OMB_PartA_Final_Update
OMB_PartA_Final_Update.docx
Research to Inform the Prevention of Asthma in Healthcare
OMB: 0920-0983
Research to Inform the Prevention of Asthma in Health Care
ICR SUPPORTING STATEMENT – PART A
Justification
DIVISION OF RESPIRATORY DISEASE STUDIES
FIELD STUDIES BRANCH
NATIONAL INSTITUTE FOR OCCUPATIONAL SAFETY AND HEALTH
Principal Investigators:
Paul K. Henneberger
Co-Project Officer/Epidemiologist
Phone: 304-285-6161
Fax: 304-285-5820
Email: [email protected]
M. Abbas Virji
Co-Project Officer/Industrial Hygienist
Phone: 304-285-5797
Fax: 304-285-5820
Email: [email protected]
Table of Contents
A. Justification
1. Circumstances Making the Collection of Information Necessary
2. Purpose and Use of Information Collection
3. Use of Improved Information Technology and Burden Reduction
4. Efforts to Identify Duplication and Use of Similar Information
5. Impact on Small Businesses or Other Small Entities
6. Consequences of Collecting the Information Less Frequently
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
8. Comments in Response to the Federal Register Notice and Efforts to Consult
Outside the Agency
9. Explanation of Any Payment or Gift to Respondents
10. Assurance of Confidentiality Provided to Respondents
11. Justification for Sensitive Questions
12. Estimates of Annualized Burden Hours and Costs
13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
14. Annualized Cost to the Government
15. Explanation for Program Changes or Adjustments
16. Plans for Tabulation and Publication and Project Time Schedule
17. Reason(s) Display of OMB Expiration Date is Inappropriate
18. Exceptions to Certification for Paperwork Reduction Act Submissions
Appendix
Appendix A: Occupational Safety and Health Act
Appendix B: 60-day Federal Register Notice
Appendix C: Response to 60 day FRN public comment
Appendix D: IRB Approval Notice
Appendix E: Initial Contact Letter
Appendix F: Recruitment Letter & Informed Consent
Appendix G: Newsletter Language
Appendix H Reminder Postcard
Appendix I: Interview Scripts
Appendix J: Survey Instructions
Appendix K: Survey
Appendix L: Spanish Language Survey
Appendix M: Survey Electronic Version
Appendix N: Draft Results Letter
Appendix O: Occupational Exposures Determined by Survey Questionnaire Responses and Job-Exposure Matrix
1. Circumstances Making the Collection of Information Necessary
Introduction
This justification document is in support of the National Institute for Occupational Safety and Health’s (NIOSH) request for Office of Management and Budget (OMB) approval for the Research to Inform the Prevention of Asthma in Healthcare project. We are seeking OMB approval for two years. This is a new Information Collection Request (ICR) and the data collection is authorized by Section 20(a) (1) of the Occupational Safety and Health Act (29 U.S.C. 669) (Appendix A).
Background
The healthcare industry provides essential services for society, both routinely and in times of great need following natural or man-made disasters. Also, healthcare is a big industry and still growing. In 2006, it was the largest industry in the United States with 14 million jobs, according to data from the Bureau of Labor Statistics (DOL 2008). Healthcare will generate 3 million new jobs during 2006-2016, more than any other industry. Ongoing efforts are needed to ensure the health and safety of workers in this critically important industry.
Asthma and work-related asthma are more common in healthcare.
The healthcare workforce in the United States has a disproportionate number of asthma and WRA cases. An analysis of data from the National Health Interview Survey (NHIS) (1997-2004) found that ‘health services except hospitals’ ranked first among 42 industries with a lifetime asthma prevalence of 11.2% (Syamlal 2008). Based on mortality data (1990-1999) from the National Center for Health Statistics (NCHS), two of the ten industries with statistically elevated proportionate mortality ratios (PMRs) for asthma were in healthcare: ‘health services not elsewhere classified’ and ‘hospitals’ (CDC 2008). From the NIOSH-sponsored Sentinel Event Notification Systems for Occupational Risks (SENSOR), healthcare workers were disproportionately represented among WRA cases, with 16% of the cases but only 8% of the workforces in the four states where surveillance was conducted (Pechter 2005). A review of accepted worker compensation claims for asthma in Washington state, 1995-2002, revealed that the ‘health services’ industry had the most claims and the ninth highest incidence among 28 industries (Curwick 2003).
Asthma and work-related asthma are associated with certain healthcare occupations.
When researchers examined NHIS data (1997-2004) for 42 different occupations, ‘health services’ and ‘health technologist and technician’ had the highest values for lifetime asthma prevalence, both with 11.5% (Syamlal 2008). Also, the same two occupations were include among the top 10 occupations for asthma attacks, with a one-year prevalence of 3.8% for ‘health services’ and 4.2% for ‘health technologist and technician’ (CDC 2008). Studies in the United States (Christiani 1993) and Canada (Dimich-Ward 2004) reported that respiratory therapists were at higher risk for post-hire asthma than physical therapists. Another Canadian study identified radiology assistants at an increased risk for asthma attacks (Dimich-Ward 2003). From the follow-up round of the European Community Respiratory Health Survey (ECRHS II), the risk of asthma was elevated for ‘personal care providers in institutions’ and ‘nursing technicians in hospitals’ (Mirabelli 2007). An analysis of United States mortality data (1990-1999) identified 22 occupations with statistically elevated PMRs for asthma (CDC 2008), with 7 (about 1/3) in healthcare: respiratory therapists; health diagnosing practitioners not elsewhere classified; dentists; clinical laboratory technologists and technicians; health aides except nursing; registered nurses; and nursing aides, orderlies, and attendants.
Asthma is associated with certain tasks and exposures in healthcare.
Exposure to dampness and mold, or other biological agents, in both homes and non-industrial indoor work environments, is a risk factor for asthma and asthma-like symptoms, and should be considered when studying asthma (Laney 2009, Park 2006, Park 2008, Sahakian 2008, Zock 2002). Dampness in buildings normally arises from building envelope deficiencies or interior sources such as plumbing leaks. Also, healthcare facilities are routinely humidified, which could contribute to dampness problems. A NIOSH Health Hazard Evaluation (HHE) investigation determined that complaints of work-related respiratory symptoms and asthma among workers in a medical center were related to water incursion and mold at work (Cox-Ganser 2001). Also, from the same study, self-reported chest, nasal, and sinus symptoms were associated with reports of water damage, visible mold, or mold odor at home (Cox-Ganser 2009).
Dr. Delclos and colleagues at the University of Texas recently investigated asthma in healthcare workers (Delclos 2007). They developed an asthma-specific job-exposure matrix (JEM) for healthcare, and a questionnaire that was completed by over 3,500 participants in four occupations: physicians, nurses, respiratory therapists, and occupational therapists. The high-risk tasks for asthma onset were using cleaning products to clean instruments (RR=2.2, 95% CI 1.3, 3.7) or surfaces (RR=2.0, 95% CI 1.2, 3.4), and implicated exposures were aerosolized medications (RR=1.7, 95% CI 1.05, 2.8) and latex during the years 1992-2000 when powdered NRL gloves were common (RR=2.2, 95% CI 1.3, 3.7) (Delclos 2007). For respiratory symptoms related to bronchial hyper-responsiveness (a finding characteristic of asthma), high-risk exposures were adhesives, solvents, or gases used in patient care (RR=1.7, 95% CI 1.2, 2.2) and accidental spills or gas releases (RR=2.0, 95% CI 1.3, 3.2). The final exposure was common, reported by 5.8% of participants.
Common causes of WRA impact several healthcare occupations.
Evidence from SENSOR WRA surveillance (1993-1997) suggests that many healthcare occupations are at risk for the adverse effects of two common exposures: cleaning agents and disinfectants (Pechter 2005). Cleaning agents were associated with WRA cases in nurses (n=26), aides and therapists (n=11), and lab workers (n=4), as well as house-keeping and food preparation staff (n=8). WRA cases attributed to glutaraldehyde or formaldehyde were more numerous in nurses (n=23) and lab workers (n=9), but also reported in aides and therapists (n=3).
Healthcare research gaps and needs.
In any future study of asthma in healthcare, it will be important to focus on occupations unique to the industry. Some occupations in the healthcare industry might be at risk for asthma, but their exposures resemble those of workers in the same occupations in non-healthcare industries. For example, food service workers in healthcare facilities probably experience similar exposures as their counterparts in other workplaces, like restaurants. Likewise, moisture-related mold problems in office buildings can increase the risk of asthma (Park 2006), but exposures for office workers in healthcare are probably similar to those experienced by office workers in other industries.
Cleaning products are common risk factors for asthma among healthcare workers. Cleaning has a different significance in healthcare than in many other settings because of the greater emphasis on preventing infections. The goal of disease prevention might be driving the use of more potent cleaning agents, which could have its own consequences in the form of asthma and related symptoms. It is important to investigate asthma among housekeepers in healthcare, even if they are a small percentage of the workforce and do not directly deliver care to patients. Also, it is important to determine if housekeepers have fewer asthma-related health problems when working with “green” cleaning products.
Exposure determinants for healthcare exposures are not well understood.
Few studies have investigated the determinants of exposures associated with asthma in the healthcare sector. A study of radiographers found ventilation, workload, and time in certain work areas were important determinants of exposure to glutaraldehyde, acetic acid and sulfur dioxide (Teschke 2002). Other studies have reported ventilation (Byrns 2000, Koda 1999) and work practices (Nayebzadeh 2007, Koda 1999) are important exposure factors in some healthcare occupations. Glove use among dental technicians was an important determinant of exposure to methyl methacrylates (Liljelind 2009). Bello and colleagues (Bello 2009) identified cleaning and disinfecting tasks and potential dermal exposures to cleaning agents among housekeepers.
Privacy Impact Assessment
Overview of the Data Collection System
Data collection for this study will be completed either online or by Computer Assisted Telephone Interview (CATI). For this study, NIOSH is partnering with the 1199SEIU (Service Employees International Union) United Healthcare Workers East in New York City. NIOSH staff will be responsible for managing the online portion of the data collection. NIOSH has initiated a contract with Research Triangle Institute (RTI) International to provide epidemiology support and conduct the telephone interviews for this data collection. In order to collect the survey data by telephone interview, RTI has subcontracted with the SEIU Communications Center (CC) (a subsidiary of our project partner 1199SEIU) to place the telephone calls and interview participants. As part of the contract between RTI and NIOSH and the subcontract between RTI and SEIU CC, RTI is required to:
provide technical oversight of the communications center throughout the data collection process;
review all required materials and plans;
prepare and provide training for the supervisory and interview staff at SEIU CC; and
monitor data collection on-site at SEIU CC.
RTI will provide 162 hours of technical assistance and review of project-related computer activities. In terms of technical oversight, RTI will be responsible for overall management of the data collection, and for ensuring that SEIU CC conducts the study and collects and delivers the data in a manner that upholds both the quality of the data itself and the integrity of the study’s design. RTI and SEIU CC will work to ensure that data is collected and stored securely on SEIU CC servers, and that access to that data is limited to a handful of authorized personnel, including the SEIU CC director and the RTI project director.
RTI’s specific duties related to reviewing computer activities include:
1. Reviewing and providing programming feedback on the SEIU CC’s Computer Aided Telephone Interview (CATI) system;
2. Reviewing and providing comments on the SEIU CC’s quality control procedures; and
3. Reviewing and providing comments on the SEIU CC’s data security
The contract specifies that RTI will provide 308 hours of onsite training and monitoring of SEIU CC supervisors and staff. Training of supervisors and staff will consist of two training sessions just prior to the start of each type of data collection: inbound calls by sample members to SEIU CC for completion of the CATI questionnaire, and outbound calls from the communications center to survey sample members who have not yet responded. This training will cover standard procedures for telephone interviews including:
A brief review of general interviewing techniques to assure basic preparedness on the part of the SEIU CC staff;
Proper procedures for providing sufficient study information and obtaining informed consent;
Instructions on and scripting for all introductory and transitional scenarios;
Instruction on the administration and completion of the survey instrument;
Techniques for asking questions, including the following requirements:
reading verbatim,
reading questions (and response choices when required) completely and in the order in which they appear,
avoiding language that is leading,
collecting responses exactly as they are given (not assuming answers),
reading all transitional and instructional text as it appears,
following instructions to interviewers as they appear, and
adhering to the skip logic (skip patterns) of the instrument as programmed;
The importance of providing sufficient information to participants before, during, and after the interview, and the importance of upholding basic respondent rights;
Correct use of neutral probing when participants provide confusing, ambiguous, inappropriate or incomplete answers;
Proper procedures for recording answers to open-ended survey questions; and
Adverse event and/or respondent distress reporting and documentation.
Onsite monitoring of telephone calls and interviews allows RTI to observe and assess supervisor and interviewer performance. This monitoring will include holding quality control meetings with supervisors and staff at the SEIU CC to review progress and address any challenges with potential solutions for reducing data collection errors.
The contract specifies that RTI will provide 210 hours of overall project management, including oversight of the data collection setup, budget, and schedule. Furthermore, RTI will review data from the first 50 telephone interviews and provide a written summary to NIOSH within one week after the last of those interviews are completed. This data review will ensure quality by detecting any challenges, allowing the team to address and resolve them before they become problematic. Data will be linked to a generated identification number to help preserve respondent confidentiality, and during data review, only anonymized data will be used. RTI and SEIU CC will also participate in weekly meetings with NIOSH to report on the data collection process, and minutes from these meetings will be delivered weekly to NIOSH.
The online survey will be hosted on the CDC website and collected data will be stored on the CDC Mid-Tier Data Center. Data from the telephone interviews will be maintained by the SEIU CC, with oversight by RTI, until the interviews are complete. Once data collection is complete, the data will be copied to portable media and mailed to NIOSH. Data collected for his project will be maintained according to CDC record schedule.
Items of Information to be Collected
All survey data will be collected by self-reporting from participants. This data includes:
Medical information
Employment information
Occupational exposures
Demographic data
No individually identifiable information will be collected.
Identification of Website and Website Content Directed at Children Under 13 Years of Age
This project involves web-based data collection methods. Study participants will have the option to complete the survey online. The online survey will be hosted on the CDC website with data stored on the Mid-Tier Data Center. Access to the website hosting the survey will be restricted and only available to participants who have been provided with an individual access code. The website hosting the survey, and the content of the survey, is not directed at children less than 13 years of age. Furthermore, the participation in the survey is limited to participants 18 years of age or older. Only CDC network administrators will have access to the website hosting the survey.
2. Purpose and Use of Information Collection
The primary objective of this project is to identify modifiable occupational risk factors for asthma, related symptoms, and exacerbation of asthma in healthcare that will inform strategies for prevention. Specific aims that support the primary objective include the following:
Aim 1. Update an existing survey questionnaire and job-exposure matrix to investigate asthma in healthcare workers.
Aim 2. Measure frequency of asthma onset, related symptoms, and exacerbation of asthma in selected healthcare occupations.
Aim 3. Assess associations between asthma outcomes and exposures to identify modifiable risk factors.
Aim 4. Communicate findings to study participants and stakeholders who can use the information for prevention.
Specific Aim # 1 Update an existing survey questionnaire and a job-exposure matrix to investigate asthma in healthcare workers.
The first aim addresses the revision and extension of the existing survey questionnaire and a job-exposure matrix (JEM) that were successfully used in the University of Texas study of asthma in healthcare (Delclos 2006, Delclos 2007). Figure 1 below presents an overview of the exposure characterization and the development and use of various exposure metrics in epidemiologic analyses to identify the potential risk factors for asthma-related symptoms and exacerbation of asthma. The survey questionnaire was extended to include exposure modules that elicit detailed information on tasks performed and the types of cleaning products (chemicals) used for the different hospital activities such as cleaning and disinfecting surfaces or work in laboratories etc. (see questionnaire items 50.1 – 55.1 in Appendix K for details). Information on the performance of tasks and products used (yes/no) and their frequency (days per week) and duration (hours) will be calculated and used as exposure metrics in epidemiologic exposure-response analyses. The exposure-response analyses with these metrics may identify tasks and/or products (chemicals) (described in Table 1, Appendix O) associated with asthma-related symptoms and exacerbation of asthma. In addition, a quantitative JEM will be developed based on environmental sampling of occupations at five medical centers. The data from these sampling efforts will be used to develop a quantitative JEM for 14 chemicals and total volatile organic compounds (TVOC) measured among the occupations included in the survey. The quantitative exposures will be stratified by exposure modifiers, such major task categories (e.g., cleaning surfaces, cleaning instruments) and used in conjunction with worker specific information from the survey questionnaire to estimate each worker’s exposures. The combination of the JEM with exposure modifiers (e.g., major task categories) facilitates the application of these exposure estimates beyond medical centers where they were measured, as the exposures are expected to be more similar within than between modifiers, and the modifiers are expected to be similar across different medical centers. The quantitative exposure metrics will be used in epidemiologic exposure-response models to identify the association between specific chemicals (described in Table 2, Appendix O) and asthma-related symptoms and exacerbation of asthma. Knowledge of the risk posed by specific chemicals may indirectly inform prevention in a broader context through the identification of products containing the specific chemicals of concern. Details of the questionnaire and JEM development are provided below.
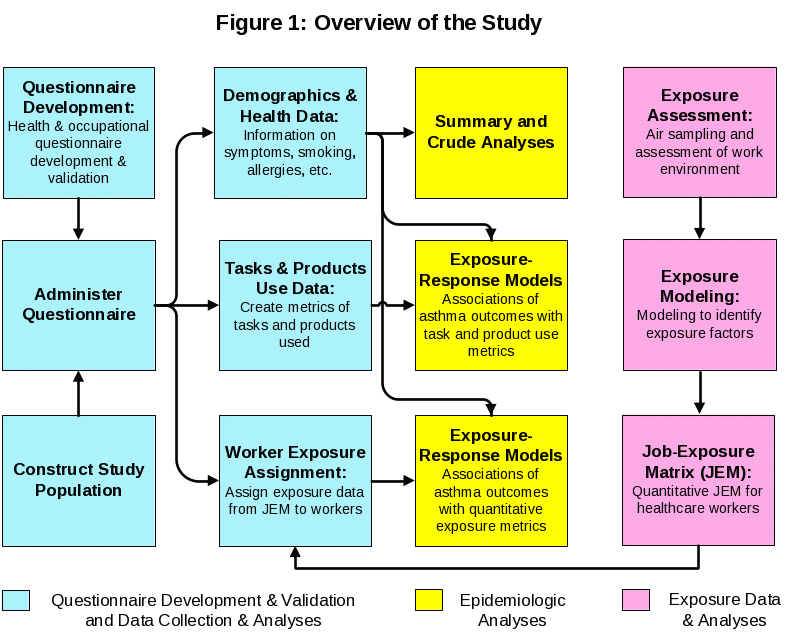
Survey Questionnaire
Study participants will complete a questionnaire that gathers information on demographics, jobs, exposures at work and home, respiratory symptoms and diseases, and other factors that can impact respiratory health (e.g., cigarette smoking). The final version of the Asthma-in-Health-Care survey (see Appendices K, L, and M) is the product of efforts completed between 2009 and 2012. It progressed through several stages of revision, each yielding a new major draft. Each major draft was prepared initially by NIOSH staff working with Dr. Delclos, followed by revisions based on feedback from other collaborators. The questionnaire progressed through: 1) formatting and cognitive review by National Center for Health Statistics (NCHS); and 2) approval by the NIOSH Human Subjects Review Board (HSRB). Two major rounds of formatting and cognitive review were conducted by NCHS behavioral scientists, using both paper and electronic forms of the questionnaire.
The Respiratory Symptoms and Asthma sections of the questionnaire are based on standardized questions, many of which were used successfully in the second European Community Respiratory Health Survey (ECRHSII). Responses to these questions will allow us to evaluate respiratory symptoms among all participants in the past 12 months, onset of asthma among those at risk in the past 10 years, and exacerbation of symptoms among those with asthma in the past 12 months. The Medical History and Asthma section will provide information on allergy status of the participant, and history of asthma and allergies in parents. The Home and Use of Hand Sanitizers section will yield information about potential confounders, including non-occupational exposure to indoor mold and moisture, renovations, and cleaning products. We have included questions on accidental spills (i.e., Accidental Chemical Spill or Release section) because 5.8% of healthcare workers in the Texas study had a history of such an exposure, and it was a risk factor for respiratory symptoms related to bronchial hyper-responsiveness (Delclos 2007). The Demographics and Smoking Status sections at the end of the questionnaire collect information to control for potential confounding when examining relationships between occupational exposures and asthma outcomes.
The Employment History section of the questionnaire focuses on current employment (i.e., the last 12 months) and later in the survey questions about employment 5 years earlier and changing jobs. The next six sections ask questions about use of products and tasks performed, and were developed based on the observations of healthcare workers during exposure assessment conducted at five facilities, as well a review of the scientific literature and material from professional societies for the occupations of interest.
Job-Exposure Matrix and Exposure Assessment
Exposure assessment studies were conducted at five facilities from 2009 to 2011. The study recruited 143 hospital workers within nine occupations: certified nursing assistants, central supply workers, dental assistants, environmental service workers, licensed practical nurses, lab technicians, operating room technicians, registered nurses, and respiratory therapists. Personal time-integrated samples for 14 specific volatile organic compounds (VOCs) and real-time measurements for TVOC were collected. These exposure data were summarized by jobs to create a JEM using average exposure as a summary measure for the 14 specific VOCs and TVOC (described in Table 2, Appendix O). The JEM will also include a measure of peak exposures obtained from the real-time TVOC monitors.
During exposure monitoring, trained NIOSH technicians observed workers for a full shift and recorded information on standardized sampling forms about: the time spent on different tasks and activities, materials or products used and their amounts, types of equipment, application methods and rates, degree of enclosure, type and quality of ventilation and work location. This approach provided systematic collection of information on factors (potential modifiers) that may affect exposure levels.
In the current study, we will
use multiple regression to model measured exposures and identify
factors affecting exposure levels. Using a repeated measures design,
the 14 specific VOCs or
the TVOC will be modeled as follows:
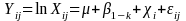 ,
where: Xij
is the natural logarithm of the exposure concentration of the ith
person on the jth
day, µ is the overall mean, β1-k
are the fixed effects of exposure determinants, χi
is the random effect of the ith
person and εij
is the random error (Burstyn
2000, Rappaport 1999).
The parameter estimates
of the significant fixed effects provide estimates of the magnitude
of change in average exposure associated with each factor (an
exposure multiplier, either increasing or decreasing exposures) and
can be used to predict exposures associated with the specific
factors. For examples, some cleaning and disinfecting tasks may
significantly influence exposures, thus the regression model can
provide estimates of exposure associated with the tasks for the
occupations of interest. Thus the job-specific exposures in the JEM
will be stratified by tasks based on the regression modeling to make
these estimates more universal in application. Additionally, the
exposure factors can also identify specific tasks, work practices, or
workplace characteristics associated with increased or decreased
exposures, thereby identifying opportunities for exposure reduction,
including tasks or conditions in need of exposure controls.
,
where: Xij
is the natural logarithm of the exposure concentration of the ith
person on the jth
day, µ is the overall mean, β1-k
are the fixed effects of exposure determinants, χi
is the random effect of the ith
person and εij
is the random error (Burstyn
2000, Rappaport 1999).
The parameter estimates
of the significant fixed effects provide estimates of the magnitude
of change in average exposure associated with each factor (an
exposure multiplier, either increasing or decreasing exposures) and
can be used to predict exposures associated with the specific
factors. For examples, some cleaning and disinfecting tasks may
significantly influence exposures, thus the regression model can
provide estimates of exposure associated with the tasks for the
occupations of interest. Thus the job-specific exposures in the JEM
will be stratified by tasks based on the regression modeling to make
these estimates more universal in application. Additionally, the
exposure factors can also identify specific tasks, work practices, or
workplace characteristics associated with increased or decreased
exposures, thereby identifying opportunities for exposure reduction,
including tasks or conditions in need of exposure controls.
Some occupational exposures are specific to workplaces and cannot be assessed via a JEM. Exposure to bioaerosols, construction materials, and accidental spills or gas releases will also be assessed qualitatively through items on the questionnaire. These occupational exposures will also be considered in epidemiologic exposure response analyses.
Specific Aim # 2 Measure frequency of asthma onset, related symptoms, and exacerbation of asthma in selected healthcare occupations.
This will be accomplished with a cross-sectional survey of current healthcare workers from nine selected occupations listed in Specific Aim #1. Data collection will be completed either online or by telephone. NIOSH staff will manage the online portion of the data collection. NIOSH has initiated a contract with RTI to provide epidemiology support and conduct the telephone interviews under subcontract with the SEIU Communications Center. Participants will be sought from members of 1199SEIU in the New York City area. Data for asthma and exacerbation of asthma will be collected using the Asthma section of the study questionnaire, while data for related symptoms will be collected in the Respiratory Symptoms section (see questionnaire in Appendix K). Details of questionnaire administration are provided above in Overview of the Data Collection System in Section 1 Circumstances Making the Collection of Information Necessary.
Specific Aim # 3 Assess associations between asthma outcomes and exposures to identify modifiable risk factors.
All statistical procedures to assess associations between asthma outcomes and exposures will be performed using standard software from the SAS Institute, Inc, Cary, North Carolina. Statistical significance will be defined as a p-value equal to or less than 0.05. There will be three major asthma-related health outcomes, each expressed as a proportion: incidence of asthma among those without asthma during the 10 years before the survey; incidence of asthma exacerbation among those with current asthma during the past year; and prevalence of asthma-related symptoms during the past year. First, we will calculate crude proportions for each outcome by exposures of interest and potential confounders. This will be followed by the calculation of prevalence or incidence ratios with robust variance using regression models that will include potential confounders (Lin 1989). Prevalence ratios are a better estimate of relative risk than odds ratios when the outcome is relatively common, which is likely the case for the last two outcomes (Thompson 1998). Job held five years before interview will be used to characterize occupational exposure in regression models for the onset of asthma. Additional information about assessing the associations between asthma outcomes and exposures, specifically epidemiologic exposure-response modeling, can be found under the data analysis plan in Section A16 of this supporting statement.
We plan to identify modifiable risk factors from these analyses. For example, if an asthma outcome is associated with manually sterilizing medical instruments (item 50.6.1 in the questionnaire Appendix K), then this identifies a potential candidate for further control. This can be based on the yes/no, frequency, or duration metrics of exposure. If a product used for instrument disinfection, such as glutaraldehyde, is associated with an asthma outcome, it can be targeted for exposure control or substitution. The same approach will be used with any of the tasks or products identified as associated with one or more asthma outcomes. Similarly, if quantitative metrics assessed by the JEM (e.g., for methyl methacrylate) are associated with an asthma outcome, products containing this chemical may be considered for exposure control or substitution.
Aim #4 Communicate findings to study participants and stakeholders who can use the information for prevention.
The current project is intended to identify modifiable risk factors that will inform the prevention of asthma, related symptoms, and exacerbation of asthma in healthcare workers. We plan to communicate study findings to several target audiences that can use this information for prevention in their own spheres of influence. These stakeholders and the desired consequences of the communications are:
1. Healthcare workers will learn about hazards in their work environment and become better prepared to participate in the development of strategies to minimize risk.
2. Health and safety staff at the facilities where participants are employed, who can potentially use the information for prevention.
3. Researchers can build on the findings to conduct additional research that will advance our understanding of asthma in healthcare and how to prevent it.
4. Clinicians will learn how occupational exposures can impact the respiratory health of their patients who work in healthcare, which should improve the care they provide.
5. Professional societies and government agencies will use findings from this and other studies to develop recommendations for preventing asthma and related symptoms in healthcare workers.
In addition to reaching out to the target audiences described, we will pursue two other approaches to communicate study findings to others who might use them for prevention. First, we will use the internet and social media to communicate the findings broadly. We will post findings on the NIOSH Asthma and Allergies website (http://www.cdc.gov/niosh/topics/asthma/), building on the experience of the Principal Investigator Paul Henneberger who has maintained the NIOSH webpage on Prevention of Occupational Asthma since 2005. Also, we will reach out to thousands of occupational safety and health experts by placing announcements on the NIOSH Twitter site and in the NIOSH eNEWS, with links to the webpage with study findings. Second, we will seek the advice of the NORA Healthcare and Social Assistance (HSA) Sector Council. This Council is composed of health and safety professionals from management, labor, academia, and government who have experience in this industrial sector. We will rely on the Leader of the NORA HSA Council to identify an Asthma Working Group composed of Council members to provide advice on communication efforts for this project.
By conducting research to identify modifiable risk factors for asthma, related symptoms, and exacerbation of asthma in the healthcare industry, this project is consistent with the mission of NIOSH, which “provides national and world leadership to prevent work-related illness, injury, disability, and death by gathering information, conducting scientific research, and translating the knowledge gained into products and services”. More specifically, it addresses one the main objectives of NIOSH which is to conduct research to reduce work-related illnesses and injuries.
Privacy Impact Assessment Information
No directly identifiable personal information (e.g., names, addresses, SSN) will be collected during this study. However, sensitive data such as information on current and past employment and health will be recorded. In addition to standard CDC safeguards, individual level data from participants will only be available to study personnel (e.g., self-reported medical information, work histories) and at no time will personal identifying information (e.g., SSN, name, address) be made available to NIOSH study personnel.
3. Use of Improved Information Technology and Burden Reduction
Data from participants in this research study will be collected by either online survey or Computer Assisted Telephone Interview (CATI). The initial method for collecting information will be by online survey. Participants will be asked to complete the online survey, however, for those participants that do not have internet access or where obtaining internet access would be difficult, a CATI will be made available. To complete the survey by CATI, participants will simply need to call into the SEIU CC. The SEIU CC will also be conducting follow-up phone calls and attempt to complete the survey by CATI with non-responding participants (i.e., union members who do not complete the online survey).
One hundred percent of the data collection will be done using technological collection techniques. We are anticipating 20% of responses from the online survey and 80% from the CATI. The same information will be collected by the online survey and CATI. Both the online survey and CATI will only collect the minimum information necessary for completing this research project
4. Efforts to Identify Duplication and Use of Similar Information
Several studies have been conducted that collected data and examined associations between work exacerbated asthma and healthcare exposures (Laney 2009, Park 2006, Park 2008, Sahakian 2008, Zock 2002). However, the data needs for this study were determined based on the results of the environmental sampling conducted by NIOSH for the JEM. Therefore, because the JEM requires corresponding environmental sampling and survey data, no similar data are available that can be used for this study.
5. Impact on Small Businesses or Other Small Entities
Small businesses or other small entities will not be involved in this study.
6. Consequences of Collecting the Information Less Frequently
All data collection for this research project will be conducted once with each participant, either by online survey or by CATI. No screening questions, besides verification of age 18 or older, will be asked prior to enrolment nor will follow-up questions be asked after participation. There are no legal obstacles to reduce the burden.
If this information is collected less frequently or not conducted we will not be able to complete the study and any identifiable risk factors for asthma will not be identified.
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This request fully complies with the regulation 5 CFR 1320.5.
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A. A 60-Day Federal Register Notice was published in the Federal Register on June 13, 2012, vol.77, No. 114, pp. 35405-35406 (Appendix B). One public comment was received in response to this notice (Appendix C).
B. Because participants in this research project will be recruited from members of 1199SEIU in New York City, discussions have been had with both the national SEIU office, the SEIU CC, and 1199SEIU to discuss the availability of data, methods for collecting data from union members, and how the study results will be disseminated to union members and stakeholders outside of SEIU.
Discussion with SEIU and 1199SEIU began in 2008. Since those discussions began, there have been no major problems and all subsequence problems have been resolved. Individuals consulted within SEIU and 1199SEIU include:
SEIU
Bill Borwegen, Health and Safety Director, 202-730-7385, [email protected]
Mark Catlin, Industrial Hygienist, 202-730-7290, [email protected]
Steve Schrag, Hazmat Program Coordinator, 203-574-7966, [email protected]
1199SEIU
Bobby Hocson, Director, 212-408-8418, [email protected]
SEIU Communications Center
Brendan Shaw, Director, 212-603-3783, [email protected]
In addition to discussions with SEIU, additional discussions regarding sample selection,epidemiology support, and completion of telephone interviews have been conducted with Research Triangle Institute (RTI) International. Individuals at RTI include:
Michael Witt, Senior Research Statistician, 919-990-8346, [email protected]
Dan Liao, Research Statistician, 301-816-4605, [email protected]
Kristina Peterson, Director Program for Occupational Safety and Health, 919-485-7722,
Matthew Strobl, Research Psychologist, 919-541-7395, [email protected]
9. Explanation of Any Payment or Gift to Respondents
There will not be payments or gifts to respondents.
10. Assurance of Confidentiality Provided to Respondents
No directly identifiable information (e.g., name, address, phone number) will be collected when participants complete the survey. 1199SEIU currently has this information, therefore; they will be solely responsible for contacting union members about participation in this study. Under no circumstances will 1199SEIU share or transmit this directly identifiable personal information to NIOSH researchers. While no directly identifiable information will be collected, the data collection will involve collecting sensitive information (e.g., medical histories, work histories). To ensure the security of the study data, access will be limited to the NIOSH research team and will be stored in a password-protected database on the CDC/NIOSH computer network. Furthermore, because the survey will be hosted on the CDC website, all necessary security protocols required by CDC will be implemented. Data collected by RTI through telephone interviews conducted by the SEIU CC, will be stored on the SEIU CC computer system under their standard security protocols, until transferred to NIOSH. Any paper records generated during the course of this study will be stored in a locked file cabinet at the NIOSH Morgantown facility and only accessible by members of the research team.
All study data and records will be securely maintained until publication of results, after which they will be archived according to federal regulations.
Privacy Impact Assessment Information
NIOSH will use standard methods to ensure the security and protection of this data. All data with sensitive information will be stored on password protected computers and any paper copies with personal identifying information will be stored in locked file rooms or cabinets. Data access will be restricted to NIOSH personnel and contractors that are involved in the study.
Approval for this project was obtained by the NIOSH Human Subject’s Review Board (HSRB) on October 25th 2012 (Appendix D). All participants will be provided written informed consent information during the recruitment process. When completing the survey online or by CATI, participants will be asked if they have reviewed the informed consent information. If they have not reviewed the information, they will be given the opportunity to read the consent information online or have the interviewer read it to them. Participants will be informed that completion of the survey either online or by telephone will constitute their consent. Please see Appendix F for copies of recruitment letter with consent information.
All participants will be informed that participation is voluntary and that they may discontinue participation at any time. There are no consequences if a participant refuses to complete the survey. The information collected using the survey will be used to examine any associations between workplace exposures in the healthcare industry and asthma or asthma symptoms. No individual level data will be shared.
11. Justification for Sensitive Questions
Some of the questions on the survey instrument may be considered sensitive. Information on race, ethnicity, gender, age, and smoking status are important covariates that are required for analysis. As a cohort study, the results will add to the general literature on this topic, but alone cannot be the basis of conclusions about the industry as a whole.. Because the outcome variable in our analysis is asthma, the survey instrument will collect information on asthma and asthma symptoms. Because of the sensitive nature of these health questions, all participants will be provided with information on informed consent in the recruitment letter (Appendix F). All participants will be asked to read the informed consent information and indicate their consent prior to completing either the online or telephone survey.
12. Estimates of Annualized Burden Hours and Costs
The target sample size for this study is 5000. Based on the 1199SEIU membership data, we know what percentage of the eligible union members fall into the nine job categories we are targeting. Therefore, we can estimate how the 5000 participants will distribute into the nine job categories. The 5000 participants will have the option to complete the survey either online or by CATI. We anticipate 20% will complete the online survey and 80% will complete survey by CATI. Completion of either the online survey or CATI will take approximately 30 minutes. Based on those numbers we estimate the total burden hours for two years to be 2503, with the annualized burden hours at 1250.
Table I. Estimated Annualized Burden Hours
Type of Respondents |
Forms (Survey Type) |
No. of Respondents |
No. of Responses per Respondent |
Average burden per response (in hours) |
Total annualized burden hours |
Certified Nursing Assistants |
Online |
297 |
1 |
30/60 |
74.3 |
Telephone |
1188 |
1 |
30/60 |
297 |
|
Central Supply Workers |
Online |
8 |
1 |
30/60 |
2 |
Telephone |
34 |
1 |
30/60 |
8.5 |
|
Dental Assistants |
Online |
18 |
1 |
30/60 |
4.5 |
Telephone |
71 |
1 |
30/60 |
17.8 |
|
Environmental Service Workers |
Online |
228 |
1 |
30/60 |
57 |
Telephone |
914 |
1 |
30/60 |
228.5 |
|
Licensed Practical Nurses |
Online |
140 |
1 |
30/60 |
35 |
Telephone |
559 |
1 |
30/60 |
139.8 |
|
Lab Technicians |
Online |
77 |
1 |
30/60 |
19.3 |
Telephone |
310 |
1 |
30/60 |
77.5 |
|
Operating Room Technicians |
Online |
27 |
1 |
30/60 |
6.8 |
Telephone |
109 |
1 |
30/60 |
27.3 |
|
Registered Nurses |
Online |
168 |
1 |
30/60 |
42 |
Telephone |
672 |
1 |
30/60 |
168 |
|
Respiratory Therapists |
Online |
36 |
1 |
30/60 |
9 |
Telephone |
144 |
1 |
30/60 |
36 |
|
Total |
|
|
|
|
1250 |
The estimated annual burden costs are calculated using the mean hourly wage rate from the Bureau of Labor Statistic’s May 2011 Metropolitan and Nonmetropolitan Area Occupational Employment and Wage Estimates for New York-Northern New Jersey-Long Island, NY-NJ_PA found at http://www.bls.gov/oes/current/oes_35620.htm (DOL 2011).We estimate the annualized burden cost for the 5000 participants to complete the survey to be $27,513.90
Table II. Estimated Annualized Burden Costs
Type of Respondents |
Forms (Survey Type) |
No. of Respondents |
Total burden hours |
Hourly Wage Rate |
Total Annualized Respondent Costs |
Certified Nursing Assistants |
Online |
297 |
148.5 |
$15.44 |
$1,146.42 |
Telephone |
1188 |
594 |
$15.44 |
$4,585.68 |
|
Central Supply Workers |
Online |
8 |
4 |
$17.53 |
$35.06 |
Telephone |
34 |
17 |
$17.53 |
$149.01 |
|
Dental Assistants |
Online |
18 |
9 |
$17.91 |
$80.60 |
Telephone |
71 |
35.5 |
$17.91 |
$317.91 |
|
Environmental Service Workers |
Online |
228 |
114 |
$14.75 |
$840.75 |
Telephone |
914 |
457 |
$14.75 |
$3,370.38 |
|
Licensed Practical Nurses |
Online |
140 |
70 |
$24.38 |
$853.30 |
Telephone |
559 |
279.5 |
$24.38 |
$3,407.11 |
|
Lab Technicians |
Online |
77 |
38.5 |
$22.63 |
$435.63 |
Telephone |
310 |
155 |
$22.63 |
$1,753.83 |
|
Operating Room Technicians |
Online |
27 |
13.5 |
$23.40 |
$157.95 |
Telephone |
109 |
54.5 |
$23.40 |
$637.65 |
|
Registered Nurses |
Online |
168 |
84 |
$39.29 |
$1,650.18 |
Telephone |
672 |
336 |
$39.29 |
$6,600.72 |
|
Respiratory Therapists |
Online |
36 |
18 |
$33.15 |
$298.35 |
Telephone |
144 |
72 |
$33.15 |
$1,193.40 |
|
Total |
|
|
|
|
$27,513.90 |
13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
There are no annual cost burdens to respondents or record keepers.
14. Annualized Cost to the Government
The annualized costs for this project are $136,890 for FY2012, $430,615 for FY2013 and $249,880 for FY2014, for a total of $712,358.
Item |
FY 2012 |
FY 2013 |
FY 2014 |
Total |
Personnel |
$84,000 |
$88,000 |
$92,000 |
$264,000 |
Equipment and supplies |
$700 |
$500 |
$900 |
$2,100 |
Contractual |
$39,890 |
$330,500 |
$35,000 |
$405,390 |
Travel |
$12,300 |
$11,615 |
$16,980 |
$40,895 |
Annualized estimate of federal costs |
$136,890 |
$430,615 |
$144,880 |
$712,358 |
15. Explanation for Program Changes or Adjustments
This data collection is new, and therefore there are no changes or adjustments.
16. Plans for Tabulation and Publication and Project Time Schedule
Recruitment of study participants will begin within a month of OMB approval. This includes a notice published in the 1199SEIU newsletter and mailing of recruitment material. The online survey and call in telephone number for completing the survey will be activated as soon as recruitment begins. Follow-up phone calls to non-responders will begin one month after the completion of recruitment. The online survey and call in telephone number will be active for 12 months. After this data collection period, a summary of the preliminary results will be drafted and sent to participating 1199SEIU members. Publications in peer-reviewed literature is anticipated no sooner than 48 months after OMB approval.
Activity |
Time Schedule |
Study notice posted in 1199SEIU newsletter |
1 month after OMB approval |
Initial Recruitment letters sent to 1199SEIU members |
1 month after OMB approval |
Online survey posted on CDC website |
1 month after OMB approval |
Call in number for phone interview activated |
1 month after OMB approval |
Primary recruitment letters sent to 1199SEIU |
1 month after OMB approval |
Reminder postcards sent to 1199SEIU members |
2 months after OMB approval |
Phone calls for recruitment and completion of survey |
2 months after OMB approval |
Results letters sent to 1199SEIU members who participated in survey |
12 - 18 months after OMB approval |
Publication in peer-reviewed literature |
48 months after OMB approval |
Data Analysis Plan
Exposure data from the Asthma-in-Health-Care survey will be combined with the JEM to generate an array of exposure metrics to cleaning and disinfecting agents, and other confounding exposure agents for each participant. Potential confounders, including non-occupational exposures to the agents of interest (e.g., cleaning and disinfecting agents as well as home characteristics that may predict exposure to bioaerosols) and other factors like cigarette smoking, age, gender, race, ethnicity, and education will also be assessed through items on the survey.
Most data management and all statistical procedures will be performed using standard software from the SAS Institute, Inc, Cary, North Carolina. Statistical significance will be defined as a p-value equal to or less than 0.05. There will be three major asthma-related health outcomes, each expressed as a proportion: incidence of asthma among those without asthma during the 10 years before the survey; incidence of asthma exacerbation among those with current asthma during the past year; and prevalence of asthma-related symptoms during the past year. First, we will calculate crude proportions for each outcome by exposures of interest and potential confounders. This will be followed by the calculation of prevalence or incidence ratios with robust variance using regression models that will include potential confounders (Lin 1989). Prevalence ratios are a better estimate of relative risk than odds ratios when the outcome is relatively common, which is likely the case for the last two outcomes (Thompson 1998).
A range of quantitative and
qualitative exposure metrics (see Appendix O) and confounders will be
used in the epidemiologic exposure-response modeling. However, the
regression model may suffer from the effects of multicollinearity
(Delclos 2007) and produce unstable parameter estimates; if
necessary, we will utilize hierarchical regression models with
empirical Bayes estimation to overcome these difficulties of modeling
multiple exposures. Specifically, the hierarchical models allow
simultaneous consideration of multiple exposures without sacrificing
the accuracy of the model parameter estimates. The empirical Bayes
estimation procedure will be used to obtain the prior distribution
and adjust the estimates to obtain the posterior parameters of
interest. This modeling will be conducted in two stages. First, the
disease outcome (asthma or symptoms) will be regressed on multiple
exposures using a model for prevalence ratios. The model will include
all exposures of interest as well as potential confounders such as
cigarette smoking. The individual coefficients (betas) from this
stage will then be modeled as outcome variables in the second stage
model, also known as the prior. In the second stage model, the betas
are modeled as a function of covariates (zi)
and coefficients ()
that are thought to determine the magnitude of the true effect
(beta). The second stage model is of the form (DeRoos 2001, Greenland
1994):
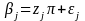 in which the i
is a random variable with mean 0 and variance (2),
the mean value ()
of all the j
is Z,
and all the j
have a common variance 2.
The distribution of
is traditionally termed as the prior distribution and the
hyper-parameters (
and 2)
are termed as the prior mean and prior variance of the distribution
of .
in which the i
is a random variable with mean 0 and variance (2),
the mean value ()
of all the j
is Z,
and all the j
have a common variance 2.
The distribution of
is traditionally termed as the prior distribution and the
hyper-parameters (
and 2)
are termed as the prior mean and prior variance of the distribution
of .
The z-matrix is constructed with rows of exposures and columns of covariates (subset or grouping variables). In this case, the matrix will consist of rows for the exposure variables and columns for the grouping covariates such as VOCs, aldehydes, and halogenated hydrocarbons (see Appendix O). This implies that all the betas within a subset/group have a common prior distribution with a common prior mean and a common prior variance. Thus all the parameters are assumed to be exchangeable within a subset/group so that the same mean and variance can be assigned to each parameter. The assumption of exchangeability of parameter estimates within a subset is important and must not include variables that are believed to have different magnitude of effect (in other words, come from a different distribution). An alternative scheme might include cleaning agents and high level disinfectants in separate groups. The posterior parameter estimates will be calculated as weighted averages of the prior mean and the parameter estimates from the model for the individual exposure agent. For example, when the VOCs grouping is used, the posterior parameter estimates for each of the exposure variables will be calculated as weighted averages of the prior VOCs mean and the parameter estimates for the individual chemical variables that make up the VOCs group. The empirical Bayes procedure has been applied in occupational and environmental epidemiology, and is shown to reduce the width of confidence intervals by factors of 2 to 10 (Greenland 1994).
The exposure-response modeling can identify specific exposures, products (including green cleaning products) or classes of exposure (e.g., quaternary ammonium compounds) associated with an increased risk of asthma outcomes. These results can then be related to the results of modeling exposure determinants to identify exposure characteristics (e.g., tasks or manner of use) or workplace factors (e.g., exposure control efforts) that can reduce exposures and, therefore, the risk of asthma symptoms. Thus, linking the epidemiologic results with the results of exposure modeling offers the potential to identify evidence-based opportunities for targeted prevention activities.
17. Reason(s) Display of OMB Expiration Date is Inappropriate
Display of OMB expiration date exemption is not requested.
18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
References
Bello A, Quinn MM, Perry MJ, Milton DK. Characterization of occupational exposures to cleaning products used for common cleaning tasks--a pilot study of hospital cleaners. Environ Health. 2009 Mar 27;8:11.
Burstyn I, Kromhout H. Are the members of a paving crew uniformly exposed to bitumen fume, organic vapor, and benzo(a)pyrene? Risk Anal 2000; 20:653-663
Byrns GE, Palatianos KH, Shands LA, et al. Chemical hazards in radiology. Appl Occup Environ Hyg 2000; 15:203-208
CDC. Work-Related Lung Disease (WoRLD) Surveillance System Report 2007. Morgantown, WV: National Institute for Occupational Safety and Health, 2008
Christiani DC, Kern DG. Asthma risk and occupation as a respiratory therapist. Am Rev Respir Dis 1993; 148:671-674
Cox-Ganser J, Rao C. Health hazard evaluation report: HETA-2000-0255-2868, Benefis Healthcare, Great Falls, Montana. Morgantown, WV: National Institute for Occupational Safety and Health, 2001; 1-101
Cox-Ganser JM, Rao CY, Park JH, Schumpert JC, Kreiss K. Asthma and respiratory symptoms in hospital workers related to dampness and biological contaminants. Indoor Air 2009: 19(4): 280-290
Curwick C BD. Work-Related Asthma in Washington State: A Review of Workers Compensation Claims from 1995-2002. Olympia, Washington: Washington State Department of Labor and Industries, 2003.
Delclos GL, Arif AA, Aday L, et al. Validation of an asthma questionnaire for use in healthcare workers. Occup Environ Med 2006; 63:173-179
Delclos GL, Gimeno D, Arif AA, et al. Occupational risk factors and asthma among health care professionals. Am J Respir Crit Care Med 2007; 175:667
De Roos AJ, Poole C, Teschke K, et al. An application of hierarchical regression in the investigation of multiple parental occupational exposures and neoblastoma in the offspring. Am J Ind Med 2001; 39:477-486
Dimich-Ward H, Wymer M, Kennedy S, et al. Excess of symptoms among radiographers. Am J Ind Med 2003; 43:132-141
Dimich-Ward H, Wymer ML, Chan-Yeung M. Respiratory health survey of respiratory therapists. Chest 2004; 126:1048-1053
DOL. Bureau of Labor Statistics, US Department of Labor (DOL). Career Guide to Industries, 2008-2009 Edition, Health Care. On the Internet at http://www.bls.gov/oco/cg/cgs035.htm (visited April 22, 2008).
DOL. Bureau of Labor Statistics, US Department of Labor (DOL). May 2011 Metropolitan and Nonmetropolitan Area Occupational Employment and Wage Estimates for New York-Northern New Jersey-Long Island, NY-NJ_PA. On the Internet at http://www.bls.gov/oes/current/oes_35620.htm (Visited May 2, 2012).
Greenland S. Hierarchical regression for epidemiologic analyses of multiple exposures. Environ Health Perspect 1994; 102 Suppl 8:33-39
Koda S, Kumagai S, Ohara H. Environmental monitoring and assessment of short-term exposures to hazardous chemicals of a sterilization process in hospital working environments. Acta Med Okayama 1999; 53:217-223
Laney AS, Cragin LA, Blevins LZ, Sumner AD, Cox-Ganser JM, Kreiss K, Moffatt SG, Lohff CJ. Sarcoidosis, asthma, and asthma-like symptoms among occupants of a historically water-damaged office building. Indoor Air 2009: 19(1): 83-90
Liljelind IE, Hagenbjörk-Gustafsson A, Nilsson LO. A method for measuring the potential dermal exposure to methyl methacrylate during two different dental technical work tasks. J J J Environ Monit. 2009 Jan;11(1):160-5.
Lin DY, Wei L. The robust inference for the proportional hazards model. Journal of the American Statistical Association 1989; 84:1074-1078
Mirabelli MC, Zock JP, Plana E, et al. Occupational risk factors for asthma among nurses and related healthcare professionals in an international study. Occup Environ Med 2007; 64:474-479
Nayebzadeh A. The effect of work practices on personal exposure to glutaraldehyde among health care workers. Ind Health 2007; 45:289-295
Park JH, Cox-Ganser J, Rao C, et al. Fungal and endotoxin measurements in dust associated with respiratory symptoms in a water-damaged office building. Indoor Air 2006; 16:192-203
Park JH, Cox-Ganser JM, Kreiss K, et al. Hydrophilic fungi and ergosterol associated with respiratory illness in a water-damaged building. Environ Health Perspect 2008; 116:45-50
Pechter E, Davis LK, Tumpowsky C, et al. Work-related asthma among health care workers: surveillance data from California, Massachusetts, Michigan, and New Jersey, 1993-1997. Am J Ind Med 2005; 47:265-275
Rappaport SM, Weaver M, Taylor D, et al. Application of mixed models to assess exposures monitored by construction workers during hot processes. Ann Occup Hyg 1999; 43:457-469
Sahakian NM, Park JH, Cox-Ganser JM. Dampness and mold in the indoor environment: implications for asthma. Immunol Allergy Clin North Am 2008; 28:485-505, vii
Syamlal G, Mazurek JM. Prevalence of asthma among youth on Hispanic-operated farms in the United States-2000. J Agromedicine 2008; 13:155-164
Teschke K, Chow Y, Brauer M, et al. Exposures and their determinants in radiographic film processing. AIHA J 2002; 63:11-21
Thompson ML, Myers JE, Kriebel D. Prevalence odds ratio or prevalence ratio in the analysis of cross sectional data: what is to be done? Occup Environ Med 1998; 55:272-277
Zock JP, Jarvis D, Luczynska C, et al. Housing characteristics, reported mold exposure, and asthma in the European Community Respiratory Health Survey. J Allergy Clin Immunol 2002; 110:285-292
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | CDC User |
| File Modified | 0000-00-00 |
| File Created | 2021-01-29 |
© 2026 OMB.report | Privacy Policy