Navajo_ Supp_Stmt A_ CLEAN_5-21-13
Navajo_ Supp_Stmt A_ CLEAN_5-21-13.docx
Prospective Birth Cohort Study Involving Environmental Uranium Exposure in the Navajo Nation
OMB: 0923-0046
Supporting Statement
For OMB Review and Approval of
Agency for Toxic Substances and Disease Registry (ATSDR)
A Prospective Birth Cohort Study Involving Environmental Uranium Exposure in the Navajo Nation
SECTION A. Justification
Date: 21 March 2013
Program Official:
Angela Ragin-Wilson, PhD
Agency for Toxic Substances and Disease Registry
Division of Toxicology and Human Health Sciences
Program Official
Phone: 770-488-3807
Fax Number: 770-488-7187
Email: [email protected]
Point of Contact:
Candis M. Hunter, MSPH
Agency for Toxic Substances and Disease Registry
Division of Toxicology and Human Health Sciences
Phone: 770-488-1347
Email: [email protected]
Table of Contents
A.1. Circumstances Making the Collection of Information Necessary
A.2. Purpose and Use of Information Collection
Privacy Impact Assessment Information
A.3. Use of Improved Information Technology and Burden Reduction
A.4. Efforts to Identify Duplication and Use of Similar Information
Review of Institutional Reports and Published Literature
A.5. Impact on Small Businesses or Other Small Entities
A.6. Consequences of Collecting the Information Less Frequently
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
A.8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A.9. Explanation of Any Payment or Gift to Respondents
A.10. Assurance of Confidentiality Provided to Respondents
Privacy Impact Assessment Information
A.11. Justification for Sensitive Questions
A.12. Estimates of Annualized Burden Hours and Costs
A.13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
A.14. Annualized Cost to the Government
A.15. Explanation for Program Changes or Adjustments
A.16. Plans for Tabulation and Publication and Project Time Schedule
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions
Attachment 1: Authorizing Legislation
Attachment 2: 60-Day Published Federal Register Notice
Attachment 3: Data Collection Instruments
3a: Mother Enrollment Survey
3b: Father Enrollment Survey
3c: Ages and Stages Questionnaire
3d: Mullen Stages of Early Development
3e: Postpartum Survey (2 months)
3f: Postpartum Survey (6, 9, 12 months)
3g: Food Frequency Questionnaire/WIC Intake
3h: Eligibility Form
Attachment 4: Consent and HIPAA Forms
Attachment 5: IRB approval
Attachment 6: Study Information and Participant Timeline
Attachment 7: Individuals Consulted
Attachment 8: Community Outreach Chronology
Attachment 9: Timeline of Interactions
Attachment 10: Recruitment Poster, Brochure, and Template Examples
Attachment 11: Letters of Support
Attachment 12: Developmental Assessment Manual and Feedback Form
Attachment 13: Survey Question Sources and Relevance
Attachment 14: Biomonitoring Table & Analyte Justification
Attachment 15: Biomonitoring Standard Operating Procedures
Attachment 16: Medical Record Abstraction Table & Form
Attachment 17: Home Environmental Assessment Protocols
Attachment 18: Biomonitoring & Home Environmental Assessment Reporting Form/Letters
A. Justification
A.1. Circumstances Making the Collection of Information Necessary
This is a new Information Collection Request (ICR) for the Agency for Toxic Substances and Disease Registry (ATSDR) “A Prospective Birth Cohort Study Involving Environmental Uranium Exposure in the Navajo Nation” (U01).
The program requests Office of Management and Budget (OMB) approval for three years.
Background
This Information Collection Request (ICR) is a new data collection request involving pregnant mothers, their infants and fathers within the Navajo Nation. The Navajo Nation encompasses 16 million acres of New Mexico, Utah, and Arizona, and is the largest Alaska Native/American Indian Reservation in the United States. From 1948 to 1986, hundreds of uranium mining and milling operations were conducted in the Navajo Nation. These mining and milling operations have left a legacy of uranium contamination through abandoned uranium mines/mills, drinking water and soil, and homes and structures built with mining waste. Uranium is a heavy metal and may cause adverse health effects due to both its radiological and chemical properties. As a heavy metal, uranium primarily damages the kidneys and urinary system. The kinetics, metabolism, and toxic effects of uranium on kidney function are well established. However, there is limited epidemiological and toxicological data regarding uranium exposure and adverse birth and reproductive health outcomes.
Uranium exposure studies have been conducted in laboratory animals. The resulting data has suggested teratogenic and reproductive toxicity resulting in decreased litter size, reduced fetal growth, and increased occurrence of congenital malformations (Domingo, 2001). Studies in rodents suggest possible modes of injury to the fetus and neonate mediated by uranium, including toxicity to the male or female reproductive organs (Domingo, 2001), damage to sperm (Malenchenko, 1978) or oocytes (Kundt, 2009), and reproductive hormone disruption (Raymond-Whish, 2007). Direct toxicity of uranium to the developing fetus is largely unstudied; its penetration of the placental barrier, uncertain (Domingo, 2001).
Human epidemiologic studies of varying quality have also found inconsistent evidence of teratogenic effects in offspring of persons exposed to depleted uranium (Hindin et al., 2005). However, due to its potential teratogenic and endocrine-disrupting effects, uranium may pose the greatest human health risks during critical windows of human development, such as the prenatal and early postnatal periods. There is a paucity of studies examining uranium exposure and subsequent maternal and child health outcomes on the Navajo Reservation.
An informal survey of more than 1,300 people who worked in the uranium industry in New Mexico after 1971 found that 30% of female uranium workers (40 of 132) and 40% (169 of 421) of female spouses of male workers reported adverse birth outcomes, defined as miscarriages, stillbirths and newborns with birth defects. The survey, by the Post-71 Uranium Workers Committee (Evers et al., 2009), also found that 13% of female workers and 15% of female spouses reported two or more adverse reproductive outcomes Although cultural and social factors may influence birth and reproductive health outcomes among Navajo women, further study is warranted to elucidate the causality of the disproportionate number of adverse child and infant health outcomes on the Navajo Nation.
The only large-scale study of uranium exposure examining human reproductive and teratogenic effects on the Navajo Nation focused on radiological effects among children born in the Shiprock Service Unit between 1964 and 1981. In examining the records of 13,329 births, Shields and colleagues (1992) reported 320 types of congenital anomalies including cleft lip and palate, Down’s syndrome, hip dysplasia and stillbirth among others. Using a nested case-control design, in which the families of 266 pairs of index and control births were interviewed, they found a significant association between adverse birth outcomes and the mother’s residence within 0.5 miles of uranium mill tailings or uranium mine waste dumps.
In the Navajo Nation, congenital anomalies remain the leading cause of infant deaths. The infant mortality rate among the Navajo people is 8.5 deaths per 1000 live births, compared to 6.9 deaths per 1000 live births among all races. The postnatal mortality rates for Navajo infants are 2.1 times higher than the US for all races. There is strong evidence linking the early and consistent use of prenatal care with positive reproductive results. However, only 61% of Navajo mothers with live births received prenatal care in the first trimester as compared to 83% of all U.S. mothers. Early and regular prenatal care is a significant predicator of positive birth outcomes.
Late initiation of prenatal care, infrequent prenatal visits, and no prenatal care has been associated with adverse birth and maternal outcomes including low-birth weight, premature births, neonatal mortality, infant mortality, and maternal mortality (Liu 2000). Since prenatal care utilization may be an important confounder in this study, monitoring of prenatal care visits and subsequent follow-ups among study participants are vital to obtaining important information on both the mother and baby at key developmental milestones during the NBCS. Study participants will be recruited from five designated Navajo Area Indian Health Service (NAIHS) clinics/Public Law 93-638 (PL-638) healthcare facilities located on the Navajo reservation. Study participants must be willing to deliver at one of the five designated study hospitals/clinics in order to be eligible to participate in the study. We recognize that we can’t require study participants to seek prenatal care due to cultural reasons. However, we will analyze for differences in quantity and quality of prenatal care, and look for inter-facility differences in study outcomes. In the Navajo Nation, prenatal care is a marker for other determinants that are correlated with education and socioeconomic status, and these factors will be also be analyzed as part of the study.
In order to increase prenatal care utilization amongst Navajo women and their families, educational outreach and community activities are planned to target this population. The project is designed with a community based participatory research approach and is consistent with Navajo Nation Human Health Review Board protocols for conducting research in the Navajo Nation. The extensive capacity building, training, outreach and education that are conducted as a part of this study may change the level of prenatal care in some of the impacted communities. Please see Part B.2 and B.3 for details on study recruitment, education, and methods to maximize response rates.
The U.S. House of Representatives Committee on Oversight and Government Reform requested that federal agencies develop a plan to address health and environmental impacts of uranium contamination in the Navajo Nation. As part of the response to the Congressional charge, ATSDR awarded a research cooperative agreement to University of New Mexico Community Environmental Health Program (UNM-CEHP) entitled “A Prospective Birth Cohort Study Involving Environmental Uranium Exposure in the Navajo Nation (U01),” in August 2010. In order to carry out the study, ATSDR and UNM-CEHP are collaborating with the NAIHS Navajo Nation Division of Health (NNDOH), Navajo Nation Environmental Protection Agency (NNEPA), and Navajo culture and language specialists to carry out the study.
Through an interagency agreement with ATSDR, NAIHS will hire project coordinators called Cohort Clinical Liaisons (CCLs) at each of the five designated clinics. The CCLs will be responsible for conducting medical record abstractions, participant recruitment, and shipment of biological samples. Through a sole-source contract with ATSDR, NNDOH Community Health and Environmental Research Specialists (CHERs) will provide survey administration, community education, recruitment, training, and outreach for the study. Table 1 details the role of the primary partners in the study.
Table 1: Affiliations and Main Roles of primary partners in the Navajo Birth Cohort Study
Affiliation |
Primary Study Role |
ATSDR/CDC |
|
University of New Mexico/ Southwest Research Information Center |
|
NAIHS/ PL-638 (CCLs) |
|
Navajo Nation Division of Health (CHERs) |
|
The study will evaluate birth and reproductive outcomes in approximately 1,500 pregnant women, follow and assess their children at birth, 2 months, 6 months, 9 months, and up to 1 year of age to investigate the potential impact of uranium exposure on biological and psychosocial endpoints. Due to the infant mortality rate in this population, we may not obtain follow-up information on all children at each age point. Also, follow-up information may be limited due to miscarriages or participant fatigue. However, extensive training and outreach will be conducted to maximize participant response rate and minimize participant fatigue (See Part B.3). The study will also provide broad public health benefits for Navajo communities through outreach and education on the importance of prenatal care, environmental prenatal risks and earlier assessment and referral for known developmental delays.
UNM-CEHP designed the data collection instruments and outreach materials in collaboration with Navajo community members and cultural and language experts. These materials were approved by NNHRRB and were determined to be culturally appropriate for this unique population. According to Navajo Nation Human Research Code, § 3253 Policy, all approved research in Navajo Nation must be “beneficial, community-based, and consistent with Navajo Nation priority and concerns.” In accordance with the Navajo Nation Human Research Code, the UNM-CEHP and its partners have utilized best practices to establish a community-based participatory research approach for this study. They have a long-standing interdisciplinary research team who has the knowledge, skills, and abilities to conduct research in this unique population and have also built trusting relationships with the community for over 15 years.
The program recognizes OMB concerns regarding participant expectations from the outreach materials. However, concerns about uranium exposure are well known and documented on the Navajo Reservation. There is also substantial coverage in the press and through regular uranium stakeholders meetings held throughout the reservation by USEPA. Although the president of Navajo Nation speaks on regular occasions about finally having a response from the US government to address what has been a long-term worry and concern of the Navajo people, CDC recognizes that this study alone does not have the power to draw such conclusions. As such, CDC is committed to working with its partners to set realistic expectations with its study participants as well as clearly articulating the limitations of the study design and, accordingly, of its conclusions when disseminating the findings to its stakeholders (including other Federal agencies, Congress, and the Navajo Nation). UNM-CEHP has done a tremendous amount of education throughout Navajo Nation over the past decade to build the understanding of research and how research is conducted and evaluated. In all of these sessions, the fact that studies need to involve exposed and unexposed people from the population, with a continuum of exposure being the most important way to assess the question.
OMB approval is requested to conduct surveys, biological sampling, developmental screenings and home environmental assessments that will be performed during the study period.
ATSDR is authorized by the Comprehensive Environmental Response, Compensation and Liability Act of 1980 and Superfund Amendments and Reauthorization Act of 1986 (SARA) [42 U.S.C. 9604(i)(1)(E), (7), (9), (15) and 9626(a)] to collect this study data. Please see Attachment 1 for Authorizing Legislation. The 60-day Federal Register Notice of the proposed information collection (IC) was published on November 22, 2011. (See Attachment 2). The 30-day Federal Register Notice was published on March 27, 2012.
Privacy Impact Assessment
Overview of the Data Collection System
The ATSDR study “A Prospective Birth Cohort Study Involving Environmental Uranium Exposure in the Navajo Nation” will be conducted by interview and by the collection of blood and urine specimens for analytical measurements. The information collection (IC) will be implemented in five phases: sampling; eligibility screening; enrollment and informed consent; risk factor assessment, health outcome assessment. The burden tables in Section A.12 reflect the data flow of the collection forms developed by the principal investigator at UNM-CEHP. The data collection system consists of the following:
Sampling: Potential Study participants must be eligible to receive prenatal care services and deliver at one of the following NAIHS/ PL-638 Clinics: Northern Navajo Medical Center, Chinle Comprehensive Health Care Facility, Gallup Indian Medical Center, Tuba City Regional Health-Care Corporation, Kayenta Health Center or Tséhootsooí Medical Center. Please refer to Part B.1 for Respondent Universe and Sampling Methods.
Eligibility screening: Interviewers will administer and document eligibility screening using paper and pencil form. Any paper copies will be stored and managed in a record keeping system in a locked file in the office of Dr. Johnnye Lewis (Principal Investigator) at UNM-CEHP. Participants are pregnant woman who are at least 14 years of age, have lived on the Navajo reservation for at least 5 years, and must plan to deliver at one of the study hospitals and clinics.
Enrollment and informed consent: Informed consent will be documented on a paper and pencil form (Attachment 4). Any paper copies will be stored and managed in a record keeping system in a locked file in the office of Dr. Lewis.
Risk Factor Assessments: The study will examine reproductive outcomes in pregnant women and follow and assess their children from birth to 1 year of age using the following (Please see Items of Information Collected and Table 2 for more information):
Survey instruments: A computer assisted personal interview (CAPI) will be conducted to ascertain the respondent’s contact information, demographic information, and to assess potential environmental and health risks. Research Electronic Data Capture (RedCap™) will be used for questionnaire responses. Survey instruments to be administered include the mother and father enrollment survey. Food Frequency Questionnaire/WIC Form and Postpartum surveys at 2, 6, 9 and 12 months.
Biological monitoring: Urine and blood samples will be collected to assess uranium and other heavy metal confounders.
Home environmental assessments: The assessment will include identifying types and condition of any heating sources, types of fuel used and source where available; physical arrangements used for conducting any in-home activities potentially using toxic materials, such as jewelry-making, in-home pesticide use, other activities using volatile compounds and other assessments detailed in “Items of Information to be Collected” below.
Outcome Assessment
Medical record abstraction: Relevant data from prenatal care medical record charts will be abstracted. Please see Attachment 16 for data to be abstracted from medical records.
Child development: Ages & Stages Questionnaire I (ASQ I), Mullen Stages of Early Development
ATSDR requests OMB approval to collect biological and survey information from pregnant Navajo women, fathers, and their children over a three-year period. Survey instruments were specifically designed to collect demographic information, assess potential environmental, health risks, and mother-child interactions. The survey instruments were developed based on previous surveys conducted by UNM-CEHP’s Dine’ Network for Environmental Health (DiNEH) Project, the National Children’s Study, and by other birth cohort studies in other indigenous populations. The final format of the survey instruments was modified based on review and input from the Navajo Nation community liaison group, and associated Navajo staff who addressed issues such as cultural sensitivity, comprehension and language translation. In addition, the Navajo Nation Human Health Research & Review Board has reviewed and approved all survey documents and outreach materials. Per NNHHRB Research Policy, no modifications to these approved materials may be made without their approval. Please see Attachment 13 for details on the sources of survey questions and their importance to the exposure assessment.
Each study participant will be given a unique study ID number. Information and/or specimens collected as part of the study will be labeled with this assigned study number. Information (without participant’s name) will be entered into RedCap™ , a secure web-based computer database. Data will be stored for three years following conclusion of the study, and then de-identified data will be turned over to the Navajo Nation per the Navajo Nation Human Research and Review Board protocols.
Items of Information to be Collected
The IC will acquire information in identifiable form (IIF) permitting sampling, screening, recruitment, and results reporting to respondents. The IIF will be stored and managed in the Principal Investigator’s record system. The categories of directly identifiable information to be collected include: names, date of birth, street address, mailing address, phone numbers, email addresses, and biological specimens. All IIF will be maintained and processed in the established record keeping system and managed by the Principal Investigator at UNM-CEHP and lead research team members. Lead research team members include the UNM Project Coordinator/ Database Manager, Navajo Nation Division of Health Project Coordinator, and the Clinical Liaisons at each of the five Navajo Area Indian Health Service (NAIHS)/ PL-638 health facilities. No other staff or trained contractors will have access to this system.
Comprehensive assessment of demographic, behavioral and environmental exposures in the target population is a principal objective of this study. The consent forms disclose all assessments (both prenatal and postpartum) administered by the NBCS. The exposure assessment includes:
Urine and blood samples from both expectant mother and father during prenatal clinic visits.
Urine and blood samples from the babies post-delivery
Administration of surveys of expectant mother and father at time of first home visit or in private space in clinics.
Home environmental assessment
The project deliverables include results for chemical analytes in blood and urine specimens as well as results from home environmental assessments. Please see Biomonitoring Table & Analyte Justification (Attachment 14) and Biomonitoring Standard Operation Procedures (Attachment 15). The laboratory analysis for the project will be provided by in-house and by the National Center for Environmental Health (NCEH), Division of Laboratory Sciences (DLS), Inorganic and Radiation Analytical Toxicology Branch at CDC. Blood and urine specimens will be labeled by study ID only. Blood and urine specimens will be stored for future analysis. The stored samples will be used to measure those analytes specified and approved in the protocol, but not yet funded through the current budget. Approved data will be stored for 3 years following conclusion of the study, and then de-identified data will be turned over to the Navajo Nation per the Navajo Nation Human Research Code (1996). As described in the consent form, participants can request to obtain remaining biological samples after laboratory requests have been conducted. If participants do not request to obtain their biological samples, the samples will be destroyed. Certificates of destruction for destroyed biological samples will be sent back to Navajo Nation. Laboratory personnel will not handle any records with IIF.
Although the primary exposure of interest is uranium, the role of other environmental contaminants such as arsenic, mercury, and lead will also be assessed. Given the strong role that demographic and lifestyle factors have been shown to play in birth outcomes, it is also essential that such information be collected and included in the epidemiological models. These factors may contribute to a higher exposure to uranium and other heavy metal confounders. The demographic and lifestyle information obtained such as: age, sex, dietary patterns, hobbies, land, water and drug use, occupational and employment history, residential history and household exposure will help to inform the biomonitoring results (Attachment 3: Data Collection Instruments and Attachment 17: Home Environmental Assessment Protocol).
The survey data will be collected by the ATSDR contractor, Navajo Nation Division of Health (NNDOH). Identifiers must be maintained because of repeated contacts needed with the parent during pregnancy and when the baby is 2, 6, 9, and 12 months in accordance with study plans to assess developmental levels of the child. However, all data will be identified by study ID# only and the list linking name and study ID # will be stored in a locked file at UNM-CEHP with access limited to the UNM-CEHP and the lead research team. Data will be de-identified by the principal investigator prior to delivery to ATSDR, through a secure and encrypted file transfer protocol that is described in Section A.10.
Consent Form
CDC/ATSDR has deferred to UNM and the Navajo Nation on the contents, style, and approach to consent. UNM IRB approval was obtained on June 17, 2011 (HRRC#: 11-310). CDC/ATSDR IRB finalized agreement to rely on UNM IRB on August 4, 2011. The Navajo Nation Human Research Board approval was obtained in December 2011. (Attachment 5). UNM research staff and study partners have undergone extensive trainings and discussions regarding the consent forms. To reduce participant bias and to be consistent with Navajo Nation Human Research Review Board policy, the following measures regarding the consent forms will need to be implemented:
Potential participants will be given a copy of the consent form to bring home and review with family members before officially deciding to consent to the study.
CHERs, Clinical Cohort Liaisons (CCLs), and field research team will be trained to administer consent forms, explain wording, and respond to any questions that participants may have. All CHERs are bilingual and will offer to discuss the consent form in whatever language the participant chooses
The wording on the Consent form about the purpose of the study is worded so that participants will know how the study will benefit them
The UNM staff has worked in this area for over 15years and based on their experience, honesty on the intent of the study, building rapport, and seeking understanding has been the best approach. Therefore, efforts have been made in the development of the consent form and outreach documents to indicate that this study will not be addressed to meet expectations of researchers, but that the study ensued due to repeated requests by the Navajo community to Congress. As a result of the Congressional testimonies of Navajo Nation members, Congress directed several federal agencies to develop a five year plan to address uranium contamination in Navajo Nation. The Navajo Birth Cohort study was funded as a part of this Congressional charge.
The consent form is not the sole method of conveying the intent of this research. The community is very much concerned about these questions, and has an expectation that they will be the focus of the research. When research studies are discussed on the Navajo Nation, trust is built through honest interactions. The UNM IRB template includes this level of detail. For openness, all partners who are involved are listed on outreach materials in order to inform potential participants that the team includes Navajo leads, and that their health care providers are equal partners
Cultural and linguistic experts have provided an extensive review of consent forms to ensure cultural sensitivity and appropriate translations.
More than 20 Navajo project staff has been meeting for months to ensure consensus in their conceptual translation of the consent forms. The development of the consent form was an iterative process. Since the Navajo language is not easily written, the dialogue regarding the consent form is more important than the written component. English in the consent form does not necessarily translate to Navajo. A co-investigator, who is Navajo, as well as members of the Native Medicine Clinical Staff units have also reviewed and approved the consent form. The development of the consent form was an iterative process. Since the Navajo language is not easily written, the dialogue regarding the consent form is more important than the written component. It is important to note that English in the consent form does not necessarily translate to Navajo. CDC acknowledges that this approach may lead to differences in how participants may understand the study and their participation; however we have deferred to the Navajo Nation IRB on the consent process.
Per Navajo Nation policy, it is important to convey that this study is being conducted because of community input, and that CDC/ATSDR used that input as the basis for their decision on the focus of the project. This is an effort to convey why the priority on birth and development was set. Also, some of the wording in the consent form is “boiler plate language” from UNM and Navajo Nation IRBs; therefore, that wording cannot be modified.
Survey Instruments
Similarly, all survey collection instruments and study outreach materials were designed in consultation with Navajo Nation linguists, community cultural experts, and Native Medicine clinicians to ensure study concept accuracy in both English and Navajo. Although CDC acknowledges that the practice of having outreach workers interpret written questions introduces inconsistencies both in how the subject responds to a given question, we have been assured by Navajo language and cultural experts who are a part of the UNM study team that the wording of the current survey instruments is appropriate and the best way to convey study concepts and questions to the target population.
The survey instruments for pregnant mothers will include the following: Enrollment Survey (Attachment 3a), Ages and Stages Questionnaire (Attachment 3c), Mullen Stages of Early Development (Attachment 3d), Postpartum Surveys (Attachment 3e, 3f, 3g) and Eligibility Form (3h). Fathers will also be given an enrollment survey (Attachment 3b).
IIF that will be maintained by the cooperative agreement study partner, University of New Mexico (UNM) includes: mailing address, medical information and notes, biological specimens, phone number, and email address. However, this information will be de-identified prior to transmission to the Research Electronic Data Capture (RedCap™). In compliance with the federal Health Insurance Portability and Accountability Act (HIPAA), all participants will sign a HIPAA Authorization to Use and Disclose Protected Health Information for Research Purposes Form (Attachment 4). This purpose of this form is to get participants’ permission (authorization) to use health information about them that is created by or used in connection with this research. Please see Section A.10 for further details.
Using the RedCap™ data entry system, CHERs will administer an enrollment survey to enrolled mothers and fathers to identify demographic information, characteristics of the home environment, land use patterns including foods locally raised, and potential confounding exposures. The enrollment survey will include questions involving use of prescription, over-the-counter, and recreational drugs, stress, physical activity, water usage, food behaviors, occupation, and activities conducted in the home which might create potential health risks. These environmental data will supplement data derived from the in-home intake survey of both mother and father, which will also occur prior to the birth of the baby. During this period, blood and urine samples will be collected from the mother and father to assess a wide range of biological markers of health. Urine, blood and meconium collected from the baby at birth will be assessed for various biological markers and radionuclides (uranium and radium in particular) to assess transport of contaminants across the placenta during pregnancy.
The post-partum and children developmental surveys will be conducted at 2, 6, 9 and 12 months after the baby is born. All of these data sources combined with specific exposure variables will be used to conduct a multifactorial statistical analysis. Please see Attachment 6: Study Information and Participant Timeline for a detailed table on study interactions and Figures A & B for a graphical description of exposure model inputs, covariates and outcomes.
Developmental assessments will utilize two standardized instruments: The Ages and Stages Questionnaire I (ASQ-I) and the Mullen Stages of Early Learning (MSEL). The ASQ-I (Attachment 3c) is a population screening measure that is used to assess a child’s development between the ages of 1 month and 5 ½ years of age. The ASQ-I uses an outcome variable to evaluate development assessment. Any relevant associations with the exposure variable (uranium) and covariates will be assessed The ASQ-I is a nationally validated assessment of a child’s function in a number of domains, including communication, gross and fine motor skills, problem solving and personal social skills. Since Native Americans were underrepresented in the ASQ-I national sample, ASQ-I has never been specifically validated in the Navajo population. However, the ASQ is routinely used for screening Navajo children. The ASQ will be used as an outcome measure when assessing the impact of exposure.
The MSEL (Attachment 3d) is a standardized measurement of early intellectual development and is used for children from birth to 5 years old. The MSEL is comprised of five (5) scales: Gross Motor, Fine Motor, Visual Reception, Expressive Language, and Receptive Language. The Fine Motor, Visual Reception, Expressive Language, and Receptive Language scales combine to form an Early Learning Composite scores. The questionnaires were developed specifically for this study and have been field tested for cultural/language appropriateness. A slight change to the consent includes wording for a data sharing agreement with NNEPA. The consent form has been approved by UNM and Navajo Nation. Attachment 12 details the Manual of Procedures for the ASQ-I and MSEL as well as the feedback forms that will be provided to participants.
CHERs will administer postpartum surveys to mothers after birth and when the baby is age 2, 6, 9, and 12 months in conjunction with the ASQ-I. The postpartum survey that is administered after birth (Attachment 3e) will assess risk factors during pregnancy such as diet, medication and change in location. The postpartum surveys at 2,6,9 (Attachment 3f) and 12 months (Attachment 3g) will assess breastfeeding behaviors, depression, and child-parent interactions using standardized instruments, including the Kessler 6 (K6) Psychological Distress Scale, (Kessler et al, 2002), the HOME screening tool (Bradley and Caldwell, 1977), and the Edinburgh postpartum depression scale (Watts et al, 2007). The questionnaires were developed specifically for this study and have been field tested for cultural/language appropriateness. More than half of the research team is Navajo and these members have worked extensively with Native Medicine staff at the participating hospitals to ensure that all materials are culturally appropriate and can be discussed appropriately, accurately, and consistently in Navajo. CDC acknowledges that the decision to allow each outreach worker translate the survey instruments as they feel appropriate will introduce variability into the responses.
The food frequency questionnaire (FFQ) will be administered to the mother in the hospital in the postpartum period following birth and not at the 12 month postnatal period. The recall period of the questionnaire is one month. While diet does change over the course of pregnancy, the FFQ is being used to provide an estimate of usual maternal intakes and dietary patterns which may be associated with environmental exposures and pregnancy / developmental outcomes. We do not have the resources available to conduct this survey at multiple time points; therefore, this time point has been determined to be a reasonable estimate of usual diet over pregnancy. Food frequency questionnaire was based on WIC survey and other sources. Please see Attachment 13 for questionnaire sources.
Home Environmental Health Assessments
The CHER and/or Research Field Staff will conduct an in-home environmental assessment with particular attention to sources of heat, types and condition of any heating sources, types of fuel used and source where available; assess physical arrangements used for conducting any in-home activities potentially using toxic materials, such as jewelry-making, in-home pesticide use, other activities using volatile compounds (noting ventilation, protective masks, etc.); place a radon canister; collect a vacuum and swipe dust sample from floor and work surfaces; and conduct a screening scan of the home to determine if any radioactive materials were used in construction.
As stated in the research protocol (Section V.i.e and Table 7), environmental sampling will include: (1) gamma radiation levels, measured in microRoengtens per hour (uR/hr) using a Ludlum Instruments Model 19 MicroR meter, both inside and immediately outside of the participant’s home; (2) indoor radon gas concentrations, measured in picocuries per liter of air over a six-day period using charcoal canisters; (3) three to four dust samples, collected in different places of the home, using 15cm-x-15cm Ghost Wipe clothes and analyzed for 24 metals at the USEPA Region 9 laboratory in Richmond, Calif.; (4) indoor hydrogen sulfide in a small subset of participants’ homes near oil and natural gas facilities using a Honeywell Single-Point Monitor gas tape meter; and (5) water quality assessment for metals and inorganic constituents in water from unregulated water sources that the participants identify as having drunk from in the past or currently. Protocols for all home environmental assessments are included in Attachment 17.
Biomonitoring
Urine samples will undergo multi-metal analyses (ICP-MS), total arsenic (ICP-DRC-MS), iodine/mercury (ICP-DRCMS), arsenic speciation (High performance liquid chromatography inductively coupled plasma mass spectrometry with Dynamic Reaction Cell [HPLC-ICP-DHC-MS]), and PAHs (GC-HRMS). Whole blood will be the source material for biomonitoring of lead, cadmium, and total blood mercury (ICP-MS); and PAHs (GC-HRMS). Serum will be assessed for copper, selenium, and zinc (ICP-DSC-MS). For efficiency, some analyses will be tiered either based on results of screening analyses or responses to the surveys. For example, arsenic speciation and mercury speciation will be done only on samples where total arsenic or mercury results were positive. Samples will be collected to accomplish all of these analyses as indicated in the Biological Specimen Table (Attachment 14). Samples will be collected in metal free containers supplied by CDC/ATSDR laboratories, frozen, batch shipped to UNM, collected and shipped to CDC for cataloguing, aliquoting and storage to ensure long-term preservation and security of samples. Sample aliquots will be returned to UNM as needed for analyses. Participants will be provided a reporting form that will disclose their biomonitoring results (Attachment 18).
Table 2 indicates the samples to be collected from mothers, fathers and babies. Mother biomonitoring samples will be collected once at enrollment/before delivery and once at delivery. Baby samples will be collected delivery. Father samples will be collected at enrollment. Please see Attachment 14 for full list of biomonitoring analytes and justification as well as Attachment 15 for the Standard Operating Procedures for the biomonitoring analysis samples.
Table 2: Biomonitoring Analytes
Participant |
Biomonitoring Panel [matrix] |
Mother |
• Multi-element (Sb, Ba, Be, Cd, Cs, Co, Pb, Mo, Pt, Ti, W, U) panel [urine] •Total arsenic [urine] •As speciation [urine]: •Cd, Pb, Mn, Hg (total), Se [blood] •Hg speciation [blood] |
Baby |
• Multi-element (Sb, Ba, Be, Cd, Cs, Co, Pb, Mo, Pt, Ti, W, U) panel [urine] •Total arsenic [urine] •As speciation [urine]: •Cd, Pb, Mn, Hg (total), Se [blood] •Hg speciation [blood] |
Father |
• Multi-element (Sb, Ba, Be, Cd, Cs, Co, Pb, Mo, Pt, Ti, W, U) panel [urine] |
Table 3 includes all variables that will be included as part of the exposure assessment, the media, and the relative data source.
Table 3: Exposure Assessment Data, Media & Sources
Environmental Exposures |
||
Environmental data |
Media |
Data Source |
Proximity to abandoned mine waste |
Existing electronic dataset of locations and surface areas |
Army Corps of Engineers/USEPA Atlases and accompanying DVDs |
Indoor dust wipe/ vacuum samples |
Collected during home assessment |
USEPA Region 9 analytical laboratory analysis |
Indoor radon |
Collected through in-home canisters placed in winter months to capture maximum in-home concentrations |
Analyzed by USEPA, staff training and support from NNEPA Radon program |
Ambient air quality |
Air monitoring data |
USEPA & NNEPA – existing datasets |
Indoor sulfur compounds |
Determined for prototypical homes in communities with high and low ambient concentrations |
Indoor monitors provided by ATSDR -- Tape readouts |
In-home radiation |
De novo screening level Ludlum scans by CHERS or NNEPA consent to release extant data if previously scanned |
USEPA Region 9 and NNEPA (training on procedures to synchronize with their program, or release of data with consent if applicable) |
Water Sources |
Surveys will identify water sources that participants utilize |
Water quality testing conducted as part of home assessment |
Historical personal exposures |
||
Data |
Media |
Data Source |
Historical & current activities bringing participants in contact with waste or other contaminants |
Survey questions on land use, water use |
Intake surveys for mom, dad (NBCS) |
Occupational exposures |
Survey questions on work history |
Intake surveys for mom, dad (NBCS) |
Biomonitoring (to confirm exposure) |
||
Data |
Media |
Data Source |
Uranium, Metals, Arsenic, Lead, Mercury |
Blood and urine samples |
CDC Environmental Health Laboratory, UNM Earth & Planetary Sciences ICP- MS/Nuclear chemistry/engineering lab |
Medical Record Abstraction
Cohort Clinical Liaisons stationed at each NAIHS hospital and PL-638 study facility will abstract medical record data from all study participants. Table of all data to be abstracted from medical record is included in Attachment 16.
Covariates and Effect Modifiers
Potential effect modification and confounding variables that may be associated with either the exposures and outcomes will be identified from prior studies based on biological plausibility and will be included as covariates in multivariate models, as appropriate. Figure A and Figure B illustrate exposure inputs, modifiers/covariates, and study outcomes. Please see Attachment 13 for additional information concerning survey questions regarding the covariates. Medical records will also provide valuable covariate information. Please see Attachment 16 for Medical Record Abstraction Data table and abstraction form.
Figure A: Potential Effect Modifiers/Covariates for Reproductive Health Outcomes
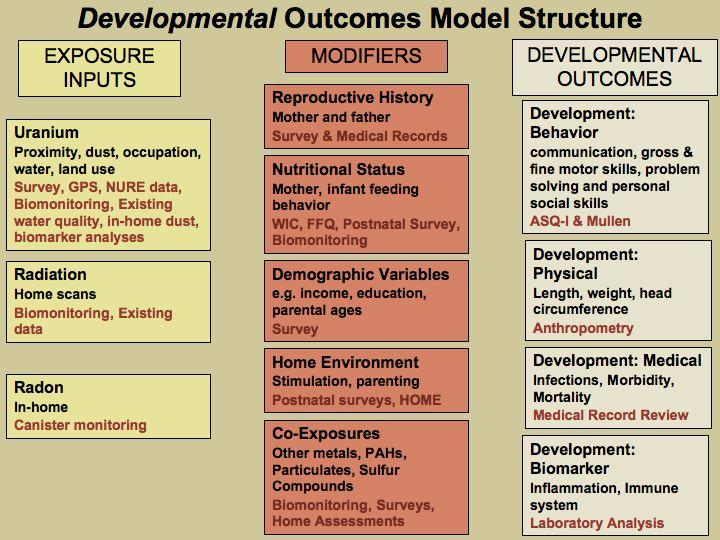
Figure B: Potential Effect Modifiers/Covariates for Developmental outcome
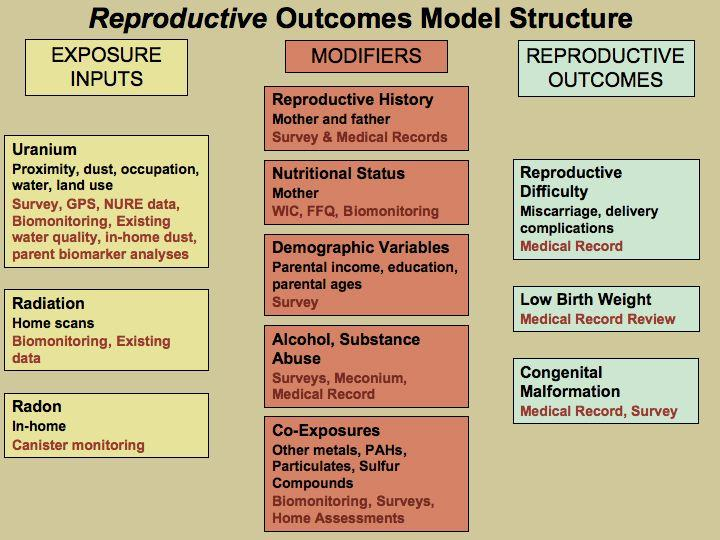
While all factors associated with prenatal development and adverse birth outcomes may not be controlled, differences in quantity of prenatal care and inter-facility differences in outcome will be assessed through surveys, medical record abstraction and biomonitoring exposure results. Should any differences be observed and not related to differences in exposures, further analysis of the type of care would be warranted. The results will inform the normalization of prenatal care as an outcome. In the Navajo Nation, prenatal care is a marker for other determinants correlated with education, socioeconomic status that will also be analyzed. Please see section B.2 for Power Sample Calculations Assumptions and potential study limitations.
Identification of Website(s) and Website Content Directed at Children Under 13 Years of Age
The information collection does involve web-based data collection methods, and there is no website with content directed at children under 13 years of age. Eligible participants must be at least 14 years of age to participate in the study. The data collection system utilizes a security controlled internal website; therefore, public access and the potential for cookies do not exist.
A.2. Purpose and Use of the Information Collection
ATSDR and its cooperative agreement partner, UNM-CEHP, will oversee the collection of this data by the contractor, Navajo Nation Division of Health. The information collected will be used to determine if Navajo mothers and their infants have elevated exposure to uranium. The Navajo Birth Cohort Study is the first prospective epidemiologic study of pregnancy and neonatal outcomes in a uranium-exposed population. The primary objective of this study is to evaluate potential exposures to environmental contaminants (i.e., uranium and other heavy metal exposures) among pregnant Navajo women. We will assess their exposure (through biomonitoring, home assessments, and surveys) at key developmental milestones, and then follow their children post-birth to investigate any associations with birth defects or developmental delays. The information generated by this study may be of value in developing programs to mitigate environmental uranium exposure. Additionally, the study will provide additional baseline data on exposures, behaviors, and chronic health that may be of value in developing health education and outreach to increase prenatal care utilization.
The study is being conducted in response to Navajo community requests conveyed to Congress, which resulted in a Congressional allocation for ATSDR to conduct an epidemiological study. Study funds were requested for the three year study period (2010-2012). The original funding proposal outlines a power and sample size calculation, though CDC acknowledges that some of the assumptions may not reflect the variability in exposure and health outcome data likely or the complex multivariate analyses that may need to be undertaken. Although environmental sampling and the measurement of risk factors associated with these developmental delays is designed to control for as much variance as possible, CDC is committed to acknowledging any potential confounders, limitations and uncertainties that remain at the end of the study for which we have not been able to control.
The results of the study will benefit the Navajo families and contribute to the knowledge base regarding the potential association between exposures to uranium wastes and other environmental contaminants and adverse birth outcomes or developmental delays on the Navajo Reservation.
Furthermore, benefits to participating families will include the potential for early identification of risks or developmental delays. Those with such risks will be referred to support services to ensure early intervention, with the hope of reducing the impacts of developmental delays. Since these studies are being conducted under Congressional mandate and include federal agencies responsible for removal of source contamination, the improved understanding of risks may help in guiding policies that prioritize remedial actions to remove contamination sources from these communities. Remedial actions to remove contamination sources will prioritize whether or not a relationship is found between uranium and study outcomes.
Privacy Impact Assessment
The IC will acquire IIF permitting sampling, screening, recruitment, and results reporting to respondents. The IIF will be stored and managed by ATSDR’s research cooperative agreement partner and principal investigator at the UNM-CEHP. IIF will be stored and managed using a record system stored in a locked file in the principal investigator’s office. The categories of directly identifiable information to be collected include: names, date of birth, street address, mailing address, phone numbers, email addresses, and biological specimens. The principal investigator at UNM-CEHP will be working in collaboration with the Navajo Area Indian Health Service (NAIHS) and Navajo Nation Division of Health (NNDOH). NAIHS and NNDOH will provide support for project activities such as recruitment and enrollment of eligible participants, scheduling appointments, administering the questionnaire, sample collection and medical consultation.
The IIF will be used for the purpose of 1) follow up of participants for home and prenatal care visits at the Indian Health Service clinics and healthcare facilities contracted with Navajo Nation Division of Health through Public Law 93-638 ( PL-638) providers and 2) recording and clarifying information that has been provided by the CHERs.
Information that might be considered sensitive by a portion of the general public is being collected, so there could be an effect on the respondent’s privacy if there were a breach of privacy. Accordingly, very stringent safeguards have been put into place as described in Section A.10.
A.3. Use of Improved Information Technology and Burden Reduction
Electronic reporting will be used to collect the majority of questionnaire data for this program. 98.5 % of burden hours will be collected by electronic reporting. The eligibility form, which constitutes 63 annual burden hours, will be conducted by paper and pencil form. This study will use the RedCap™ CAPI development tool which is provided through the University of New Mexico Virtual Private Network (VPN). RedCap™ allows for design of a data collection form which can be used to rapidly collect and store questionnaire data in the field. Data collected can be aggregated, reported and exported using a variety of formats including XML and Microsoft Excel. Trained Community Health Environmental Representatives (CHERs) will administer surveys in the field and record responses in RedCap™. The RedCap™ CAPI will be deployed on laptop computers to collect data. The NCEH/ATSDR Information Systems Security Officer (ISSO) has approved the Data Privacy & Security Plan to ensure measures are in place to protect participant data while using RedCap™ software.
A.4. Efforts to Identify Duplication and Use of Similar Information
ATSDR efforts to identify duplication of the proposed IC included reviews of existing reports, peer reviewed publications, abstracts presented at international, national and tribal meetings. Please see section A.1 Background for a review of the literature on environmental uranium exposure and its association with reproductive and birth outcomes.
ATSDR also worked with the principal investigator at UNM-CEHP to identify whether the proposed IC is duplicated for 1) proposed population of interest; 2) specific area of concern; and 3) proposed chemical contaminants.
There is a paucity of studies focused on uranium contamination in the Navajo Nation; therefore, the U.S. House Committee on Oversight and Government Reform requested that government agencies prepare a plan to address Health and Environmental Impacts of Uranium Contamination in the Navajo Nation. This study is a result of the House Committee’s Request for an epidemiological study focused on environmental uranium contamination in the Navajo Nation.
Literature searches, data base searches, and consultations with Navajo Nation Division of Health, Navajo Nation Environmental Protection Agency, New Mexico Department of Health, and Arizona Department of Health were also conducted to determine that a similar data collection is not being conducted by another institution. This is the only epidemiological study that combines both uranium biomonitoring of pregnant Navajo women and fathers with the applied public health benefit of prenatal educational outreach.
A.5. Impact on Small Businesses or Other Small Entities
No small businesses will be involved in this data collection.
A.6. Consequences of Collecting the Information Less Frequently
In order to gather data relevant to prenatal, perinatal, and postnatal time periods, data must be collected during pregnancy, as well as, post-pregnancy. When studying developmental endpoints it is necessary to collect data during multiple critical developmental time periods.. Please see Attachment 6: Study Information and Participant Timeline which details frequency of participant interactions. There are no legal obstacles to reduce the burden.
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This request fully complies with the regulation 5 CFR 1320.5.
A.8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A. 60-day Federal Register Notice was published on November 22, 2011 in Volume 76, page 72206 (Attachment 2). We received no public comments.
B. Efforts to consult with persons outside of the agency
In September 2009, ATSDR representatives met with several Navajo agency and department representatives (NNDOH, NNEPA), NAIHS, EPA, community members, and local university researchers to gain further understanding of previous research activities conducted in the Navajo Nation, to summarize current activities, and to discuss knowledge gaps in environmental uranium exposure and potential health effects. In addition, NNEPA staff led ATSDR representatives on a tour through parts of the reservation to observe some of the abandoned mine areas and three of the four milling sites. The study coincides with the Health and Environmental Impacts of Uranium Contamination in the Navajo Nation 5 Year Plan requested by the House of Representatives Committee on Oversight and Government Reform. Therefore, we have conducted yearly congressional briefings to the Rep. Henry A. Waxman Committee in consultation with the Environmental Protection Agency, Bureau of Indian Affairs, Nuclear Regulatory Commission, Navajo Area Indian Health Service, and the Department of Energy. The following primary individuals were consulted to obtain their views on the availability of the data, the clarity of instructions, disclosure, questionnaire development, language interpretation, analyte selection, cultural sensitivity regarding the study. A full detailed list of individuals consulted is in Attachment 7. Please see Attachment 8 for Community Outreach Chronology and Attachment 9 for ATSDR Timeline of Interactions.
University of New Mexico and Affiliates |
|
LEWIS, Johnnye L., PhD. , DABT (PI) Director, Community Environmental Health Program (CHEP) 1 University of New Mexico, MSC09 5360 Albuquerque, NM 87131-0001 Phone (505) 991-3489 Fax (505) 925-4549
|
SHUEY, Chris, MPH (Co-PI) Director, Uranium Impact Assessment Program SRIC P.O.
Box 4524 Phone: 505-262-1862 Fax: 505-262-1864 |
ETTINGER, Adrienne, ScD, MPH (Co-PI) 60 College Street P.O. Box 208034 New Haven, CT 06520-8034 Phone (203) 785-6232
|
BEGAY, David, PhD (Co-PI) UNM-CEHP Phone (928) 607-0365
|
Navajo Area Indian Health Service |
|
PETER , Douglas, MD NAIHS Chief Medical Officer P.O. Box 9020 Window Rock, AZ 86515-5004 Phone (928) 871-5811
|
ALLEE, Lisa, CNM Program Director, Community Uranium Exposure / Women’s Health Northern Navajo Medical Center Box 160 Shiprock, NM 87420 Phone (505) 368-6311
|
Navajo Nation Environmental Protection Agency
|
|
ETSITTY, Stephen Executive
Director, NNEPA Phone:
(928) 871-7692
|
QUINTANA, Eugenia Department Director, Air and Toxics P.O.
Box 339 [email protected] Fax: (928) 871-6757
|
Navajo Nation Division of Health |
|
POUDEL, Madan, PhD Health
Services Administrator, Navajo Nation [email protected]
|
BEGAY, Mae- Gilene Department Manager, Community Health Representatives/ Outreach Program Tribal Administration Building 2 Window Rock Boulevard Window Rock, Arizona 86515 Phone (928) 871- 6785
|
A.9. Explanation of Any Payment or Gift to Respondents
In consultation with the Community Liaison Group, (see Attachment 7 –Individuals Consulted), the following incentive plan was determined to be most appropriate. A program for token of appreciation has evolved through discussions with community members, staff, and clinicians in the participating facilities who also provide tokens of appreciation for various health programs. In our effort to remain consistent with these activities and with community consideration of the value of their time, the following plan has evolved based on success with a similar incentive card structure within the Special Diabetes initiative at Kayenta Health Center:
Enrollment: $10 gift card + skin care products valued at $15 donated by a company in partnership with Navajo Nation. This will be given to moms and dads during initial home visit after consent and HIPAA completed and home-visit scheduled, and prenatal labs completed.
Delivery: $35 gift card + bag with in-kind donations of baby care products and CD of Navajo language stories for baby. Delivery gift will be presented when punch card is completed for in-home environmental assessment, enrollment survey, nutritional delivery survey, mom’s delivery blood and urine samples, cord blood, and baby urine.
• One-year follow-up: $75 gift card when 2, 6, 9, 12 mo. Home visits, ASQs, post natal lab work and MSEL completed. This card will be presented as final gift when the 2nd punch card is completed at one year.
These cash card values are consistent with payment provided in our previous work where those completing surveys received $10 cash cards, and those providing blood and urine samples and clinical screens received $35. In this study, multiple blood draws, plus multiple assessments warrant an increase in those numbers. Budgetary limitations, however, meant that cash gifts have been supplemented by full or partial in-kind donations to allow us to more appropriately recognize the time that participants are committing to this effort. The punch cards allow us to both more readily manage the process with respect to tracking and distribution of gifts, plus help in motivating participants to self-identify when attending clinics. This program has been used successfully at the participating Kayenta Health Center and been recommended by both the clinicians and participants in the special diabetes projects that have used it.
A.10. Assurance of Confidentiality Provided to Respondents
This submission has been reviewed by the NCEH/ATSDR Privacy Officer who determined that the Privacy Act does apply. Data will be primarily collected by the ATSDR contractor Navajo Nation Division of Health (NNDOH) and identifiers must be maintained because of repeated contacts needed with the parent during pregnancy and when the baby is 2, 6, 9, and 12 months old in accordance with study plans to assess developmental levels of the child. The applicable Privacy Act System of Records Notice (SORN) is 09-19-0001, “Records of Persons Exposed or Potentially Exposed to Toxic or Hazardous Substances.”
Surveys questions that involve information in identifiable form (IFF) include name, mailing address, medical information and notes, biological specimens, phone number, and email address. The contact information is necessary for scheduling and follow-up of prenatal visits and home visits. Biological specimens are critical for evaluating uranium and other contaminant levels. Information and /or specimens collected as part of the study will be labeled with an assigned study ID number.
The master list linking participant identifiers and project numbers will be stored and maintained in the Principal Investigator’s (Dr. Johnnye Lewis) locked files, maintained at the University of New Mexico. Dr. Lewis and her research associates will maintain access to participants study information by participant ID number. Dr. Lewis and the lead research team (UNM Project Coordinator/Database Manager, Navajo Nation Division of Health Project Coordinator, and the Clinical Liaisons at each of the five Navajo Area Indian Health Service / PL-638 health facilities) will be the only ones with access to information linking participants’ names to their participant numbers. IIF will be used for the purpose of 1) follow up of participants for home and prenatal care visits at the Indian Health Service clinics and healthcare facilities contracted with Navajo Nation Division of Health through Public Law 93-638 (PL-638) providers and 2) recording and clarifying information that has been provided by the CHERs for data quality and control purposes.
IIF will be provided to CHERs for home and prenatal care visits and follow-ups. IIF will be provided in a limited manner sufficient to accomplish the task, and destroyed once the task is complete. Lead research team members may also use IIF to verify correct data entry by CHERs during periodic data quality and control checks. Data will be stored for 3 years following conclusion of the study, and then de-identified data will be turned over to the Navajo Nation per the Navajo Nation Human Research Code (1996).
For scheduling and medical record access, lead research team members will need linkage information for individual participants and entry of data. They will be asked to destroy that linked information on completion of the interview, and no data collected in the field will be entered into the electronic data files by any identifier other than participant number. All members of the research team will be required to complete UNM (or NAIHS) privacy protection, confidentiality, and health information security trainings, as well as CITI training for research with human participants.
There should be no infringement on the privacy of the volunteer participants as recruitment will be through self-identification to a member of the research team. Consents will be obtained either in the home at an appointment scheduled with the participant, or in a private room in the clinic setting. If any consents are administered at other locations due to subject contact with the research team in a different venue, all staff will be trained to ensure the consent process is done in a private space beyond hearing of others. Follow-up will be conducted through the research team scheduling home appointments, and at 1 year arranging for a parent or guardian to bring a child into a designated location for testing. Again, these tests will be administered in a private space. Clinical staff providing care or accessing medical records will have access to identifiers in order to access the medical record. However, abstractions from the records will only be documented by participant ID numbers.
Staff will be trained in the protection of private medical information. Training will be through the on-line trainings mandatory for all UNM staff or through approved NAIHS training protocols as appropriate. For staff not affiliated with either of these institutions, the UNM HIPAA and CITI trainings as well as other mandatory trainings for Health Sciences Center staff on security and control of data and protection of PHI will be required. A RedCap™ scheduling tool will be used to create prompts for research team members to schedule appointments for individual participants in a timely manner, and this notification will contain contact information linked to the participant number in order for the research to enter data correctly. Again, these staff will be trained on participant privacy, and entry of data into the database or onto forms in their computers will be done only by participant ID numbers. The research team will be asked to destroy notifications linking names and numbers upon completion of the appointments.
All study partners will adhere to all federal, HHS, and/or CDC IT security policies. Systems development work shall comply with the HHS Enterprise Performance Life Cycle (EPLC) Framework as an IT Project Management requirement and shall perform Enterprise Performance Life Cycle (EPLC) requirements, objectives, responsibilities, and standards for managing information technology (IT) projects in conjunction with the government or on the behalf of the government. More details about the EPLC are available at: http://www.hhs.gov/ocio/eplc/.
Adequate administrative, operational, and technical security controls will be implemented to prevent unauthorized access to or disclosure of any personally identifiable information (PII) that will be accessed by the contractor. Electronic transfers of PII via Internet or portable media must utilize FIPS 140-2 compliant encryption. The final de-identified dataset with data collected on all participants will be delivered to CDC/ATSDR, UNM, and NNDOH using excel files with encrypted, password coded spreadsheets through a password protected data sharing facility.
IRB Approval
UNM IRB approval was obtained on June 17, 2011 (HRRC#: 11-310). CDC/ATSDR IRB finalized agreement to rely on UNM IRB on August 4, 2011. The Navajo Nation Human Research Board approval was obtained in December 2011. (Attachment 5).
A.10.1 Privacy Impact Assessment
Subject to Privacy Act
This submission has been reviewed by the NCEH/ATSDR Privacy and Confidentiality Officer who determined that the Privacy Act does apply.
Describe how information will be secured
Access to information will be tiered to the roles and responsibilities of members of the research staff. Data will be collected using Research Electronic Data Capture (RedCap™) . The NCEH/ATSDR Information Systems Security Officer (ISSO) has approved a Data Privacy & Security Plan to ensure measures are in place to protect participant data while using RedCap™ software.Field research staff will have access to enter data based on participant study number; the database manager will have full privileges; statisticians and modelers will have authorization to read and export data; while team members with no data entry or manipulation responsibilities will have read-only privileges. The lead research team members who provide Quality Assurance/Quality Control (QA/QC) for the data will have time-limited access to edit specific datasets until QA is completed. Reports to team members with no responsibilities for data entry, QA, or analysis will be exported through requests to the database manager to ensure adequate metadata accompanies the report.
Electronic reporting will be used to collect all questionnaire data for this program. This study will use the RedCap™ CAPI development tool. The University of New Mexico has secured a license for RedCap™ software, and UNM will develop RedCap™ based CAPI. RedCap™ is a secure application for building and managing surveys and databases. RedCap™ is currently utilized by other Centers for Disease Control and Prevention programs such as the Division of Global HIV/AIDS. Data collection using RedCap™ will occur using the following:
Direct on-line connection to the RedCap™ system: This online connection occurs through a secure virtual private network (VPN) tunnel that disables any other network connections on machines. Data transfer when an internet connection is available occurs by utilization of the encrypted transfer protocol inside the RedCap™ system, again inside the VPN secure tunnel. The system generates an email notification of an available file transfer, followed by a second notification of a password necessary to open that encrypted file package.
Off-line collection RedCap™ system process: This offline collection generates an encrypted file stored in a truecrypt basket on the machine. That encrypted file basket is closed on removal of the drive. UNM staff performs regular QA of new data on a weekly basis, and will conduct field review and follow-up with NNDOH staff monthly.
The RedCap™ CAPI will be deployed on laptop computers to collect data in designated clinics and field sites. Trained CHERs will administer surveys in the field and record responses in RedCap™. Data collected via RedCap™ can be aggregated, reported and exported using a variety of formats including XML and Microsoft Excel. CHERs will receive extensive training on data entry and laptop security and will follow all guidelines outlined in the approved RedCap Data & Security Plan.
Opportunities for obtaining respondent consent
Because more than 20% of births on Navajo occur to women under 18, and the inclusion criteria include mothers as young as 14, consent will require a tiered process. For minors, regardless of state of residence, the consent of one parent for participation of their minor child as a parent in the birth cohort will be required, whether for a minor mother or minor father. A signature acknowledging the assent from the minor will also be obtained. However, the minor parent will be asked to consent for their child to be enrolled in the study and to be followed for a minimum of one year. Should the minor parent reach 18 during the study, she or he will be re-consented if additional data collections are scheduled. Copies of consent forms for the mother and the father are included in Attachment 4.
For parents over 18, the study consent will be required from the mother and from the father for their own participation, and the mother will be asked to consent for her child’s participation through the first year. Because we are working to develop a sustainability plan for the cohort, the parents will also be informed that their data will be retained for up to three years past the end of the current project period. The sustainability plan will be developed once the study is underway and the working relationships/capabilities of the various partners have been established. UNM has submitted several partnership grants with Navajo Nation Division of Health as an initial step in building the capacity of Navajo Nation to continue this effort, either alone or in partnership with the academic partners at UNM. Navajo Nation Division of Health has identified Environmental Health as a key initiative in the strategic plan developed this year to move the Division to a Department of Health.
Participants will be asked for consent to note in their medical and Growing in Beauty records that they are participants in the Navajo Birth Cohort Study, and to note whether they consent to be re-contacted. They will be retained in the active data system with successive 5-year consents for 20 years, but will be able at any time to refuse continuation in the cohort. Refusal will be noted in their files, and they will be withdrawn from any subsequent aspects of the project. In addition to these criteria, all participants will be informed of their right to refuse participation in particular components of the study without compromising their participation in others.
Meconium and umbilical cords have used in some Navajo traditional ceremonies, and some cultural traditions stress the importance of not destroying leftover blood Therefore, destruction of unused biological samples and collection of meconium may conflict with cultural practices. Study participants will be able to indicate on the consent form if they choose to have their remaining samples returned to them instead of destroyed. If participants indicate on the consent form that they would like their unused biological specimens, then the unused specimens will be sent to them instead of being destroyed after analysis. Consents will be administered by clinical staff dedicated to the project in each clinical service unit, by NNDOH field research staff in the home, or by the UNM DiNEH Project field staff. All team members administering consents will be mandated to maintain active CITI, HIPAA, and confidentiality trainings as provided by UNM, or their institutional equivalents submitted to the PI for review. Because UNM-CEHP have dedicated clinical staff on the project within the service units, we anticipate no difficulties in having the consents linked to the medical records ― a process UNM-CEHP is currently doing in a collaborative project with NAIHS.
There will be two consent forms, one for the father and one for the mother of the unborn child (Attachment 4). Once the consent form is read and discussed with the potential participant, they will be asked to sign as appropriate on the consent/assent page where the following options will be available:
For participants 18 and over:
Consent for participation of self and baby (mother) or self (father)
For participants under 18:
Assent of interest to participate (mother and father)
Consent of parent for participation of their minor child (mother and father)
Consent of minor mom for participation of unborn child (mother only)
At the time of consenting, participants will also be given a brochure that outlines details and flow of the project with respect to each family member and endpoint, relative to prenatal, postnatal, and neonatal collections of data. Phone numbers for Navajo IRB, UNM IRB, DiNEH Project, NNDOH-CHR program, NNEPA and other agencies will be included in the brochure to enable participants to obtain information or to access available services (Attachment 10).
The informed consent procedures include a detailed description of the study as well as an assurance of the subject’s freedom to withdraw from the study at any time without prejudice of any kind. Eligible subjects will be assured that their level of care will not be affected by participation or non-participation in the study. We will take all appropriate measures to ensure safeguarding of the data in order to minimize participant distress. Data collection and management will be carried out in a manner that ensures that sensitive data are handled appropriately with the utmost attention to privacy and security. Participation in the study involves answering questions about sensitive information of a personal nature. Interview questionnaires will be administered in a neutral and private setting to ensure the comfort of the participant. A list of available community social service resources will be provided to all participants for support and assistance. Research staff will be trained to administer interviews in a sensitive manner and to identify participants that may need referrals to outside agencies. Collaborating members of the research team include psychologists from the Center for Development and Disabilities and the Department of Pediatrics at UNM, as well as Navajo Nation’s Growing in Beauty Program. Ongoing services will also be available through the Navajo Area Indian Health Service and PL-638 programs collaborating on the study and through Navajo Nation’s Growing in Beauty Program which provides case management to families to ensure access to all available services.
Finally, the study partners include several Navajo language and culture experts who will ensure that all questions are asked in a manner that is culturally appropriate, and that staff conveys professional and cultural sensitivity to increase the confidence in safeguarding and professional management of information. Navajo is a descriptive language and traditionally not a written language – very few Navajos actually read Navajo although many are fluent speakers. Therefore, the Navajo team members, as noted above, have worked for several months to establish consistency in translation of materials introducing the study and the consent and HIPAA forms. The actual participants in the study are all likely to speak English, and therefore, we anticipate the actual survey administration will be in English. However, when clarification is requested, the team will have the background provided through these working session to convey concepts in Navajo in a consistent manner. This method has been used by this team in field surveys before. The project staff are also involved in ongoing, multidisciplinary training programs to ensure consistency regardless of the direct employment or reporting status. All staff are also completing HIPAA training and certification as well as completing the security and confidentiality trainings provided on line by the University of New Mexico for those in contact with patients.
Indicate whether respondents are informed about the voluntary or mandatory nature of their response
The consent and assent forms indicate that participation is completely voluntary and there is no untoward effect on the respondent if they decide not to respond to the data collection request. There are no plans to share identifiable data.
A.11. Justification for Sensitive Questions
Sensitive survey questions involve current medication and substance abuse, alcohol use, tobacco use, and stress. These questions provide information about critical confounders to identify any activities that would also result in adverse birth and reproductive health outcomes.
The purpose of the study is to explore the association between the short- and long-term health and developmental effects and uranium exposure to mothers and infants living on the Navajo Nation. In order to characterize the effects of environmental exposures, it is essential to collect and control for information relating the individual and contextual factors that may be related to the exposures and outcomes of interest. To estimate the burden of uranium toxicity on the population-level, we need to collect individual-level information about complex, interdependent social variables and apply analytical approaches to model and control for the effects of these covariates. Please see “Items of Information to be Collected” in A.1 for specifics on exposure assessments as well as Attachment 13.
The survey response data collected under this research study protocol that are generally considered sensitive by a majority of the population are central to evaluating the research questions and study hypotheses. Exposure to toxic chemicals, such as uranium, and other stressors commonly co-occur in disadvantaged populations. It is now recognized that contextual social factors may not only confound the relationship between environmental exposures and negative health outcomes, but may also determine the degree of susceptibility (Cooney, 2011).
Animal studies have demonstrated that the joint impact of psychosocial stress and environmental exposure is synergistically more toxic than either factor alone. In addition, these studies also suggest that a positive social environment can mitigate toxicity of environmental exposures. Therefore, collecting information about the social environment is paramount to being able to appropriately and fully investigate the effects of environmental exposures, such as uranium, on maternal and child health. It is essential to simultaneously account for chemical environmental exposures as well as social conditions of the family which occur during critical windows of fetal and infant development. Such information includes, but is not limited to: parental education, family environment, psychosocial stress (parenting stress, depression, exposure to violence, chemical use and/or dependency), as well as educational and socio-economic information.
IFF such as name, mailing address, phone number, and email address will be collected. This contact information is necessary for scheduling and follow up of prenatal visits and home visits. Social security numbers will not be collected.
A.12. Estimates of Annualized Burden Hours and Costs
A. Burden hours are included in Table 1. Participants will include Native American mothers from age 14 to 45 with verification of pregnancy who have lived in the study area for at least 5 years. Also, participants must consent to deliver at one of the healthcare facilities (Northern Navajo Medical Center, Chinle Comprehensive Health Care Facility, Gallup Indian Medical Center, Tuba City Regional Health-Care Corporation, or Tséhootsooí Medical Center) that are taking part in the study. Fathers will be included in the study with consent regardless of age or residence. We estimate that 550 pregnant women and fathers per year must be enrolled in the study to obtain adequate statistical power. A 10% pregnancy loss will be assumed, which would result in 500 live births per year. Therefore, the total anticipated sample size is 1,500 mother-infant pairs over the three years of the study.
Table 1: Estimate of Annualized Burden Hours
Type of Respondent |
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Average Burden Response (Hours) |
Total Burden (Hours) |
Mother |
Eligibility Form (Screening Form) |
750 |
1 |
5/60 |
63 |
|
Enrollment Survey |
550 |
1 |
2 |
1100 |
|
Home environmental Assessment |
550 |
1 |
1 |
550 |
|
Ages and Stages Questionnaire (2,6,9 12 months) |
500 |
4 |
15/60 |
500 |
|
Mullen Stages of Early Development |
500 |
1 |
20/60 |
167 |
|
Food Frequency Questionnaire/ WIC Intake Form |
500 |
1 |
45/60 |
375 |
|
Postpartum Survey (2 months) |
500 |
1 |
1 |
500 |
|
Postpartum Survey (6,9, 12 months) |
500 |
3 |
15/60 |
375 |
Father |
Enrollment Survey |
550 |
1 |
90/60 |
825 |
|
|
|
|
Total |
4455 |
B. Burden costs are included in Table 2. The study participants will be members of the general public in the Navajo Nation. Recent government estimates of hourly-wages for the entire Navajo Nation were not yet available in the 2010 Census. Therefore, 2000 Census data was used. According to 2000 Census data, the average weekly wage within the Navajo Nation is $406 or $10.15/hour assuming a 40 hour work week. This data is based upon the 2000 Census Demographic Data (http://censtats.census.gov/data/AZ/280042430.pdf).
Table 2: Estimate of Annualized Burden Costs
Type of Respondents |
Total Burden Hours |
Hourly Wage Rate |
Total Burden Cost(s) |
Mothers |
3630 |
$10.15 |
$36,844.50 |
Fathers |
825 |
$10.15 |
$ 8,373.75 |
A.13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers
There are no capital or maintenance costs incurred by respondents other than travel to their designated prenatal visits. There are no costs or burden to respondents for recordkeeping.
A.14. Annualized Cost to the Federal Government
The total estimated cost to the government is $6 million, based on $2 million Congressional allocation projected for the 3 year project study period. The estimated average annualized cost of the program is ~ $2 million.
NNDOH Sole source contract: ~$250,000 a year
UNM Research Coop: ~$1 million a year
Navajo Area IHS interagency agreement: $375,000 a year
ATSDR personnel and travel total costs: $168,102
A.15. Explanation for Program Changes or Adjustments
This is a new study data collection.
A.16. Plans for Tabulation and Publication and Project Time Schedule
A.16-1 Project Time Schedule |
|
Activity |
Time Schedule |
Raise awareness of the study through outreach and education
|
1-2 months after OMB approval |
Conduct study recruitment |
2-12 months after OMB approval |
Data collection, cleaning, merging and analysis |
5-36 months after OMB approval |
Continue data analysis and report preliminary study findings to Navajo community and at scientific meetings |
12-36 months after OMB approval |
Prepare reports and publications as analyses are completed |
24-36 months after OMB approval |
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate
Exemption from displaying the expiration date for the OMB approval of forms is not requested.
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | hlb8 |
| File Modified | 0000-00-00 |
| File Created | 2021-01-29 |
© 2026 OMB.report | Privacy Policy