Change Request OMB 0920-0004 4-5-13
Change Request OMB 0920-0004 4-5-13.docx
National Disease Surveillance Program - II. Disease Summaries
Change Request OMB 0920-0004 4-5-13
OMB: 0920-0004
0920-0004 National Disease Surveillance Program II-Disease Summaries
(expiration 8/31/14)
Change Request
April 5, 2013
Amy McMillen
CDC/NCEZID
1600 Clifton Rd
Atlanta GA 30333
404-639-1045
List of Attachments
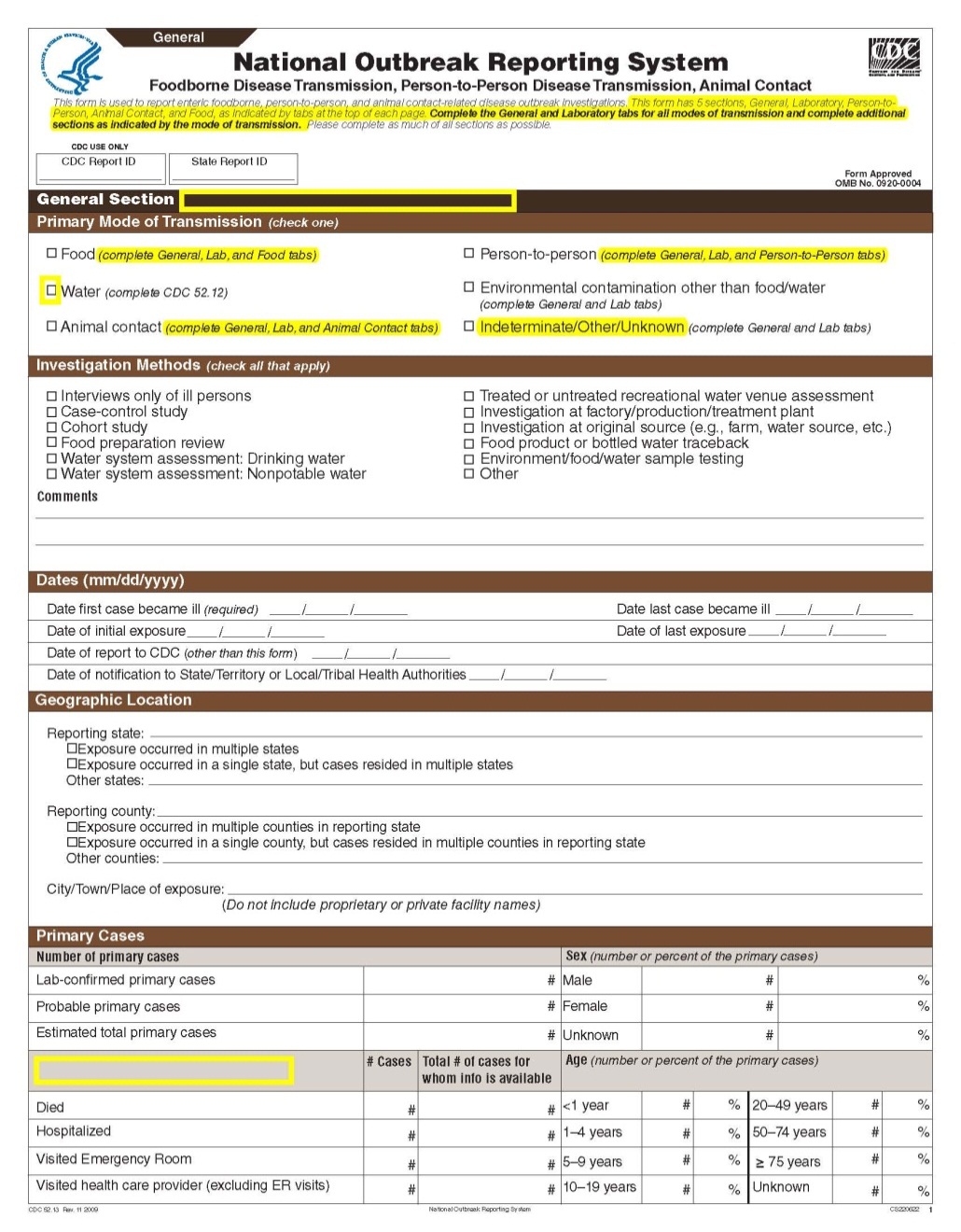
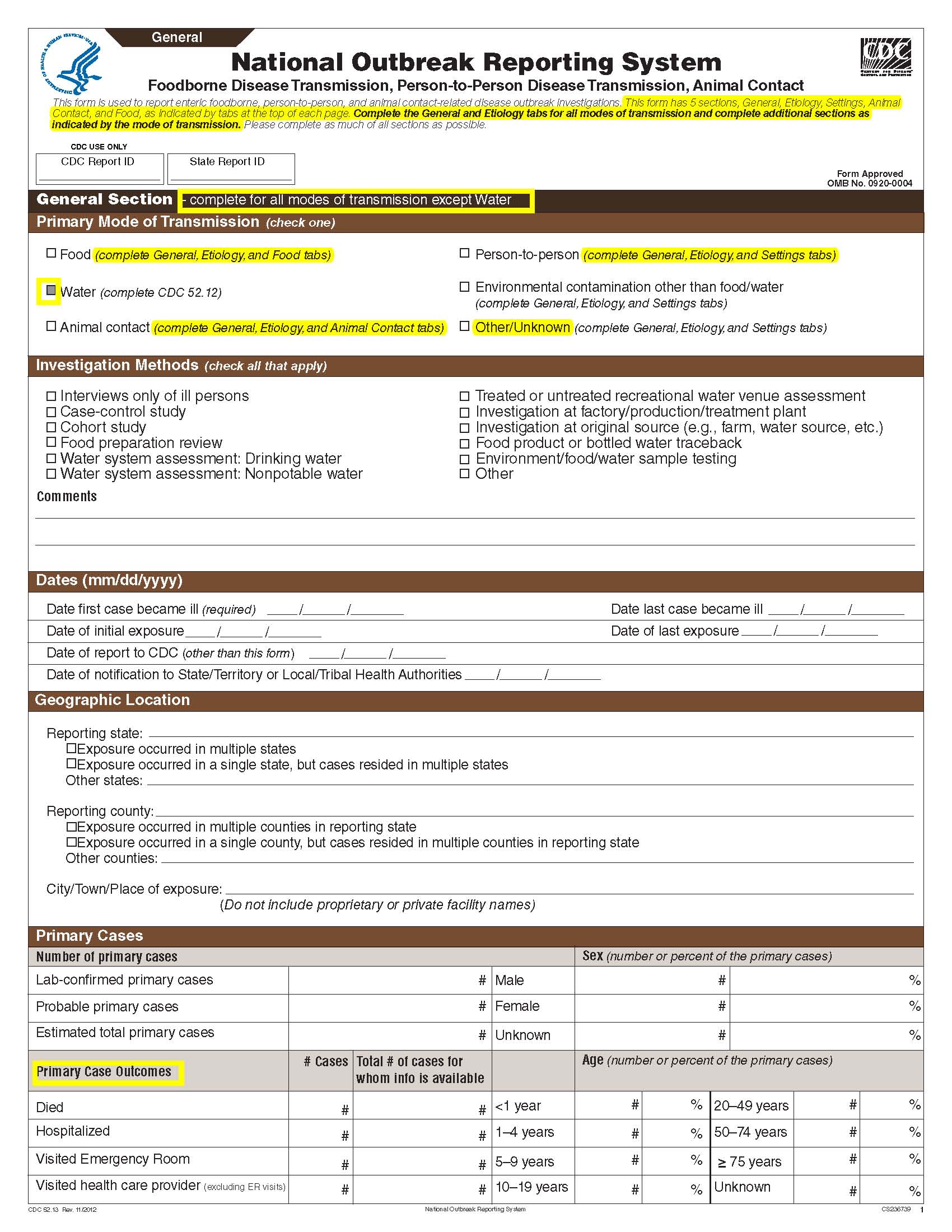
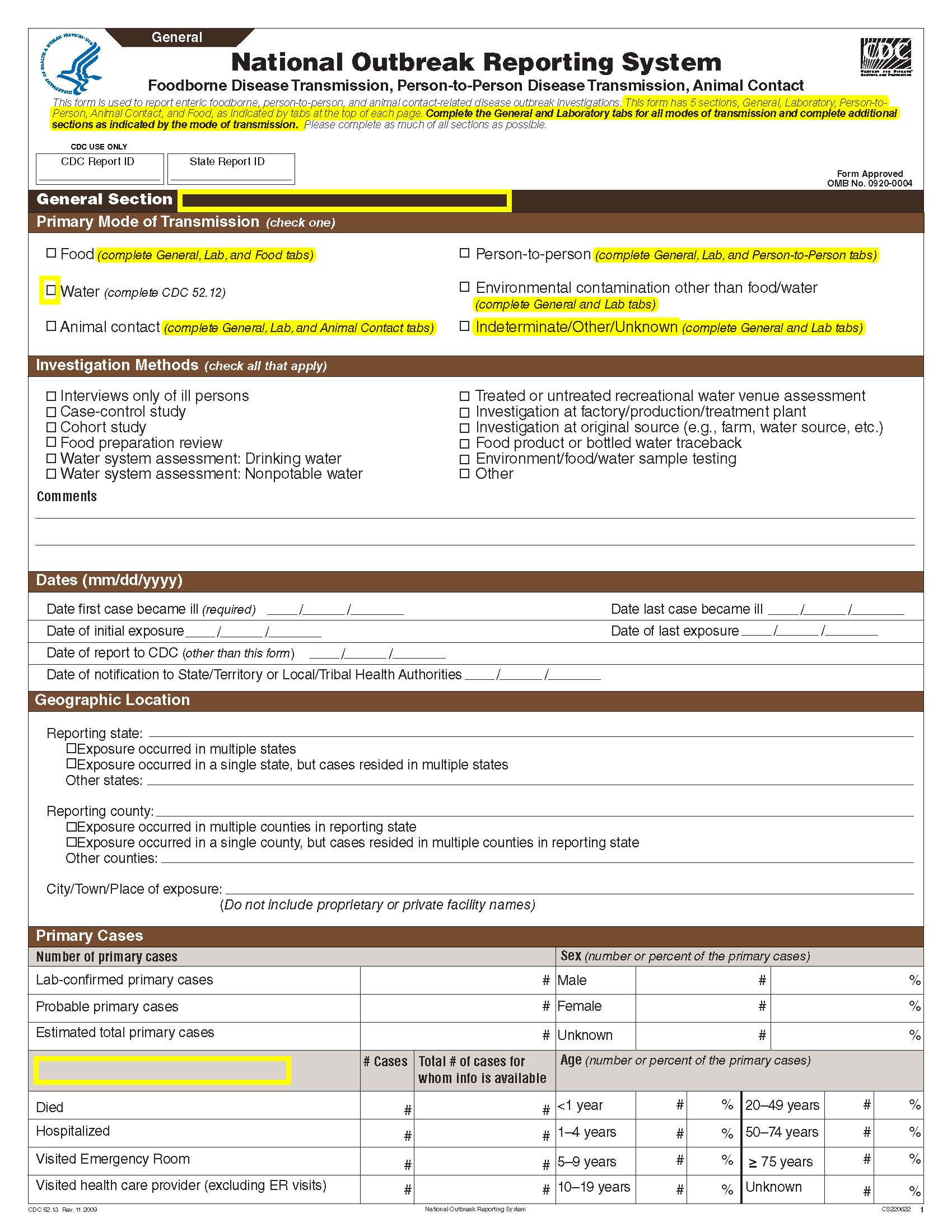
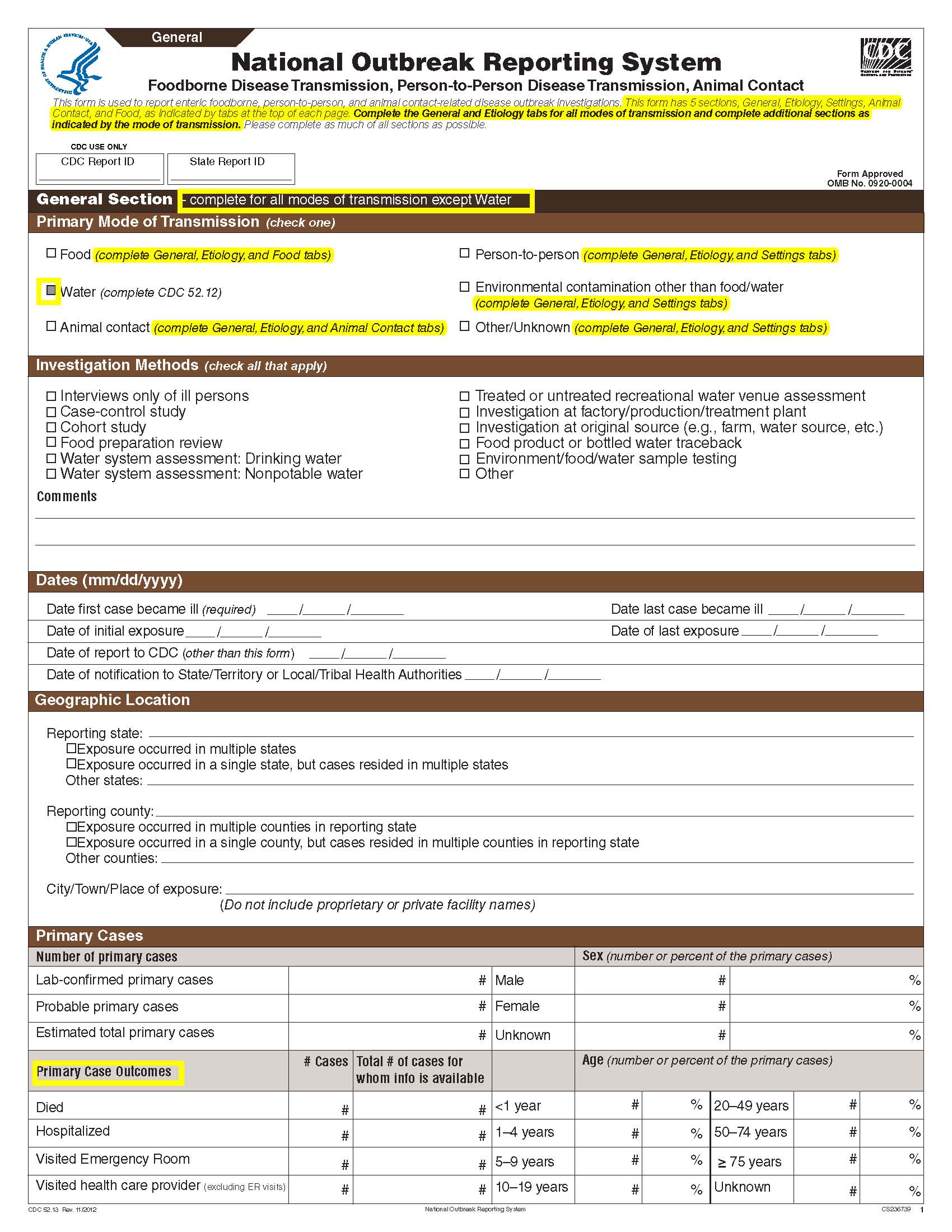
National Outbreak Reporting System (NORS) – CDC 52.13 form
National Outbreak Reporting System (NORS) – CDC 52.12 form
Human Infection with Novel Influenza A Virus Case Report Form
Antiviral-Resistant Influenza Case Report Form
National Outbreak Reporting System (NORS) – CDC 52.13 form, Circumstances of Change Request for OMB 0920-0004
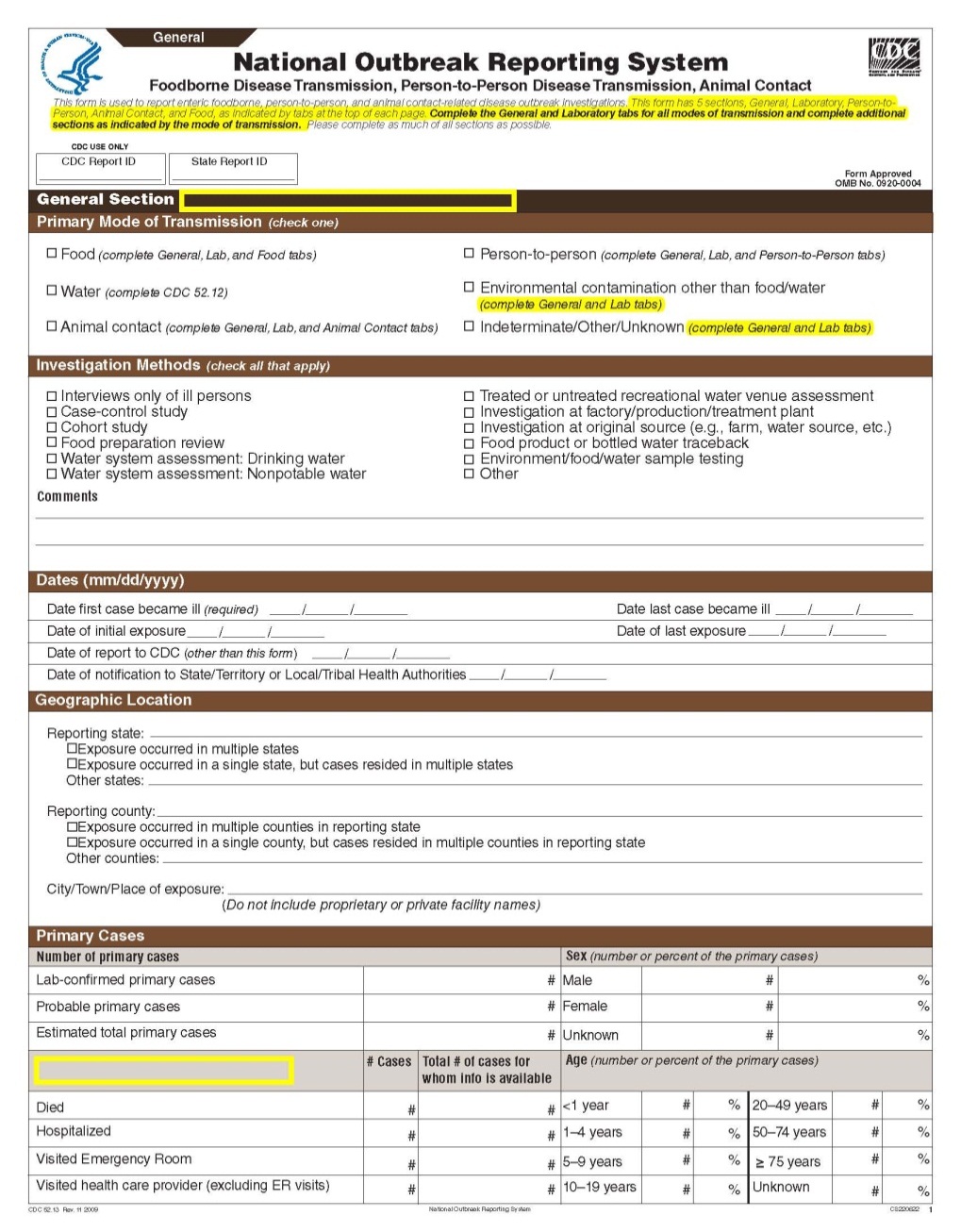
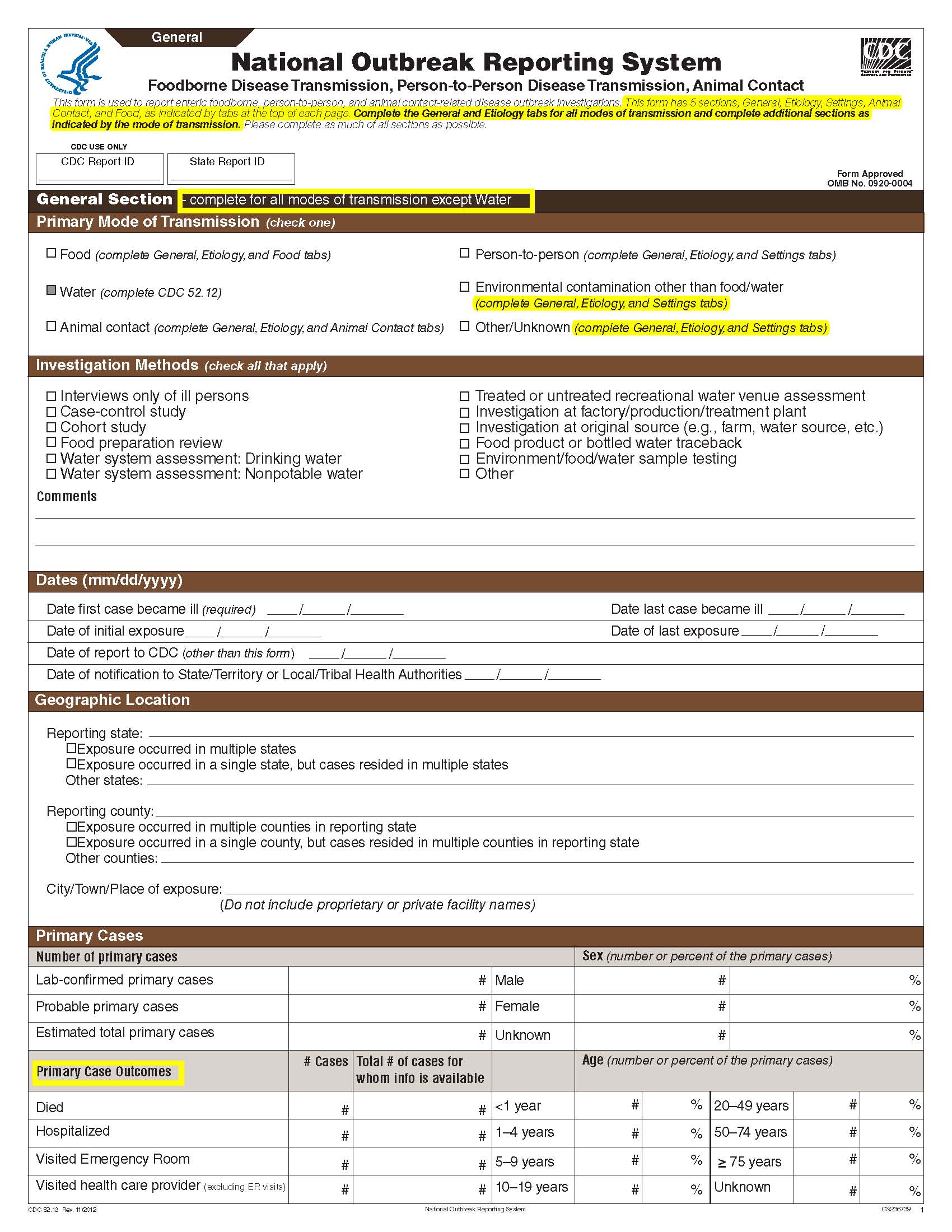
This is a nonmaterial/non-substantive change request for #0920-0004, which received a 3-year extension through August 2014 for the reporting of foodborne, enteric person-to-person, animal contact, environmental contamination other than food/water, and other/unknown modes of transmission outbreak data from 59 reporting jurisdictions (50 states, and 9 territories) to the National Outbreak Reporting System (NORS).
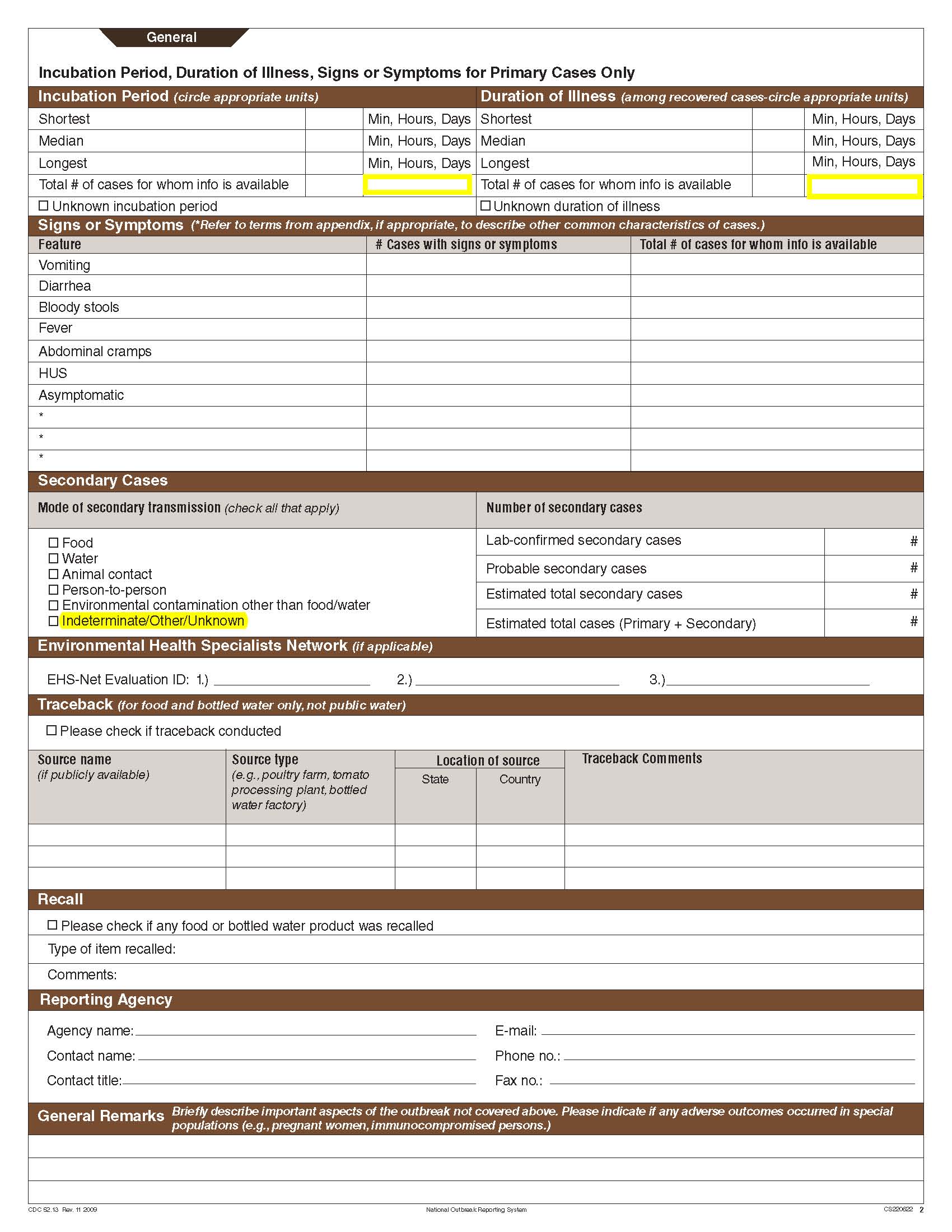
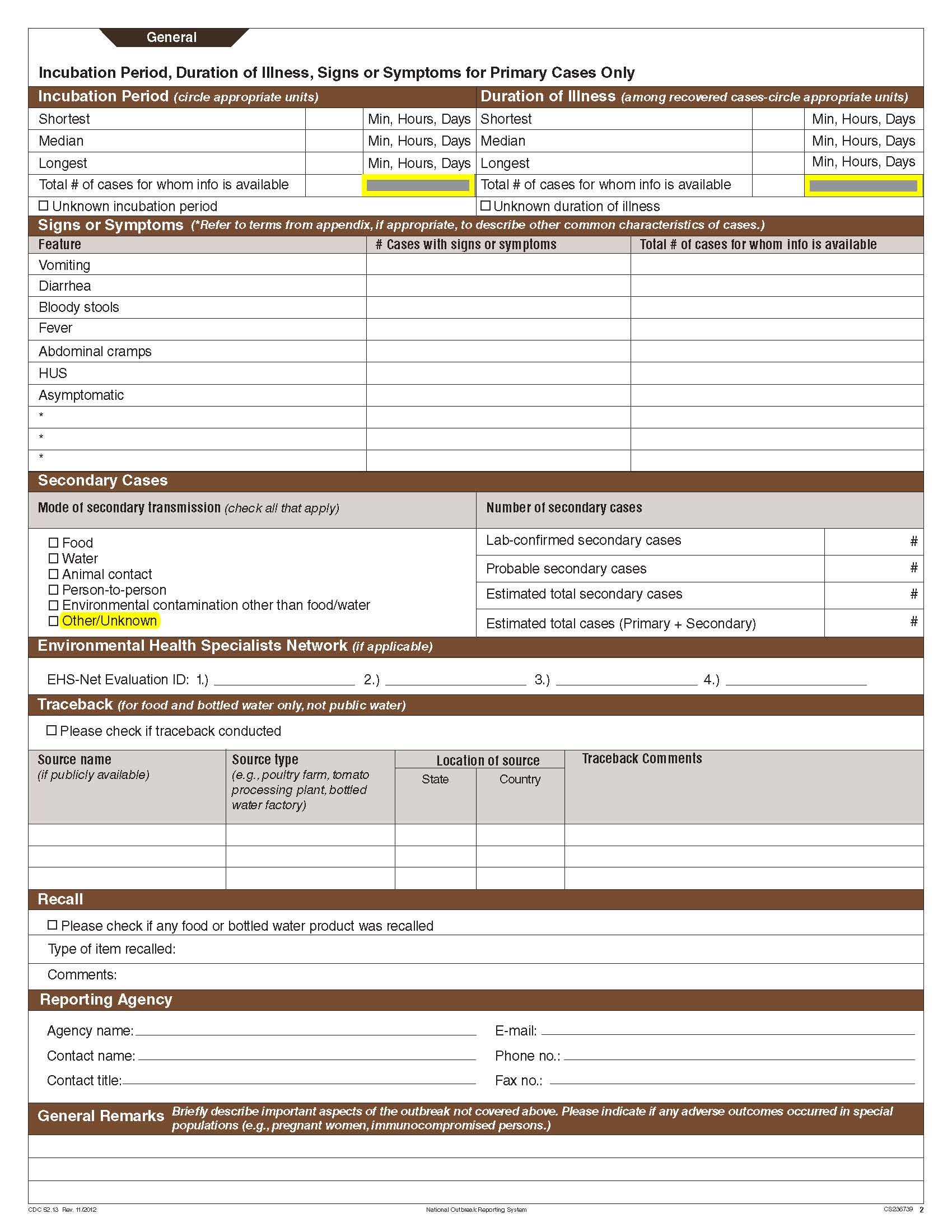
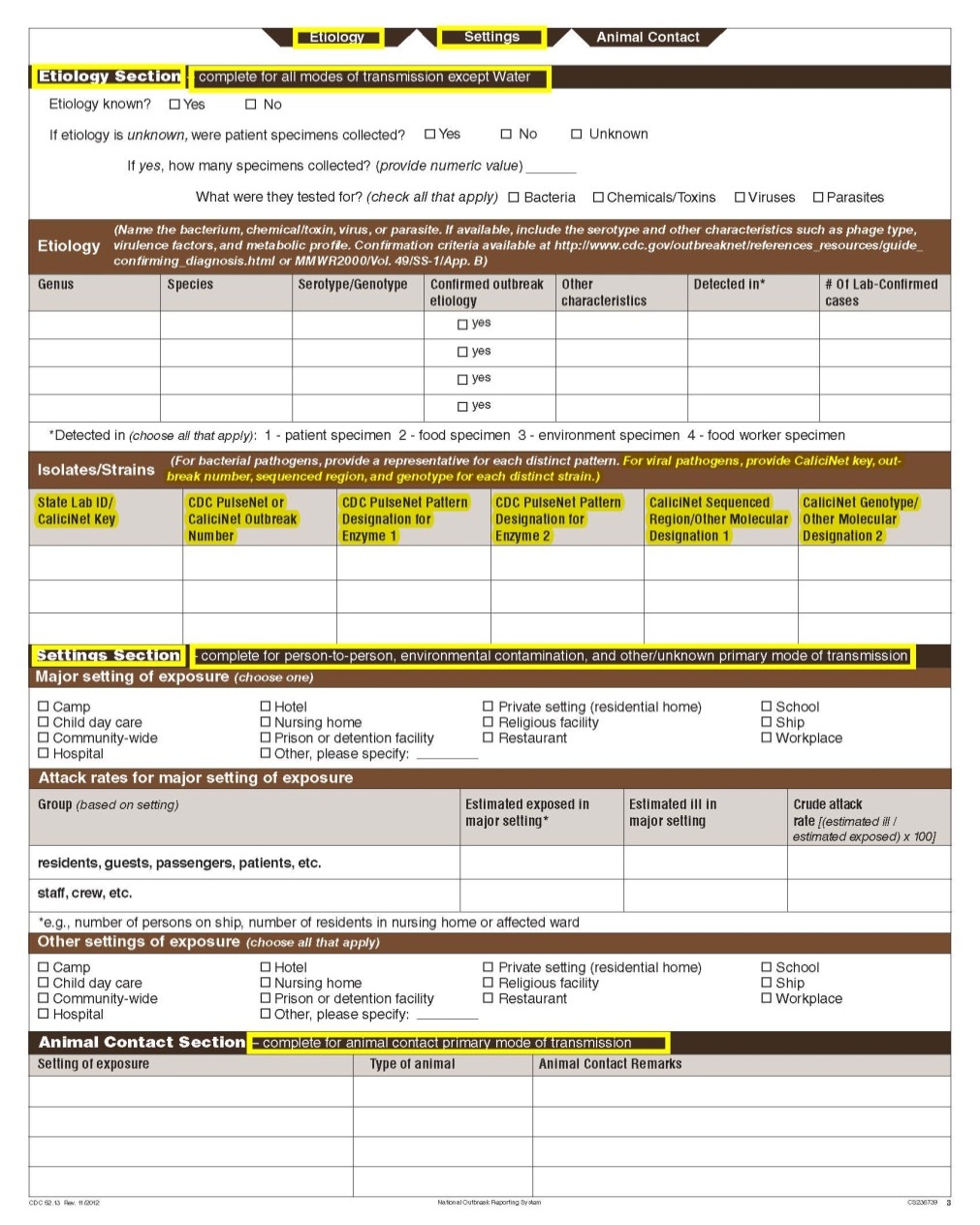
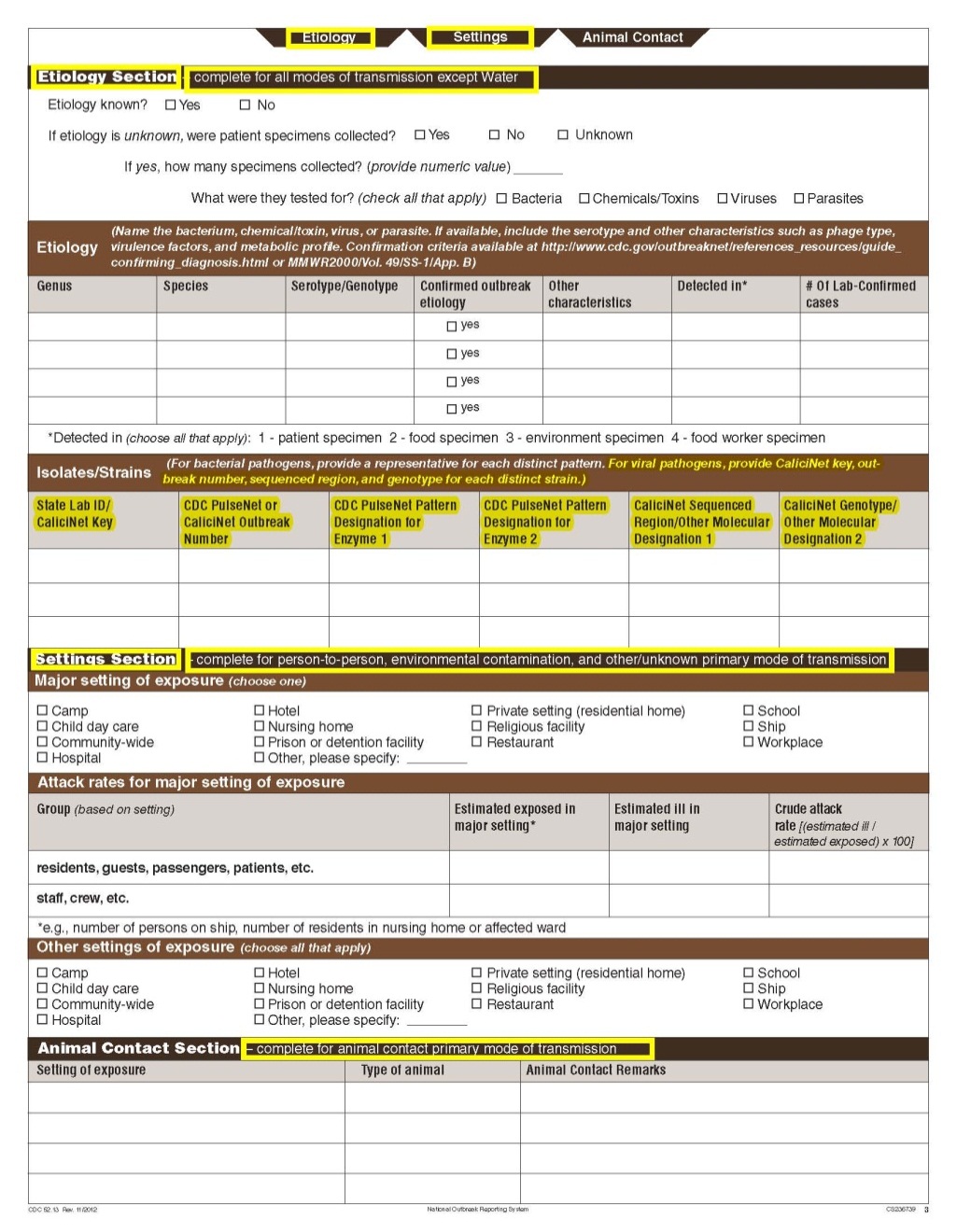
Although foodborne outbreaks surveillance has occurred since the 1970’s, NORS was launched in 2009 as the CDC Form 52.13, and collects aggregate outbreak data on foodborne, enteric person-to-person, animal contact, environmental contamination other than food/water, and other/unknown modes of transmission outbreaks. Data elements requiring change will improve clarification and readability of the data collection form; see table below. The settings or locations of the outbreak are routinely summarized in annual summaries and have been the subject of inquiry from the reporting agencies as well as US federal regulatory agencies. The setting or location where the outbreak occurred is important and essential to inform targeted intervention strategies, regardless of the primary mode of transmission. Currently, settings or locations of the outbreak are collected for animal contact, foodborne, and person-to-person outbreaks. However, the settings for environmental contamination other than food/water and other/unknown outbreaks are not collected. In response to the importance of the settings or locations of those outbreaks, reporting sites will be asked to complete an additional section, ‘Settings’, (formerly named ‘Person-to-Person’); no data collection fields will be added or changed to this section. The data collection changes are summarized in the table below.
Changes for clarification and readability |
|||
Section |
Current Question/Item |
Requested Change |
|
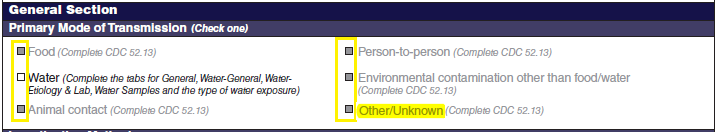
General Section, Primary Mode of Transmission |
|
|
|
No additional questions have been added
|
|||
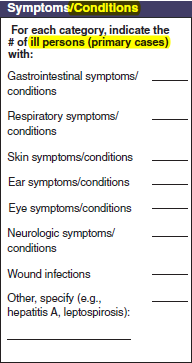
Primary Cases |
|
|
|
|
No additional questions have been added
|
||
Incubation Period and Duration of Illness |
|
|
|
|
No additional questions have been added
|
||
Secondary Cases |
|
|
|
No additional questions have been added
|
|||
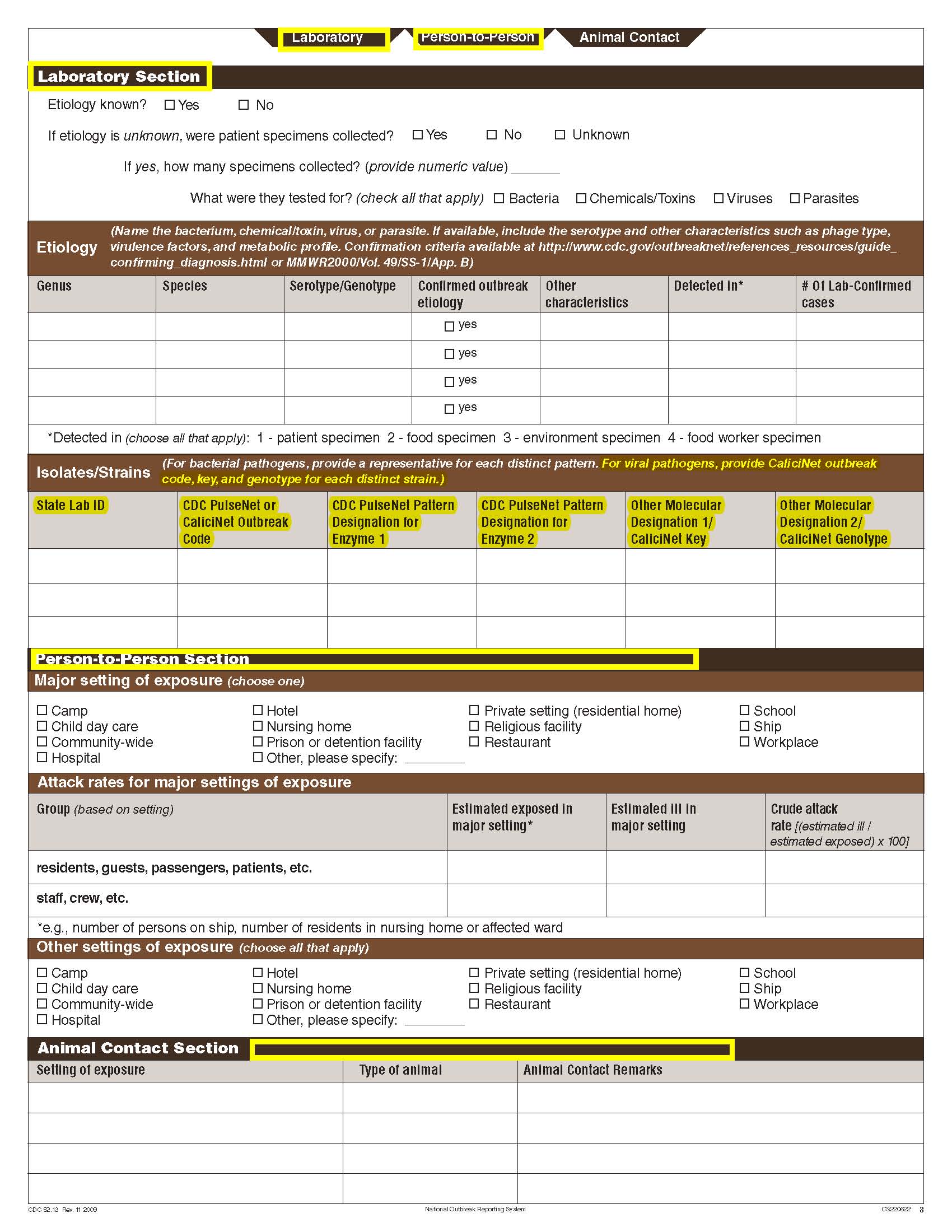
Etiology Section and page headers |
|
|
|
|
No additional questions have been added
|
||
Isolates/ Strains |
|
|
|
|
No additional questions have been added
|
||
Animal Contact |
|
|
|
|
No additional questions have been added
|
||
Food |
|
|
|
|
No additional questions have been added
|
||
|
|
|
|
|
|
|
|
Changes in data collection related to Settings |
|||
Section |
Current Question/Item |
Requested Change |
|
Primary Mode of Transmission |
|
|
|
|
No additional questions have been added
|
||
Page 3 Header |
|
|
|
|
No additional questions have been added
|
||
Settings Section |
|
|
|
|
No additional questions have been added
|
||
At the national level, the outbreak surveillance data are used to describe outbreaks and their characteristics through publications and data inquiries, identify trends in common exposures (including foodborne attribution and burden of illness estimates), inform public health policies, determine reporting metrics, and grant funding allocation.
Burden
The annualized burden hours and cost to reporting agencies to submit this data to CDC will not change significantly, if at all, from the estimates providing during the 2008 Paperwork Reduction Act Change Worksheet OMB 83-C (E) for OMB #0920-0004. The change to the annualized burden hours and cost is minimal because the reporting agencies currently collect these data elements for foodborne, person-to-person, and animal contact outbreaks. In addition, the setting or location where the outbreak occurred is a common data element reporting agencies track for internal documentation. Therefore, the effort to include these additional data elements does require a minimal up-front cost in hours. In addition, the changes to the annual submissions to CDC are not expected to change after these changes are implemented. The burden hours were based on the average time to complete the common data collection fields by multiple team members. In addition, the burden cost was based on the form being completed by master-level staff at the reporting site.
Estimates of Annualized Burden Hours (no change)
Type of Respondents |
Form name |
Number of Respondents |
Number of Responses per Respondent |
Average Burden Per Response (in hours) |
Total Burden (in hours) |
State or local governments |
CDC Form 52.13 |
50 |
33.5 |
20/60 |
558 |
Territories |
CDC Form 52.13 |
9 |
2.9 |
20/60 |
9 |
Total |
|
|
|
|
567 |
Estimates of Annualized Cost Burden (no change)
Respondents |
Number of Respondents |
Number of Responses per Respondent |
Average Burden Per Response (in hours) |
Hourly Wage Rate |
Respondent Cost |
State or local governments |
50 |
33.5 |
20/60 |
$19.92 |
$11,122 |
Territories |
9 |
2.9 |
20/60 |
$19.92 |
$173.30 |
Total |
|
|
|
|
$11,295.30 |
Privacy Impact Assessment
No individually identifiable information is being collected.
National Outbreak Reporting System (NORS) – CDC 52.12 form, Circumstances of Change Request for OMB 0920-0004
The Waterborne Disease and Outbreak Surveillance System (WBDOSS) is a collaboration between the Centers for Disease Control (CDC), the Council of State and Territorial Epidemiologists (CSTE), and the Environmental Protection Agency (EPA). This system tracks and analyzes waterborne disease outbreaks in the United States. WBDOSS has received disease outbreak reports through the electronic National Outbreak Reporting System (NORS) since a revised form was approved by the Office of Management and Budget (OMB) in 2009. NORS variables correspond to variables in the CDC 52.12 form. The CDC 52.12 (rev 01 2010) has been revised to improve overall data quality and usability by local, state and national partners. Feedback from state and federal epidemiologists was solicited prior to making these changes. The most substantial changes on the revised form (CDC 52.12, rev 02 2013) are: 1) the simplification of an existing section that is used to report outbreak etiology; 2) the consolidation of questions about water sampling; and 3) the addition of a checkbox to better characterize exposures that cause waterborne disease outbreaks. Changes to the layout of the form are detailed in the table below. Neither the actual annual number of reports nor the burden hours for users are expected to increase or decrease as a result of the changes present in the CDC 52.12 (rev 01 2010), however a change has been made to how the numbers are calculated. This change is also described below.
Section |
Current Question/Item |
Requested Change |
General |
|
|
|
||
General Section, Primary Mode of Transmission |
|
|
No additional questions have been added
|
||
Primary Cases |
|
|
No additional questions have been added
|
||
General Section, Incubation Period |
|
|
No additional questions have been added
|
||
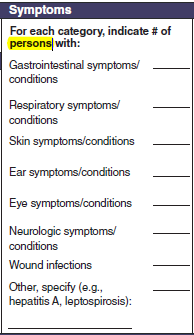
Signs or Symptoms |
|
|
No new questions have been added
|
||
Secondary Cases |
|
|
No additional questions have been added
|
||
Environmental Health Specialists Network |
|
|
No new questions have been added
|
||
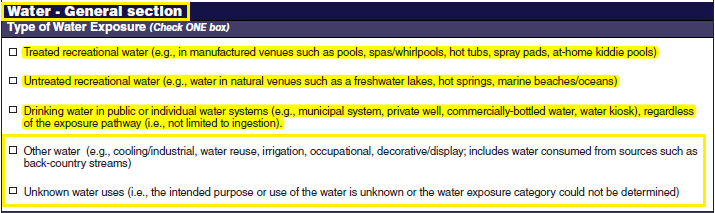
Waterborne Disease and Outbreaks - General |
|
|
|
||
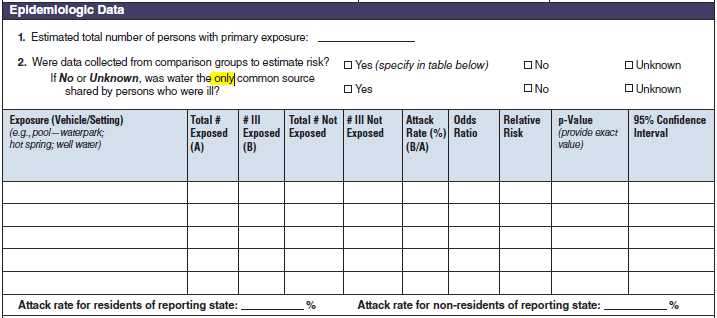
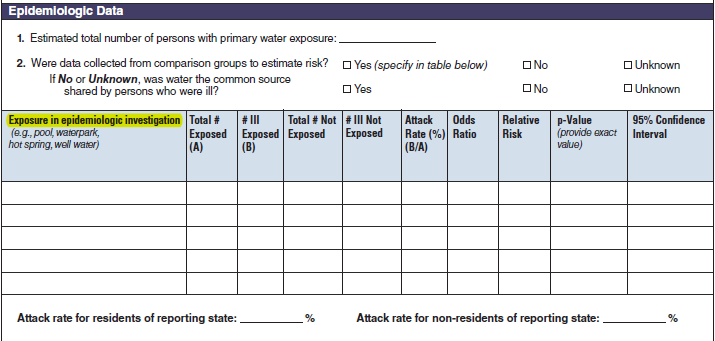
Epidemiologic Data |
|
|
No additional questions have been added
|
||
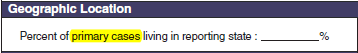
Geographic location, Symptoms
|
|
|
No additional questions have been added
|
||
|
|
|
|
||
Page 4 Header
|
|
|
No additional questions have been added
|
||
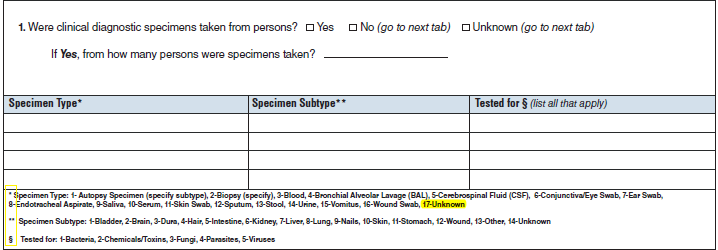
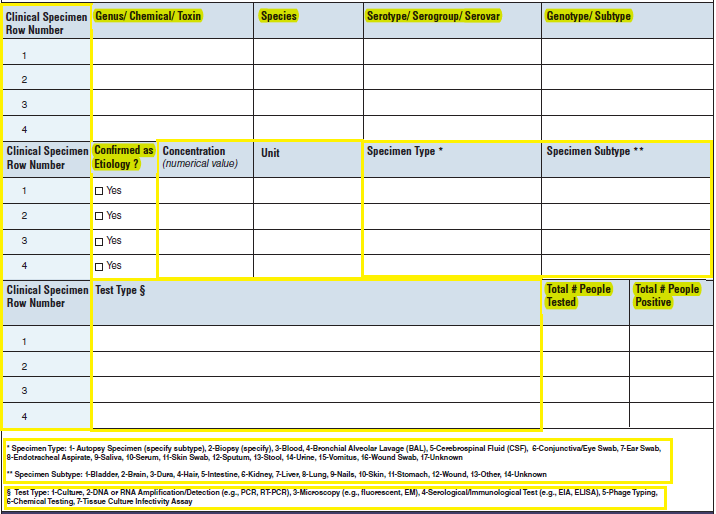
Clinical Specimens – Laboratory Results |
|
|
No additional questions have been added
|
||
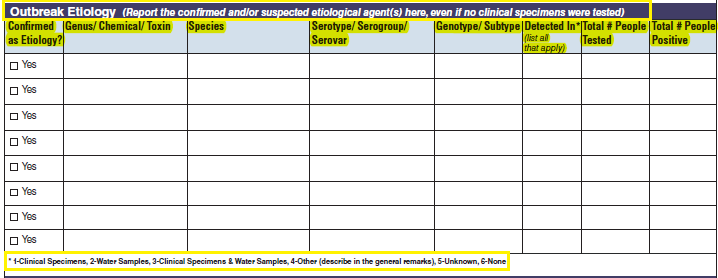
Etiology |
|
|
No additional questions have been added
|
||
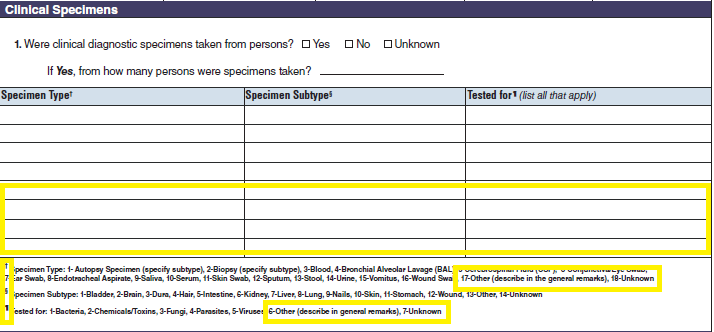
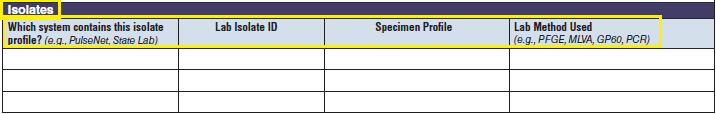
Isolates |
|
|
|
||
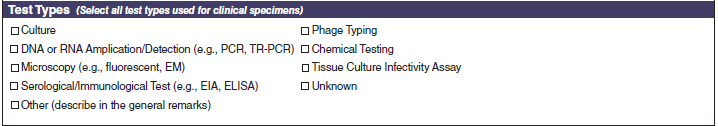
Test Types |
|
|
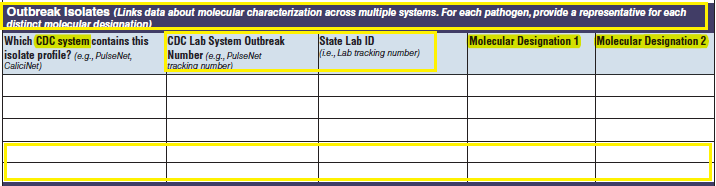
A section called Test Types has been added to the bottom of page 4.
|
||
Laboratory Section |
|
|
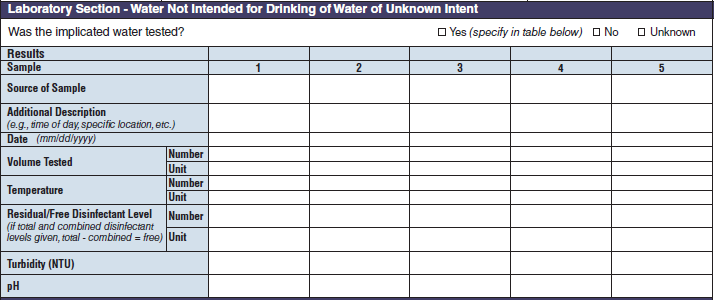
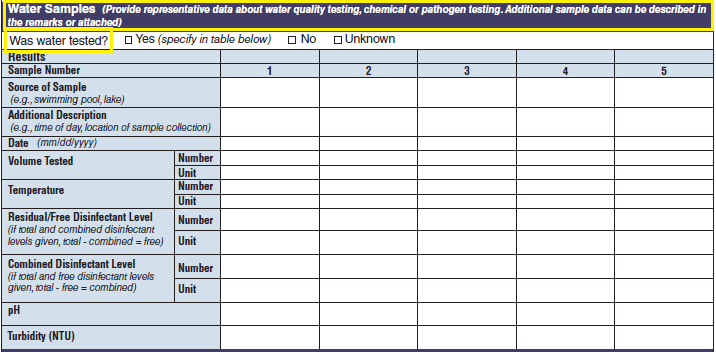
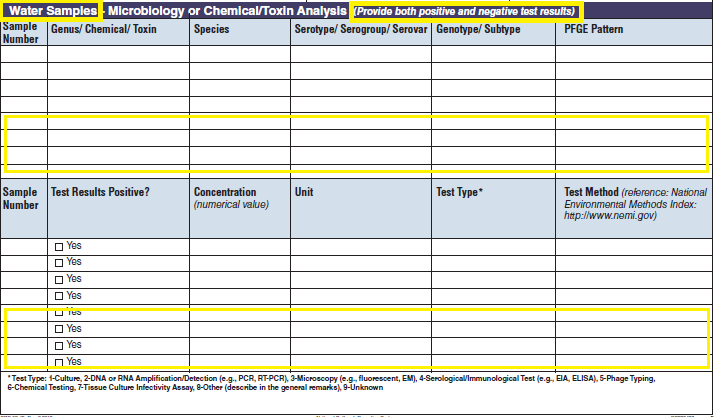
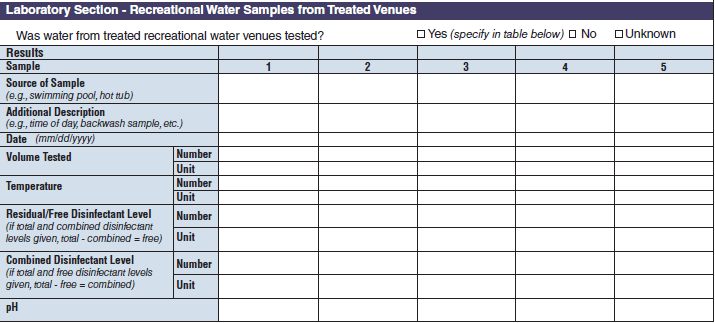
No additional questions have been added • Previously, there were four different sections about water sampling in the form. One section would be filled in per report, depending on the type of water exposure selected at the top of page 3 (i.e., Recreational water – treated, Recreational water – untreated, Drinking Water, and Water Not Intended for Drinking or Unknown). Each water sample section collected the same type of information. As a result, the sections were very similar. The four sections have been removed and consolidated into one section and page. The consolidated section and page have been labeled as a new tab called Water Samples. The Water Samples page, which can be used for any outbreak report, has been placed on page 5 so that it precedes sections of the form that are specific to the type of water exposure categories.
The four water sample tables (below, at left) have been combined into one (below, at right):
|
||
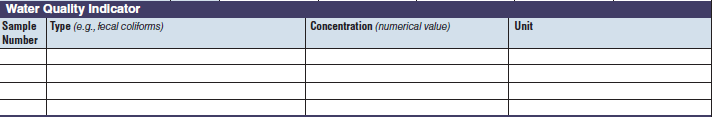
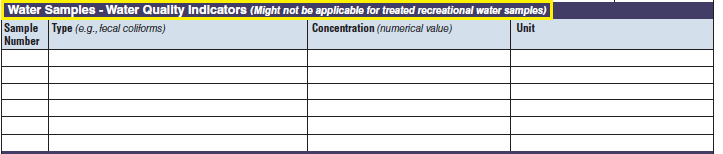
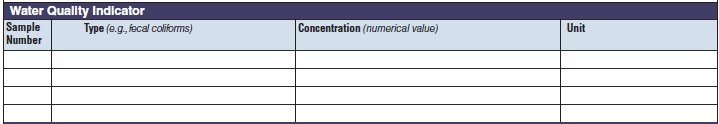
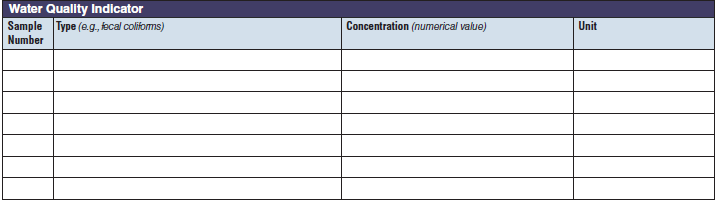
Water Quality Indicator |
|
|
The three water quality indicator tables have been combined into one (below, at right). The left side of table shows an example of the original tables, because all three tables are the same:
|
||
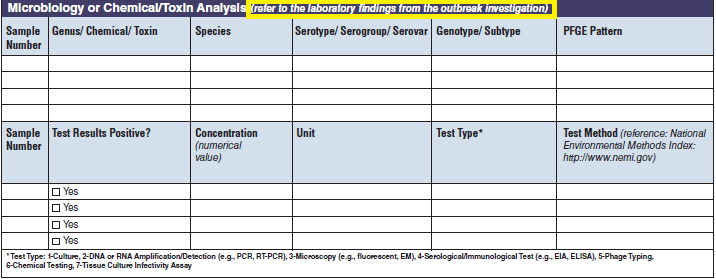
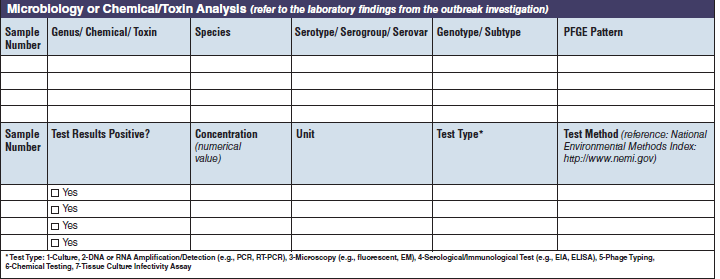
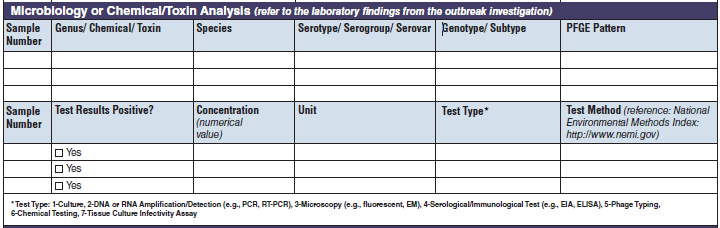
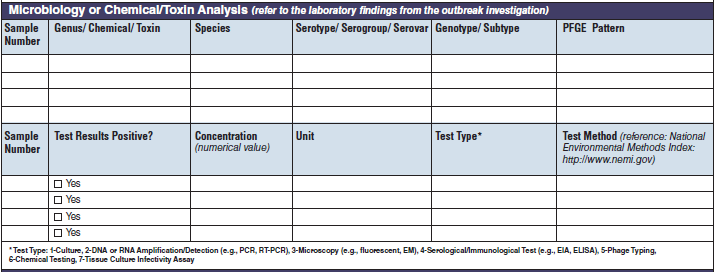
Microbiology or Chemical/ Toxin Analysis |
|
|
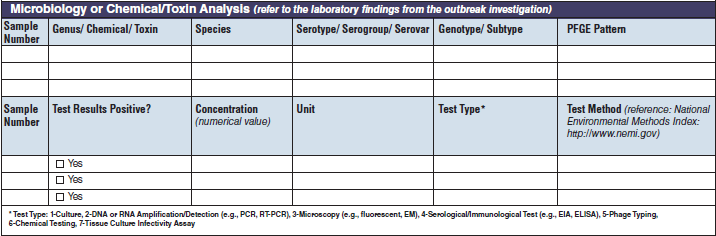
The four microbiology or chemical/toxin analysis tables have been combined into one (below, at right). The left side of table shows an example of the original tables, because all four tables are the same:
|
||
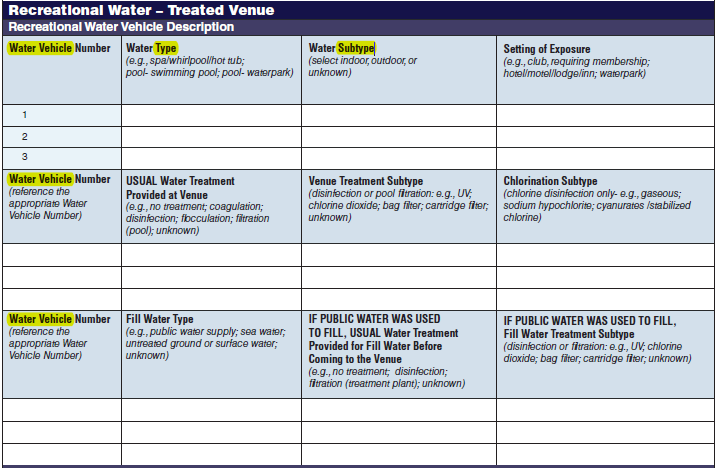
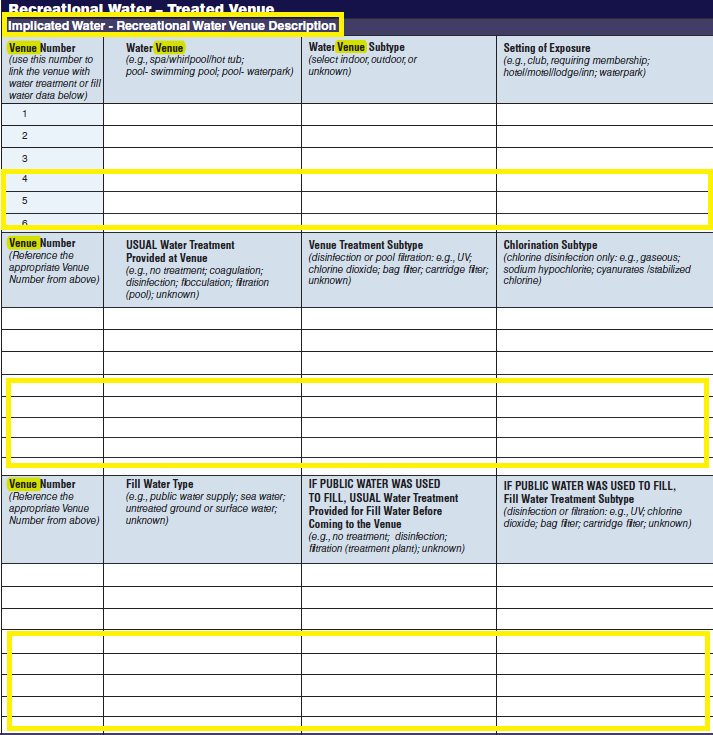
Recreational Water – Treated Venue, Recreational Water Vehicle Description |
|
|
No additional questions have been added
Five additional rows have been added to both the second and third tables to allow for the submission of more data. More rows were added to these two tables because for a single row in the first table, multiple rows of data may be filled out in second and third tables. |
||
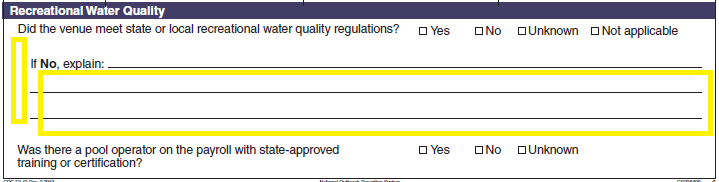
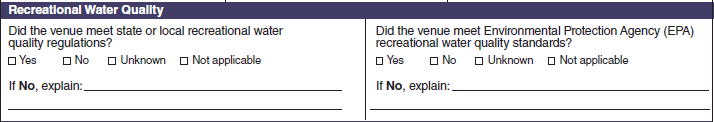
Recreational Water Quality |
|
|
|
||
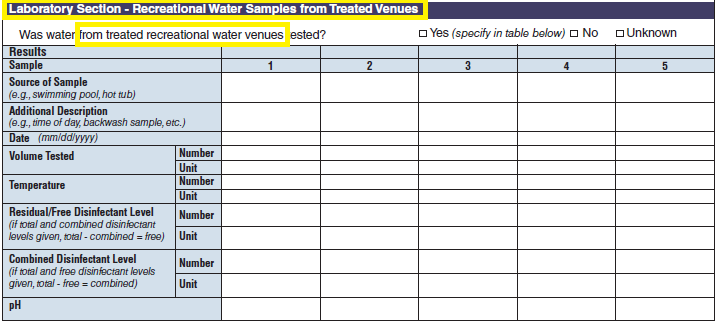
Laboratory Section – Recreational Water Samples from Treated Venues |
|
|
|
||
Microbiology or Chemical/ Toxin Analysis |
|
|
|
||
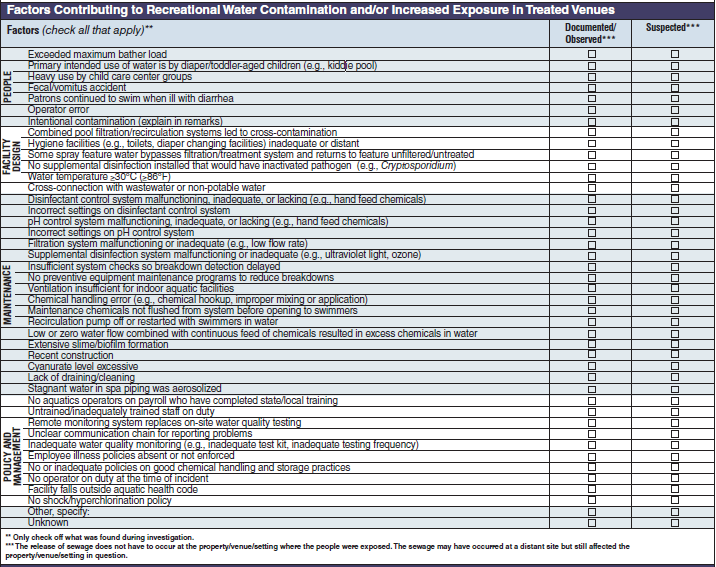
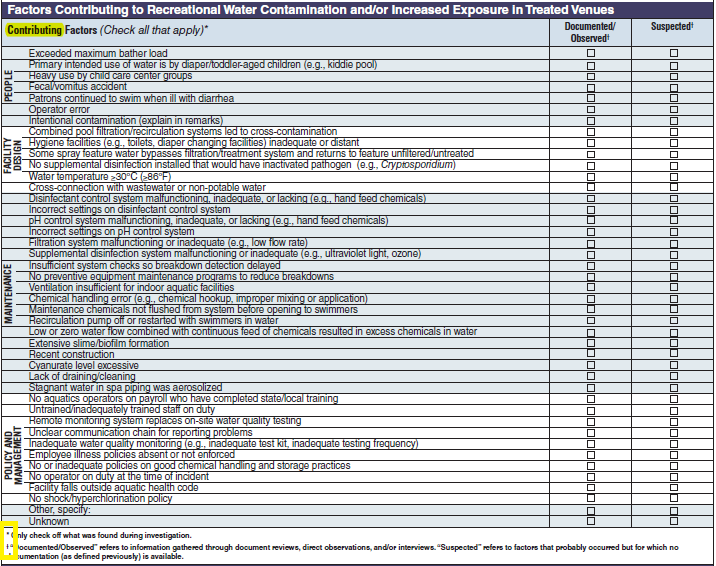
Factor Contributing to Recreational Water Contamination and/or Increased Exposure in Treated Venues |
|
|
No additional questions have been added
|
||
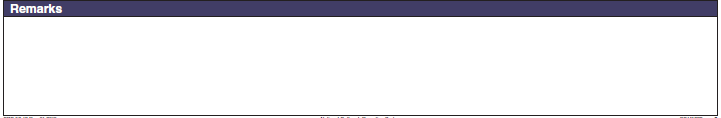
Remarks |
|
|
No additional questions have been added
|
||
Recreational Water – Untreated Venue. Recreational Water Vehicle Description |
|
|
No additional questions have been added
|
||
Recreational Water Quality |
|
|
No additional questions have been added
|
||
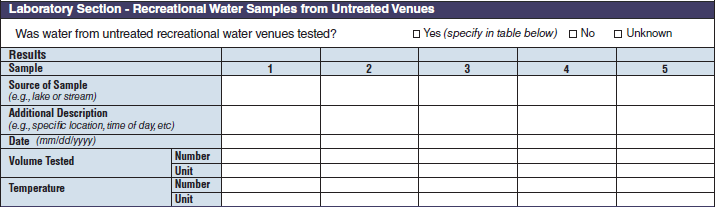
Laboratory Section – Recreational Water Samples from Untreated Venues |
|
|
|
||
Water Quality Indicator |
|
|
|
||
Microbiology or Chemical/ Toxin Analysis |
|
|
|
||
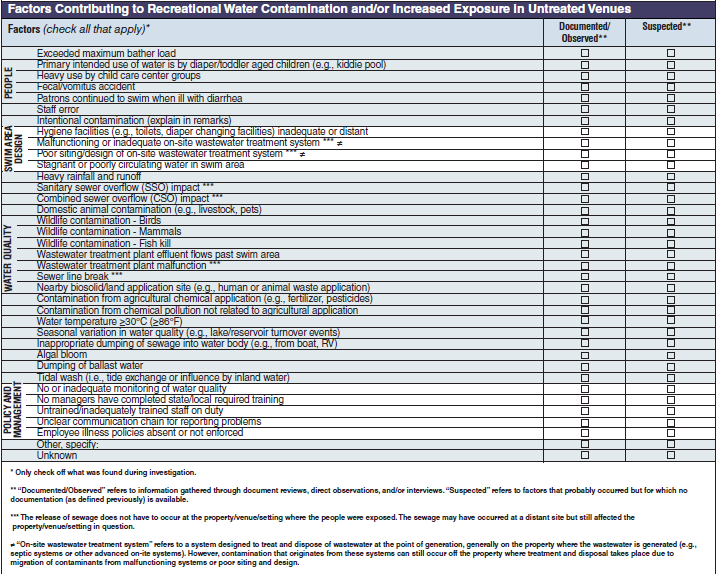
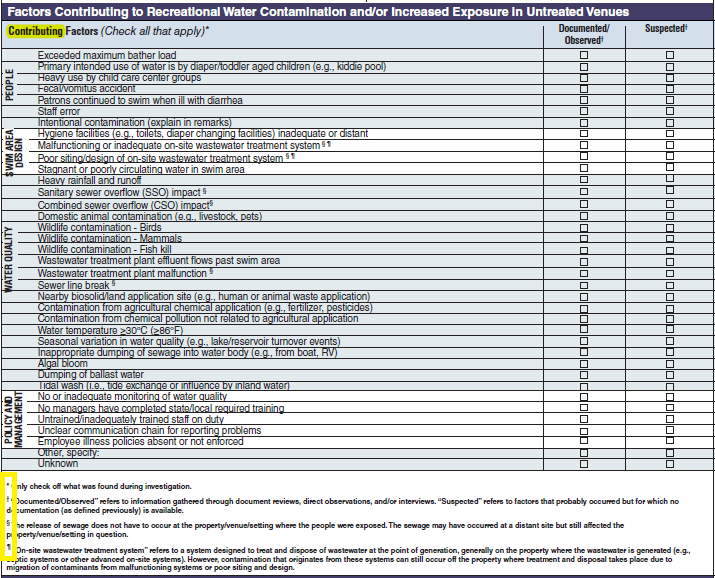
Factors Contributing to Recreational Water Contamination and/or Increased Exposure in Untreated Venues |
|
|
No additional questions have been added
|
||
Remarks |
|
|
No additional questions have been added
|
||
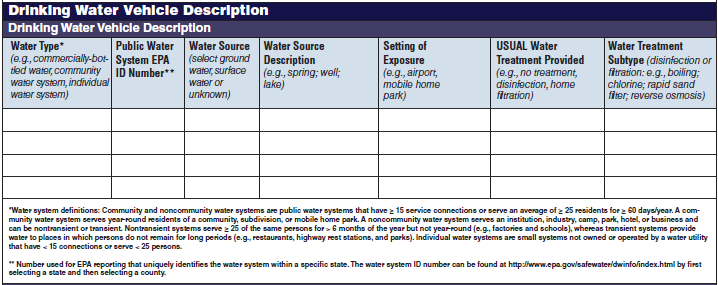
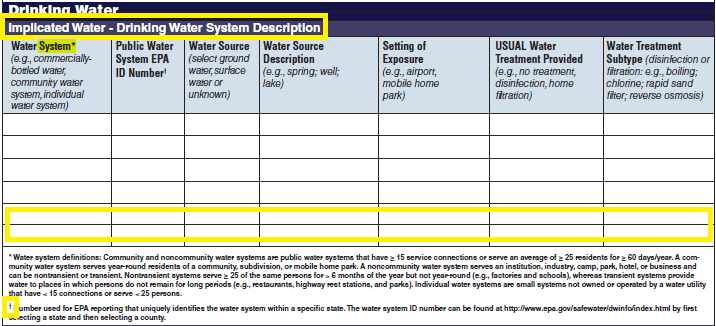
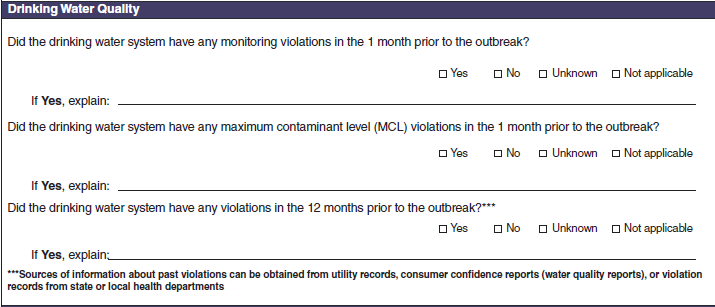
Drinking Water Vehicle Description |
|
|
No additional questions have been added
|
||
Drinking Water Quality |
|
|
No additional questions have been added
|
||
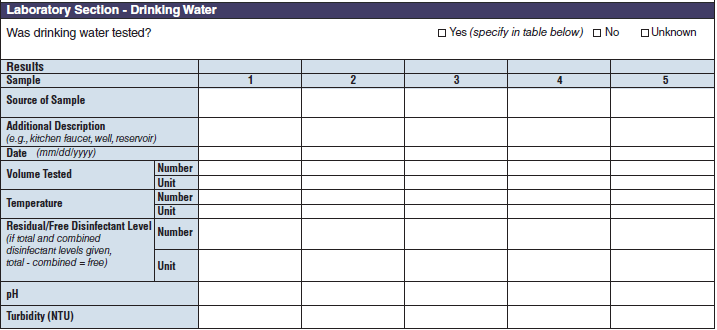
Laboratory Section – Drinking Water |
|
|
|
||
Water Quality Indicator |
|
|
|
||
Microbiology or Chemical/Toxin Analysis |
|
|
|
||
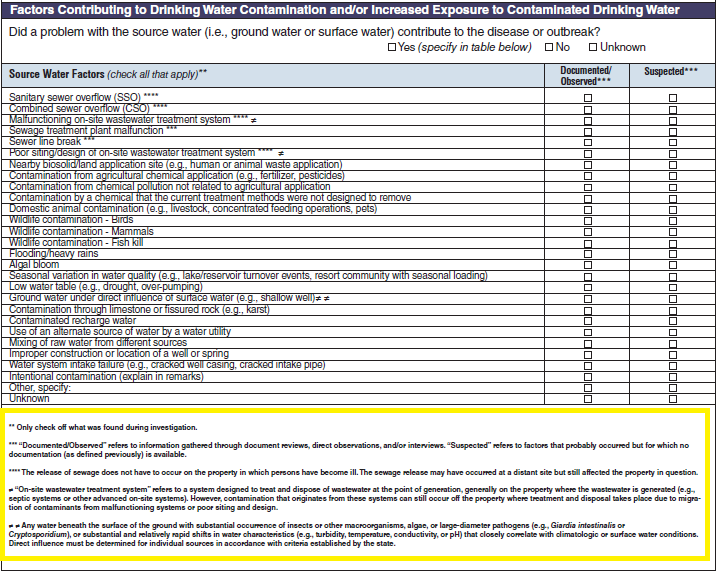
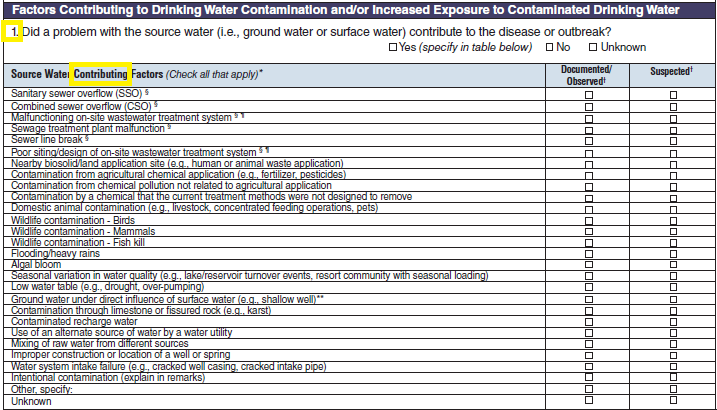
Factors Contributing to Drinking Water Contamination and/or Increased Exposure to Contaminated Drinking Water
Source Water Factors |
|
|
No additional questions have been added
|
||
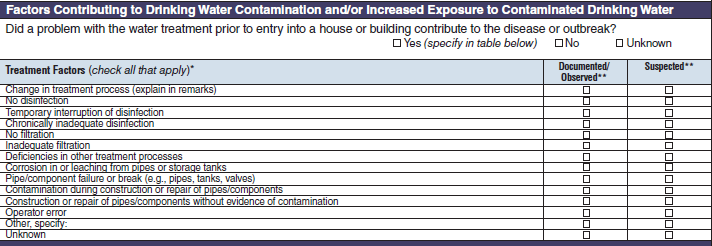
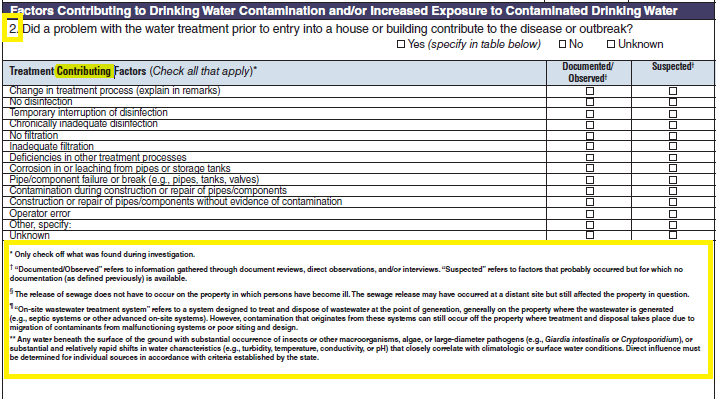
Treatment Factors |
|
|
No additional questions have been added
|
||
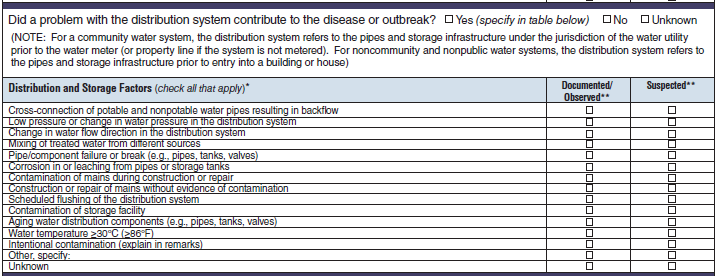
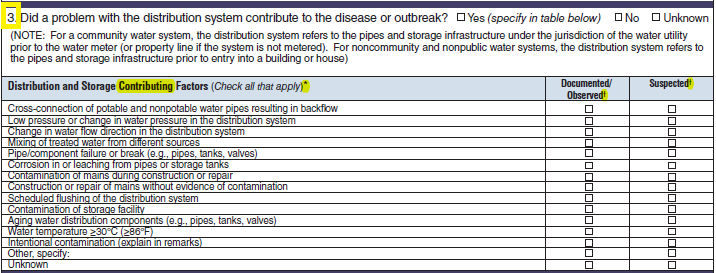
Distribution and Storage Factors |
|
|
No additional questions have been added
|
||
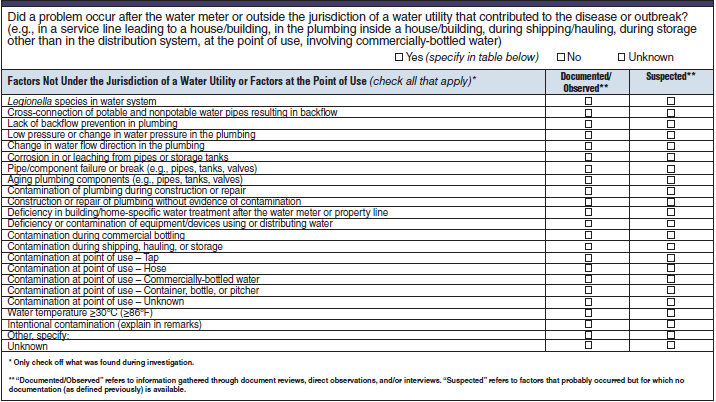
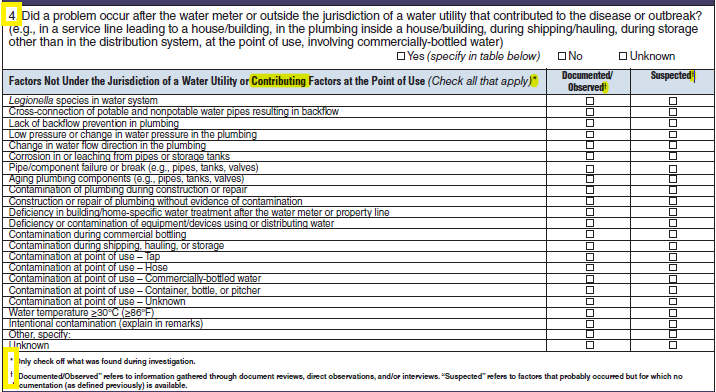
Factors Not Under the Jurisdiction of a Water Utility or Factors at the Point of Use |
|
|
No additional questions have been added
|
||
Remarks |
|
|
No additional questions have been added
|
||
Section |
Current Question/Item |
Requested Change |
Water Not Intended for Drinking or Water of Unknown Intent |
|
|
No additional questions have been added
|
||
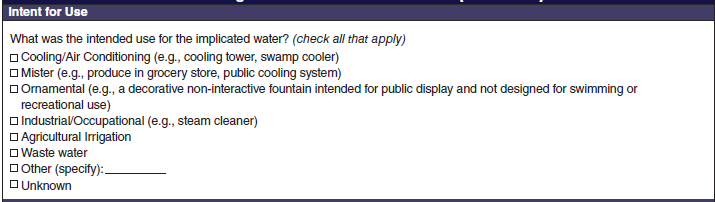
Intent for Use |
|
|
No additional questions have been added
|
||
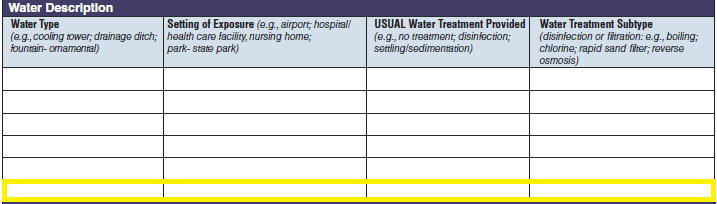
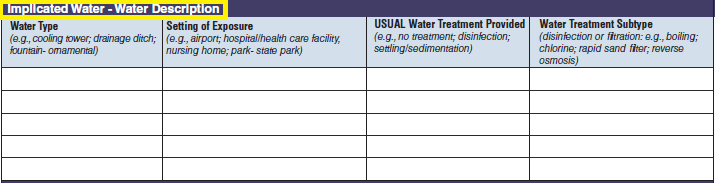
Water Description |
|
|
No additional questions have been added
|
||
Laboratory Section – Water Not Intended for Drinking of Water of Unknown Intent |
|
|
|
||
Water Quality Indicator |
|
|
|
||
Microbiology or Chemical/Toxin Analysis |
|
|
|
||
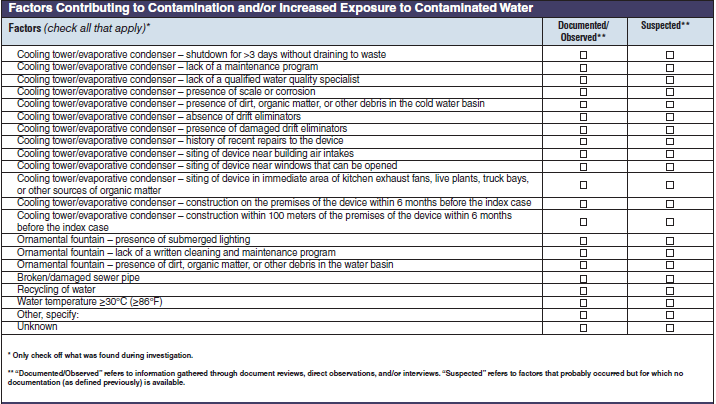
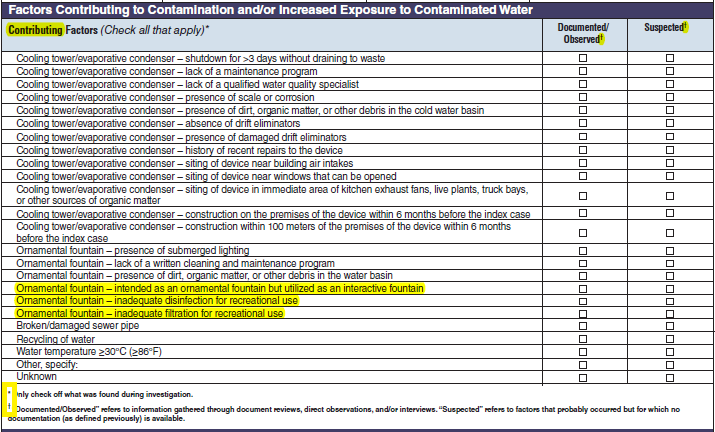
Factors Contributing to Contamination and/or Increased Exposure to Contaminated Water |
|
|
|
||
Remarks |
|
|
No additional questions have been added
|
||
Purpose and Use of Information Collection
At the national level, waterborne outbreak surveillance data are used to describe outbreaks and their characteristics through publications and data inquiries; identify trends in common exposures; and inform public health policies and interventions. WBDOSS has collected data since 1971. No other United States public health surveillance system collects aggregate data about waterborne disease outbreaks and human illness at a national level.
Burden
The annualized burden hours and cost to reporting agencies that submit waterborne disease outbreak data to CDC will not change significantly, if at all, from the estimates provided previously in 2010. The change to the annualized burden hours and cost is minimal because the form asks the same questions but has been revised to be easier to use. Additional fields or data table rows have been added based on form user feedback, reporting practices, or for convenience where extra space was available on a page. In addition, the form has been shortened by two pages, therefore, if any change in burden and cost were anticipated, the result would likely be a lower cost and burden. The number of annual submissions to CDC is not expected to change as a result of the modifications to form.
The burden hours and cost below are based on the calculations from the previous CDC 52.12 form OMB submission in 2010.The tables have been revised. The 2010 OMB paperwork described 57 respondents (50 states and 7 other reporting jurisdictions). NORS currently supports outbreak reporting by 59 sites (50 US states, the District of Columbia, five US territories, and three Freely Associated States), however, not all states or other reporting jurisdictions report waterborne disease outbreaks each calendar year. The burden hours have therefore increased from 19 to 23 but this represents a maximum value. The cost burden has also increased from $1,322.40 to $1,368.80 but this also represents a maximum value.
Privacy Impact Assessment
No individually identifiable information is being collected.
Estimates of Annualized Burden Hours (change to the total number of reporting sites from NORS, but no actual change in burden hours because not all reporting sites submit an outbreak report annually)
Type of Respondents |
Form name |
Number of Respondents |
Number of Responses per Respondent |
Average Burden Per Response (in hours) |
Total Burden (in hours) |
State governments |
CDC Form 52.12 |
50 |
1 |
20/60 |
17 |
Territories, District of Columbia, Freely-associated states |
CDC Form 52.12 |
9 |
1 |
20/60 |
3 |
Total |
|
|
|
|
20 |
Estimates of Annualized Cost Burden (change to the total number of reporting sites from NORS, but no actual change in cost burden because not all reporting sites submit an outbreak report annually)
Respondents |
Number of Respondents |
Number of Responses per Respondent |
Average Burden Per Response (in hours) |
Cost Per Response |
Respondent Cost |
State governments |
50 |
1 |
20/60 |
$23.20 |
$386.67 |
Territories, District of Columbia, Freely-associated states |
9 |
1 |
20/60 |
$23.20 |
$69.60 |
Total |
|
|
|
|
Influenza – Revision of one form, addition of 1 form.
Novel influenza A virus:
In 2007, the Council of State and Territorial Epidemiologists (CSTE) adopted a position statement making human infection with a novel influenza A virus a nationally notifiable condition. Novel influenza A virus infections include all human infections with influenza A viruses that are different from currently circulating human influenza H1 and H3 viruses. These viruses include those that are subtyped as nonhuman in origin and those that are unsubtypable with standard methods and reagents. Rapid reporting of human infections with novel influenza A viruses will facilitate prompt detection and characterization of influenza A viruses.
From 2005 to early 2012, only 36 cases of variant (v) influenza virus infection were reported to the Centers for Disease Control and Prevention (CDC). From July–September 2012, however, 306 cases of H3N2v were reported in 10 states, representing the largest outbreak of human infections with a variant influenza virus since the 2009 H1N1 pandemic. A majority of cases had self-limited illness, but hospitalizations were more prevalent among those with young age and the presence of underlying medical conditions. Most cases reported prolonged and direct exposure to swine at an agricultural fair, suggesting that was the primary risk factor for illness.
This outbreak highlighted the assertion that every case of variant influenza virus infection has epidemic potential and must be investigated thoroughly and rapidly. Therefore, a working group was convened to identify and incorporate additional data elements that will be instrumental in the efficient and rapid investigations of all variant influenza virus infections. The additional elements include new sections to assess the signs and symptoms associated with the illness, the clinical course of the illness, the exposures to agricultural fairs and animals prior to illness onset, and the potential for human-to-human transmission, especially among household members and healthcare workers. These additional elements will accelerate the understanding of the basic epidemiology of new variant influenza viral infections and the implementation of effective public health responses, thereby preventing additional morbidity and mortality.
The Human Infection with Novel Influenza A Virus Case Report Form, is a standardized case questionnaire which contains detailed questions on relevant clinical and epidemiologic features of influenza, was developed by CSTE and CDC. State or territorial influenza surveillance epidemiologists report these data over the Internet on the Secure Data Network (SDN). The title of this form has been slightly revised from its original title of the Novel Human Influenza A Virus Infection Case Report Form.
Privacy Impact Assessment
Personal identifiers are collected by state or local public health officials and maintained at the state or local health department before submission to CDC.
Estimated Burden
The annualized total burden hours did increase from the previous approval. A significant increase in the number of human infections from novel influenza A virus were identified during 2012, compared to previous influenza seasons. The increase in the number of responses per respondent was needed to more accurately portray the burden on respondents. The annualized burden to complete one case report form did not change from the previous approval.
Human Infections with Novel Influenza A Virus
Type of Respondents |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Hrs/response |
Total Burden in hrs. |
State and Local Governments |
Human Infection with Novel Influenza A Virus Case Report Form |
57 |
6 |
30/60 |
171 hours |
Antiviral resistance form
Antiviral drugs are the second line of defense against influenza viruses. Currently, only 2 drugs are licensed for use and active against circulating viruses, oseltamivir and zanamivir; oral oseltamivir is used for almost all infections in the US. There are limited treatment options for an infection with an oseltamivir-resistant viruses, experimental drug use would e required; thus widespread circulation of resistant viruses is a public health emergency requiring special guidance and testing. After a resistant virus is identified by the laboratory, it is necessary to obtain key information from the infected patient to determine whether the resistant virus was circulating in the community or whether the resistant virus developed during treatment. This information is critical to antiviral recommendations and guidance. Over the past several seasons since the pandemic 2009 virus began circulating, we have seen a small but steady increase in the circulation of oseltamivir-resistant viruses. Any additional and significant increase will require new guidance and health alerts. This new form, Antiviral Resistant Influenza Infection Case Report Form, will be critical to the collection of information that is essential to antiviral use guidance. Since circulating viruses are constantly changing, annual monitoring is needed.
Privacy Impact Assessment
Personal identifiers are collected by state or local public health officials and maintained at the state or local health department before submission to CDC.
Antiviral Resistant Influenza Infection
Type of Respondents |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Hrs/response |
Total Burden in hrs. |
State and Local Governments |
Antiviral Resistant Influenza Infection Case Report Form |
57 |
3 |
30/60 |
86 hours |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Roberts, Virginia (CDC/OID/NCEZID) |
| File Modified | 0000-00-00 |
| File Created | 2021-01-29 |
© 2026 OMB.report | Privacy Policy