NHANES_24-Hour_Urine_Pilot_Supporting_Statement_01152013_Final
NHANES_24-Hour_Urine_Pilot_Supporting_Statement_02282013.docx
National Health and Nutrition Examination Survey
NHANES_24-Hour_Urine_Pilot_Supporting_Statement_01152013_Final
OMB: 0920-0950
National Health and Nutrition Examination Survey
OMB No. 0920-0950
(Expires November 30, 2015)
Nonsubstantive Change to Conduct 24-Hour Urine Pilot Study
Contact Information
Vicki L. Burt, ScM RN
Chief, Planning Branch
NCHS/CDC
3311 Toledo Road, Room 4211
Hyattsville, MD 20782
Telephone: 301-458-4127
FAX: 301-458-4028
E-mail: [email protected]
February 28, 2013
Supporting Statement (Change)
National Health and Nutrition Examination Survey (0920-0950)
This is a request for a non-substantive change to the approval of the National Health and Nutrition Examination Survey (NHANES) (OMB No. 0920-0950, exp. November 30, 2015), conducted by the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention, to conduct a 24-hour urine pilot study. Burden for this project has already been approved; thus, no change to the burden is requested.
If the pilot test is successful a revision request to add a 24-hour urine component to the 2014 NHANES will be submitted.
A. Justification
Circumstances Making the Collection of Information Necessary.
There is compelling evidence indicating that excess dietary sodium intake is detrimental to cardiovascular health (e.g., hypertension, left ventricular hypertrophy, congestive heart failure, excess risk of coronary heart disease, stroke) for almost all population groups regardless of age, ethnicity, sex, or health status. Because of this evidence efforts are underway in the United States to adopt a population strategy to reduce dietary sodium intake by reducing sodium in the food supply.1 It is important to obtain population baseline data on sodium intake prior to any population based efforts to reduce sodium in the food supply and to monitor trends in intake at the national level subsequent to changes in the food supply.
In the clearance approved in November 2012, the summary of changes in Supporting Statement A (page 5) states: “Request for continued permission to conduct pilot or methodological testing for future NHANES will be submitted through a non-substantive change request.” This request is to conduct a 24-hour Urine Pilot Study.
The 24-Hour Urine Pilot Study is designed to assess the feasibility and test all procedures related to a 24-hour urine collection in the 2014 NHANES. Undertaking this pilot study will provide information that will be useful in determining the feasibility of the component and aspects of implementing a 24-hour urine collection as part of NHANES in 2014. The major objectives of the pilot study are as follows:
• To assess the feasibility and test all procedures related to the 24-hour urine collection
• Including the weighing, aliquoting and shipping procedures for the collected urine
• To estimate response rates
• To evaluate completeness of the urine collection
Study Protocol
The proposed pilot study will occur in three NHANES locations in early spring, 2013. The locations were selected based on the date of occurrence. Because the order and dates of NHANES locations are selected more than a year in advance with the intent of minimizing travel time from location to location for the field staff and avoiding extreme winter weather during the winter months the selection is primarily based on what three consecutive locations could be completed in time to inform decision making for 2014. The three locations are also expected to have high response rates and cover urban and rural populations. These three locations will have large numbers of non-Hispanic blacks and non-Hispanic whites. Hispanics will be in high numbers at only one location. None of these three locations is expected to have high proportions of Asians. A high NHANES response rate is beneficial in maximizing the available number of participants in the target age group who can be recruited for the pilot study. Ending the pilot study by the end of the third selected location will allow us to assess the feasibility and conduct analysis in time to determine whether the 24-hour urine component will be included in the main NHANES in 2014 and have sufficient time to plan for its implementation.
The pilot study will be conducted with a random half sample of NHANES participants, ages 20-69 years, who were examined in the mobile exam center (MEC) (n=250). NHANES survey statisticians estimate that there will be 500 examined sample participants aged 20-69 at the three pilot locations. The selection of the one half random sample of NHANES examinees for the 24-hour urine will be done with the existing NHANES subsamples mechanism. That mechanism is based on dividing the Sample Person ID by 12 and selecting one-half of the integer remainders between 0 and 11. Participants whose Sample Person ID has one of those remainders is asked to participate in the 24-hour urine collection. The remainders for the 24-hour subsample collection are: 1, 4, 6, 7, 9, and 11. Participants will be asked to collect urine samples over a 24-hour period.
A random half of NHANES examinees in the three pilot locations ages 20-69 years will be asked to participate in the 24-hour urine pilot. He/she will be scheduled to return to the Urine Pilot Study MEC (UMEC). Upon arriving for their appointment, a urine collection kit will be given to the participant to use for collecting urine samples over a 24-hour period. The kit will contain instructions on how to collect and return the urine specimen. It is desirable to have urines collected on all days of the week because it is known that dietary intake is different on weekend days versus week days. It has been observed that in studies where participants are not encouraged to collect urines on weekdays most opt to collect the urine on a weekend day. To increase week day collections, a random one-half of the pilot participants will be asked to collect on a week day. If the participant indicates it is impossible for them to collect during the assigned interval they will not be excluded from participation and will be allowed to schedule during the alternative time interval. Participants will be encouraged to start urine collection the following day if that day meets the weekday/weekend criteria and if the individual’s schedule allows it. Participants will also be asked to end their urine collection in the UMEC. When scheduling them to return their urine sample, participants will be asked to void at the UMEC to end the 24-hour collection. If not possible, they will be asked to bring the urine samples back within 24-48 hours of the start of collection. A set of completion questions will be used to assess the completeness of the urine collection.
One-half of the participants who successfully completed the first collection will be invited to collect a second 24-hour urine (n=125 of 250). The selection of participants to do the second 24-hour urine collection will not utilize the standard NHANES subsampling because of the desire to select half of the participants that are compliant with the first 24-hour urine collection. Since NHANES subsamples are determined by the Sample Person ID, there is no way to ensure that half of the compliant participants are selected. Instead of the standard NHANES subsampling, the application will use a random number between 1 and 100, to determine the half sample.
If the participant agrees, the second 24-hour urine collection will be scheduled 3-10 days later, but not on the same day of the week as the first 24-hour urine collection. Dietary intake varies significantly from day to day not just from week day to weekend day. Therefore to better characterize individuals intake it is desirable to have the two 24-hour urines on different days of the week. The day of the week assigned will not be based on the weekend/week day randomization above and will not be an absolute requirement of participation.
The selection of random one-half sample to do the second 24-hour urine collection will use a random number between 1 and 100, to determine the half sample. This will be a different random number than that used to determine who is asked to do a second 24-hour urine. Of those participants selected for the second 24-hour urine collection, half (n=63) will be randomly selected to begin the second urine collection at home and half (n=62) will be asked to return on another day to begin collection at the UMEC to test the feasibility of either protocol. This will assist us in assessing whether a protocol for 2014 that only involves three visits to the UMEC would be as successful in obtaining a high rate of complete urine collections as four visits to the UMEC.
A remuneration of $100 will be given to the participant at the end of both the first and second urine collections.
A flowchart in Figure 1 describes the 24-hour urine collection process. The approved ERB protocol is attached. It is amended to remove references to sodium and salt in the recruitment script.
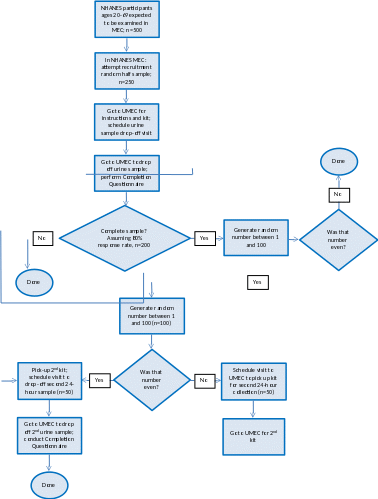

Figure 1. 24-Hour Urine Pilot Flow
Purpose and Use of the Information Collection
The Institute of Medicine, the Pan American Health Organization, and a National Heart Lung and Blood Institute (NHLBI) expert work group all strongly recommended measurement of 24-hour urinary sodium excretion as the most accurate means of monitoring mean sodium intake in the U.S. population. In addition, NHLBI recommended collecting a second 24-hour urine sample in a subsample of those with one 24-hour urine sample to estimate the population distribution (e.g., proportion of the population who exceeds recommended limits) accounting for day-to-day variation in sodium intake and excretion. The Institute of Medicine report1 specifically recommended the following:
“Federal agencies should ensure and enhance monitoring and surveillance relative
to sodium intake measurement, salt taste preference, and sodium content of foods, and should ensure sustained and timely release of data in user-friendly formats.
Ensuring Monitoring
(5.1) Congress, HHS/CDC (Centers for Disease Control and Prevention), and
USDA authorities should ensure adequate funding for the National Health
and Nutrition Examination Survey (NHANES), including related and supporting
databases or surveys.
Expanding and Enhancing Monitoring
(5.2) CDC should collect 24-hour urine samples during NHANES …”
Sodium reduction plays a crucial role in the prevention and treatment of hypertension and Cardiovascular Disease (CVD). NHANES currently provides extensive information related to hypertension and CVD. Existing NHANES data on prescription medication use, blood pressure, diabetes, CVD and chronic kidney disease in combination with the proposed data from 24-hour urine collections can be used to assess whether those with diagnosed hypertension or other chronic diseases have obtained appropriate treatment at the population level.
The addition of 24-hour urine collection to NHANES will allow us to estimate the prevalence of the U.S. adults who meet the recommendations for sodium reduction based on the Dietary Guidelines for Americans 2010 which indicate all Americans should reduce their sodium intake to <2300 mg daily and specific subpopulations to <1500 mg daily to prevent CVD. These specific subpopulations include African-Americans, individuals with hypertension, diabetes, and chronic kidney disease, and older aged individuals. Several randomized controlled trials show that reducing sodium to about 2300 mg/d (measured using 24 hour urine sodium) reduces blood pressure (BP), and one trial in adults with elevated BP showed that reducing sodium to 1500 mg/d reduces BP further. These recommended levels are also supported by observational studies using sodium intake measured by either 24 hour urine sodium or one of several dietary intake assessment methods (e.g., 24h recall) and examining risk of heart disease and stroke.
The addition of 24-hour urine collections to NHANES will provide population-based estimates of total dietary sodium intake (and other dietary nutrient intake such as iodine). If this pilot is successful, other measures may be added to the laboratory testing component of the 24-hour urine in 2014 to obtain additional information on other chronic conditions such as chronic kidney disease.
Previous studies have shown that there is high day-to-day variation in dietary intake for most nutrients. For estimates of mean usual sodium intake and the prevalence of individuals above and below current recommended levels, 24-hour urine collections adjusted for within individual day-to-day variance in excretion are considered the gold standard for sodium and related nutrients (e.g. potassium). For sodium, within-individual variance in 24-hour urine excretion was estimated in one study to be about 40% of the overall variance. This protocol is similar to that used to estimate usual intake from 24-hour dietary recalls and estimates of within-individual variance will be assessed using a second 24-hour urine specimen in a subset of the population using the same measurement error models used for 24-hour recall data. Thus, as part of the NHANES pilot study to add 24-hour urine collection to NHANES, we will test the feasibility of collecting two 24-hour urine samples on a subset of those who participate.
We need half of the people to collect two twenty-four urine samples to accurately measure within person day-to-day variance in excretion. One of the objectives of the pilot is to test the feasibility of collecting two 24-hour urine samples in a subset of participants. For the overall survey, estimates of within person variability require a minimum sample size of 30 participants in each demographic subgroup of interest. In some subgroups, e.g., male, Mexican-Americans, aged 45-69 years, the sample size in the actual survey will be 60 or less. Thus, if the pilot is successful, a minimum of 50% of this group will need to collect two 24-hour urine samples. Thus, we wanted to test this scenario in the feasibility study. Additionally, there are practical issues. There is almost no difference in cost between having ½ the sample or a smaller percentage (e.g., 30%) collect two 24-hour urine samples.
If a 24-hour urine from sodium in included in the 2014 NHANES and a portion of the participants provide two 24-hour urine collections on non-consecutive days the usual dietary sodium intake can be estimated using methods similar to those used for usual intake from 24-hour dietary recalls. Having an estimate of usual sodium intake can be used to estimate the proportion of the US population meeting the recommended limits of usual daily sodium intake, that is, 2,300 or 1,500 mg per day. More than one day’s intake of sodium as measured in the 24- hour urine is necessary from a substantial proportion of the sampled population to provide these estimates.
Ongoing research is examining alternative approaches to estimate the intake of dietary sodium in the U.S. population. NCHS and collaborators initiated a 24-Hour Urine Calibration Study that compared 24- hour excretion of sodium and related nutrients estimated from one or more spot urine samples with observed excretion from a 24-hour urine collection.
One study has completed data collection and analysis is ongoing. From June to August 2011, 481 volunteers aged 18-39 years old living in the Washington DC metropolitan area were recruited to collect urine for a 24-hour period, placing each void in a separate container. Among them, 407 (85%) persons returned a complete 24-hour collection of urine specimens. Of the participants with a complete 24-hour urine collection, 133 (33%) completed a second 24-hour urine collection 4-11 days later. Of those who completed 24-hour urine collections about one-half were female and one-half were black. Mean 24-hour sodium excretion was 3,540±1,513 mg for males and 3,088±1,262 mg for females. Among all race-gender groups, overnight specimens had the largest volume and lowest sodium concentrations, compared with other timed-spot urines. The preliminary results from the study indicated 24-hour sodium excretion predicted from a single spot urine sample estimates mean observed 24-hour sodium excretion reasonably well. However, the differences between predicted and observed 24-hour sodium excretion indicate substantial bias at the low and high ends of the distribution. Subsequent analyses are focusing on developing new calibration equations to estimate population-level 24-hour sodium from multiple spot urines with adjustment for within-person variance. Thus, we do not yet know if we can estimate 24-hour sodium excretion distributions in younger adults. In addition, we do not know if estimation equations will estimate means or distributions in older adults (i.e., 40 years and older). This is especially important given that many older adults have hypertension and sodium excretion has been shown to differ between people with and without hypertension. A second calibration conducted by NHLBI will begin in March and end in September. If it is not possible to estimate means and distributions in older adults and distributions in younger adults, we want to be positioned to begin collection of a 24-hour urine component in 2014. Because we need to assess feasibility, we want to pilot the 24-hour urine collection in 2013.
Although these two efforts are underway to develop less burdensome methods to monitor sodium intake in the US population it will be some time before all results are analyzed, published in peer reviewed journals and validated in data collection effort such as NHANES or similar to NHANES. In the interim we are following the Institute of Medicine reports recommendation to plan a 24-hour urine for the NHANES.
In addition, preliminary results suggest that using more than one spot urine specimen to capture a greater amount of total sodium intake may improve the prediction of 24-hour sodium excretion. Based on these preliminary results NCHS has changed the Home Urine Collection protocol in the 2013 NHANES. In 2011-12, a spot urine that was an overnight void was collected on all examined persons 6 and older who had provided a urine specimen during their examination. In 2013 NHANES is collecting from adults 20-69 who had provided a urine specimen during their NHANES examination a full void late in the evening and a full overnight void. This urine is being stored for potential future use to estimate 24-hour sodium intake in the population if calibration equations are developed and validated among US adults aged 18-39 years and aged 40 years and older. The older age group is of particular importance as the diurnal sodium excretion pattern differs between persons with and without high blood pressure.
Criteria for evaluating pilot test success
The following are the pre-established criteria for evaluating the success of the pilot for possible inclusion in the 2014 NHANES.
Response rates (completion rates) for the 24-hour urine collection
The pilot will be considered successful if 70 percent of eligible participants agree to participate and successfully complete the first 24-hour urine collection.
For those asked to collect a second 24-hour urine, a 70 percent response rate will be considered a success.
Rationale for these selected completion rates
It is ideal to have an 80% or higher response rate to minimize non-response bias. Because of the complicated nature and strict requirements to consider a 24-hour urine complete, a slightly lower rate was selected as the success rate for this pilot test.
Those who successfully complete the first urine collection and agree to the second have already proven they are cooperative and able to follow the directions. We did not think it would be unrealistic that we would be able to get the majority of this cooperative subgroup to successfully complete a second 24-hour urine collection.
Non response bias analysis
The NHANES examination experiences significant differential non-response related to geography, race and Hispanic origin, and age to the NHANES examination. The NHANES examination weights are adjusted related to these factors and others to minimize non-response bias in estimates obtained from the NHANES examination components. The NHANES contains numerous planned subsamples. These planned subsamples have separate weights to use for analysis. Because each of these experiences some missingness of data among the examined population subsample specific weights are produced to minimize non-response bias and to maintain the representativeness of the data. For NHANES components that experience high rates of missing data at the examination it is recommended that non-response bias be assessed. If there is an indication of non-response bias the data analyst should make appropriate adjustments or disclaimers.
In the context of a pilot test there are limits to the potential to evaluate non-response bias. However the following aspects of participation in the pilot will be looked at by location, gender, age and race and Hispanic origin: willingness to collect the 24-hour urine, success in meeting the criteria for a complete urine collection, and willingness to collect a second 24-hour urine specimen.
If the overall response rate is in the 70’s and the pattern of non-response is highly skewed within one or more subgroups defined by age, gender, language or race ethnicity this information would be taken into account in making a decision to implement the component in 2013.
A complete urine collection is judged by responses to the Completion Questionnaire (included in the attached protocol).
The urine specimen will be considered “Incomplete” and will not be processed if:
The start and end time of the collection was not recorded and cannot be ascertained
The length of collection time is <20 hours
The total volume of urine is less than 500 ml
A female participant was menstruating during the urine collection (Item FQ1 on Completion Questionnaire)
More than a few drops of urine were missed during collection (Items FQ2-FQ8 on Completion Questionnaire)
All urine specimens collected over a period of at least 20 hours which meet other criteria for completeness will be processed (weighed, aliquoted and shipped). NCHS will determine whether specimens collected over a 26+ hour time frame will be included in the analytic sample.
Effect on other post-examination content (A telephone dietary recall (DR) occurs 3-10 days after the NHANES MEC examination and a physical activity monitor (PAM) that is given to participants during the examination is to be mailed back on the 8th day after the examination.)
If either the DR or PAM response rate decreases more than 15 percentage points at the first location the pilot will be stopped during the second location. If the response rate decreases between 10 and 15 points for the first 2 locations we will end the pilot during the third location. Because the locations overlap and these components occur after the MEC examination the possible stop times must reflect this timing. The justification is that we want to limit adverse effects on the PAM and DR response rates but have enough information to make a decision about the feasibility of the pilot. For all NHANES pilot tests the current NHANES content takes precedence over the pilot data. While somewhat arbitrary, these are reasonable cut points given our examination of possible variation by chance alone in response rates to these two post-examination components over the last 29 locations. We randomly divided the examinees ages 20-69 into two groups. By chance alone some of the differences between the two groups were as high as 13%. Selecting the cut off of 15% should avoid stopping based on a difference that might occur by chance. Additionally, given an alpha of 0.05 and a sample size of 83 participants at each location, the statistical power would be 61% to detect a 15 percentage point difference (88% vs. 73%).
If the response rate for the entire duration of the pilot (3 locations) for either of these components decreases by more than 10 percentage points (e.g. from 88 to less than 78 percent) the pilot will be considered unsuccessful. If the response rate for both of these components decreases by 5 or fewer percentage points, the pilot will be considered a success. A reduction of greater than 5 up to 10 percentage points in response rate for one or both components will require further evaluation of the impact of the pilot on the post-examination components.
Note that the comparison group to evaluate changes in response rates to post-examination components will be the random one-half sample of NHANES examinees not asked to participate in the 24-hour urine collection.
Two home urine collections (HUC) are part of the NHANES protocol for those ages 20-69. Because of the similarity of the protocols a decision was made to not offer the HUC to participants agreeing to collect the 24 hour urine until after they have done the 24 hour urine collections(s). Because the HUC is so much less burdensome to the participant DHANES feels that the participants completing the 24-hour urine collection(s) will continue on and supply the HUCs.
There are other qualitative aspects of the pilot that will be considered in evaluating the feasibility of the pilot however no explicit criteria have been established. These items will be considered in fine-tuning protocol details to maximize the response rates and quality of the data collected in 2014. For example, we will track our success in getting participants to randomly collect their 24-hour urines on a week day or weekend day. Additionally, we will track our success in getting those who complete a second urine to collect it on a different day of the week. Data about time to complete the first and second visit to the UMEC, patterns and frequency of broken appointments and time to process the urine specimens will be tracked within the NHANES computer system. Data on appointment times for the urine pilot study (including broken and re-scheduled appointments) and reminder calls made to participants will automatically be generated. The system will also record the time at which the reminder calls were made. Participants will receive a phone call to remind them of their appointment to start the urine collection (unless their start date is the following day). If a participant does not have a phone where he/she can be reached, an NHANES staff member will make a personal visit to remind them of their appointment to start urine collection. All this information will be considered when reviewing the pilot results.
Explanation of any payment or gift to respondents.
Participants in the 24-Hour Urine Pilot Study will be remunerated $100 for each 24-hour collection. Remuneration will be provided at the time of urine specimen delivery. Past remunerations for urine collections related to NHANES include the Home Urine Collection (HUC) and the 24-hour calibration study done in mid-2011. In 2012 when we collected one HUC, the remuneration was $40. In 2013 we are collecting two HUCs and remunerating $50 total for those two. In the 2011 24-hour urine calibration study we remunerated an average of $125 for each 24-hour urine returned.
12. Estimates of Annualized Burden Hours and Cost.
The 24-Hour Urine Pilot Study has been budgeted for 1.5 hours for those providing one 24-hour urine and questionnaire, and 3 hours for those providing two 24-hour urine collections. The first collection will be done at an NHANES location. Participants will be given supplies for the remaining collection. The maximum number of respondents would be 250 (ages 20 -69) and the maximum burden 563 hours (250 respondents*(1.5 hours) + (125 repeat respondents*1.5 hours) = 563)).
The total burden is 563 hours. This time was already budgeted and approved in line 2 (Special study/pretest participants), of the original submission. No additional burden is sought.
15. Explanation for Program Changes and Adjustments. There are no changes in this package from the previous approved clearance. The burden hours were approved by OMB in the full clearance.
Attachment:
NHANES 24-Hour Urine Pilot Study Protocol
Reference: 1Jane E. Henney, Christine L. Taylor, and Caitlin S. Boon, Editors; Committee on Strategies to Reduce Sodium Intake; Institute of Medicine. Strategies to Reduce Sodium Intake in the United States. The National Academies Press, Washington, D.C. 2010.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | National Health and Nutrition Examination Survey |
| Author | vlb2 |
| File Modified | 0000-00-00 |
| File Created | 2021-01-29 |
© 2026 OMB.report | Privacy Policy