Medicare Part C and Part D Data Validation (42 C.F.R. 422.516g and 423.514g) - (CMS-10305)
Medicare Part C and Part D Data Validation (42 C.F.R. 422.516g and 423.514g)
App4_Instructions for FDCF
Medicare Part C and Part D Data Validation (42 C.F.R. 422.516g and 423.514g) - (CMS-10305)
OMB: 0938-1115
Appendix 4: Instructions for Findings Data Collection Form, Version 4.0
Medicare Part C and Part D Reporting Requirements
Data Validation Procedure Manual
Instructions for Findings Data Collection Form for Data Validation Reviewers
Version 4.0
Prepared by:
Centers for Medicare & Medicaid Services
Center for Medicare
Medicare Drug Benefit and C & D Data Group
Table of Contents
1 Overview of Findings Data Collection Form and Evaluation Process 1
1.1 Record Findings at the Reporting Section Level or the Reporting Section’s Data Element Level 1
1.2 Structure of Findings Data Collection Form 2
2 Appendix: Data Elements for Part C and Part D Reporting Sections 3
List of Exhibits
1Overview of Findings Data Collection Form and Evaluation Process
The Findings Data Collection Form (FDCF) is a tool for reviewers to record their data validation findings for each contract included in the scope of the data validation review. The form mirrors the content of the Data Validation Standards document, but allows the reviewer to record notes, data sources referenced, and findings for the different standards and criteria specified for a given reporting section. An overview of this process is depicted in Exhibit 1, below.
Exhibit 1: Overview of Findings Data Collection Process and Pass/Not Pass Determination
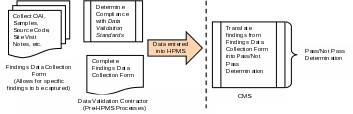

Using the FDCF, the reviewers will conduct the review and record reporting section-level, and in some cases data element-level, findings for each reporting section’s standards. Once the findings have been finalized the reviewer will share them with the organization and then submit the completed FDCF to CMS via the Health Plan Management System (HPMS). CMS will then evaluate the reporting section- or data element-level findings for each reporting section’s standards to derive an overall “Pass” or “Not Pass” determination.
Please note that all revisions since the 2013 data validation cycle are identified by underlined and/or strikethrough text.
1.1Record Findings at the Reporting Section Level or the Reporting Section’s Data Element Level
While most data validation standards and criteria are assessed at the reporting section-level (e.g., Standard 1, a review of source documents indicating that all source documents accurately capture required data fields and are properly documented), some are assessed at the data element-level (e.g., Standard 2.e examines each data element for compliance with reporting section criteria). Depending on the level of assessment for each standard and criteria, reviewers will record results in the FDCF at the reporting section-level or at the reporting section’s data element-level.
The standards and criteria that involve data element-level reviews are Standards 2.e and 3.a, specifically, as they assess the accuracy of reported results that may vary across data elements reported by the organization. Reviewers must refer to the reporting section criteria for Standard 2.e in their evaluation.
1.2Structure of Findings Data Collection Form
Each Part C and Part D reporting section’s FDCF is included in a corresponding worksheet within the overall FDCF Microsoft Excel file. The content in each reporting section’s form mirrors the data validation standards and includes space for the reviewer to record data sources, review results, and findings for a given reporting section. Reviewers should only complete areas displayed in white for data sources, review results, and findings. Areas displayed in grey are not applicable and should not be completed. In the "Data Sources and Review Results:" column, the reviewer will enter the review results and/or data sources used for each standard or sub-standard. In the adjacent "Findings" column, select "Y" if the requirements for the standard or sub-standard have been completely met. Select “N” if any requirement for the standard or sub-standard has not been met. The reviewers can also quickly reference the appropriate data element details provided in Section 2.
2Appendix: Data Elements for Part C and Part D Reporting Sections
Data elements have been updated to reflect their data element names as they appear in the Part C and Part D Reporting Requirements Technical Specifications, rather than the supporting data element definitions or descriptions.
2.1Part C Reporting Section Data Elements
2.1.1Serious Reportable Adverse Events (SRAEs)
Element Number |
Data
Elements for Serious Reportable Adverse Events (SRAEs) Reporting
Section |
3.1 |
Number of total surgeries |
3.2 |
Number of surgeries on wrong body part |
3.3 |
Number of surgeries on wrong patient |
3.4 |
Number of wrong surgical procedures on a patient |
3.5 |
Number of surgeries with post-operative death in normal health patient |
3.6 |
Number of surgeries with foreign object left in patient after surgery |
3.7 |
Number of Air Embolism events |
3.8 |
Number of Blood Incompatibility events |
3.9 |
Number of Stage III & IV Pressure Ulcers |
3.10 |
Number of fractures |
3.11 |
Number of dislocations |
3.12 |
Number of intracranial injuries |
3.13 |
Number of crushing injuries |
3.14 |
Number of burns |
3.15 |
Number of Vascular Catheter-Associated Infections |
3.16 |
Number of Catheter-Associated UTIs |
3.17 |
Number of Manifestations of Poor Glycemic Control |
3.18 |
Number of SSI (Mediastinitis) after CABG |
3.19 |
Number of SSI after certain Orthopedic Procedures |
3.20 |
Number of SSI following Bariatric Surgery for Obesity |
3.21 |
Number of DVT and pulmonary embolism following certain orthopedic procedures |
2.1.2Grievances (Part C)
Element Number |
Data Elements for Grievances (Part C) Reporting Section |
5.1 |
Number of Grievances for Fraud |
5.2 |
Number of Grievances for Enrollment/Disenrollment |
5.3 |
Number of Grievances for Benefit Package |
5.4 |
Number of Grievances for Access |
5.5 |
Number of Grievances for Marketing |
5.6 |
Number of Grievances for Customer Service |
5.7 |
Number of Grievances for Privacy Issues |
5.8 |
Number of Grievances for Quality of Care |
5.9 |
Number of Grievances for Appeals |
5.10 |
Number of Grievances for Other |
5.11 |
Number of Grievances for Enrollment/ Disenrollment for which the Sponsor provided timely notification of its decision |
5.12 |
Number of Grievances for Benefit Package for which the Sponsor provided timely notification of its decision |
5.13 |
Number of Grievances for Access for which the Sponsor provided timely notification of its decision |
5.14 |
Number of Grievances for Marketing for which the Sponsor provided timely notification of its decision |
5.15 |
Number of Grievances for Customer Service for which the Sponsor provided timely notification of its decision |
5.16 |
Number of Grievances for Quality of Care for which the Sponsor provided timely notification of its decision |
5.17 |
Number of Grievances for Appeals for which the Sponsor provided timely notification of its decision |
5.18 |
Number of Grievances for Other for which the Sponsor provided timely notification of its decision |
2.1.3Organization Determinations/Reconsiderations
Element Number |
Data Elements for Organization Determinations/ Reconsiderations Reporting Section |
6.1 |
Number of Organization Determinations – Fully Favorable |
6.2 |
Number of Organization Determinations – Partially Favorable |
6.3 |
Number of Organization Determinations – Adverse |
6.4 |
Number of Reconsiderations – Fully Favorable |
6.5 |
Number of Reconsiderations – Partially Favorable |
6.6 |
Number of Reconsiderations – Adverse |
2.1.4Special Needs Plans (SNPs) Care Management
Element Number |
Data Elements for Special Needs Plans (SNPs) Care Management Reporting Section |
13.1 |
Number of new enrollees |
13.2 |
Number of enrollees eligible for an annual reassessment |
13.3 |
Number of initial assessments performed on new enrollees during reporting period |
13.4 |
Number of annual reassessments performed on enrollees eligible for a reassessment |
2.2Part D Reporting Section Data Elements
2.2.1Medication Therapy Management Programs
Field Names for Medication Therapy Management Programs Reporting Section (Data Upload) |
Contract Number |
HICN or RRB Number |
Beneficiary First Name |
Beneficiary Middle Initial |
Beneficiary Last Name |
Beneficiary Date of Birth |
Met the specified targeting criteria per CMS – Part D requirements |
Long Term Care (LTC) facility resident |
Beneficiary identified as cognitively impaired |
Date of MTM program enrollment |
Date met the specified targeting criteria per CMS – Part D requirements |
Date of MTM program opt-out, if applicable |
Reason participant opted-out of MTM program (Death; Disenrollment from Plan; Request by Beneficiary; or Other). Required if Date of MTM program opt-out is applicable. |
Offered Annual Comprehensive Medication Review (CMR) |
If Offered a CMR, date of (initial) offer |
Received Annual CMR with written summary in CMS standardized format |
Number of CMRs received with written summary in CMS standardized format |
Date(s) of CMR(s) with written summary in CMS standardized format |
Date(s) of CMR(s) with written summary in CMS standardized format, second date of CMR |
Date(s) of CMR(s) with written summary in CMS standardized format, third date of CMR |
Date(s) of CMR(s) with written summary in CMS standardized format, fourth date of CMR |
Date(s) of CMR(s) with written summary in CMS standardized format, fifth date of CMR |
Method of delivery for the annual CMR |
Qualified provider who performed the initial CMR |
Recipient of CMR |
Number of targeted medication reviews |
Number of drug therapy problem recommendations made to prescriber(s) as a result of MTM services |
Number of drug therapy problem resolutions made as a result of MTM recommendations |
2.2.2Grievances (Part D)
Element Number |
Data Elements for Grievances (Part D) Reporting Section |
A |
Total number of Enrollment, Plan Benefits, or Pharmacy Access Grievances |
B |
Number of Enrollment, Plan Benefits, or Pharmacy Access Grievances for which the Sponsor provided timely notification of its decision |
C |
Total number of Customer Service Grievances |
D |
Number of Customer Service Grievances for which the Sponsor provided timely notification of its decision |
E |
Total number of Coverage determinations/Exceptions and Redeterminations process (e.g. untimely decisions) Grievances |
F |
Number of Coverage determinations/Exceptions and Redeterminations process (e.g. untimely decisions) Grievances for which the Sponsor provided timely notification of its decision |
G |
Total number of Grievances related to CMS issues |
H |
Number of Grievances related to CMS issues for which the Sponsor provided timely notification of its decision |
I |
Total number of Other Grievances |
J |
Number of Other Grievances for which the Sponsor provided timely notification of its decision |
2.2.3Coverage Determinations and Exceptions
Element Number |
Data Elements for Coverage Determinations and Exceptions Reporting Section |
A |
Number of pharmacy transactions |
B |
Number of pharmacy transactions rejected due to non-formulary status |
C |
Number of pharmacy transactions rejected due to prior authorization (PA) requirements |
D |
Number of pharmacy transactions rejected due to step therapy requirements |
E |
Number of pharmacy transactions rejected due to quantity limits (QL) requirements based on CMS approved formulary |
F |
Indicate if the plan had high cost edits for compounds in place during the time period above |
G |
If yes to element F, indicate the cost threshold used. |
H |
Indicate if the plan had high cost edits for non-compounds in place in the reporting period |
I |
If yes to element H, indicate the cost threshold used |
J |
Number of claims rejected due to high cost edits for compounds |
K |
Number of claims rejected due to high edits for non-compounds |
L |
Total
number of PA |
M |
Number of timely PA decisions in the reporting period |
N |
Number of favorable PA decisions (PA requirements satisfied) in the reporting period |
O |
Number of decisions for PA exceptions made in the reporting period |
P |
Number of timely PA exception decisions in the reporting period |
Q |
Number
of favorable PA exception |
R |
Number of decisions for exceptions to step therapy requirements made in the reporting period |
S |
Number of timely step therapy exception decisions in the reporting period |
T |
Number of favorable step therapy exception decisions in the reporting period |
U |
Number of decisions for exceptions to quantity limits (QL) requirements made in the reporting period |
V |
Number of timely QL exception decisions in the reporting period |
W |
Number of favorable QL exception decisions in the reporting period |
X |
Total number of decisions for tier exceptions made in the reporting period |
Y |
Number of timely tier exception decisions in the reporting period |
Z |
Number
of favorable tier exception |
AA |
Total number of decisions for formulary exceptions made in the reporting period |
BB |
Number of timely formulary exception decisions in the reporting period |
CC |
Number
of favorable formulary exception |
2.2.4Redeterminations
Element Number |
Data Elements for Appeals Reporting Section |
A |
Number of Redeterminations made in the reporting period |
B |
Number of Redeterminations made within required timeframes |
C |
Number of partially favorable Redeterminations |
D |
Number of fully favorable Redeterminations |
2.2.5Long-Term Care Utilization
Element Number |
Data Elements for Long-Term Care Utilization Reporting Section |
A |
Number of network LTC pharmacies |
B |
Number of network retail pharmacies |
C |
Number of beneficiaries in LTC facilities for whom Part D drugs have been provided under the contract |
D |
For
each network LTC pharmacy in the service area: |
E |
In
aggregate, for all retail pharmacies in the service area: |
| File Type | application/msword |
| File Title | AppJ_Instructions for FDCFV4 |
| Subject | Instructions for Findings Data Collection Form for Data Validation Contractors |
| Author | CMS |
| Last Modified By | Terry Lied |
| File Modified | 2013-11-13 |
| File Created | 2013-11-13 |
© 2026 OMB.report | Privacy Policy