Project Management IT/Database Design Plan
Attachment B. 3-14-13.docx
Enhancing Substance Abuse Treatment Services to Address Hepatitis Infection Among Intravenous Drug Users Hepatitis Testing and Vaccine Tracking Form
Project Management IT/Database Design Plan
OMB: 0930-0300
Attachment B
Project Management IT/Database Design Plan
Enhancing Substance Abuse Treatment to Promote Healthy Lifestyles through Addressing Hepatitis Infection among Injection Drug Users HHSS283200700053I/HHSS28342001T (Reference No. 283-07-5302)
Version 1.0 draft 1
Prepared by Randolph Edmead
DB Consulting Group, Inc.
January 2011
Table of Contents
1. Introduction............................................................................................................3
2. Project Management Database..............................................................................4
3. Hepatitis Vaccine Program Mamgement..............................................................5
4. Software Use and Website Development .............................................................9
Project Management IT/Database Design Plan
Introduction
A data system is extremely critical to efficient execution of the tasks of this project. It significantly reduces the labor required to manage project activity and technical assistance delivery as well as assembles information for reports to the TOO. A project management information system (MIS) enables DB to share information about technical assistance activities quickly and completely, assuring we miss no deadlines or confuse assignments. For this project, DB will implement and operate a project MIS in accordance with the following principles:
The Project MIS supports the needs of the TOO for information about project activities: Routine progress reports (monthly and annual), analytic reports, and special inquiries by the TOO regarding the volume, type, cost, location, and method of technical assistance delivered under the contract must be supported by the data system.
The Project MIS is easy to use: Users tend to avoid software that is hard to learn, difficult to interpret, or cumbersome to navigate. When users substitute paper systems, even temporarily, data quality suffers, with attendant loss of utility of reports.
The Project MIS is accessible to staff both onsite at DB and remotely: A web-based system greatly reduces the effects of distance, avoids redundant data entry, and helps ensure coordination of activities.
The Project MIS supports decision making by project staff: Data systems succeed when their perceived burden, in the form of labor for data entry, is offset by their perceived value, in the form of useful information. This value is contained in reports, particularly reports for use by the project managers and the TOO. We identify these as reports that support:
T
 racking
each action or assignment through each stage of delivery
racking
each action or assignment through each stage of deliveryPrioritizing project activities and needs
Analyzing critical performance data
Developing budgets and tracking costs for each assignment
Assessing progress in meeting project schedule and objectives
Identifying emerging issues and the consultants, training approaches, documents, and other materials needed to address those issues.
Project Management Database
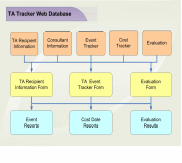
We will adapt our web-based Technical Assistance Management System to support the further implementation and maintenance of this project. Our easy-to-use Technical Assistance Management System, which we developed to manage technical assistance and training for SAMHSA contracts, is specifically designed to meet the needs of this CSAT-funded project. It will only require minor modifications to adapt its functions to the requirements of this task order. As illustrated in Exhibit 2.2, the Technical Assistance Management System combines event/action tracking and a consultant resource database in a single web-accessible system that permits us to manage project events and our consultant pool simultaneously. The database consists of five main tables: consultants, technical assistance recipients, events, costs, and evaluations. To ensure data integrity, none of the tables is accessible to anyone other than the system administrator. Users employ online screens to enter data to the appropriate table. Historical data are not overwritten, so changes can be tracked when necessary. A full set of reports is available, including data for monthly progress reports and the project's annual reports.
As required for this project the database will be created to efficiently plan, collect, organize, manage, and maintain project-related information, and they will use this information to track and monitor project activities and prepare reports. As project activities unfold, project staff will enter data, including the name, address, telephone number, title, and affiliation of the requestor; the nature of the technical assistance requested; specific information related to all requests (e.g., date of request, date of approved on-site visit, summary of actions responding to the request); timetables for actions; and any other pertinent information. Staff also will use the project MIS to plan, organize, track, and manage project activities. We will use the system to establish and maintain complete records on participating OTPs, service-associated costs, project events, event evaluations, and consultants. Summary reports are included with each monthly and annual progress report, and updates of these records will be provided to the TOO upon request.
We use the Cost Tracker to establish budgets, monitor expenditures, and record the total cost of each intervention. This allows us to evaluate the cost effectiveness of the demonstration, as well as provide any necessary cost data to CSAT. The Event Tracker allows us to track information on all project activities (e.g., date of event, date of approved actions, summary of actions responding to the request, etc.); timetables for actions; and other pertinent information. The Event Tracker can track multiple activities for each organization. This allows us to monitor and report all the resources assigned to a single recipient.
The Evaluation Table allows each individual action or intervention to be evaluated as well as aggregates the evaluation data over time. The data for each event are entered into the table as they are submitted.
DB will maintain both electronic and hard copies of the consultant database and upon the TOO's request provide a hard copy of the consultant database list. The Consultant-Expert table will contain information about areas of specialization and a history of the individual's experience with the project. The system allows us to monitor all the assignments given to a particular consultant. It is also used to generate consultant contracts, monitor vouchers, and fulfill reporting requirements. This feature allows us to closely monitor all expenses to ensure that technical assistance is being provided in a cost-effective manner.
Our Technical Assistance Management System allows us to use a systematic feedback process to ensure the project team is performing quality work within the work plan requirements.
The Senior Project Director and IT staff will ensure that SAMHSA and IT standards and guidelines are met. The database design will incorporate quality assurance and control processes. All modifications are based on the TOO’s comments and will be submitted prior to implementation.
Hepatitis Vaccine Program Management
DB has developed a system to support the main components of the medical management of hepatitis infection. This system was developed to manage SAMHSA hepatitis support program for opioid treatment clinics. These components include:
Testing for hepatitis A, B and C infections;
Education and counseling regarding at-risk behavior and hepatitis transmission, acute and chronic hepatitis infection, liver disease and its care and treatment;
Vaccination against hepatitis A and B infection; and
Integrative primary care as part of the comprehensive treatment approach for recovery from opiate abuse and dependence.
The bulk of DB’s support of these programs is the management of vaccine purchasing, shipping, and tracking. The following high-level diagram illustrates linked processes from a clinic’s point of entry through to the clinic support and feedback. The supply of vaccination begins with a careful of assessment of clinics’ needs. This assessment forms the basis of the order to the pharmaceutical supply companies. The tracking of vaccine usage facilitates clinic feedback and support.
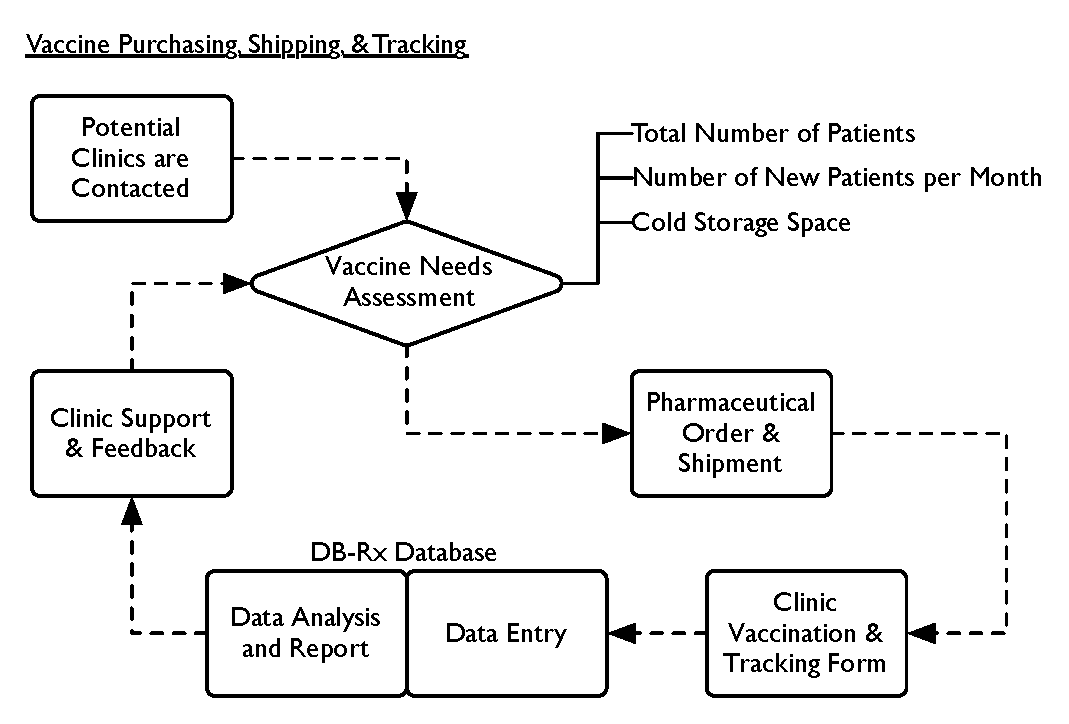
DB’s just-in-time shipping process uses intensive follow-up and feedback to sites. The communication loop maintains the supply chain and accountability among the clinics.
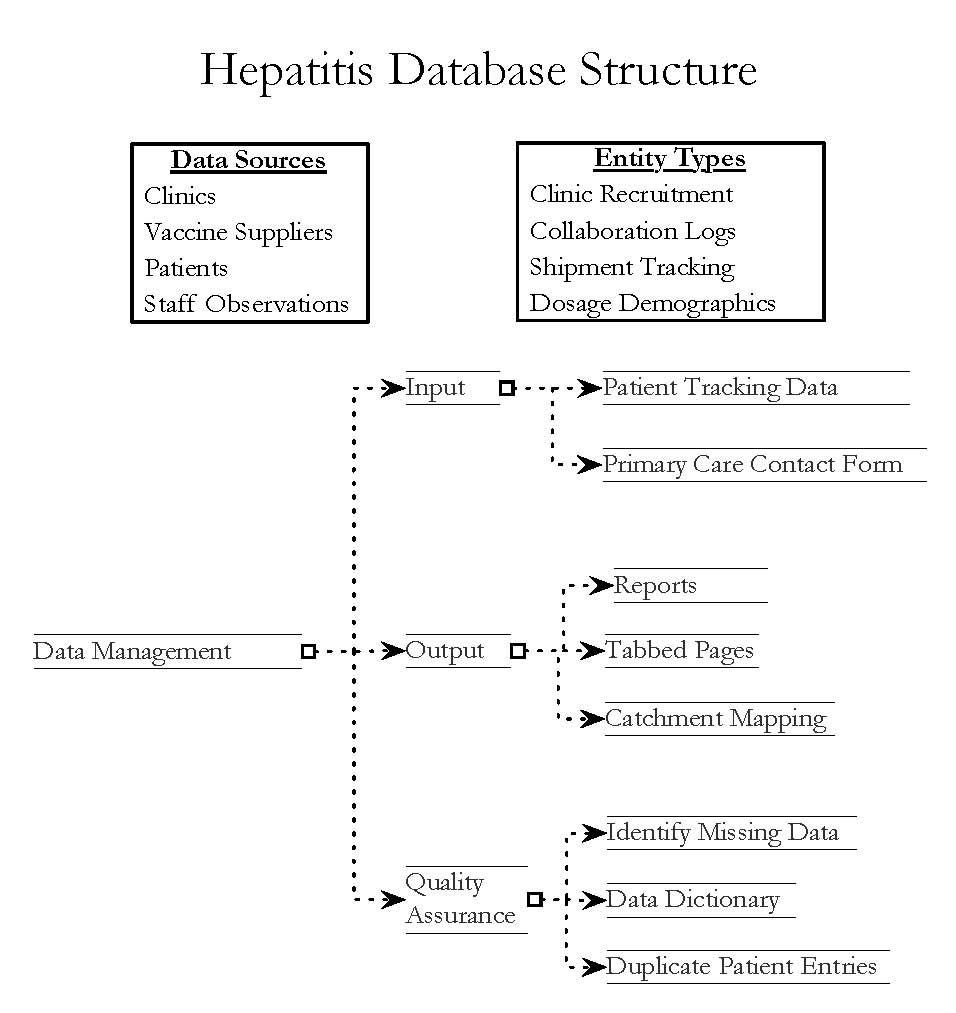
The database is designed to:
Manage site recruitment
Collect information from enrolled sites
Track SAMHSA clearance
Report on the sites enrolled
Track shipping of vaccines
Record prices paid for vaccines
Track patient demographics
Track vaccine dosage administration
Track hepatitis test administration
Create various reports on clinics and patients
Data collection:
DB data collection process minimizes respondent burden. Each respondent is asked to respond three times. The first time the respondent is asked to provide demographic information, vaccination history, and hepatitis testing information. The second and third times the respondent is asked to provide the date and lot number for subsequent vaccinations and/or HCV test. One month after intake, the clinics are asked to send the Hepatitis Test and Vaccine Tracking Form. This timeframe captures all of the information except for the date and lot number of the third dose. Consequently, after the third dose is administered (approximately six months after intake), the clinics are asked to send the third dose’s date and lot number.
Accurate data collection is the foundation to the tracking and vaccine supply processes. DB received OMB clearance for the data collection forms. The form captures information about dosage, testing, demographics, clinic identifiers, and risk factors. This data is the foundation of DB’s just in time delivery process. The design of the Hepatitis Test and Vaccine Tracking Form encourages the use of automation to reduce burden on participating opioid treatment programs. The form can be submitted using any of the four methods listed. The form can be completed within Microsoft Word and then emailed. The form can be completed by hand and faxed. The form can be completed by hand and mailed in pre-paid envelopes. Additionally, if the program previously collects the information needed on the form, they can send the information in the format in use. The use of information technology and the methods for transmitting the form will be determined by program based on the least burden for the opioid treatment program staff.
DB developed a routine data input process to insure correct data input. The input routine has three stages: 1) data entry, 2) control applied to inserted data, 3) correction of mistakes. Data entry was assigned to a dedicated staff member by clinic. This consistent assignment helps staff become familiar and therefore less likely to misinterpret changes between the submitted clinic forms. Along with the involvement of single data-managers per clinic, DB constructed controls applied to inserted data, including: combo-boxes and filters that prevent fields being in logical contradiction to other values. These controls reduce transcriptional mistakes. Routine 20 percent random sampling of the database provided quality assurance with manual comparison of the entered data to the original forms.
Data will be collected from:
Phone calls: site recruitment and site enrollment
Forms from the clinics
Output reports include:
DB developed an efficient reporting system for monthly reporting, summary reporting, total cost charting, graphs, and progression charts. These reports help staff and stakeholders track tasks, progress and accomplishments, planned work, issues and delays, financial budgets, OTP reporting, and site visits. Items tracked include:
Sites enrolled
# of Vaccines Shipped
# of Vaccines Used
Client Dosage and Testing
Database Diagram
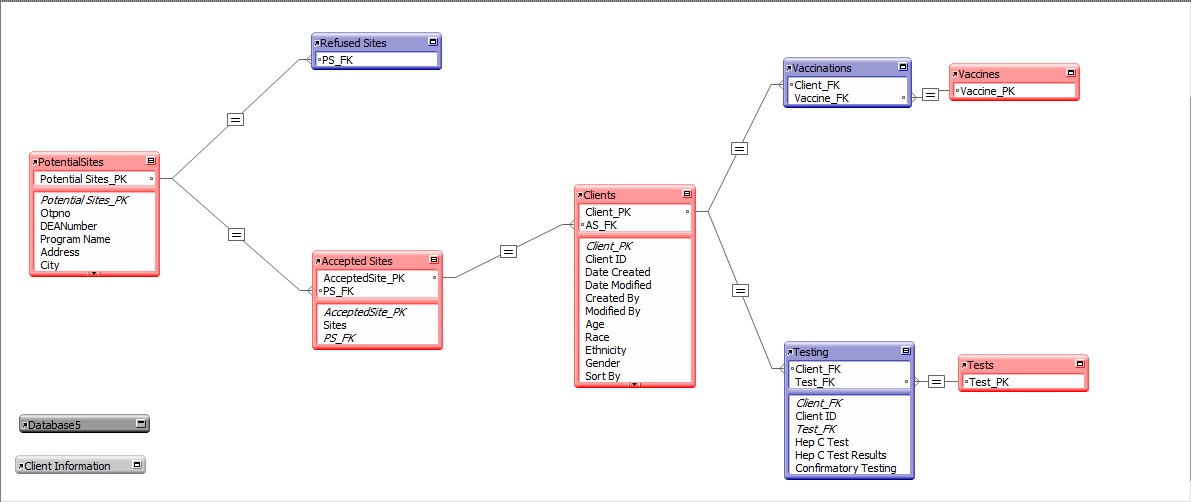
The vaccine program recruits clinics from across the country. Each clinic is associated with one location. All clinic-related information is stored in the site tables. Each clinic site has an identification number, DEA number, site name, and address. Some patients at the clinic participate in the hepatitis vaccine program. All patient related information is stored in the Clients table.
The entities required include:
Potential Clinic Sites
Enrolled Clinic Sites
Not-enrolled Clinic Sites
Assessment of Need
Shipping Orders
Vaccine and Testing Products
Suppliers
Clients
Doses
Testing
The Entities are related as follows:
A Customer can have One or Many Orders
An Order can contain One or Many Products (Order Lines)
A Product can be associated with One or Many Orders
A Supplier can supply One or Many Products
The design allows a Customer to place zero, one or many Orders. An Order can be for one or many Products, and this Product can be associated with zero, one or many Orders (the reason for the Linked Order Product table)
When asking questions of the database we need to know:
How many products have been ordered by a clinic
What date/time was a particular shipment made
How much vaccine and test kits has a clinic received to date
How much vaccine and test kits has a clinic used to date
What is the total cost for a certain shipment
Software Use and Website Development
DB uses software that meets SAMHSA Guidelines. Specifically, the systems are PC compatible, operate in a Windows environment, and use Microsoft Office Suite (Word; Excel; PowerPoint; Access; and Project), or other software consistent with SAMHSA/DMS-IT standards. DB, at all times, maintains compliance with current DMS-IT standards, which may change over the duration of this contract.
DB follows SAMHSA Internet/Web Site Policy guidance. Any development and production of Internet/web applications, including Intranets and Extranets shall comply with SAMHSA policy and procedures. These policies and procedures cover websites, web page linkages, and web development; and agency programmatic, concept, and technical clearances.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | anthony.campbell |
| File Modified | 0000-00-00 |
| File Created | 2021-01-30 |
© 2026 OMB.report | Privacy Policy