Cover Memo
Cover_Memorandum_NCS_Request_for_Nonsubstantive_Change.docx
Provider-Based Sampling Feasibility Study for the Vanguard (Pilot) Study and Data Collection Updates for the National Children's Study (NICHD)
Cover Memo
OMB: 0925-0593

DATE: February 5, 2013
TO: Dr. Margo Schwab, Ms. Julie Wise
Office of Management and Budget
Office of Information and Regulatory Affairs
FROM: CDR Colleen Lee
THROUGH: Dr. Steven Hirschfeld
SUBJECT: Request for Non-Substantive Change to National Children’s Study, Vanguard (Pilot) Study (OMB Control #0925-0593, Expiration Date: August 31, 2014) – Pregnancy Loss, Stillbirth and Neonatal Death Instruments, Alternate Mode Request, Satisfaction Questions, Dietary Assessment in Infants and Young Children, Specimen Collection in the Provider-Based Sampling
CC: Dr. Sarah Glavin, Ms. Jamelle Banks, Ms. Seleda Perryman
We request non-substantive changes to the most recent revision of the National Children’s Study (NCS) Vanguard (Pilot) Study protocol approved by the Office of Information and Regulatory Affairs (OIRA).
Vanguard Study Collections
1. Justification for Including the Pregnancy Loss, Stillbirth, Neonatal Death (PLSND) Instruments
The NCS proposes to add the PLSND instruments to the protocol approved for use in the NCS Vanguard (Pilot) Study (approved by OIRA on 8/31/2012). Information about pregnancy losses and neonatal deaths, from women enrolled in the Study is important to test as the Main Study seeks to investigate the relationships between perinatal loss and environmental factors in a longitudinal cohort study. The PLSND Interview (Attach A1.) and SAQ (Attach A2.) will be initiated when NCS field contractors learn that a woman enrolled in the NCS experiences a loss of her child through miscarriage (including if the information is learned at Pregnancy Visit 1 or 2 [PV1 or PV2]). Please see the section below “Initiation of the instrument” (paragraph 3, page 3) for more detailed information. Among the additional PLSND related materials (Attach A3. – Attach A10.) are scripts and such items as medical record and death certificate release forms.
Description
The PLSND Interview is designed to collect information on pregnancy losses (e.g., miscarriages and stillbirths) and neonatal deaths from women enrolled in the Vanguard Study, and cover the following topics: (1) outcome of most recent pregnancy; (2) prenatal care; (3) pregnancy complications; (4) causes of pregnancy loss and/or baby’s death; (5) support resources; and, (6) obstetric history. The instrument also asks women to review and sign a medical records release form (Attach A3.) and (if applicable) death certificate release form (Attach A4.) for the deceased baby. These release forms are requested in order to gain more detailed information from the woman’s and, if applicable, the baby’s healthcare providers about the loss and/or death. The data collected through the PLSND Interview will provide the NCS with self-reported and medically-based information about the characteristics of pregnancies that end in a loss (e.g., miscarriage, stillbirth, or infant death), as well as the reproductive history of women who experience pregnancy loss, and/or whose infants experience neonatal death.
Development & Design
During the design of the PLSND Interview, a literature review and a review of existing surveys was conducted to identify questions that would address the topics of interest for this instrument. This review found relevant questions in the following surveys: (1) National Pregnancy and Health Survey: Drug Use Among Women Delivering Live Births, 1992; (2) National Maternal and Infant Health Survey, 1988, Mother’s Questionnaire; (3) National Maternal and Infant Health Survey, 1988, Hospital Questionnaire; (4) National Survey of Family Growth, Cycle VI, 2002; (5) National Violence Against Women Survey; and (6) National Youth Survey, Wave VII, 1987. Several questions in the PLSND Interview are based on questions found in these surveys, but the exact wording from the original sources could not be maintained because none were brief, stand-alone instruments on this topic. All required re-wording for use in the PLSND Interview in order to succinctly capture all data needed for the NCS. None of these data sources provided adequate information on pregnancy losses and/or neonatal deaths to replace the need for this instrument’s inclusion in the Vanguard Study protocol.
We anticipate that a subset of women enrolled in the Vanguard Study will lose their pregnancies or experience the death of an infant. The NCS will enroll approximately 750 women in the Provider Based Sampling arm of the Vanguard Study on who we will monitor pregnancy outcomes. The estimated rate of miscarriage is about 15-20% of pregnancies1, the estimated rate of stillbirth is approximately 0.6% (or 6.2 stillbirths per 1000 pregnancies)2, and the estimated rate of infant death is 0.7% (or 7 infant deaths per 1000 infant births)3. We expect that these rates will be similar among Vanguard Study participants.
Best practices in asking sensitive questions were incorporated into the design of this instrument. We conducted interviews with experts on pregnancy loss and infant death and reviewed literature on asking sensitive questions to develop the instrument. Best practices incorporated into the instrument include: (1) the use of lead-in language to provide some background information about why the questions are being asked, acknowledge the sensitivity of the topic, and remind respondents of relevant confidentiality assurances and options to opt-out; (2) avoidance of certain sensitive terms such as “fetus”; and, (3) offering multiple mode options for instrument administration, so that women can select the option with which they are most comfortable.
Also incorporated into the development of the instrument is consideration given to the possibility that some questions might not be answered by participants. Other surveys that include questions prone to ‘non-response’ have analyzed these questions in terms of percentage response and developed a range of ‘non-response’ rates. To address this anticipated issue of ‘non-response’ to specific items in this instrument, PLSND Item NR Rates (Attach A5.) provides item non-response rates for the source questions from these data sets (indicated by an asterisk), as well as non-response rates for similar questions asked across the data sets. As shown in Attach A5., PLSND Item Non-response Rates , rates of missing data due to respondents’ refusal or not knowing the answer (“don’t know”) are extremely low for these questions despite their sensitive nature. Data indicate that there will likely be very low levels of non-response to the survey questions on these topics that are included in the proposed instruments.
Delivery of Instrument – Operational Considerations
During a pilot study of 9 participants who tested the instrument and related forms, we found:
Overall, respondents were willing and able to answer all questions. Participants did not experience any significant problems in understanding the questions and did not express any major difficulties in discussing their losses.
Participants noted that they might have felt some discomfort discussing the loss soon after it occurred, and several suggested waiting 6 weeks to 3 months before asking for medical record releases.
About half of the respondents felt that a telephone interview may be easier to complete than an in-person interview.
Two participants said they would likely not give permission for access to their medical records related to their pregnancy.
In light of these findings, we propose two main operational procedures: (1) Initiation of the Instrument and (2) Administration of the Instrument.
Initiation of the Instrument
The PLSND Interview will be initiated when NCS field contractors learn that a woman who has already been enrolled in the Study experiences a loss of her child through miscarriage (voluntary or involuntary), stillbirth, or infant death. Instead of continuing with the study visit, NCS field contractors will ask participants if they are willing to complete the PLSND Interview. If they agree, they will be given the opportunity to select their preferred mode of administration for the instrument (in-person, telephone, or mailed self-administered). We anticipate that NCS field contractors will usually be apprised of the pregnancy loss or infant death near the start of a study visit. The NCS Vanguard Study has already had enrolled pregnant women who lose pregnancies, and each field contractor has handled follow-up with these women differently. Sometimes, however, as was suggested in the pilot study, it may be more effective to wait several weeks or months after the loss before contacting the women in order to increase their willingness to respond. To this end, participants who refuse will be given a “cooling off” period before any attempts at refusal conversion are made. The duration of the period will be tailored to the specifics of each case as there will be variability in when the NCS is made aware of the death.
Administration of the Instrument
The NCS requests the PLSND Interview to be offered in three modes of administration (in-person, telephone, and mail). Request of multiple modes allows women to select the mode in which they are most comfortable answering sensitive questions about their pregnancy loss and/or infant death. Computer-assisted (web-based) interviewing systems will be employed for in-person and telephone data collection activities. Self-administered forms will be designed in user-friendly formats to reduce the amount of time necessary to complete them. The wording of the questionnaires followed plain language guidelines and were pre-tested using cognitive testing to ensure readability and comprehension followed by pilot testing the instrument under actual in-person survey conditions and providing input for any final revisions that might be needed.
Overall, we anticipate the inclusion of the PLSND instruments into the Vanguard (Pilot) Study will provide important information as to the experience and impact of fetal and/or infant loss.
List of Attachments
Attach A1. PLSND Interview
Attach A2. PLSND Follow-Up SAQ
Attach A3. PLSND Medical Records Release Form
Attach A4. PLSND Death Certificate Release Form
Attach A5. PLSND Item NR Rates
Attach A6. PLSND Follow-up SAQ and Release Forms Letter
Attach A7. PLSND Telephone Medical Records Release Forms Letter
Attach A8. PLSND Telephone Medical Records and Death Certificate Release Forms Letter
Attach A9. PLSND Mail Reminder Call Script
Attach A10. PLSND Telephone Reminder Script
Attach A11. Multi-Mode Introductory Script
2. Alternate Mode Administration and minor changes for Pregnancy Visits 1 and 2
We propose that the following instruments approved for use in the Vanguard Study be administered in alternate modes as listed below (Table 1). Offering several modes would maximize flexibility for the participants and investigators while ensuring scientifically robust data collection for the Vanguard Study. There is no anticipated increase in participant burden.
Table 1: Proposed Alternate Modes of Instrument Administration, by Phase 2 Instrument |
||||
Item |
Instrument Name |
Currently Approved |
Proposed Change |
|
|
|
Mode |
Clearance |
Mode |
1 |
Pregnancy Visit 1 Interview |
In-Person |
8/31/2012 |
Plus Phone/Mail/Web |
2 |
Pregnancy Visit 2 Interview |
In-Person |
8/31/2012 |
Plus Phone/Mail/Web |
We also propose to add follow-up questions about the father’s knowledge of the pregnancy and participation to Pregnancy Visit 2. These questions initially arise in the Pregnancy Visit 1 SAQ.
List of Attachments
Attach A12. Pregnancy Visit 1 Interview
Attach A13. Pregnancy Visit 2 Interview
3. Participant Satisfaction Questions added to the Validation Interview and the Tracing Interview
We propose to include three questions that aim to determine participant satisfaction with the Vanguard Study into the Pregnancy Visit 1 SAQ (Attach A14.) and to the Validation Interview (Attach A15). To date, these questions have not been administered in the Vanguard Study. The questions consist of two close-ended questions concerning the participant’s overall experience with the Study and the participant’s view on interview length, and one open-ended question asking participants to tell us about their own or their child’s experience with the Vanguard
We also propose an additional question in the Tracing Interview (Attach A16.) that collects any additional comments the participant may have about their experience with the NCS.
Data collection on participant satisfaction enables the NCS to learn from past experience, adjust study visit assessments (as necessary), and, in turn, improve participant retention rates. Upon re-timing the instrument, there was no change to the estimated participant burden.
List of Attachments
Attach A14. Pregnancy Visit 1 SAQ
Attach A15. Validation Instrument
Attach A16. Tracing Interview
4. Provider Frame Questionnaire Refusal Conversion
The Provider-Based Sampling “arm” of the Vanguard Study (approved for three Study Centers) requires information from providers of prenatal care about practice parameters In order to compute a measure of size (e.g., the number of first prenatal care visits or the number of births) for each provider location, two of the three Study Centers are collecting this information from prenatal care providers through administration of the Provider Based Sampling Frame Questionnaire (PFQ). The third site is obtaining the needed information via birth records, and thus, is not interacting with providers for this component of the study. Since obtaining clearance from OMB/OIRA on 8/31/2012, the two PBS Feasibility Study locations have had moderate success in completing the PFQ; however, of the 109 PFQs distributed to prenatal care provider offices, 42 remain pending. Table 2 below indicates the number of provider locations that completed the PFQ and the number of provider locations that remain pending. Some PFQs pending completion may represent soft refusals from the providers. In order to obtain a sufficient sample of provider locations with completed PFQs, the NCS proposes to send a letter (Attach A17.) asking provider locations that refuse to complete the PFQ to reconsider their decision about providing information to help this research effort.
Table 2: PFQ Refusal Conversion Status |
||||||
Study Center |
PFQs sent |
Completed |
Pending |
Final Refusals |
Pending Refusals |
Final Ineligible |
Jefferson County, KY |
36 |
23 |
10* |
1 |
0 |
2^ |
Worcester County, MA |
75 |
42 |
26 |
0 |
0 |
7* |
Jefferson County, KY
*Still waiting to receive 4 PFQs; waiting for clarification on 6.
^Location #003 – no prenatal visits; location #020 closed.
Worcester County, MA
*Includes the 2 locations that participated in the NCS Provider-Based Recruitment strategy in Providence County, RI.
The NCS also spoke with staff from the National Center of Health Statistics who lead the National Ambulatory Medical Care Survey (NAMCS) (OMB #0920-0234) to determine methods used in that survey to recruit physicians and physician offices. Of note, their unweighted and weighted response rates were 62.1% and 62.4%, respectively, but the NAMCS is a more in depth and burdensome data collection than is the PFQ (an estimated 20 minutes of burden per response) that is being administered as part of the NCS. NAMCS staff suggested that an advance letter be sent describing the data collection effort. They recommend following this with a call to make an appointment to visit the office to complete the form. They emphasized that the best results occur with a visit to the office to collect the information in a face-to-face interview. If it is not possible to make an appointment, they suggested that study staff stop by the office, preferably in the morning when practices tend to be less busy, and introduce the data collection effort to identified key personnel. The NCS is already implementing many of these procedures but we will re-emphasize these best practices to our Study Centers.
List of Attachments
Attach A17. PFQ Refusal Letter for Providers
Formative Research Projects
1. LOI2-QUEX-14-Phase 2 Dietary Assessment in Infants and Young Children
One proposed formative research project as described below (Table 3) aligns with the current scope of work for the NCS Vanguard Study. The NCS Vanguard Study is designed to assess the feasibility (technical performance and reliability), acceptability (impact on study participants and study infrastructure), and cost (level of effort, personnel, resources, and money) of recruitment, study visit measures, and study logistics to inform the NCS Main Study. Each of these proposed formative research projects would evaluate the methods to be considered for the NCS Main Study at minimal participant burden and cost when compared with direct implementation within the NCS Vanguard Study overall.
Additionally, formative research projects do not feature aspects that would interfere with the comparison of recruitment and retention strategies described in the Alternate Recruitment Substudy and the Provider Based Sampling Substudy of the NCS Vanguard Study. Language in the NCS Phase 2 Vanguard Study Supporting Statement A (approved by OIRA on 4/13/2011) supports formative research recruiting either NCS Vanguard Study participants or their demographically similar peers.
Phase 2 of LOI2 QUEX-14, a multi-site formative research project, will help inform the NCS on the feasibility, acceptability and costs of using the ASA24 Dietary Assessment in infants and toddlers, compared to 24-hour dietary recall, the current gold standard for dietary assessment. ASA24 was developed by the National Cancer Institute (http://riskfactor.cancer.gov/tools/instruments/asa24/), is self-administered and non-proprietary. Evaluating feasibility, acceptability and cost of ASA24, reducing burden on participants and evaluating the scientific robustness of the proposed measures by acculturation status will be goals of Phase 2 of LOI2 QUEX-14. The table below provides the purpose of the study, the annual burden and other important information regarding LOI2-QUEX-14 Phase 2: Improving Dietary Assessment in Infants and Children.
Table 3: LOI2-QUEX-14-Phase 2 Summary |
||||
Project Title |
Purpose |
Proposed Information Collection |
Rational for Inclusion in Vanguard Study |
Respondent Burden Hours (annual) |
Improving Dietary Assessment in Infants and Children |
To compare feasibility acceptability and cost of the Automated Self-Administered 24-hour Recall (ASA24) methods and Infant and Child Feeding Questionnaires. Additionally, measures of acculturation status will be evaluated. |
Multiple days of 24-hour dietary recall and ASA 24 |
Utilization of the Web-based ASA24 tool may reduce participant burden and will improve accuracy in participant response compared to the paper-based Infant and Child Feeding Questionnaires. |
1,322 |
List of Attachments
Attach B1. LOI2-QUEX-14 OIRA Template
Attach B2. LOI2-QUEX-14 Exemplar Consent Form
Attach B3. LOI2-QUEX-14 SocioDemographics Questionnaire
Attach B4. LOI2-QUEX-14 ASA24 Instructions
Attach B5. LOI2-QUEX-14 ASA24 Protocol
Attach B6. LOI2-QUEX-14 Food Diary Instructions-Parent
Attach B7. LOI2-QUEX-14 Infant Acceptability #1
Attach B8. LOI2-QUEX-14 Infant Acceptability #2
Attach B9. LOI2-QUEX-14 Child Acceptability #1
Attach B10. LOI2-QUEX-14 Child Acceptability #2
Attach B11. LOI2-QUEX-14 Infant Feeding Questionnaire
Attach B12. LOI2-QUEX-14 Child Food Questionnaire
Attach B13. LOI2-QUEX-14 IRB Approval Letters
Attach B14. LOI2-QUEX-14 Manual of Procedures
Attach B15. LOI2-QUEX-14 Exemplar Recruitment Flyer
Attach B16. LOI2-QUEX-14 Recruitment Screener
2. Specimen Collection in the Provider-Based Sampling Cohort
The Provider-Based Sampling (PBS) Feasibility Study is expected to yield an N=750 births from across the three Study Centers participating) (60-day FRN published on 1/30/2012 and 30-day FRN published on 4/26/2012; approved by OIRA on 8/31/2012). The primary rationale for inclusion of biospecimen samples during the birth and immediate post-birth period in the PBS cohort is to expand our understanding surrounding the feasibility, acceptability, and cost of these collections in hospitals / birthing centers during or after the birth event.
The NCS will be recruiting two groups of women: women prenatally at the provider location and women during their birthing event at hospital/birthing centers. The NCS will compare the rates of consent for specimen collection for women recruited in the provider setting compared to those women recruited in the hospital/birthing centers. In the Initial Vanguard Study, our prior experience of biospecimen sample collection during the birth and immediate post-birth period (maternal blood, cord blood, placenta and umbilical cord, child heel stick and meconium) examined specimen collection primarily from women enrolled in the study prior to or during pregnancy with only about 1% recruited perinatally.
Yet, data and experience is lacking for specimen collection for those women enrolled at the hospital/birthing center for the birth event. We need to recruit both groups in PBS in order to compare specimen collection rates for those consented in the hospital to those consented in the provider setting.
Additionally, this pilot study will guide the development of refined acquisition SOPs, coordination pathways between NCS and hospital / birthing center staff, and standardization surrounding transport and long-term storage.
We propose collecting maternal blood, maternal urine, a second blood card from the child heel stick, cord blood and placenta tissue (or the entire placenta, if possible) from 100 mother/baby pairs, for a total of 100 specimens of each specimen type (for more details, please see Attachment B17. Vanguard Pilot Study Biospecimen Collection Chart). After 100 specimens of each type are acquired, specimen collection in PBS will cease. Each of these collections will be performed by either the participating hospital or birthing center staff (default) or Study staff during routine collection procedures. The NCS will rely upon the hospital and birthing center processes currently in place and will receive the specimens ‘as is’. Participating NCS Study Centers will develop formal agreements with participating hospitals or birthing centers that will define the respective roles, expectations and potential burden on hospital / birthing center staff and Study Center staff in the processes of identification of eligible participants, participant provision of study related information, obtaining informed consent, collection of interview data, collection of medical record data, and collection of biospecimens. Through the formal arrangements made by the study center with the hospitals, burden can be assessed by secondary sources such as invoices; these figures can then be factored into the analysis of the feasibility and acceptability of collecting sample in hospitals at the time of birth. We expect there to be variations in the case of individual Study Center and hospital combinations and are prepared to deal with this by tailored contractual agreements. These may vary in the case of individual Study Center and hospital combinations.
Consent Procedures: Specimen Collection
Informed consent for sample collection will be administered during the birth hospitalization after the labor in the hospital/birthing center or when previously recruited in the provider setting. Informed consent will be administered in-person if administration of the Provider-Based Sampling Eligibility Screener - Hospital (Approved by OIRA on 8/31/ 2012) identifies a woman who recently gave birth and is eligible to participate in the NCS. Once an eligible woman is identified, data collectors will administer the PBS Participant Verification Birth Cohort Interview (adapted from Participant Verification Interview, approved by OIRA on 8/31/2012; Attach B25.) to ensure the eligible woman is the legal guardian of the child, and, therefore, is able to provide informed consent for the child. The appropriate consent forms will be obtained during recruitment in the provider’s office for those participants recruited prior to the birth event. Consent rates from the administration of the Parental Permission for Child’s Participation: (With Samples) (Approved OIRA on 8/31/2012, Revised this Submission Attach B18.) conducted in the hospital/birthing center will be compared to consent rates from the provider setting.
To maintain privacy, data collectors will identify a private setting for the administration of the informed consent process and for all data collection done at the hospital / birthing center. Biospecimens will only be obtained if the enrolled woman provides written informed consent to collect specimens from herself and the enrolled child. If the participant does not provide written informed consent for some or all specimen collections, then the specimen(s) will not be collected.
If biospecimens are not collected by the hospital / birthing center, data collectors will obtain written informed consent for the woman’s participation using the New Adult Informed Consent Form (Approved by OIRA on 8/31/2012) either at the hospital/birthing center or previously during enrollment in the provider’s office. When administering the New Adult Informed Consent Form (Approved by OIRA on 8/31/2012), data collectors will also administer any required HIPAA authorizations. Data collectors will then obtain written informed consent for the child’s participation through 6 months of age using the Parental Permission for Child’s Participation: Birth through 6 Months of Age (Without Samples), (Approved by OIRA on 8/31/2012). Lastly, data collectors will present the Sample Collection Visit Information Sheet Scripts (OMB 0925-0593, Approved by OIRA on 8/31/ 2012; Revised this Submission Attach B24.) to obtain verbal permission for the PBS Birth Cohort Birth Event data collection activities.
If biospecimens are collected by the hospital / birthing center, data collectors will obtain written informed consent for the woman’s participation and sample collection permissions using New Adult Informed Consent Form (Approved by OIRA on 8/31/2012) when the participant is enrolled and will administer any required HIPAA authorizations. Secondly, data collectors will present the Sample Collection Visit Information Sheet Scripts to obtain verbal permission for the PBS Birth Cohort Birth Event data collection activities. Data collectors will then obtain verbal consent for sample collections from the mother (i.e., adult blood and urine) using the Sample Collection Visit Information Sheet Scripts. Finally, data collectors will obtain written informed consent for the child’s participation through 6 months of age and permissions for child sample collections (i.e., cord blood, placenta, and infant heel stick) using the Parental Permission for Child’s Participation: (With Samples).
Collection Procedures: Child Specimens
Placenta and Cord Blood
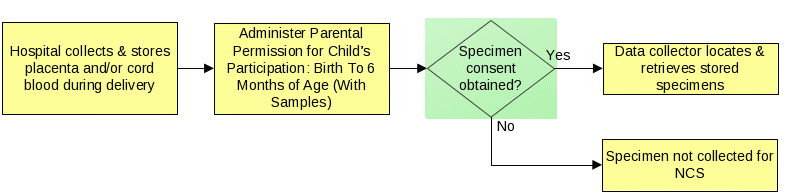
The placenta and cord blood specimens will only be collected retrospectively from hospitals routinely collecting and storing these specimens as part of clinical practice; data collectors will recover specimens collected by hospital staff during delivery. Data collectors will locate and pick up stored placenta and cord blood specimens. If stored placenta and/or cord blood specimens are unavailable, then the specimen(s) will not be collected for the NCS. Additionally, cord blood samples will not be obtained from women who are banking cord blood. The figure below describes this process.
Infant Heel Stick
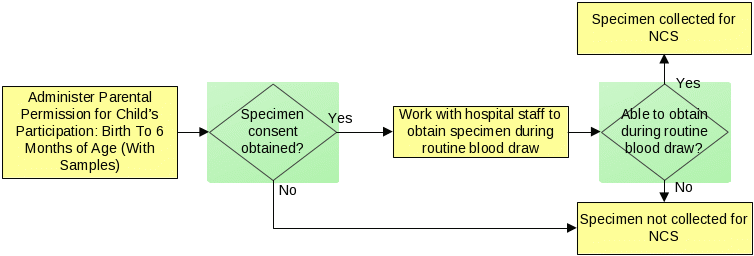
The infant heel stick specimen will only be collected as part of routine hospital procedures; no additional heel punctures will be performed to collect this specimen. Data collectors will work with hospital staff to obtain about 7 additional drops of the child’s blood during a routine heel stick. If this specimen cannot be obtained during routine specimen collection, then this specimen will not be collected for the NCS. The figure below describes this process.
Collection Procedures: Maternal Specimens
Maternal Blood
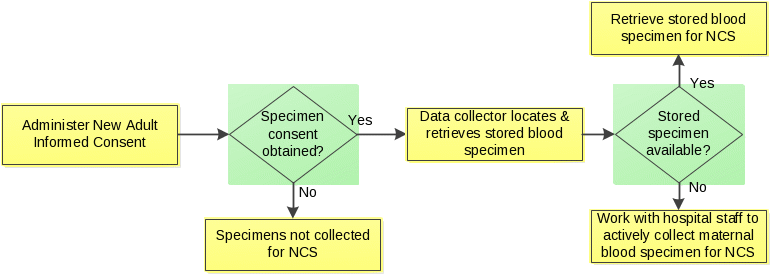
Blood may be obtained either from stored hospital samples collected during the labor and delivery event or by collection of new specimens. If a specimen the hospital collected before the baby’s birth is available, then data collectors will retrieve that specimen. If an existing blood sample is not readily available, a new blood draw will occur to obtain about 2 tablespoons of blood. Depending upon contractual arrangements with a given hospital, the blood specimen will be collected either by an NCS data collector or paid hospital staff during the birth hospitalization. The figure below describes this process.
Maternal Urine
Urine may be obtained either from stored hospital samples collected during the labor and delivery event or by collection of new specimens during the birth hospitalization interview. If a urine specimen is not readily available at the hospital, then participants will be provided with a collection cup and directions for collecting a fresh specimen. The figure below describes this process.

Interview Data Collection
The NCS proposes to administer a series of questionnaires pertaining to each specimen collected that will total 42 minutes of interview data from the mother (Attach B19.-B23.). If the specimen is unable to be collected, then the interview will not be administered to the participant. The Biospecimen Adult Blood Instrument (Attach B19.) and Biospecimen Adult Urine Instrument (Attach B20.) collect necessary information including recent food consumption, previous issues with blood draws, and other information that could impact the specimen or the collection of it. The Biospecimen Infant Blood Spot Instrument (Attach B21.), Biospecimen Cord Blood Instrument (Attach B22.), and the Biospecimen Placenta Data Collection Instrument (Attach B23.) will be completed by the NCS data collector regarding the sample collected (if no sample is collected, instrument will not be completed).
Estimates of Annual Hour Burden
Data Collection Activity |
Type of Respondent |
Estimated Number of Respondents |
Estimated Number of Responses per Respondent |
Average Burden Hours Per Response |
Estimated Total Annual Burden Hours |
Questionnaires* |
Mothers |
100 |
1 |
42/60 |
70 |
Specimen Collection – Blood and Urine |
Mothers |
100 |
1 |
10/60 |
17 |
Specimen Collection – Heel Stick |
Infants |
100 |
1 |
5/60 |
8 |
Specimen Collection, Storage, and Retrieval** |
Hospital staff |
100 |
1 |
15/60 |
25 |
TOTAL |
|
300 |
|
|
120 |
*The PBS Participant Verification Birth Cohort Interview is a shortened version of the Participation Verification Questionnaire, for which sufficient participant burden has already been requested.
**We have requested the maximum potential burden to account for formal arrangements that result in the hospital staff member collecting and storing samples being paid by the NCS.
Annualized Cost to Respondents
Data Collection Activity |
Type of Respondent |
Estimated Total Annual Burden Hours |
Hourly Wage Rate |
Respondent Cost |
Questionnaires |
Mothers |
70 |
$10/hr |
$700.00 |
Specimen Collection – Blood and Urine |
Mothers |
17 |
$10/hr |
$170.00 |
Specimen Collection – Heel Stick |
Infants |
8 |
$0/hr |
$0.00 |
Specimen Collection, Storage, and Retrieval |
Hospital staff |
25 |
$10/hr |
$250.00 |
TOTAL |
|
120 |
|
$1120.00 |
List of Attachments
Attach B17. Vanguard Pilot Study Biospecimen Collection Chart
*Attach B18. Parental Permission for Child’s Participation – Birth Through 6 Months of Age (with Samples)
Attach B19. Biospecimen Adult Blood Instrument
Attach B20. Biospecimen Adult Urine Instrument
Attach B21. Biospecimen Infant Blood Spot Instrument
Attach B22. Biospecimen Cord Blood Instrument
Attach B23. Biospecimen Placenta Data Collection Instrument
*Attach B24. Sample Collection Visit Information Sheet Scripts
Attach B25. PBS Participant Verification Birth Cohort Interview (extracts 3 questions – child’s name, sex, and legal guardian – from the approved Participant Verification)
* Revised this Submission
Table 4. Respondent Burden Table for Candidate Projects (OMB Collection # 0925-0593, Expiration Date 8/31/2014) |
||||||
Formative Research Project Number |
Project Title (Abbreviated) |
Type of Respondent |
Number of Respondents |
Responses per Participant |
Burden per Response (in hours) |
Total Hour Burden |
LOI2-QUEX-14 |
“Dietary Assessment in Young Infants and Children” |
Mother / Child / Infant |
610 |
14 |
9 / 60 |
1,322 |
PBS BV First Specimen Collection |
“Biospecimen Collection at Birth Visit in PBS Hospital-Based Cohort” |
Mother / Infant / Hospital Staff |
300 |
1.33 |
18 / 60 |
120 |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Nolen Morton |
| File Modified | 0000-00-00 |
| File Created | 2021-01-30 |
© 2026 OMB.report | Privacy Policy