Form FDA 3744 FDA 3744 Electronic Form FDA 3744 Antimicrobial Animal Drug Distr
Antimicrobial Animal Drug Distribution Reports Under Section 105 of the Animal Drug User Fee Amendments of 2008 (ADUFA 2008)
0910-0659 FDA 3744a ADUFA eForm Screenshots Dec 2012
Annual Reports for Sponsors with Inactive Applications (e-form FDA 3744a)
OMB: 0910-0659
Section 1: eSubmitter Interface
Screenshots for the Electronic Form FDA 3744 -
Antimicrobial Animal Drug Distribution Report for ADUFA Section 105
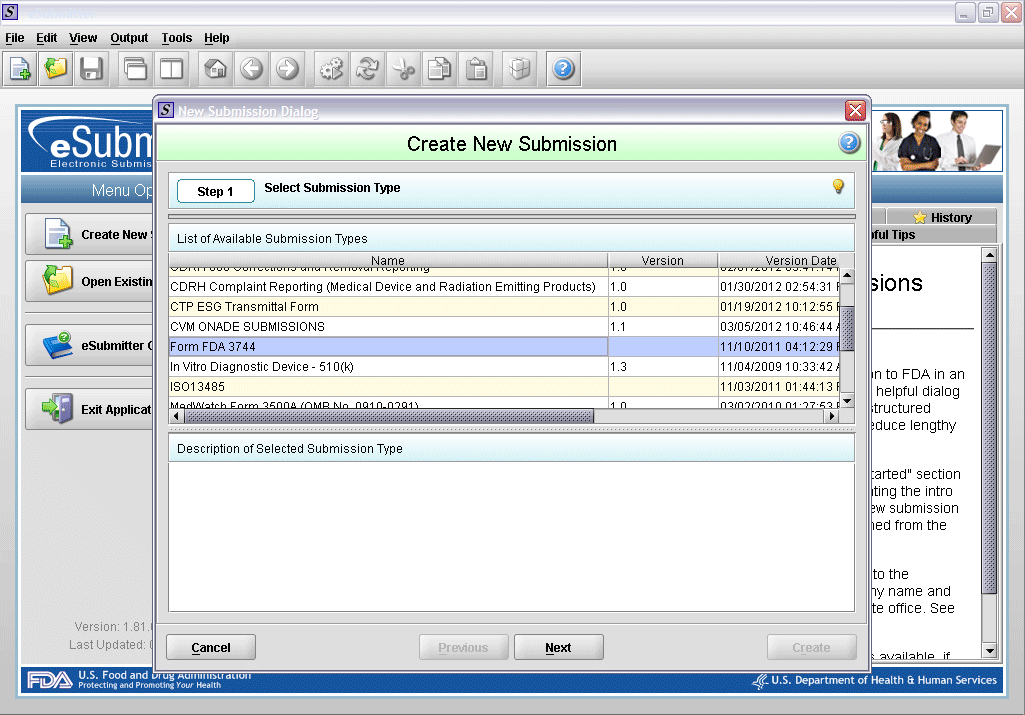
1. Create new submission.

2. Enter description.
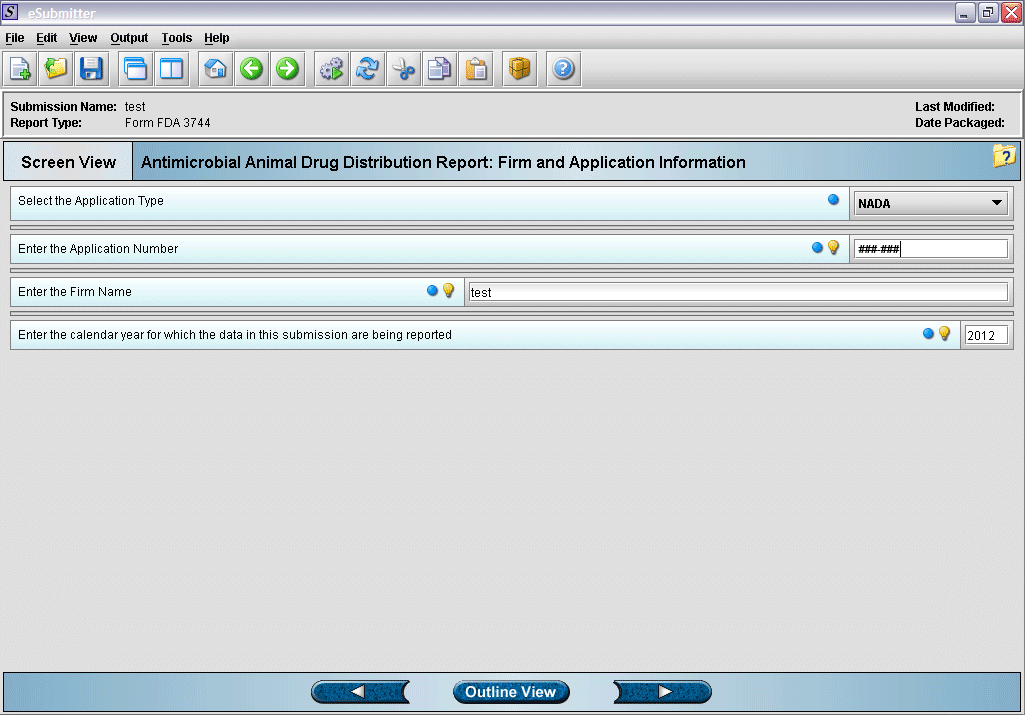
3. Enter identifying information for application.
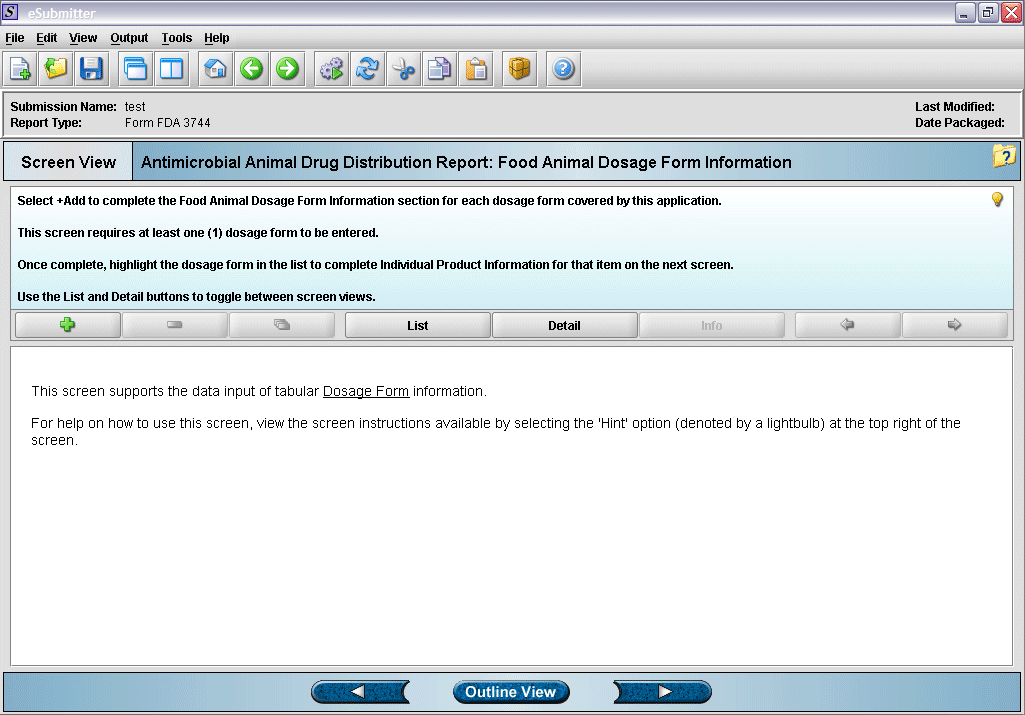
4. “Dosage Form” overview screen.
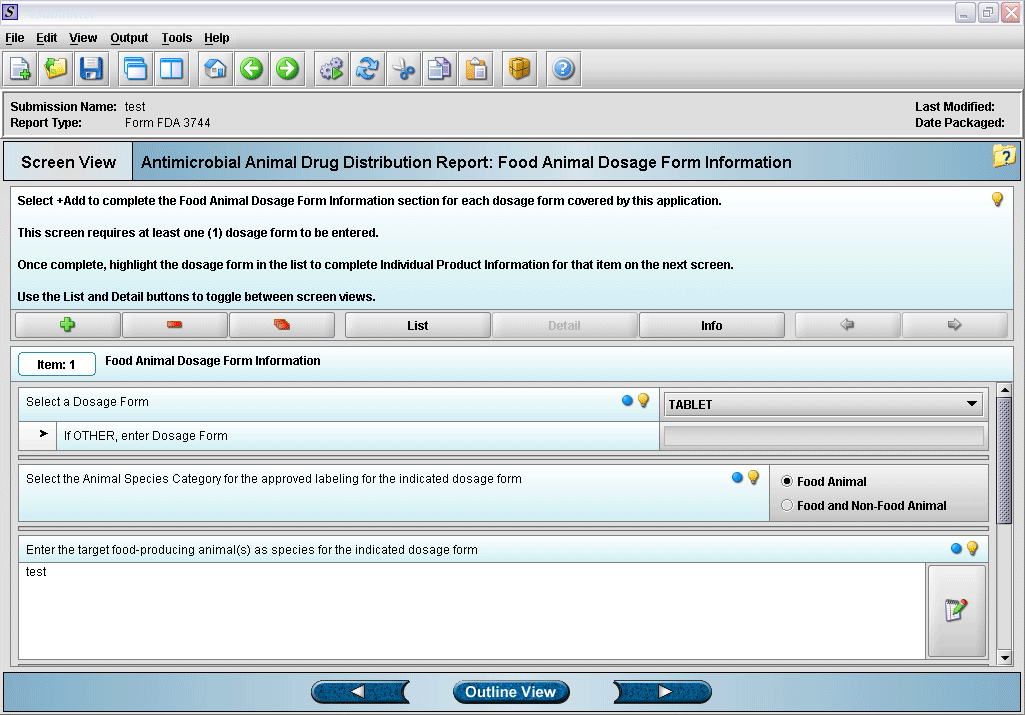
5. Entering dosage form information.
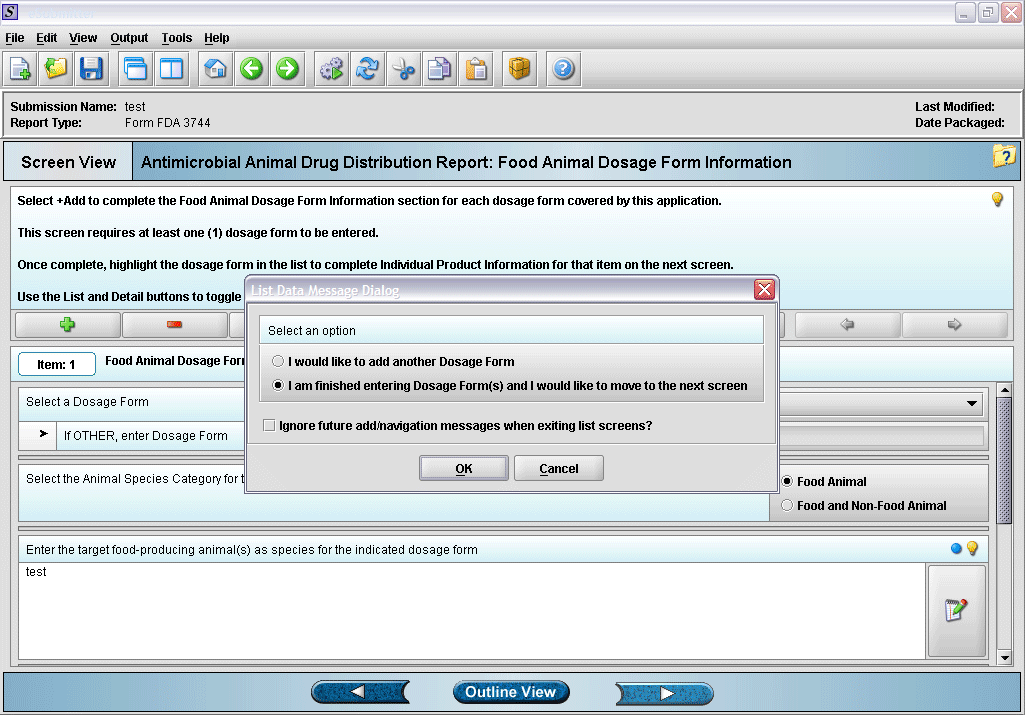
6. Prompt after finishing with dosage form information entry.
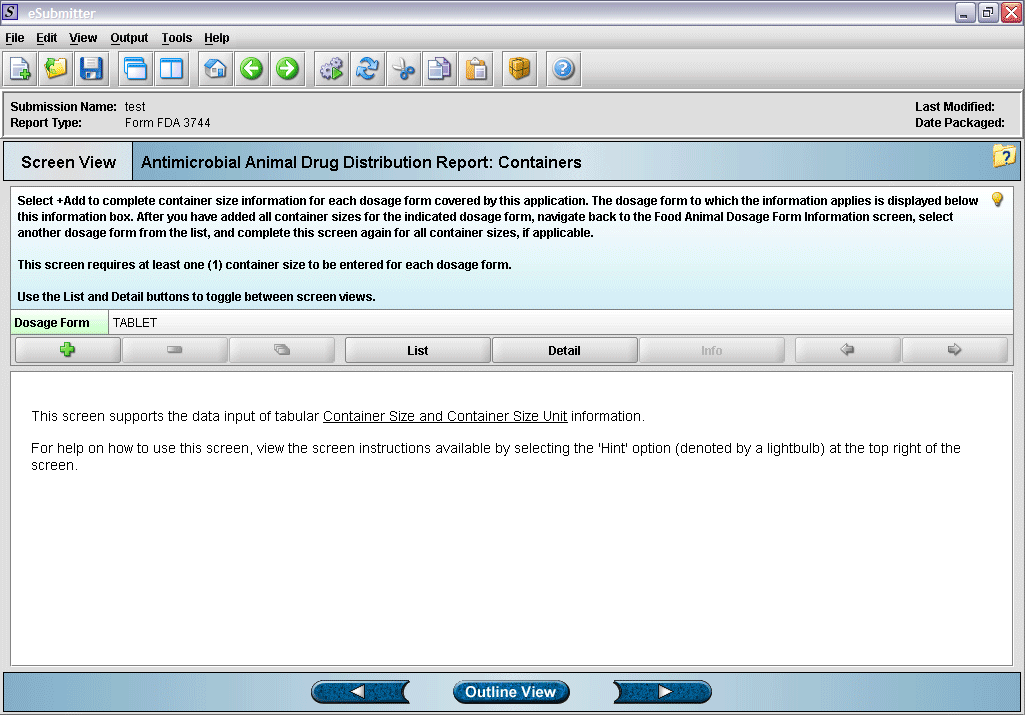
7. Container size and unit overview screen.
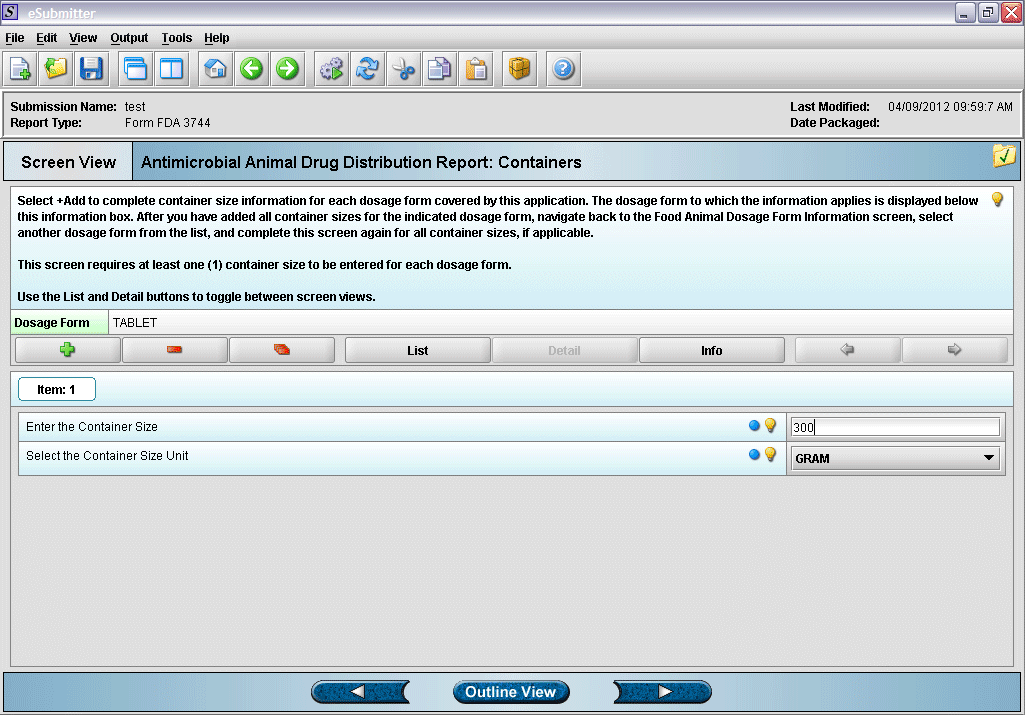
8. Entering container size and unit information (click green plus to add a container).
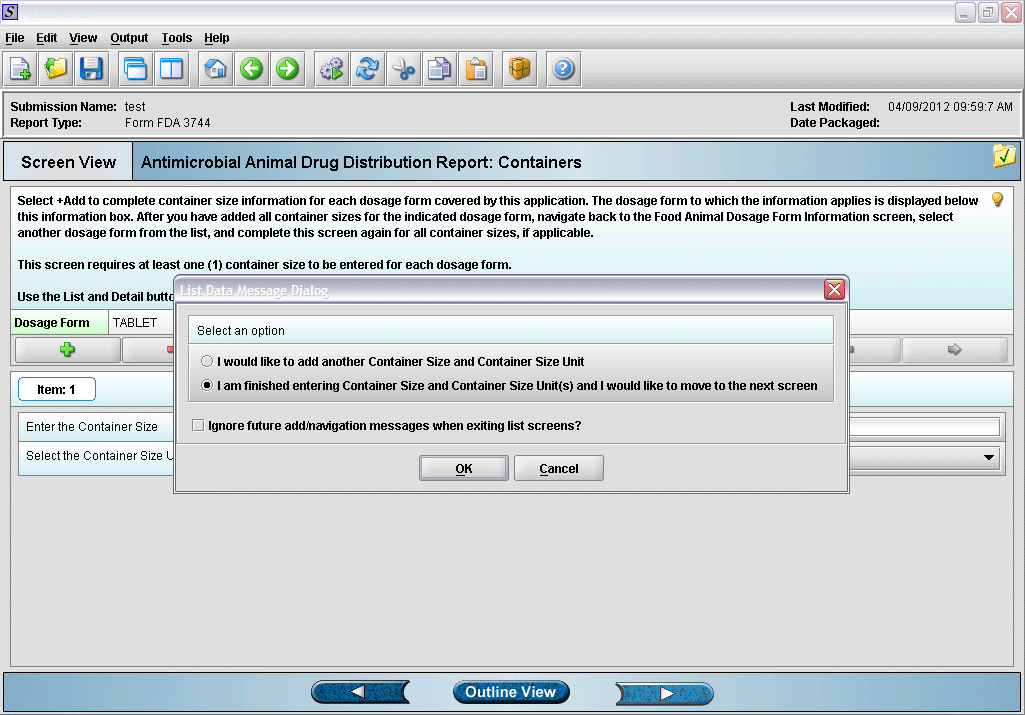
9. Prompt after finishing with container size and unit information entry.

10. “Active Ingredient” overview screen.

11. Entering active ingredient information.
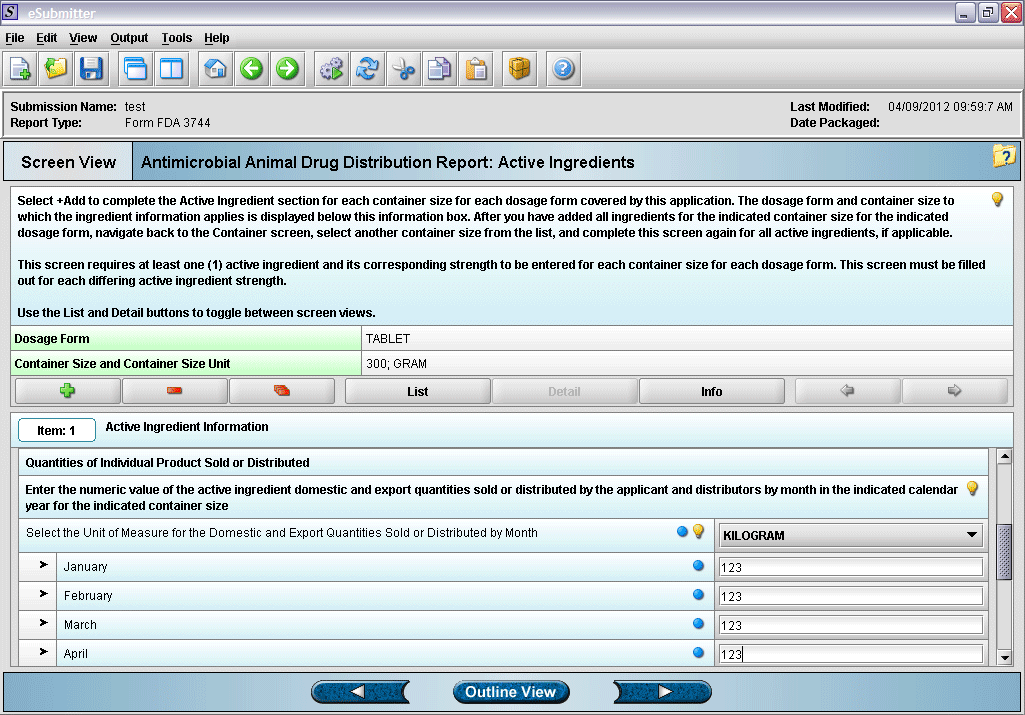
12. Entering active ingredient information (continued).
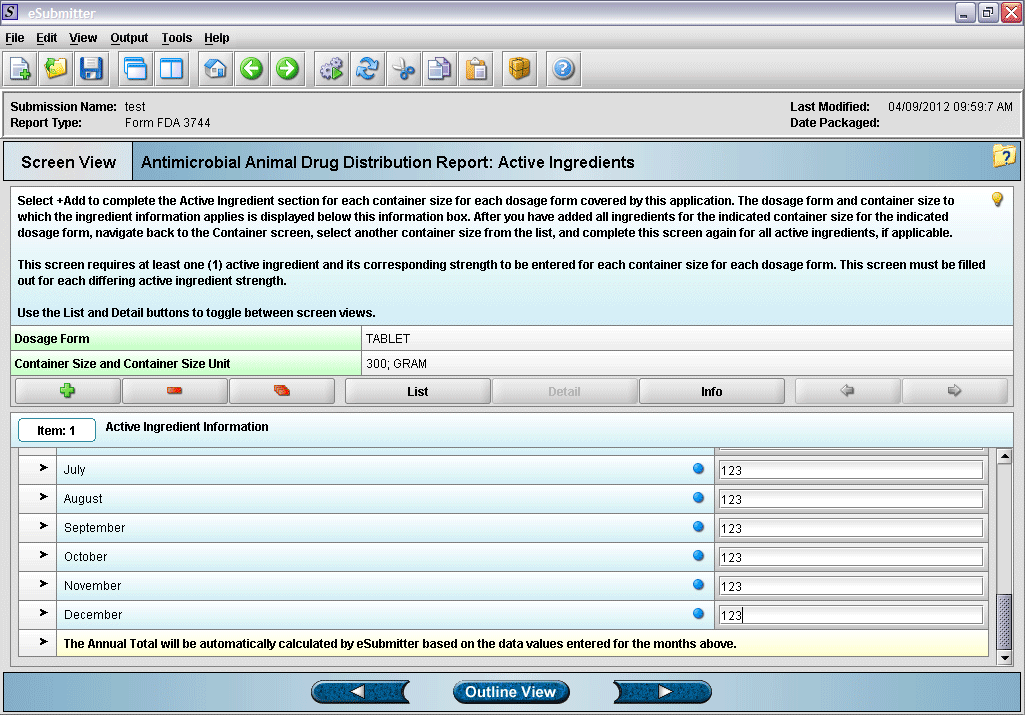
13. Entering active ingredient information (continued).
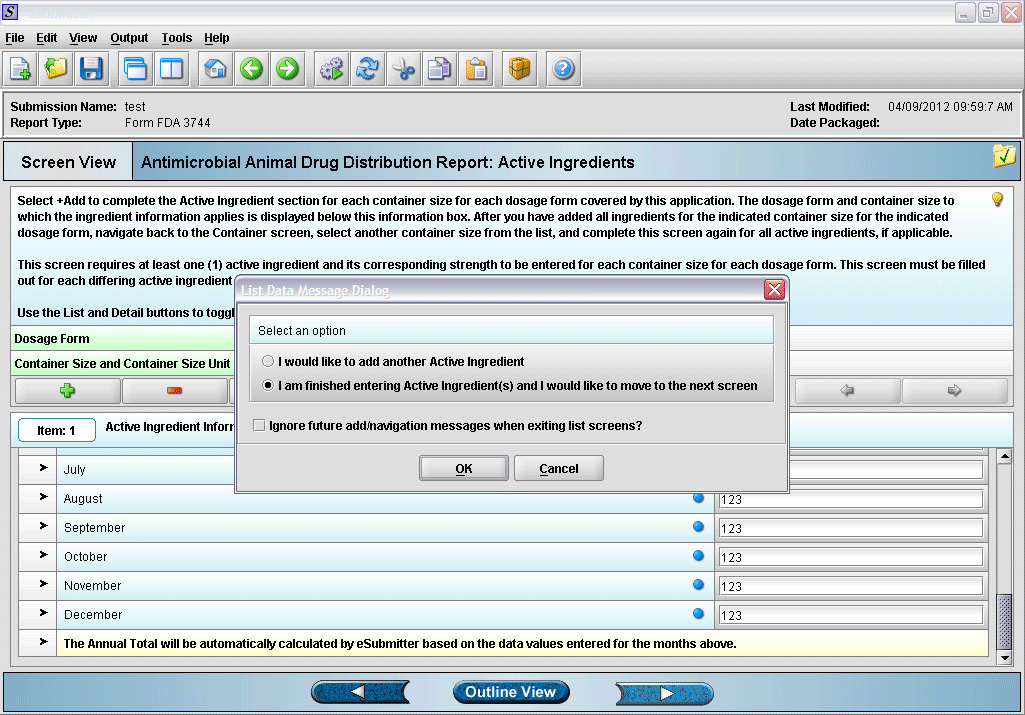
14. Prompt after finishing with active ingredient information entry.
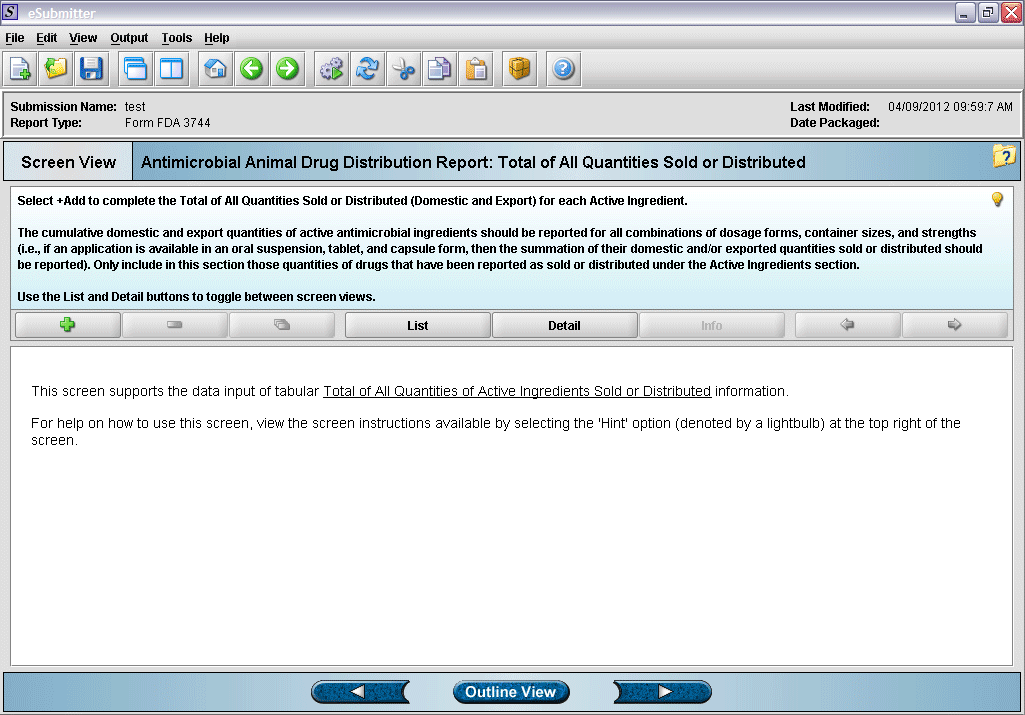
15. “Total of All Quantities Sold or Distributed” overview screen.

16. Entering total quantity sold information.
17. Entering total quantity sold information (continued).
18. Form completed.
Page
| File Type | application/msword |
| File Title | Screenshots for the Electronic Form FDA 3744 - |
| Author | wyowell |
| Last Modified By | Capezzuto, JonnaLynn |
| File Modified | 2013-01-03 |
| File Created | 2013-01-03 |
© 2026 OMB.report | Privacy Policy