Saliva Collection
Adoption, Health Impact and Cost of Smoke-Free Multi-Unit Housing Policies
Att 10A(e) Protocol for Saliva Collection_8 29 2012
Resident Saliva Collection
OMB: 0920-1004
Attachment 10A Form Approved
OMB No. 0920-xxxx
Exp. Date xx/xx/xxxx
Saliva Collection Protocol for
Multi-Unit
Housing Survey

To Be Used by Field Data Collectors to Collect Specimens
Public reporting burden of this collection of information is estimated to average 10 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (0920-xxxx)
Table of Contents
Important phone numbers …………………………………………………………….. 3
Materials for saliva collection ………………………………………………………… 4
Sample collection preparation ……………………………………………………….. 4
Sample collection techniques
Sample storage procedures ………………………………………………………….. 8
Transporting the samples …………………………………………………………….. 8
Important Phone Numbers
Lisa V. Smith ………………………………………………………………… (323) 231 – 7640 (main)
.……………………………………………………………….. (323) 333 – 2135 (cell)
.……………………………………………………………….. (213) 240 – 8324 (work)
Mary Lou Gilbert…………………………………………………………….(310) 801 - 2524 (cell)
Carol Kawecki ………………………………………………………….. (301) 524 – 5078 (cell)
The purpose of this protocol is to guide you through the process of collecting the saliva sample for those participating in the Multi-Unit Housing Policy Study. Contact Lisa V. Smith, Mary Lou Gilbert, or Carol Kawecki immediately if you have any questions regarding data collection.
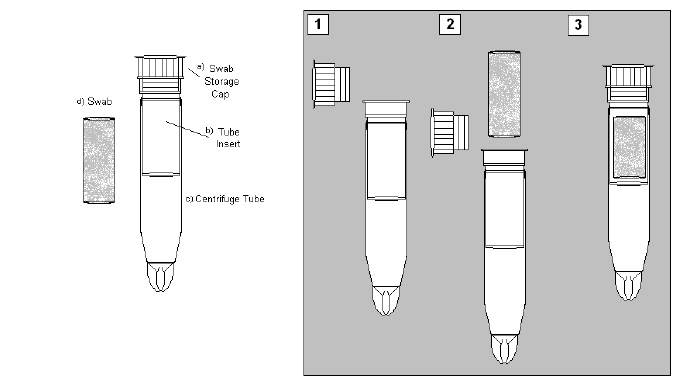
Materials for Saliva Collection:
Before you start the day, ensure that you have all of the supplies necessary to complete saliva collection including:
|
|
|
|
|
|
|
|
|
|
Sample Collection Preparation:
Collection of the sample should be completed near the end of the interview process. This will ensure that participants have not consumed any foods that may contaminate or invalidate the saliva sample.
At the beginning of the interview, let the participant know that you will be collecting a saliva sample for the study shortly. The saliva will only be used to look at the levels of cotinine present, which is a byproduct that is present in saliva after exposure to nicotine. Their saliva will not be used for any other purpose.
Ask the participant to rinse their mouth out with clean water from the tap. This should be completed 10 minutes before sample collection.
Advise the participant to not ingest any other foods or liquids before completing the sample collection.
Sample Collection Techniques:
For persons older than 6 years of age:
Get all materials prepared for sample collection:
Salimetrics Oral Swab (SOS) – for persons aged 6 years or older
Swab Storage Tube (SST) – to place sample after collection
Hand sanitizer
Gloves for yourself and the participant
Ziplock bag – to place collected samples into cooler for transport
Timer – to time sample collection
Labels
Label the Swab Storage Tube (SST) with the Westat-provided respondent ID label and Salimetric’s bar-coded label. Since the Salimetrics Oral Swab (SOS) can be used with anyone over age 6, be sure that you use the appropriate Westat label – the ID that ends in “A” is for an adult (individual aged 18 or older); the ID that ends in “C” is for the child’s sample (individual aged 2 – 17). The label should be set on the tube with the Salimetric barcode placed horizontally (see Figure 1). Ensure that the ID label matches the ID number on the participant’s paperwork.

Figure 1: Placement of the barcode label on the SST.
Clean your hands using the provided hand sanitizer.
Offer hand sanitizer to the participant.
Ask participant to put on gloves. (If he/she refuses, you may still continue with the sample collection, but be sure that his/her hands are cleaned thoroughly.)
Open the package for the Salimetrics Oral Swab (SOS), leaving the swab in the package.
Instruct the participant to place the swab underneath his/her tongue, in the front of his/her mouth (see Figure 2).
Figure 2: Placement of the
Oral Swab in the mouth for saliva testing.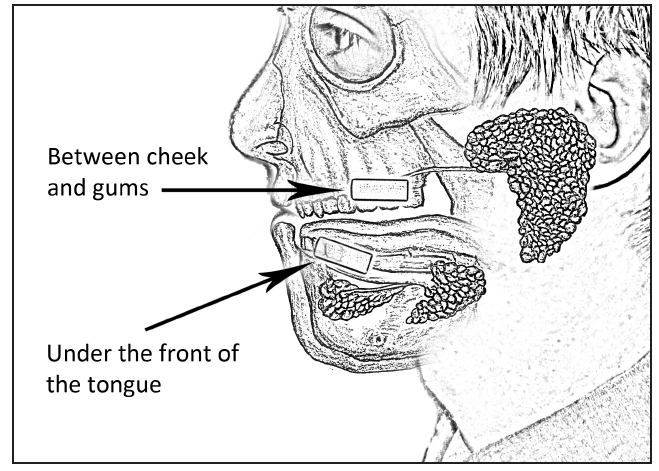

Once the participant has placed the SOS in his/her mouth, start the timer for two minutes.
If the individual is a smoker or has a dry mouth, instruct him/her to leave the swab in the mouth until it “feels full,” but for at least 2 minutes. Smokers are more likely to take longer to collect enough saliva for the sample.
Remove the swab storage cap from the SST.
After two minutes have elapsed, instruct the participant to remove the swab from his/her mouth and place it into the SST (see Figure 3).

For persons 2 to 6 years of age:
Get all materials prepared for sample collection:
Salimetrics Children’s Swab (SCS) – for persons aged 2 - 6 years
Swab Storage Tube (SST) – to place sample after collection
Hand sanitizer
Gloves for yourself and the parent
Ziplock bag – to place collected samples into cooler for transport
Timer – to time sample collection
Labels
Label the Swab Storage Tube (SST) with the Westat-provided respondent ID label and Salimetric’s bar-coded label. Be sure that you use the appropriate Westat label – the ID that ends in “C” is for a child (individual aged 2 – 17). The label should be set on the tube with the Salimetric barcode placed horizontally (see Figure 4). Ensure that the ID label matches the ID number on the participant’s paperwork

Figure 4: Placement of the barcode label on the SST.
Clean your hands using the provided hand sanitizer. Offer the parent the use of the sanitizer.
Put gloves on. Ask the parent to put gloves on. (If the parent refuses, you may still continue with sample collection, but be sure that his/her hands have been cleaned thoroughly.)
Open the package for the Salimetrics Children’s Swab (SCS), and remove the device.
Ask the parent to securely hold one end of the swab. Ask him/her to place the other end underneath the child’s tongue, in the front of the mouth.
Once the SCS is in the child’s mouth, start the timer for 90 seconds. Re-introduce the swab in the child’s mouth as needed until the lower third of the swab appears saturated. If the child cannot keep the swab under his/her tongue, ask the parent to assist with this.
Ask the parent to remove the swab from the child’s mouth.
Remove the swab storage cap from the Swab Storage Tube (SST).
P
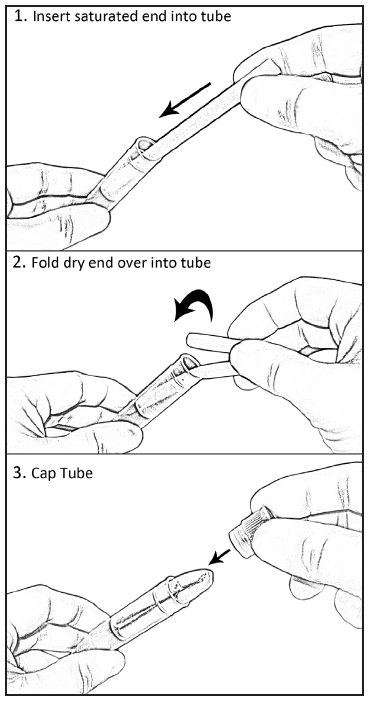
 lace
the saturated end of the swab into the SST and then fold the dry end
into the tube (see Figure 5).
lace
the saturated end of the swab into the SST and then fold the dry end
into the tube (see Figure 5).
Figure 5: Placement of the
Oral Swab in the SST for storage.
Sample Storage Procedures for all age groups:
Replace the swab storage cap on the SST tightly to ensure that no evaporation will occur.
Place the SST into a Ziplock bag. Multiple samples from a single household may be stored in the same bag.
Place the sealed Ziplock bag into the cooler with gel packs for storage. Place one gel pack under the samples and one gel pack over the samples to keep the contents cool.
Remove gloves.
Place all trash from the saliva collection into the trash bag. Seal the bag and place it into the trash receptacle.
Complete the Saliva Sample Collection Log (see Figure 6). Place the appropriate Westat-provided label in the first column. Record the date the sample was collected and check the box for whether the SOS or the SCS was used. If you encountered a problem during collection (such as a smoker’s need to hold the sample under the tongue for longer than two minutes), record this in the Notes section.
After all interviews have been completed for the day, place the samples into the freezer provided to you by the study team as soon as possible. Failure to freeze samples in a timely fashion may result in loss of samples/data quality.
Update the web-based Study Management System on the day the samples were collected.
Transporting the samples:
Transport the samples in a cooler with one gel back on top and one underneath the samples. Return samples on Monday and Thursday to the Los Angeles County freezer located at:
Veterinary Public Health
313 N. Figueroa Street, Room 1132
Los Angeles, CA 90011
11th Floor Laboratory
Bring your identification so that you will be allowed to enter the building.
If you arrive during normal business hours, check in with the Veterinary Public Health staff in room 1127. They will grant you access to the Laboratory to store the samples.
If you arrive after normal business hours, or no one is available in the Veterinary Public Health office, report to Security in the lobby of the building. They will need to let you into the Laboratory in Room 1132.
Remove saliva samples from the ziplock bags and place the SSTs in the cryobox provided by LACDPH before being placed in the freezer. Make sure that SSTs are stored with the lid on top.
Place the cryobox into the freezer.
Check the Saliva Collection Log (Figure 6) to be sure it is fully completed. Sign it and note the date you returned the samples to LACDPH. Turn in the log in the designated folder near the freezer.
Contact Lisa V. Smith (phone numbers on Page 3 of this manual) to let her know the number samples that have been transferred to the freezer and what date they were transferred.
If you have any difficulty accessing the Laboratory, contact Lisa V. Smith immediately.
Remember to update the web-based Study Management System with the date that the samples were returned to LACDPH.
Smoke-Free MUH Resident Saliva Sample Collection Log |
||||
Field Data Collector Name: |
||||
Date Samples Returned to LACDPH: |
||||
Westat Respondent_ID (Place ID label here) |
Date Sample Collected |
Check if Salimetrics Oral Swab (SOS) Used (Participant older than age 6) |
Check if Salimetrics Child Swab (SCS) Used (Participant 2-6 years old) |
Notes |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 6: Example of Saliva Sample Collection Log
NOTES
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Draft Protocol 1 |
| Author | jpiron |
| File Modified | 0000-00-00 |
| File Created | 2021-01-30 |
© 2026 OMB.report | Privacy Policy