a document explaining data collection activities planned for the current visit. This change to the VIS development process change is an operational, technical one to account for the modular structure of visits, beginning with the 30-Month Interview.
This approach to VIS development reduces additional burden to participants by reducing redundancy across VISs and between the VIS and the consent, and reducing overall time for VIS administration across visits. This approach also simplifies field procedures, and reduces potential for error and protocol deviations for data collectors, as there is uniformity of VIS administration across all postnatal visits. This consolidation of VIS language into a single document, from which visit-specific collection VIS may be tailored, will minimize the administrative burden associated with review of multiple modifications to a template VIS as data collection procedures are phased into the protocol. With this revised Specifications document, as additional procedures and future visits are developed and added to the Study protocol, NCS oversight and regulatory bodies' review will only involve the single specifications document as opposed to multiple VISs.
Finally, since the specifications are intended to be used for postnatal visits at this time, we have provided a revised Birth VIS which describes the 1-and 3-month breast milk self-collections.
B.2.2 Quality Control
Informatics Model
The NCS recognizes the valuable contribution informatics play in many aspects of research studies, particularly those with the complexity and longevity seen with the NCS. Our approach to informatics has been informed by several trends, including the use of open, modular and flexible architecture, the leveraging of standards-based terminologies and transmission specifications, interoperability, and established development communities. Overall, this approach fosters innovation while adapting to the ever-evolving field of informatics.
The Initial Vanguard Study utilized a centralized model of data management where NCS case management systems and data capture systems utilized the same approach across field contractors. This centralized approach is common in large scale data collection, even in multi-center studies. In the Initial Vanguard Study experience, it was determined that data capture systems and case management systems used successfully by other studies did not meet the particular needs of a study as complex and dynamic as the National Children’s Study. Therefore, a new solution was sought. In particular the NCS sought a non-proprietary open source, modular and interoperable solution.
The NCS Program Office used a facilitated decentralization model to support informatics in the Alternate Recruitment Substudy. Like in the Initial Vanguard Study, the NCS Program Office developed evaluation questions and plans; data fields, tables and relationships; formatting and transmission standards; a central data archive; and specifications and guidelines for data security, participant confidentiality, and regulatory compliance. Distinct from the centralized model, however, the facilitated decentralization model allowed field contractors to select case management systems, data acquisition platforms, and as appropriate, data collection tools to acquire data whose specifications including content, format and security requirements have been established by the NCS Program Office. All data systems were certified and accredited per the Federal Information Security Management Act of 2002 (FISMA) and related regulatory compliance. This model aided in identifying the costs, acceptability and feasibility of implementing and maintaining myriad systems and processes. Based on the lessons learned, the NCS has recently begun a process of convergence whereby the number of IMS systems has been reduced to a limited number of solutions and the infrastructure management will leverage a secure, remote, centrally-hosted (“Hub”) model. The selected systems and the currently contracted “Hub” provider are:
Comprehensive Research Informatics Suite (CRIS) - University of Arkansas
NCS Navigator - Northwestern University
Research Electronic Data Capture-REDCap™ - Vanderbilt University
Sugar Customer Relationship Management/Data Collection Application Suite (Sugar/DCAS) – Booz Allen Hamilton
Data Management Quality Assurance Process
Quality Control and Quality Assurance (QA/QC) procedures and metrics have been included in each component of the NCS. As related to data collection and data management, the QA/QC effort is focused on three major task areas, including instrument development/design; interviewer training and oversight; and data monitoring. Each task area is described below.
Instrument Development
All study instruments were designed centrally at NICHD for implementation by the field contractors. Instruments were designed with standardized variable names, question text, response categories, skip patterns, range checks, and interviewer and programmer instructions. Adherence to these requirements is mandatory, and their implementation will be monitored through a variety of methods, including, but not limited to, site visits, attendance at interviewer training sessions, and interviewer debriefing sessions.
Field Staffing and Interviewer Training and Oversight
Field contractors will be provided guidance on interviewer recruitment, training and ongoing monitoring. Centrally developed training materials have been provided for specific tasks, and site visits are planned to monitor training sessions at local field contractors. The Study has a Manual of Procedures that is posted on the Study Portal with a Wiki format to facilitate use of these detailed documents. Field contractors are required to track and report the various trainings and certifications completed by individual interviewers, as well as the frequency and outcome of validation calls to participants aimed at identifying potential falsification of study data. Field contractor supervisory staffs are expected to hold, at a minimum, weekly calls with individual field interviewers to identify issues and areas of concern, and provide strategic guidance. The Validation Interview included in this Information Collection Request will also be used to provide information on interviewer performance and to identify instances of data falsification.
The NCS produces guidelines and recommendations for the hiring of local field staff. Depending upon the measures being collected the required levels of experience and necessary certifications are variable. The specific roles, responsibilities, and requirements for biospecimen collection, supervision, training, and quality assurance/quality control (QA/QC) are NCS Biospecimens Standard Operating Procedures (SOPs). The required levels of experience and necessary certifications depend upon the measures being collected (the most stringent criteria are for pediatric phlebotomists who must meet state licensing requirements for phlebotomists and have specific current experience (2 years of experience within the past 4 years or completion of 100 successful venipunctures on infants and children within that time period)). All data collectors complete training in NCS procedures for biospecimen collections prior to certification and field data collection. Training includes remote, self-directed training followed by in-person training, observation, and certification. Master trainers complete certification using direct observation combined with an objective checklist of critical steps and activities that must be completed successfully. Re-training and re-certification is completed on a regular basis.
SOPs are developed for each data collection activity and include step-by-step activities, diagrams, and quick guides to provide the data collector with a standardized approach to the data collection. SOPs for each data collection are developed by method specialists, reviewed and approved by the NCS Program Office, and subsequently incorporated into the flow of activities for the entire visit. SOPs include information about preparation, implementation, and follow-up, in addition to processing and shipment to a repository or laboratory, if applicable.
Study Centers are encouraged to have weekly individual monitoring sessions with each data collector. The intent is to review the current cases and collect qualitative information on recently completed Study visits. During these sessions, supervisors are encouraged to probe for information related to participant concerns and reactions to the protocol. Study Centers may also hold group debriefing calls with all field staff to address particular topic of interest; for example, the introduction of any new biospecimens or environmental samples is an appropriate topic for this venue. Similarly, if the NCS Program Office receives feedback that some measures are appearing to be problematic, our Field Support contractor may hold a series of these debriefings with multiple study locations to get a more global perspective on any issues.
Data Monitoring
Monitoring and analyses of operational and study data will occur on an ongoing basis at both local field contractors and centrally at the NCS Program Office. The NCS has (and will continue to) develop standardized file layouts for all data collected for the Alternate Recruitment Substudy, and Provider-Based Sampling. Field contractors will be required to transform their “raw” production-level data to meet these common specifications. Layouts are available for each instrument and for all operational data that describe the process of collecting the data, and specify required formats, labels, and code frames. Most importantly, the data layouts specify handling of various types of item nonresponse distinguishing between legitimately missing data versus those missing in error.
The NCS will conduct ongoing reviews of these transformed data, comparing “national” and field contractor-specific outcomes against expected values. The centralized review will occur through the development of standardized reports as well as ad hoc queries. Standardized reports will be made available to field contractors to assist in their field monitoring efforts and any resolution of known issues or concerns. Examples of operational data to be examined include, but are not limited to, characteristics of contact attempts; refusal conversion patterns; interviewer-effects on recruitment; and questionnaire timing data. As needed or on a schedule still to be determined, the NCS Program Office will conduct audits of “raw” production data that have not yet been transformed to meet NCS specifications, and all programming code developed to complete such transformations.
Adverse Event and Other Required Reporting
The National Children’s Study Program Office has implemented monitoring and reporting procedures to ensure that human subject protections are followed, participant confidentiality is maintained, and study protocols are implemented correctly. Within this structure, field contractors are responsible for reporting to the NCS PO using a standard format within 24 hours of knowledge of serious adverse events, unanticipated problems, suspected or confirmed confidentiality breaches, or failure to obtain legally effective consent. The NCS Program Office then adjudicates and responds to incidents reported by NCS field contractors at least once per week (or more frequently, depending on the nature of the event and associated regulatory requirements). The NCS Program Office reports adverse events systematically in accordance with guidance from the Office of Human Research Protections to the NICHD IRB and to the Independent Study Monitoring and Oversight Committee (iSMOC) for their review.
NCS field contractors are also expected to report adverse events to their local IRB(s) in accordance with local reporting requirements. Should field contractors sign the Memorandum of Understanding to join the (IRB) Federation, they will retain the responsibilities of reporting adverse events to the IRB of record. Depending upon their level of participation as a member of the Federation, field contractors will report adverse events either to the NICHD IRB or the appropriate local IRB. In reporting adverse events, field contractors do not ask additional questions of study participants; therefore, the NCS does not request permission for additional respondent burden hours for this activity.
B. 2.3 Special Statistical Considerations
Statistical methodology for stratification and sample selection within the Provider-Based Sampling Feasibility Study
We will select provider locations with PPS to support a self-weighting design. Using characteristics from the frame file, the provider locations will be stratified using agreed upon variables deemed to be important within each PSU. The variables may not be the same in each PSU given the different population characteristics. Details are provided the “Stratified Probability Sampling of the Provider Locations” subsection on page 10 in Section B.1.
A sample of eligible women (stratified by date and time) within each selected provider location will be selected. A set of alternative protocols will be developed to support the potentially different operational configurations and requirements/preferences of provider locations. Details are alternative protocols are outlined above in Section B.2 on page 12.
Estimation procedure
As with the Alternate Recruitment Substudy, Provider-Based Sampling is an operational feasibility pilot where most assessment/evaluation measures will be descriptive in nature and not intended to provide inferences of the general population. The potential scope of such assessments/evaluations is outlined below in Section B.3
Degree of accuracy needed for the purpose described in the justification
The inclusion of approximately 60 provider locations is sufficient to develop an understanding of whether provider location participation under Provider-Based Sampling is as viable as that observed in Provider-Based Recruitment. Inviting participation of about 1,200 women across the 3 PSUs will be sufficient to determine the operational viability of successfully enrolling into the NCS through the Provider-Based Sampling design and operational mechanisms.
Unusual problems requiring specialized sampling procedures
As stated above, we will need to develop a set of alternative sampling protocols from which to choose to support the selection of eligible women in each selected provider location. We will determine the particular sanctioned sample schema to employ through discussions with the provider location.
Any use of periodic (less frequent than annual) data collection cycles to reduce burden
Not applicable
B.3 Methods to Maximize Response Rates and Deal with Nonresponse
Community Outreach and Engagement
Each field contractor will employ a community outreach and engagement plan that benefits from core content generated centrally from the NCS Program Office, employs themes reflecting the sampling/recruitment strategy enrollment approaches, and is adapted to fit local interests and needs. Additionally, the three field contractors engaged in Provider-Based Sampling will have the advantage of having access to those strategies that were found to be most useful for engaging providers in the Provider-Based Recruitment strategy of the Alternate Recruitment Substudy.
Core NCS content includes the presentation of the purpose, goals, design, and management of the study. This content will be used consistently throughout outreach and engagement activities across the three field contractors. All study locations are expected to use media and community activities, tailored to local circumstances, to increase public awareness of the NCS and aid with recruitment of participants. These methods will include but will not be limited to messages transmitted through local media (for example, newspapers, radio, and television), distribution of various NCS materials (for example, brochures, question and answer sheets, and newsletters), and secure use of electronic modes, such as Internet and social media. Provider-Based Sampling efforts will need to utilize materials to enhance working relationships with provider locations in addition to materials to enhance relationships with study participants.
Other Methods to Promote Response and Deal with Nonresponse
The experience of the Initial Vanguard Study has yielded several important “lessons learned” that have informed the design and implementation of the proposed Provider-Based Sampling. We have learned that using the same data collectors to interact with participants over time builds rapport and promotes response. For this reason, field staff should be cross-trained to administer multiple instruments and visits. Obtaining supplementary contact information (such as cell phone numbers), varying callback days and times, and setting up each subsequent meeting at the preceding visit have also proved successful strategies. Finally, the experience of the Initial Vanguard study also demonstrated that professionalizing enumeration staff – and employing different staff with different skills and characteristics for enumeration compared to interviewing – can promote successful responses. These findings have informed all strategies proposed for Provider-Based Sampling, as appropriate.
B.4 Tests of Procedures or Methods to be Undertaken
The analytic aim of the Provider-Based Sampling Feasibility Study is a quantitative description of the feasibility, acceptability, and cost of Provider-Based Sampling the association of Provider-Based Sampling with retention outcomes, and testing of selected study visit measures that have been revised based on Initial Vanguard Study and Vanguard (Pilot) Study experience (Phase 2) to date. Overarching research questions, and research questions specific to Provider-Based Recruitment, were presented in Part A of this supporting statement. This section describes the decision rules that form the basis of the evaluation plan for Provider-Based Sampling.
Sampling
The Provider-Based Sampling Feasibility Study will identify factors associated with the success or failure of this approach. Specific sampling questions to be addressed in this study include:
Can a sampling frame of prenatal care and birth providers be constructed?
Is the feasibility of construction of the sampling frame consistent across the three study locations, and, if not, what factors contribute to success or failure?
Are the measures of size of number of first prenatal care visits sufficiently reliable for an efficient sample design? If not, what alternative measures can be used?
Of providers selected into the sample, what proportion agrees to participate? Is this proportion comparable across the three field contractors? If not, what factors are associated with rates of participation?
Will there be differential success in engaging hospital and birthing center providers versus prenatal care providers?
Provider Location List and Frame Building
Coverage Assessment
A critical component in evaluating PBS is to assess the completeness of the list of provider locations. Undercoverage of provider locations, especially if it is differential by certain critical subpopulations, can introduce additional biases. There are a few ways to assess the coverage of the list/frame of provider locations that provide “first prenatal care” to women. These may include:
Comparing the total MOS across all provider locations on the list to historically known number of births in the sample PSU.
Where possible (particularly at Baylor & UMass), comparing a historic birth certificate attendant-based list of provider locations to the list built from administrative records identified as “first prenatal care” provider locations from the Frame Questionnaire.
Reviewing birth records from all or a subset of birthing hospitals/centers from the recruitment period to determine if attendant name and address identify provider locations not on the provider location list. This can also be used to assess the degree to which no prenatal care births could be covered through the sampling of birth records from birthing hospitals/centers. And though possibly less critical, it could also be used to determine if births associated with provider locations deemed to be too small for the women to be efficiently sampled can be picked up at the hospital/birthing center.
Where possible, acquiring a file of birth certificates from state or county Vital Statistics, corresponding to recruitment period and compared to the list of provider locations.
Provider Location Measure of Size (MOS) Accuracy
The MOS for PBS is the number of first prenatal care visits to a prenatal care provider for women who reside in the sample PSU. The efficiency of the PBS probability based sampling relies on an accurate MOS, both in terms of determining the provider location selection probability and the within provider location sampling rate of women. The accuracy of the MOS can be assessed in a couple of ways:
Comparisons of MOS as derived from processing birth certificate based information versus MOS as reported in the PBS Frame Questionnaire by provider locations. This is of particular interest in providers located outside or near sample PSU boundaries as it may be difficult for provider locations to provide an MOS properly restricted to only those patients who reside in the sample PSU.
For sampled provider locations (originals and recruited substitutes), the actual sample yields of eligible women will be compared to the MOS from the frame.
Related to 1.d) above, the actual sample yields could be compared birth certificate records corresponding to the recruitment period to assess the MOS, but possibly more broadly, the coverage of eligible women.
Provider Location Characteristics Comparisons
The provider location characteristics are used to stratify the provider locations into homogenous groups from which one or more provider locations are selected from each stratum. The efficiency in this process to reduce variances in the sample is impacted by the accuracy of the measures of these characteristics. To the extent possible, comparisons will be made between birth certificate based information and similar dimensions (see potential list of variables in Section B.1) collected in the Frame Questionnaire completed by provider locations.
Response/Completion Rates of the Frame Questionnaire
By SC and mode of administration
NCS Staff Debriefing Questionnaire and Discussion
Frame Questionnaire Assessment (Instrument and Operational)
Use of birth certificate records data files
Costs and required staff resources
Recruitment
Feasibility will be evaluated in terms of recruitment and retention of participants. Recruitment goals for Provider-Based Sampling are based on the experience gained from the Alternate Recruitment Substudy. The outcome of interest for Provider-Based Recruitment is to understand the entire process and achieve acceptable feasibility of Provider-Based Sampling.
Accordingly, outcomes that will be used to assess recruitment include:
The rate at which the study learns of potentially eligible women through the participating provider
The rate at which the study can successfully contact potentially eligible women
The rate at which the study can complete the screening for eligibility
The rate at which eligible women consent to entering the study after being contacted and screened for eligibility
The distribution of gestational age at time of enrollment of pregnant women
Note that where appropriate, American Association for Public Opinion Research (AAPOR) standards will be applied in the calculation of response, consent/enrollment, and retention rates.1
For the Main Study, a customized recruitment strategy may be required for different types of study locations. The Provider-Based Recruitment experience, along with other extant data and resources, will help guide the choice of recruitment strategies for the Main Study.
Sampled Provider Location Recruitment
One of the key measures in evaluating the operational feasibility of PBS is whether sampled provider locations are willing to participate. For those not willing to participate, assess the ability to recruit substitute provider locations. Relevant measures are outlined below:
Provider Location Recruitment Response Rate

Response rates need to remove the out-of-scope units from the denominator, in this case, the out-of-scope original provider locations (Recruitment_Status = 5). This rate is used when “officially” reporting on the PBS Provider Location Recruitment Response Rate.
Provider Location Recruitment Response Rate with Substitution.
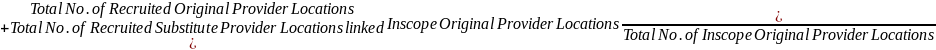
As above, the out-of-scope original provider locations (PR_Recruitment_Status = 5) are removed from the denominator. Participating substitutes “linked” to in-scope non-participating original provider locations are included in the numerator. Note that this rate should not be thought of as a representation of the PBS’s provider location recruitment response rate, instead to the degree that substitute provider locations are like the original provider locations they are replacing. It provides some indication of the degree to which non-response bias at the provider location level may be mitigated.
Special PBS Provider Location Recruitment Yield Rate (inclusion of subs for out-of-scope)
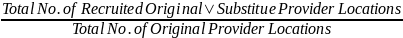
Conventionally, substitutes are only brought in for in-scope non-participating units. However, to ensure sufficient sample yields in the PBS Pilot Study, we are allowing substitute provider locations to be recruited for out-of-scope original provider locations, as well. This special PBS Feasibility Study protocol may result in yield rates that are higher than would otherwise be achieved under more conventional or Main Study protocols.
Conventional Provider Location Recruitment Yield Rate

As stated above, the Special Provider Location Recruitment Yield Rate may overstate a yield rate that one might otherwise expect under moreconventional substitution protocols. This Conventional Yield Rate excludes those cases in the numerator that under conventional substitution protocols would not have allowed a substitute to have been recruited. This rate would be expected to be less than or equal to the Special Yield Rate, but may be more applicable under a Main Study protocol that correctly only allows substitutes to be recruited for in-scope non-participating provider locations.
Differential Rates by
Study Location
Provider location type/characteristics/size
Analysis of Contact Log
Number of contacts
Distribution of length of recruitment time periods
Collect information on contributing circumstances: e.g., IRB delays, any special requirements imposed by the IRB, and processes for obtaining a HIPAA waiver.
Reason for non-participation
NCS Staff Debriefing Questionnaire and Discussion
Operational Evaluations of Methods for Sampling Women
There is a wide range of operational characteristics associated with the sampled provider locations. These differences in provider location characteristics can very across several dimensions, including size, willingness to allowing eligibility screening to take place in the office, to maintain a list of patients, or to prescreen patients on eligibility; and the method for identifying and contacting sampled patients (i.e., release of PII, PHI, and possible HIPAA requirements).
In order to support the process of selecting a sample of women from provider locations with varied characteristics and operational constraints, alternative sampling methods with corresponding sets of protocols were developed. Guidance materials and training were provided to assist in the determination and use of an appropriate sampling method.
An evaluation of the operational aspects of the sampling methods may include a review of the following dimensions:
Sampling method selection process
What methods chosen?
Women listing procedures by
Provider Location Staff
NCS Staff
Prescreening
Frequency and pattern of use
Prescreened ineligibles listed with reason, listed only, only counts, or no information on number
Proportion of women prescreened as ineligible
Systematic Subsampling
Frequency of use
Operational assessment, especially when (if) combined with prescreening
Contacting Sampled Women
Real-time
Retrospectively
HIPAA requirements
Time interval between sampled visit and first interview
NCS Staff Debriefing Questionnaire and discussion
Sampling Method Protocols and Guidance Materials
Recruitment, Application of Eligibility Screener, and Consenting/Enrolling of Sampled Women
Eligibility Screener Response Rate (ESRR)
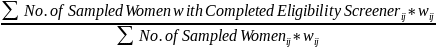
Where wij = (No. of women listed and eligible for sampling + No. of women prescreened as ineligible (Pre_Screened_Status = 2))/No. of women listed and eligible for sampling for each provider location and sampling visit. The weighting factor wij is needed in order to compute the Eligibility Screener Response Rate properly. There needs to be an accounting of the women who are prescreened as ineligible and not even asked to complete the Eligibility Screener. If no eligibility prescreening of women was conducted at a particular sampling visit, then wij = 1. This response rate is a weighted proportion across all provider location visits of all the women approached to complete the Eligibility Screener that are willing to do so.
Eligibility Rate (ER)

As above, in order to compute the Eligibility Rate properly, there needs to be an accounting of the women who are prescreened as ineligible and not asked to complete the Eligibility Screener; otherwise, the computed rate will overstated the true Eligibility Rate. In particular, the estimated number of women who would have been asked to complete the Eligibility Screener but did not due being prescreened as ineligible need to be accounted for in the denominator.
Eligible Women Consent/Enrollment Rate (EWCR)
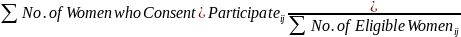
From the completed Eligibility Screener, this rate is the proportion of all the women eligible for enrollment into the NCS that consent to do so.
Overall Yield Rate
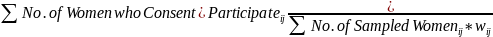
In other words, the overall yield rate is the product of the 3 individual rates above: Overall Yield Rate (OVR) = Eligibility Screener Response Rate (ESRR) * Eligibility Rate (ER) * Eligible Women Consent Rate (EWCR).
Differential Rates by
Study Location
Provider location type/characteristics/size
Roles played by provider staff
Real-time vs. retrospective contact
Employment of prescreening
HIPAA requirements
Stage of pregnancy, if possible
Reasons for non-response/non-participation by real-time vs. retrospective (e.g., failure to locate)
Observed/Listed Number of First Prenatal Care Women vs. MOS (Birth Certificate/Frame Questionnaire)
Distribution of Stage of Pregnancy (Gestational Age) at Time of Enrollment and First Study Visit
Enrolling women and beginning data collections as early as possible in the pregnancy is a major operational goal of the NCS to support efforts to understand the impact of environmental exposures on women and their children. In particular, this would inform the Main Study about realistic gestational age targets for a provider-based recruitment strategy in the Main Study. Various measures can be considered:
Proportion of women enrolled within 2 months of becoming pregnant (gestational age is <=2 months)
Proportion of women enrolled in the 1st trimester, 2nd trimester, 3rd trimester, or at birth
Proportion of first study visits occurring in the 1st trimester, 2nd trimester, 3rd trimester, or at birth
Retention
A key goal for the NCS Main Study is to obtain information on the health and developmental outcomes of subjects as they move through adolescence and early adulthood. To answer many of the central scientific questions, it will be essential to retain a sample of sufficient size throughout the course of the Main Study. Determining expected rates of retention of participants through pregnancy to birth and beyond is a key part of the analytic plan for the Vanguard (Pilot) Study. Retention of participants from visit to visit will be carefully monitored.
Specifically, the NCS will monitor:
The proportion of consented women who participate in at least one data collection study visit.
The proportion of women enrolled during pregnancy and participating in all data collection visits through the birth of a child that is enrolled into the study.
The proportion of women who receive a pre-birth data collection visit that also receive a successful birth visit.
The proportion of women enrolled during pregnancy and participating in all data collection visits through age 30 months of the child that is enrolled.
Retention challenges and solutions will likely vary by the nature of the visit, the length of time between visits, and the participant’s stage in the study cycle. Information collected from field data collectors represents a critical source of data from which to evaluate the feasibility and acceptability of the NCS Vanguard Study. Our ability to utilize these data to inform subsequent decisions requires coordination of several operational efforts, including hiring, training, and monitoring of field staff (for more information on this, please see B.2.2 Quality Control), and the development of instruments, study procedures and case management documentation. For example, unit nonresponse – both initial and due to attrition - will be assessed systematically through the administration of the Nonrespondent Questionnaire. For more information on this questionnaire, please see page 12 of Supporting Statement A. Additionally, our understanding of participant reactions to introducing the collection of biospecimens from infants specifically will be informed by these multiple sources.
The NCS has developed data collection forms associated with each unique specimen collection. These forms will capture any item nonresponse and any stated reasons. For example, the Biospecimen Child Blood Collection Instrument has a question that records the reason the participant chose not to participate in the collection. The SOP has a statement notifying the data collector to record a reason in this scenario. There are similar questions in each of the data collection instruments where the data collector must record the reason why the participant did not wish to participate.
To ensure the collection of standardized, quantitative information on Study processes, case management, and nonresponse, NCS IT systems also include multiple options to record negative (or positive) reactions from participants and field staff. These may occur and be recorded at any point within a Study Visit, from administration of consent and beyond.
All visits involving questionnaire administration begin with the reading of the Multi-Mode Visit Introductory Script (VISCR). For visits where specimens will be requested, there are written visit information sheets (VIS) describing the specimen collections requested of each participant at that visit. For more details on the VISCR and biospecimen collection VIS, please see B.2.1 Informed Consent.
The VIS associated with the referenced child visits includes a description of the proposed biospecimen collections (e.g. urine, saliva, blood). Any concerns or comments from the participant may be recorded in the IMS tables associated with the VIS administration. The NCS has intentionally provided open-ended fields to allow for the collection of this information. If a participant chooses to decline a particular biospecimen collection, this information is captured in the IMS. When applicable, a Reconsideration Script for data collections that can be captured at one of multiple visits is administered as part of the VIS administration to recognize that participants may choose not to provide specimens during a particular visit, but may choose to provide the specimen at a subsequent visit.
In the case of a participant who previously provided written consent for willingness to participate in biological and environmental specimen collections but indicates that she/he is not willing to provide any further biological (or environmental ) sample for subsequent visits, data collectors are required to (1) re-administer the appropriate written consent form so that participants can indicate in writing their new preference regarding specimen collection and (2) enter a new record noting the withdrawal of consent for these types of measures. The IMS tables associated with the Informed Consent process is one way the NCS can measure ongoing participant satisfaction and retention.
Field data collectors are required to document additional case management information. Specifically, all contacts with participants are to be recorded with the associated outcome or disposition. If the contact is associated with the administration of a Study Visit, additional information on the event and each instrument is required. Systems have been developed to allow data collectors to provide both coded and open-ended responses for all of these areas; allowing the NCS to systematically track participant refusals and stated concerns. If the outcome of a specific data collection visit is a refusal, data collectors are required to complete an additional form, not administered to the participant but to the data collector. This form – designed to immediately assess unit nonresponse – collects reasons for participant refusals, and is one additional tool for the NCS to assess participant attrition.
It is essential to recruit a broad range of enrollees to the Main Study so that the NCS can provide valid information about the population of births in the United States. Further, the receptiveness of particular women to particular recruitment strategies, including Provider-Based Sampling may be associated with demographic factors, such as race, ethnicity, age, marital status, primary language, employment status, and level of education. The Vanguard (Pilot) Study will examine the demographic and general medical characteristics of screened and recruited women and compare those characteristics among the three recruitment strategies and Provider-Based Sampling, and, as measures permit, the cohort for the Initial Vanguard Study. Additionally, these distributions will be compared to population level data such as U.S Census or birth data for the geographic region. Recruitment via Provider-Based Sampling may also be influenced by factors related to the provider, such as the size of the practice or specific operational aspects of participant recruitment.
Cost
As described above, respondent burden and impact on study location and Program Office infrastructure will be evaluated as well. All else being equal, methods that reduce respondent burden will be given priority over methods that reduce impact on study infrastructure.
Evaluation of cost will consider level of effort, equipment and materials for data collection. Study locations and the NCS Program Office will track these data; the NCS Program Office will evaluate the combined data. Evaluation will consider stationary or repeated costs, and investment in infrastructure that may reduce costs in the Main Study. Cost alone will not be the basis for retaining or rejecting a recruitment strategy.
Costs and effectiveness of these approaches will be evaluated systematically. Contractors are asked to provide detailed estimates of labor hours spent on key PBS tasks. These data will allow us to understand the labor costs and other expenses associated with each stage of sampling and recruitment of provider locations and NCS-eligible women. We hope to assess several critical issues, including but not limited to the following:
What is the response rate of the selected provider locations?
The provider location response rate is the number of participating original provider locations (numerator) divided by the number of eligible original provider locations (denominator). If possible, these rates will be examined by PSU and other provider location type characteristics (e.g., MOS, patient demographics, etc.). Substitute provider locations will be recruited to replace eligible non-responding original provider locations in order to maintain sample size yields, but, they will not be included in the calculation of the provider location response rate.
Which approaches and materials are most effective in engaging providers?
What is the consent/enrollment rate of women?
The consent/enrollment rate is the number of sampled women enrolling in the NCS (numerator) divided by the number of sampled eligible women (denominator).
What is the retention rate of enrolled women to birth?
The retention rate is the number of observed birth visits (numerator) divided by the number of enrolled women (denominator).
To what degree are provider locations willing to engage in outreach to potential study participants?
Are participants more likely to enroll in the study if the study is introduced to them by a provider of prenatal care?
Is it feasible to conduct outreach (e.g. provision of study brochures) in provider offices?
Is it more effective to have study personnel on-site at provider offices to conduct outreach and enrollment activities?
Are general media campaigns cost-effective in this setting?
What is the cost per delivered message in media campaigns (exact or approximate)?
Are particular efforts more or less successful with persons with particular demographic traits (race, ethnicity, age, marital status, primary language, employment, or education)?
Are particular efforts associated with more effective retention?
B.5 Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
The sampling and data collection strategies proposed for Provider-Based Sampling have been presented to, and benefited from, comments received from staffs from several federal agencies, advisory committees, and scientific experts including: representatives from the National Center for Health Statistics (NCHS), the Census Bureau, the Bureau of Labor Statistics, the National Institutes of Health (NIH) including the Division of Epidemiology, Statistics, and Prevention Research (DESPR) at the National Institute of Child Health and Human Development (NICHD), and the National Human Genome Research Institute (NHGRI), the NCS Federal Advisory Committee [April 2011 and July 2011]; representatives from the Office of Management and Budget, the NCS Interagency Coordinating Committee, comprising EPA, CDC, NIEHS and NICHD; Mr. Warren Strauss of the Battelle Institute, Dr. Graham Kalton and David Hubble from Westat™, Dr. Michael Elliott at the University of Michigan, Dr. George Rhodes at the University of Medicine and Dentistry of New Jersey. In addition, each of the NCS field contractors is also staffed with appropriate statistical expertise.
1The American Association for Public Opinion Research. 2008. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 5th edition. Lenexa, Kansas: AAPOR.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | NICHD-TECH |
| File Modified | 0000-00-00 |
| File Created | 2021-01-30 |
© 2026 OMB.report | Privacy Policy