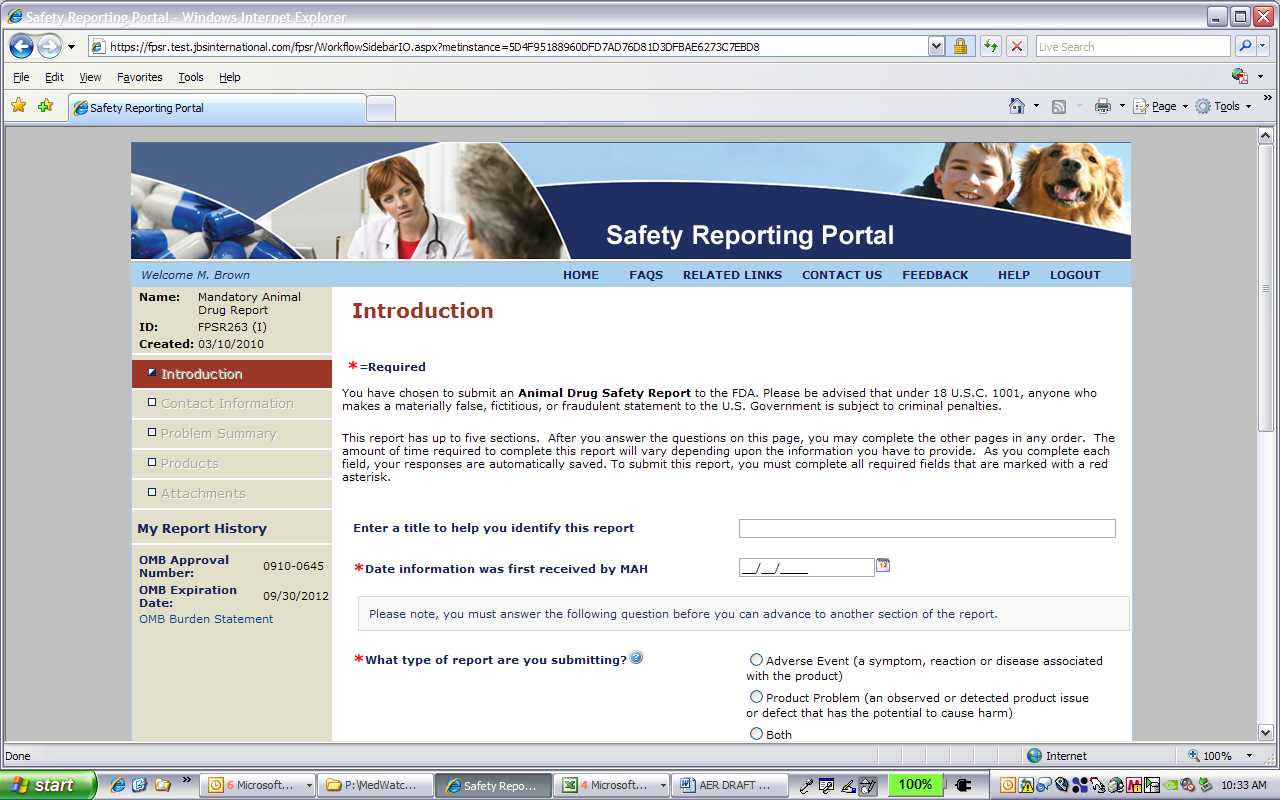
Mandatory Adverse Event Report via the SRP
Electronic Data Collection Using MedWatchPlus Portal and Rational Questionnaire--21 CFR 310.305, 314.80, 314.98, 514.80, 600.80, 1271.350 and Part 803
0645_Mandatory_pet_screenshot_3-11-2010[1]
Mandatory Adverse Event Report via the SRP
OMB: 0910-0645
⚠️ Notice: This form may be outdated. More recent filings and information on OMB 0910-0645 can be found here:
© 2026 OMB.report | Privacy Policy