Voluntary Adverse Event Reporting via the SRP
Electronic Submission of FDA Adverse Event Reports and Other Safety Information Using the Electronic Submission Gateway and the Safety Reporting Portal
0910-0645 Livestock Food Reporting screen shots 2013 Sept 6
Voluntary Adverse Event Reporting via the SRP
OMB: 0910-0645
⚠️ Notice: This form may be outdated. More recent filings and information on OMB 0910-0645 can be found here:
Document [doc]
Download: doc | pdf
Electronic Submission of Food and Drug Administration Adverse Event Reports and Other Safety Information Using the Electronic Submission Gateway and the Safety Reporting Portal - Control No. 0910-0645
Sample Screen Shots for Livestock Food Reports
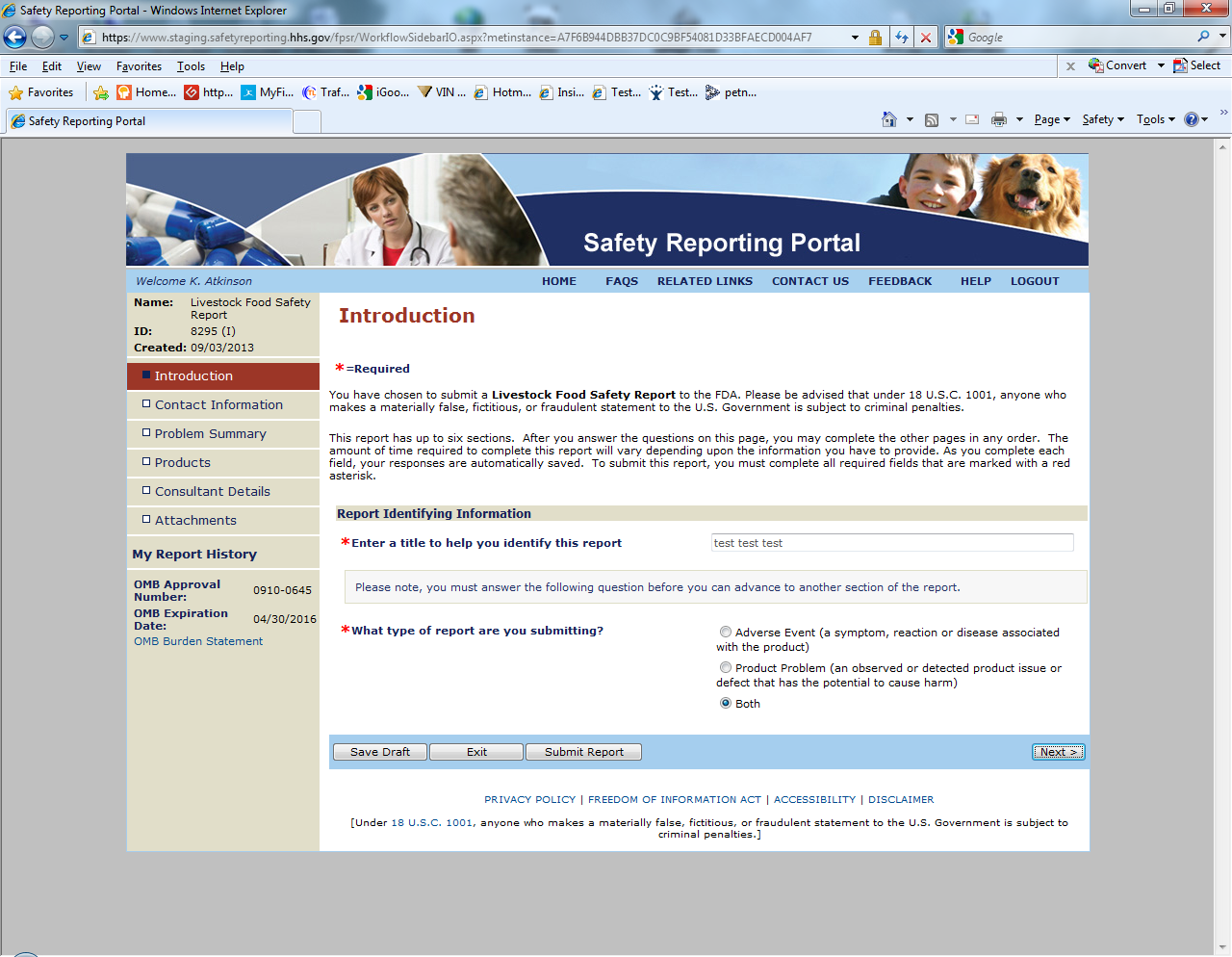
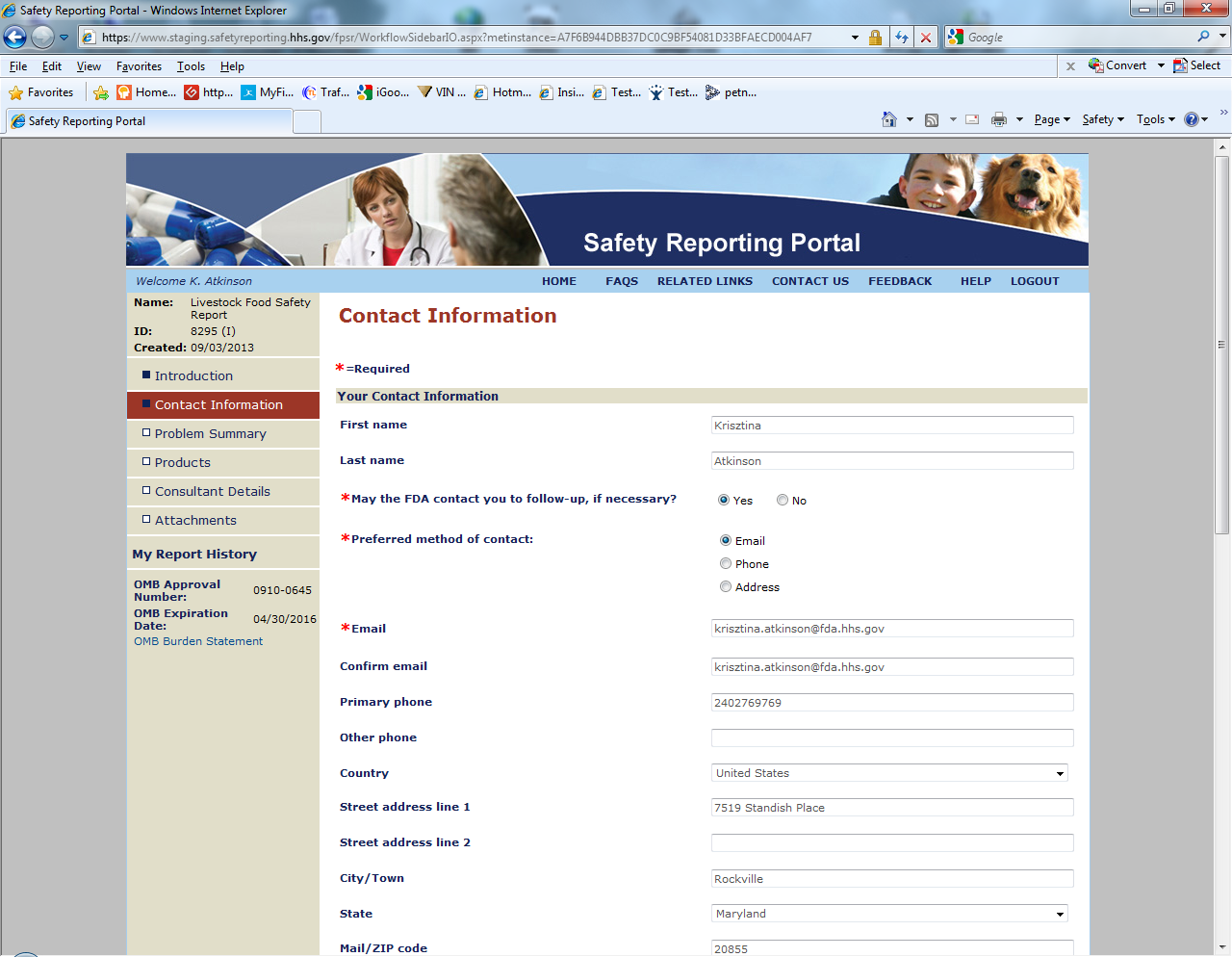
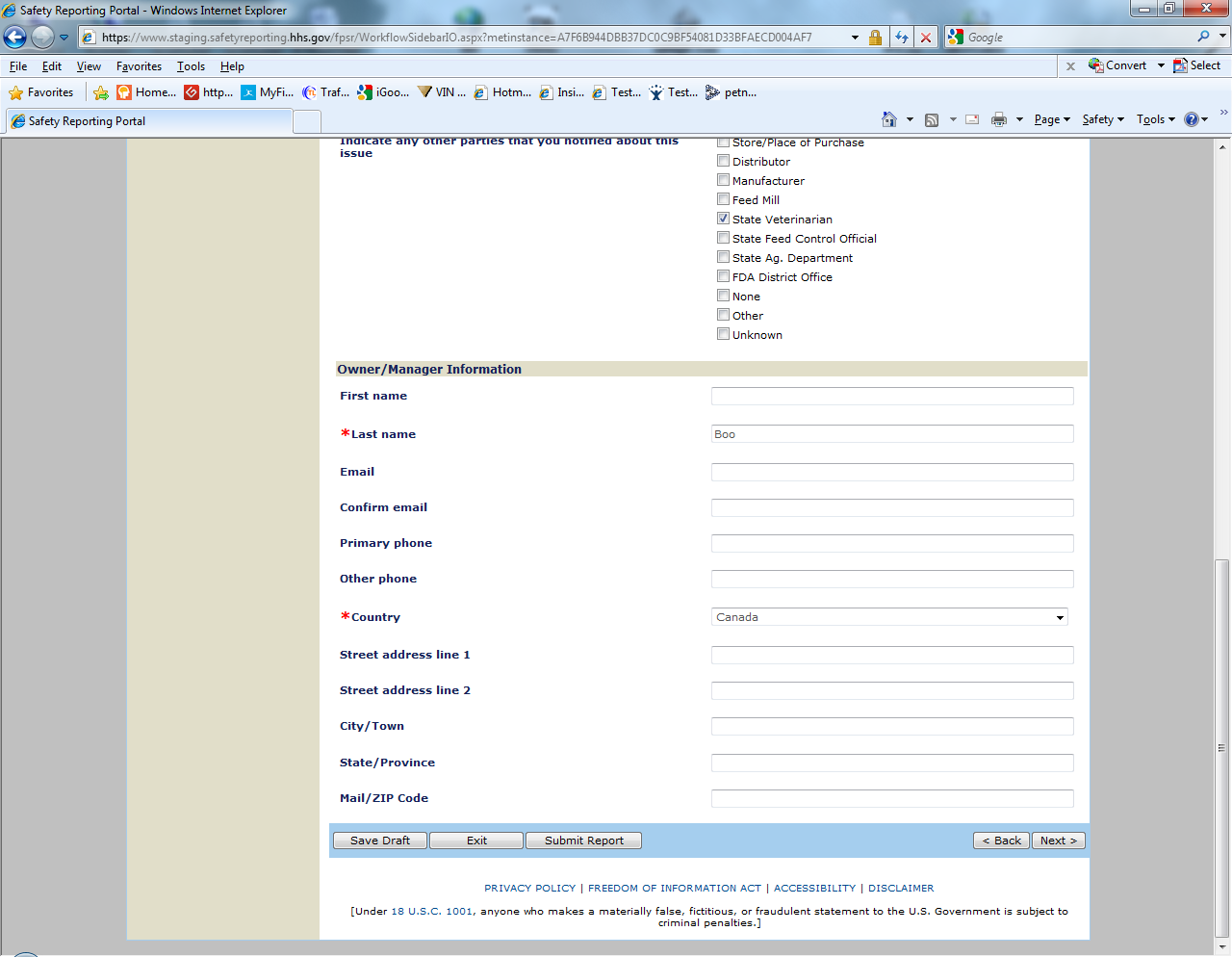
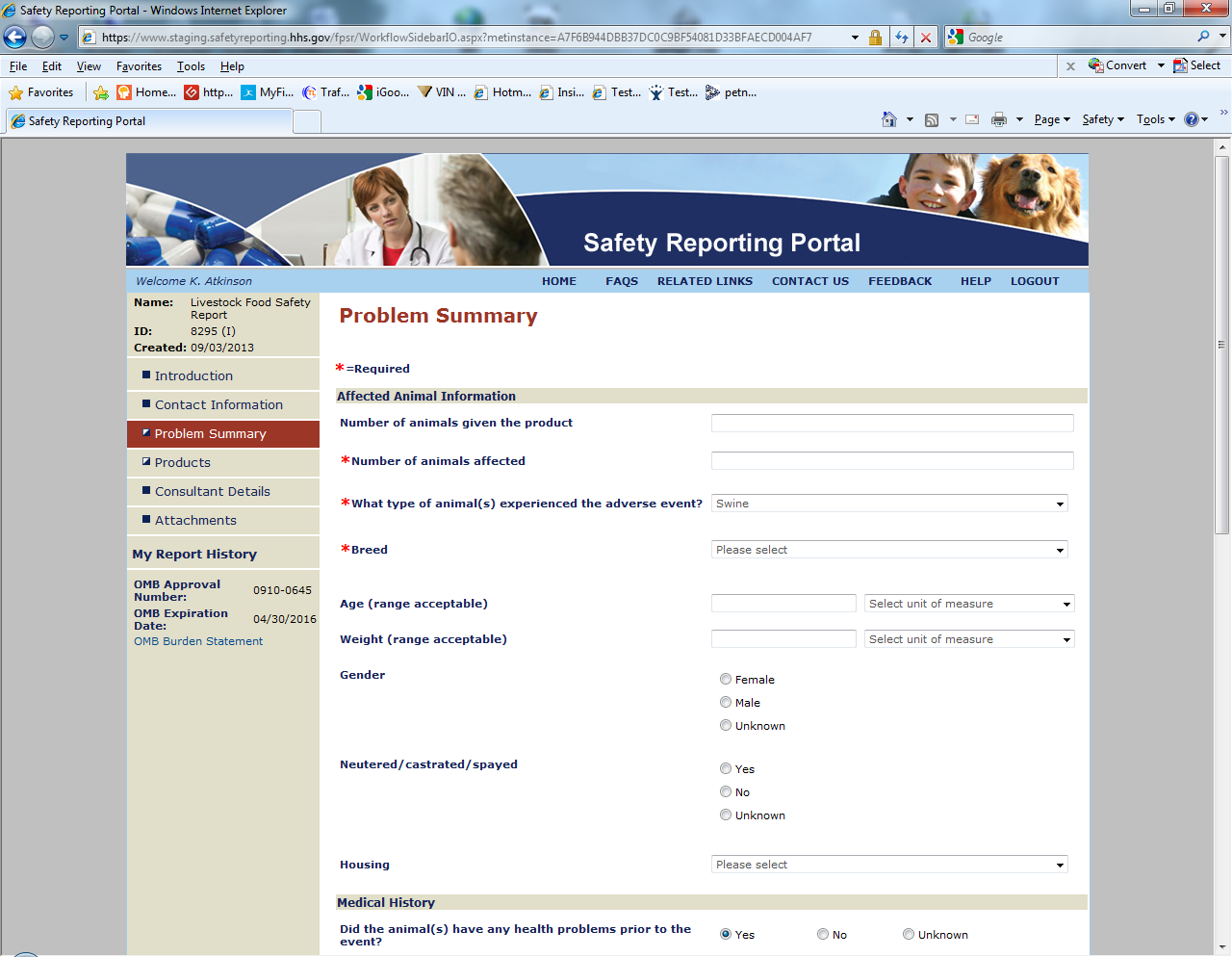
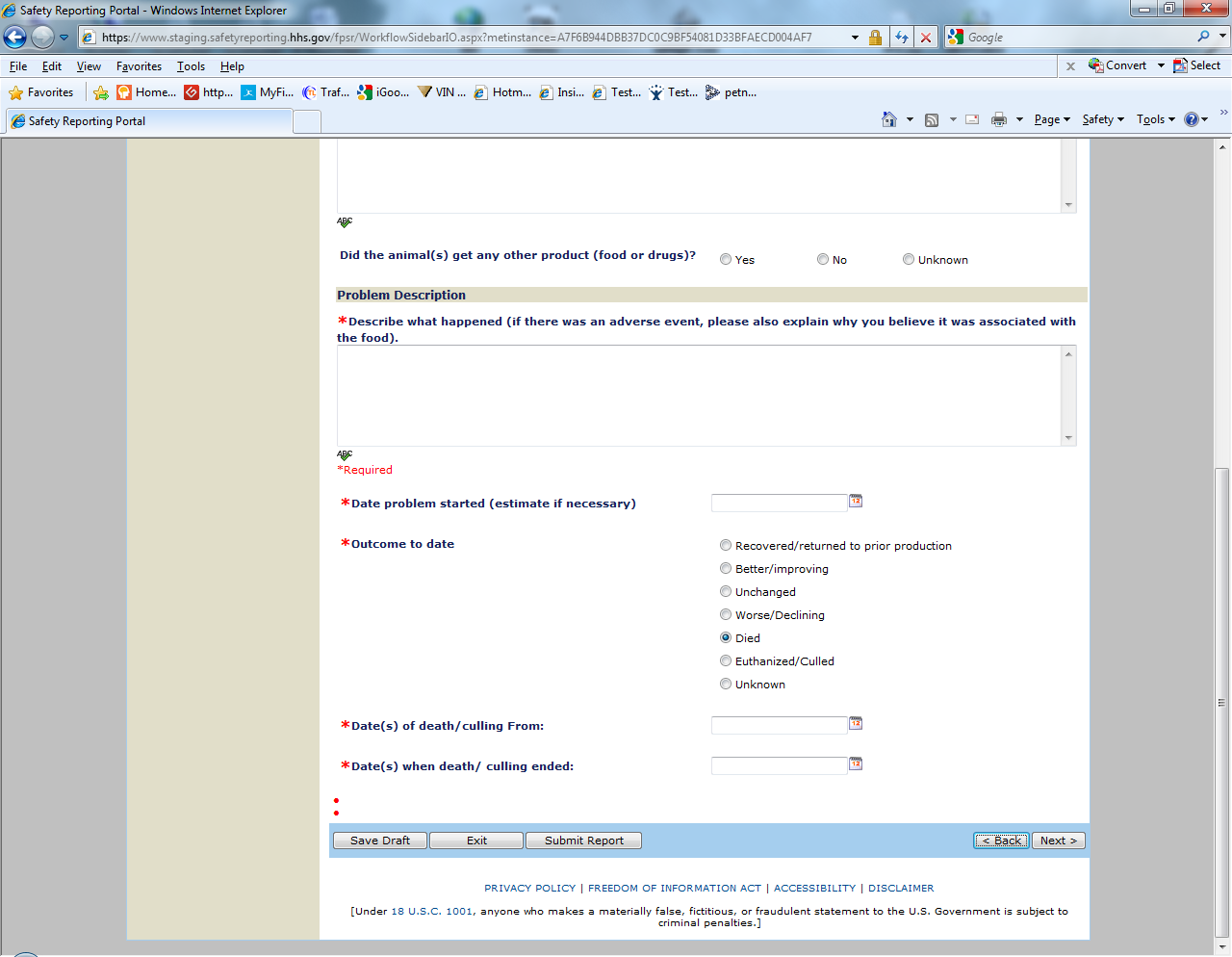
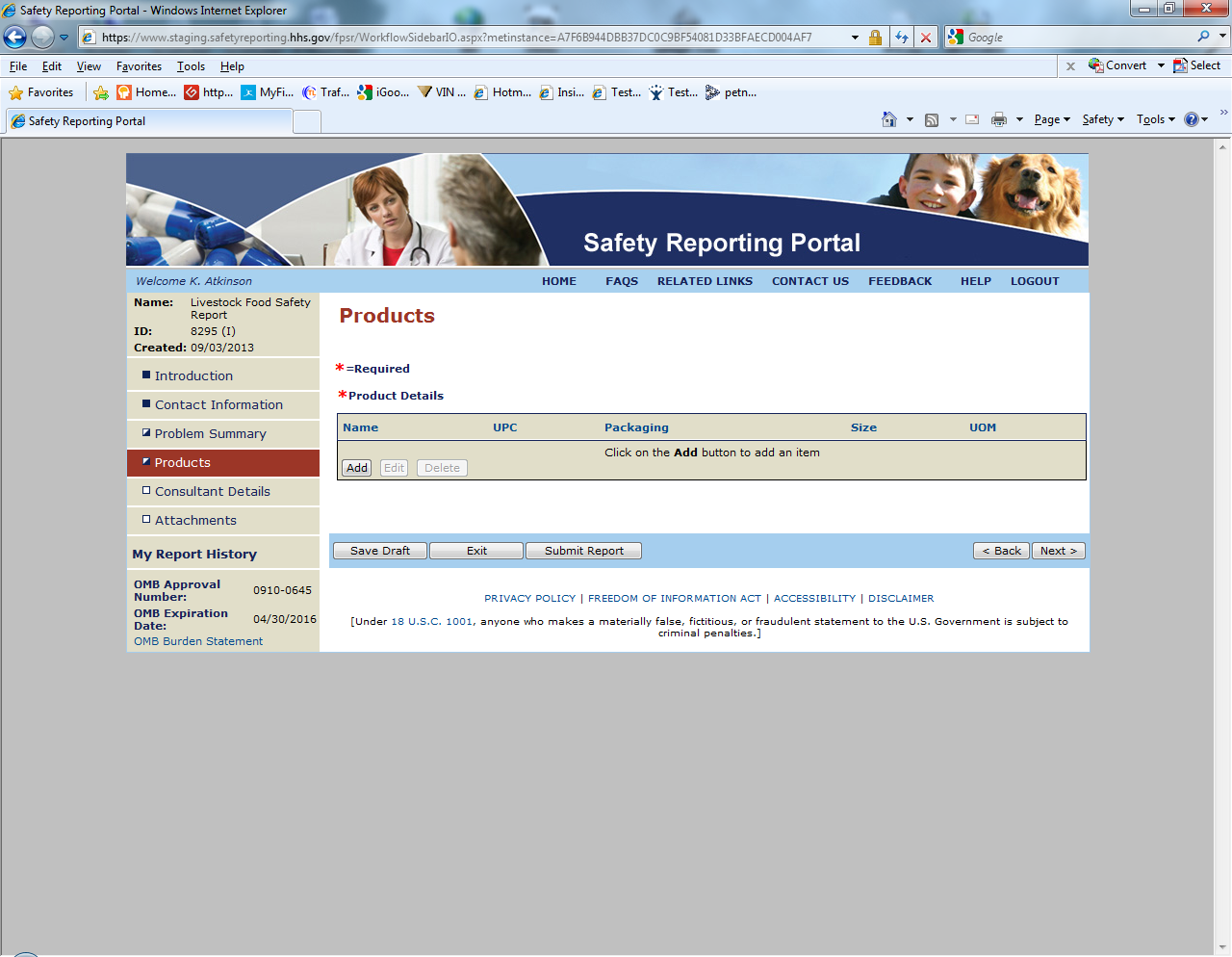
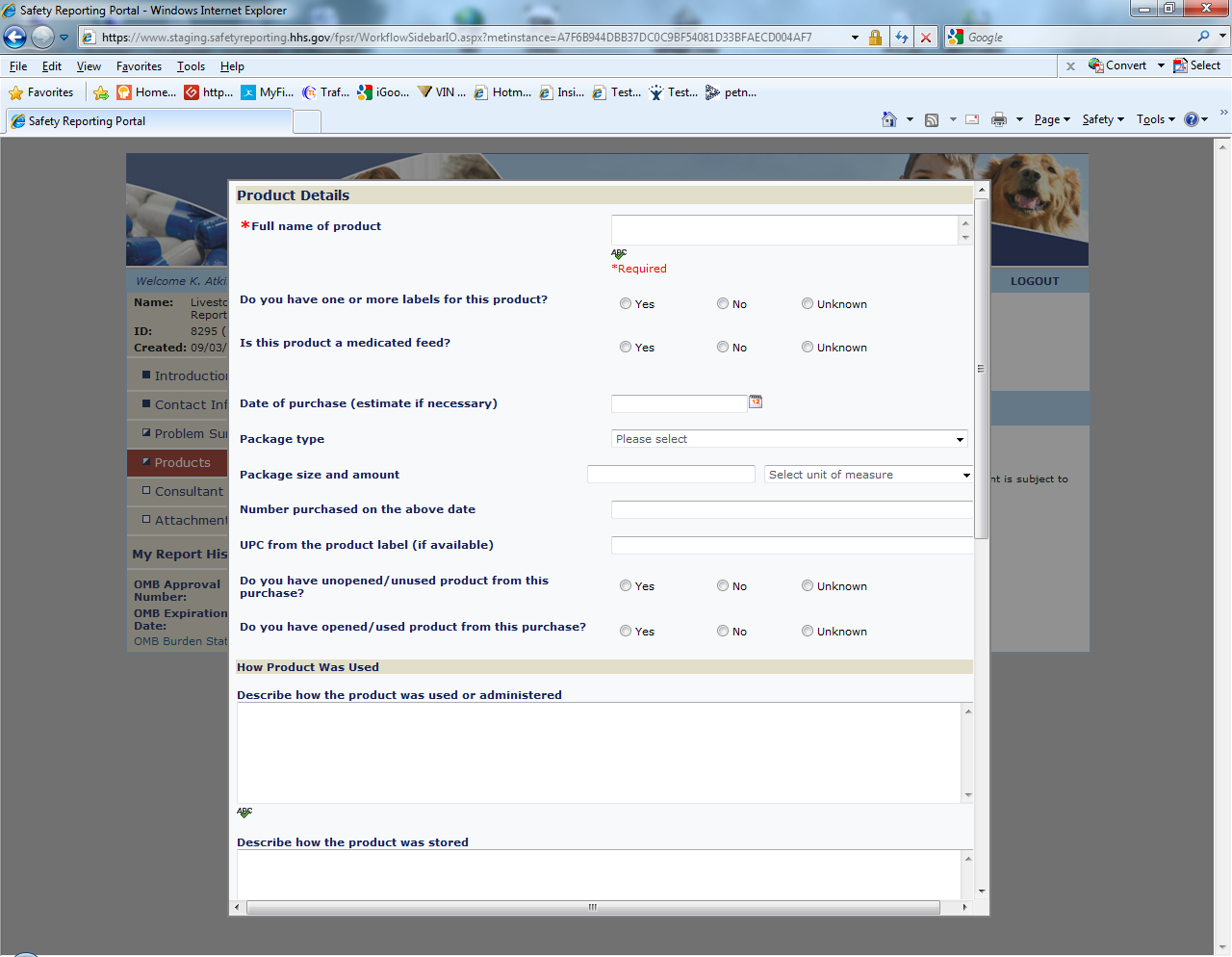
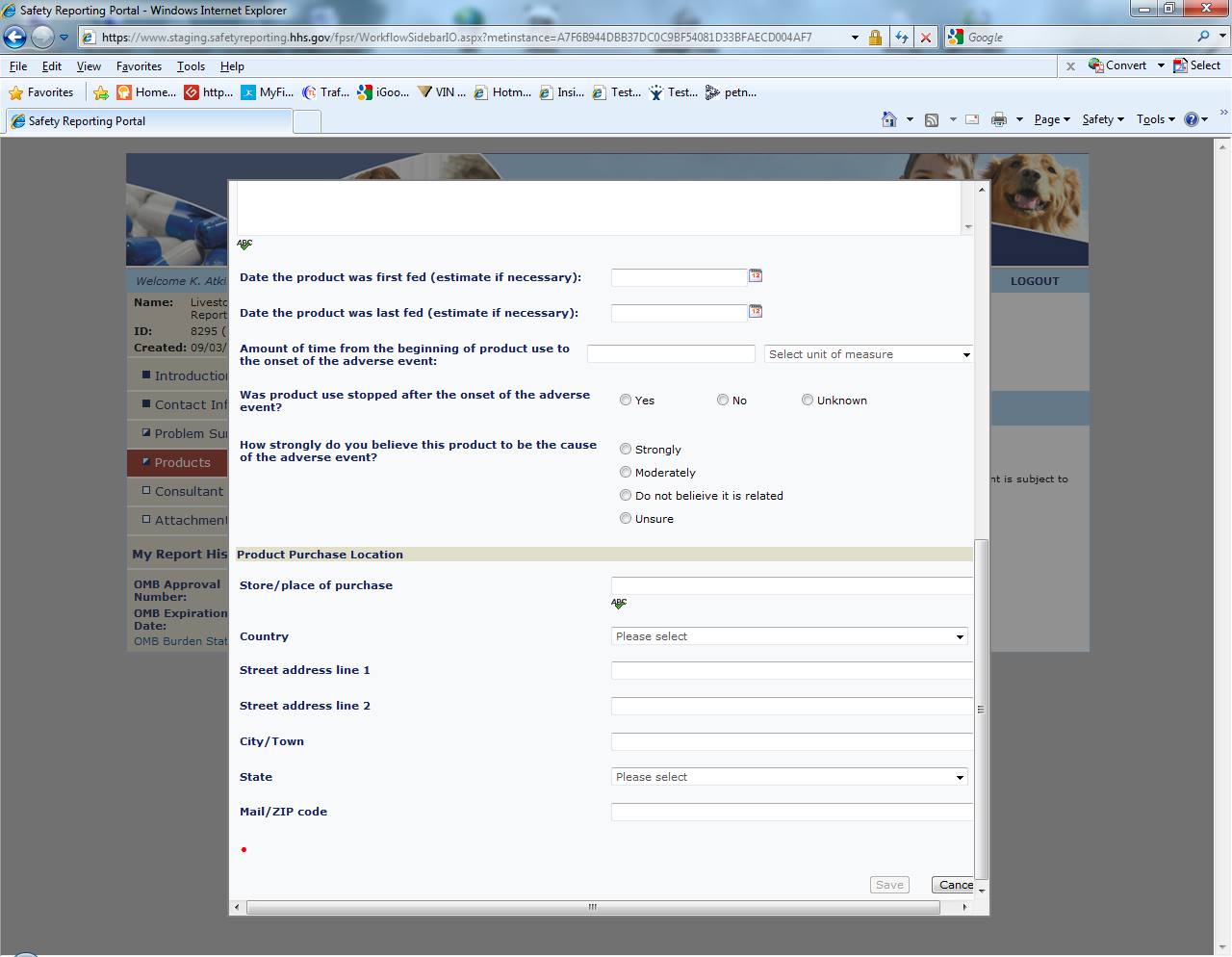
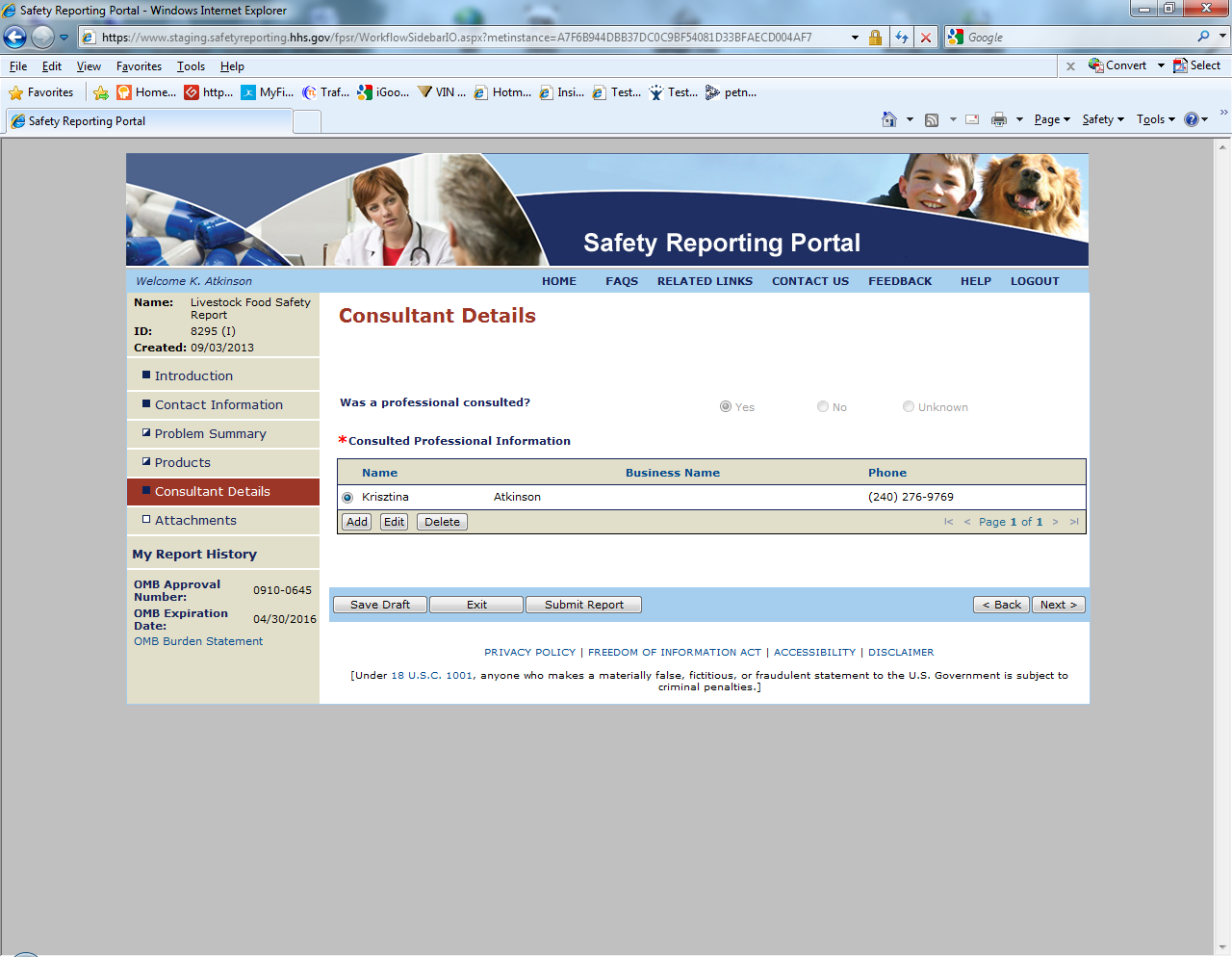
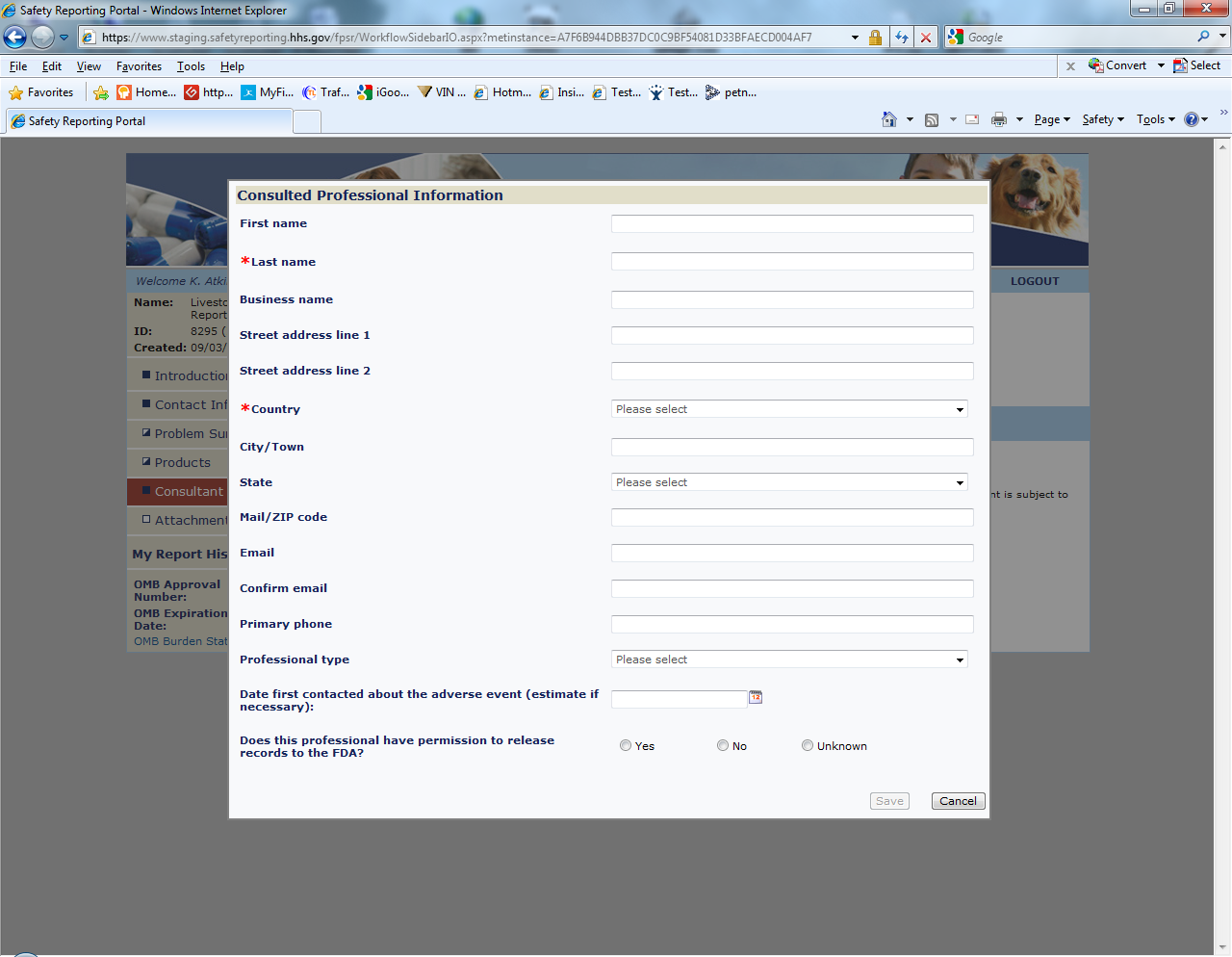
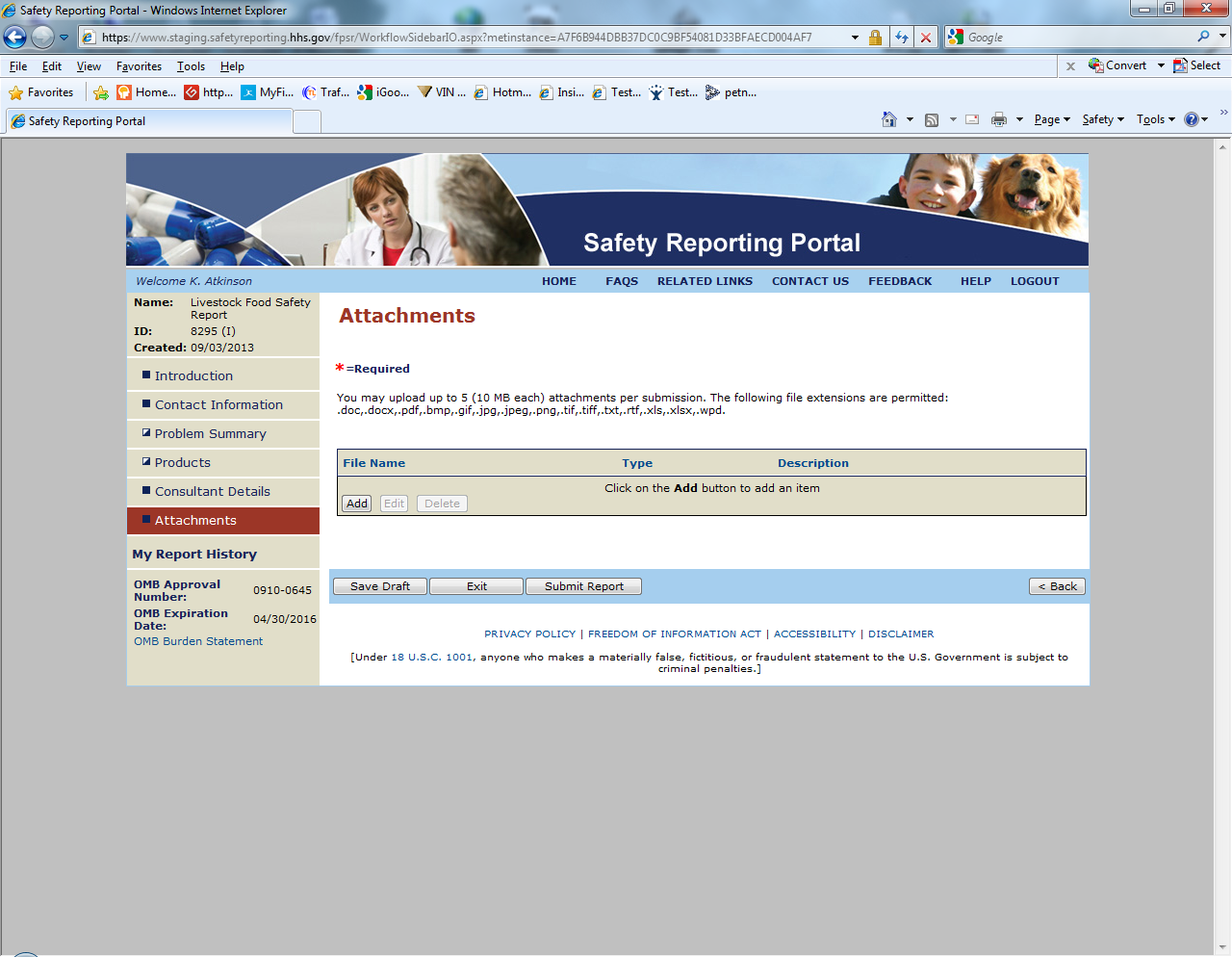
Sample Screen Shots for Livestock Food Reports
Page
| File Type | application/msword |
| Author | Atkinson, Krisztina Z |
| Last Modified By | Taylor, Anne |
| File Modified | 2013-09-06 |
| File Created | 2013-09-06 |
© 2026 OMB.report | Privacy Policy