App.H 1_Eng Post Birth HIPAA Letter
App.H 1_Eng Post Birth HIPAA Letter.V2.docx
WIC Infant and Toddler Feeding Practices Study-2
App.H 1_Eng Post Birth HIPAA Letter
OMB: 0584-0580
OMB
Approval No. 0584-XXXX Approval
Expires: XX/XX/20XX

Appendix H.1
Post-Birth HIPAA Letter – ENGLISH
[Recipient Name]
[Street Address]
[City, ST ZIP Code]
Dear [Recipient Name]:
Congratulations on the birth of your baby [NAME] on [DATE]! The WIC Feeding My Baby study team is looking forward to talking with you over the next two years to learn more about your WIC experiences and the choices you make about how and what to feed your baby. As you know, you will receive $20 for each telephone interview you complete.
This package includes the following:
HIPAA form [If core, postnatal group] that authorizes us to get records about your baby’s birth from the hospital where you gave birth, and the information about your baby’s height and weight from your baby’s pediatrician. Please sign and return the form to us in the enclosed postage-paid return envelope.
A calendar on the yellow sheet that shows the four-week period when we will call you to complete each followup interview. It also has a toll-free number, 1-888-888-8888, which you can use to call us during each interview period to complete an interview. You may find it helpful to place the calendar on your refrigerator.
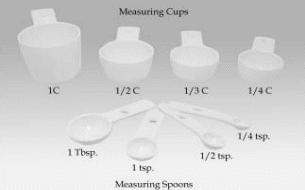
A set of measuring guides including measuring cups and spoons, a household teaspoon and tablespoon, and a ruler to help you with the next interviews that ask about the type and amount of food the child eats on a given day.
If you have any other questions, please contact your study liaison, [STUDY LIAISON NAME]. She can be reached by e-mail at ______________, and you can also call or text her at _________________.
Thank you for your continued participation in the WIC Feeding My Baby study.
Enclosures
HIPAA Form (Core, Postnatal Group Only)
Study Calendar
Measuring Guides
Postage-paid Return Envelope (Core Group Only)
Permission to Get Information from Medical Records
WIC Feeding My Baby Study
Food and Nutrition Service, U.S. Department of Agriculture
If you sign this document, you are giving permission to (1) the hospital or medical facility where you gave birth to your child, and (2) your child’s doctor, to release health information that identifies you to Westat for the WIC Feeding My Baby Study. The health information that we will use for the Feeding My Baby Study includes both your medical records and your child’s medical records from the hospital stay when you gave birth to your child; and, your child’s weight, length, and health status information from your child’s doctor up until your child is two years old. Westat will use this health information, along with information you give during your interviews and information from your WIC records, to learn more about the health and feeding choices of WIC families.
Both the hospital or medical facility where you gave birth, and your child’s doctor, are required by law to protect your health information. The Health Insurance Portability and Accountability Act of 1996 (HIPAA) prevents them from releasing your health information without your permission. Once your information is released to Westat it is no longer protected by HIPAA, but the same privacy protections Westat takes with your other information will also apply to your medical records. Your name and your child’s name will not be used in any research reports, and Westat will not share personal information about you with WIC or with anyone else who is not on the study staff.
The hospital, medical facility, or your child’s doctor may not refuse to treat you because of your decision to sign or not sign this authorization. You can change your mind and take back this authorization at any time by contacting the Feeding My Baby study by phone at XXX-XXX-XXXX or in writing at [Address]. The Feeding My Baby study would not seek any more records about you or your child, but would still use any records that had already been released.
By signing this document, you are authorizing the hospital or medical facility where you gave birth, and your child’s doctor, to release your health information to Westat for this research. The health records are for care provided only during the study period of November 1, 2012, to April 1, 2016.
I am voluntarily giving permission for my medical records and my child’s medical records, as described above, to be released to Westat for the Feeding My Baby Study.
Patient’s Name (Mother):
Please Print Your Full Name
Date of Birth: ____ / ____ / ____
Month Day Year
Patient’s Name (Child):
Please Print Your Child’s Full Name
Date of Birth: ____ / ____ / ____
Month Day Year
Patient’s Signature (Mother):
Date Signed:
If the mother is a minor, her parent’s or guardian’s signature is also needed:
Parent or Guardian Signature (for Mother):
Signer’s Relationship to Mother:
Date signed:
A parent or guardian’s signature is needed for the child’s records:
Parent or Guardian Signature (for Child):
Signer’s Relationship to Child:
Date signed:
Follow up Interview Calendar for Core Participants
(Baby born on March 15, 2013 and enrolled at age <1month)
Thank you for your participation in the WIC Feeding My Baby study.
We will be contacting you for follow up interviews during the times listed below.
You will receive $20 for each interview.
You can also call us at [toll free number] during the interview times to complete the interview.
[DATES TO BE DETERMINED AFTER OMB APPROVAL]
First Follow up Interview |
|
Second Follow up Interview |
|
Third Follow up interview |
|
Fourth Follow up Interview |
|
Fifth Follow up Interview |
|
Sixth Follow up Interview |
|
Seventh Follow up Interview |
|
Eighth Follow up Interview |
|
Ninth Follow up Interview |
|
Final Follow up Interview |
|
Please let us know of any changes in your address or phone number by contacting your Study Liaison [STUDY LIASIONNAME] at [STUDY LIASION PHONE NUMBER AND EMAIL ADDRESS] or[toll-free number]
Follow up Interview Calendar for Supplemental Participants
(Baby born on March 15, 2013 and enrolled at age <1month)
Thank you for your participation in the WIC Feeding My Baby study.
We will be contacting you for follow up interviews during the times listed below.
You will receive $20 for each interview.
You can also call us at [toll free number] during the interview times to complete the interview.
[DATES TO BE DETERMINED AFTER OMB APPROVAL]
First Follow up Interview |
|
Second Follow up Interview |
|
Third Follow up Interview |
|
Final Follow up Interview |
|
Please let us know of any changes in your address or phone number by contacting your Study Liaison [STUDY LIASIONNAME] at [STUDY LIASION PHONE NUMBER AND EMAIL ADDRESS] or[toll-free number]
PICTURES OF THE MEASURING GUIDES


According
to the Paperwork Reduction Act of 1995, no persons are required to
respond to a collection of information unless it displays a valid
OMB number. The valid OMB control number for this information
collection is 0584-XXXX. The time required to complete this
information collection is estimated to average 3 minutes per
response, including the time for reviewing instructions, searching
existing data sources, gathering and maintaining the data needed,
and completing and reviewing the collection of information.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Janice Machado |
| File Modified | 0000-00-00 |
| File Created | 2021-01-29 |
© 2026 OMB.report | Privacy Policy