Coordination of Benefits between Part D Plans and Other Prescription Coverage Providers (CMS-10171)
Coordination of Benefits between Part D Plans and Other Prescription Coverage Providers
PDBM-Chapter 14 08202013
Coordination of Benefits between Part D Plans and Other Prescription Coverage Providers (CMS-10171)
OMB: 0938-0978
CMS Manual System |
Department of Health & Human Services (DHHS) |
|
Pub. 100-18 Medicare Prescription Drug Benefit Manual |
Centers for Medicare & Medicaid Services (CMS) |
|
Transmittal 17 |
|
Date: August 23, 2013 |
SUBJECT: Chapter 14-Coordination of Benefits
I. SUMMARY OF CHANGES: Updated information reflects changes in statute and regulation to Part D coordination of benefits requirements, new policy on beneficiary cash purchases and direct member reimbursement, updated procedures related to CMS systems changes, SPAPs/ADAPs and automated TrOOP balance transfer, and updates to NCPDP electronic transaction standards.
NEW/REVISED MATERIAL - EFFECTIVE DATE*: June 7, 2010
IMPLEMENTATION DATE: January 1, 2011, unless otherwise specified.
Disclaimer for manual changes only: The revision date and transmittal number apply to the red italicized material only. Any other material was previously posted to http://www.cms.hhs.gov/PrescriptionDrugCovContra/12_PartDManuals.asp#TopOfPage or http://www.cms.hhs.gov/manuals/ and disseminated via the Health Plan Management System (HPMS). However, if this revision contains a table of contents, you will receive the new/revised information only, and not the entire table of contents.
NOTE: The Medicare Prescription Drug Benefit Manual can be accessed at http://www.cms.hhs.gov/PrescriptionDrugCovContra/12_PartDManuals.asp#TopOfPage or http://www.cms.hhs.gov/manuals/. All revisions to Pub. 100-18 will be issued via HPMS.
II. CHANGES IN MANUAL INSTRUCTIONS: (N/A if manual not updated.)
(R = REVISED, N = NEW, D = DELETED)
R/N/D |
CHAPTER/SECTION/SUBSECTION/TITLE |
R |
14/Table of Contents |
N |
14/Index of Acronyms |
R |
14/20/Overview |
R |
14/30/CMS Requirements |
R |
14/30.1/Enrollment File Sharing |
R |
14/30.2/Validation of Information about Other Payers |
R |
14/30.3/Establishing the Order of Payment for Part D Coordination of Benefits (COB) |
R |
14/30.4/Contracting with a Part D Transaction Facilitator |
R |
14/30.4.1/Part D Transaction Facilitation Process |
R |
14/30.4.2/Enhancements to E1 Transactions |
R |
14/30.4.3/Real-time Versus Batch Processing |
R |
14/30.4.4/Enhancements to Nx Transactions |
R |
14/30.4.5/TrOOP Accounting |
R |
14/30.5/Assessment of COB User Fees |
R |
14/40.1/Providing Information to Sponsors on Other Coverage |
R |
14/40.2/Using On-line Processing |
R |
14/40.3/Submitting Documentation for Off-line Processing on a Timely Basis |
R |
14/50.1/Providing 4Rx Data on Primary Coverage |
R |
14/50.2/Notifying Beneficiaries Regarding Other Prescription Drug Coverage on File and Transmitting Updated Information to CMS |
R |
14/50.3/Connecting to Systems Supporting COB |
R |
14/50.4/Processing Claims and Tracking TrOOP |
R |
14/50.4.1/Receiving an Nx Transaction, Without Supplemental Payer on File |
R |
14/50.4.2/Beneficiary Cash Purchases |
N |
14/50.4.3/Direct Member Reimbursement |
R |
14/50.5/Use of Standardized Technology |
R |
14/50.5.1/Primary Payer Use of Fields to Support COB |
R |
14/50.6/Accepting Payment of Premiums from Other Payers |
R |
14/50.7/Coordinating Payment of a Lump Sum for Supplemental Coverage |
R |
14/50.7.1/Lump Sum Per Capita Approach |
D |
14/50.7.2/The Non-Risk-Based Lump Sum Payment with Claims Reconciliation Approach |
R |
14/50.8/Transferring TrOOP Balance When a Beneficiary Changes Part D Sponsors |
N |
14/50.8.1/Automated TrOOP Balance Transfer Process |
N |
14/50.8.2/TrOOP Balance Transfer When CMS Terminates a Part D Sponsor Contract |
R |
14/50.9/Special Transition Period for Retroactive Enrollment Situations |
R |
14/50.10/Sharing Formulary Information with Other Payers |
R |
14/50.11/Sharing Claims Data |
R |
14/50.12/Applying Medicare Secondary Payer (MSP) Requirements |
N |
14/50.12.1/Workers’ Compensation |
N |
14/50.12.2/Flexible Savings Accounts (FSAs), Health Savings Accounts (HSAs), Archer Medicare Savings Accounts (MSAs), and Health Reimbursement Accounts (HRAs) |
R |
14/50.13/Executing Business Associate Agreement (BAA) with Part D Transaction Facilitator |
D |
14/50.13.1/Worker’s Compensation |
D |
14/50.13.2/Flexible Savings Accounts (FSAs), Health Savings Accounts (HSAs), Archer Medicare Savings Accounts (MSAs), and Health Reimbursement Accounts (HRAs) |
R |
14/50.14/Payment Reconciliation |
N |
14/50.14.1/Plan-to-Plan Reconciliation During Transition Period |
N |
14/50.14.2/Other CMS-Defined Reconciliation Processes |
N |
14/50.14.3/Retroactive Claims Adjustments |
N |
14/50.14.4/Resolution Directly with Other Payers |
N |
14/50.14.5/Re-adjudication Versus Pharmacy Reprocessing |
N |
14/50.14.6/Timeframes for Claims Filing |
D |
14/50.15/Payment Reconciliation |
D |
14/50.15.1/Plan-to-Plan Reconciliation During Transition Periods |
D |
14/50.15.2/Other CMS-Defined Reconciliation Processes |
D |
14/50.15.3/Retroactive Claims Adjustments and Resolution Directly with Other Payers |
D |
14/50.15.4/Re-adjudication Versus Pharmacy Reprocessing |
D |
14/50.15.5/Claims Filing Timeframes |
R |
14/60.1/Reporting the Existence of Prescription Drug Coverage Provided to Enrollees |
R |
14/60.2/Obtaining and Reporting Rx Identifiers |
R |
14/60.3/Supplying Claims Information When a Supplemental Payment Is Made |
R |
14/60.4/Coordinating with Part D Sponsors for Payment of Premiums |
R |
14/60.5/Following MSP Laws and Order of Payment Standards |
R |
14/Appendix A/Transaction Facilitation Process |
R |
14/Appendix B/COB-related Web Sites |
R |
14/Appendix C/Part D Sponsor Guidance—Automated TrOOP Balance Transfer |
R |
14/Appendix D/Automated TrOOP Balance Transfer Implementation Guidance-- PACE Addendum |
R |
14/Appendix E/Issues for Other Entities Providing Prescription Drug Coverage |
R |
14/Appendix F/Part D Requirements Waived for PACE Organizations |
N |
14/Appendix G/NCPDP White Paper- Overview of the Medicare Part D Prescription Drug Coordination of Benefits Process, Section 4 |
N |
14/Appendix H/Glossary |
III. FUNDING: No additional funding will be provided by CMS; contractor activities are to be carried out within the annual bid process.
IV. ATTACHMENTS:
|
Business Requirements |
X |
Manual Instruction |
|
Confidential Requirements |
|
One-Time Notification |
|
One-Time Notification -Confidential |
|
Recurring Update Notification |
*Unless otherwise specified, the effective date is the date of service.
Medicare Prescription Drug Benefit Manual
Chapter 14 - Coordination of Benefits
Table of Contents
(Rev. 17, 08-23-13)
30.2 – Validation of Information about Other Payers
30.4 - Contracting with a Part D Transaction Facilitator
30.4.1 – Part D Transaction Facilitation Process
50.4.3 – Direct Member Reimbursement
50.5 – Use of Standardized Technology
50.6 – Accepting Payment of Premiums from Other Payers
50.7.1 – Lump Sum Per Capita Approach
50.8 – Transferring TrOOP Balance When a Beneficiary Changes Part D Sponsors
50.8.1 – Automated TrOOP Balance Transfer Process
50.8.2 – TrOOP Balance Transfer When CMS Terminates a Part D Sponsor Contract
50.9 – Special Transition Period for Retroactive Enrollment Situations
50.10 – Sharing Formulary Information with Other Payers
50.12 – Applying Medicare Secondary Payer (MSP) Requirements
50.12.1 – Workers’ Compensation
50.13 – Executing Business Associate Agreement (BAA) with Part D Transaction Facilitator
50.14 – Payment Reconciliation
50.14.1 – Plan-to-Plan Reconciliation During Transition Periods
50.14.2 – Other CMS-Defined Reconciliation Processes
50.14.3 – Retroactive Claims Adjustments
50.14.4 – Resolution Directly with Other Payers
50.14.5 – Re-adjudication Versus Pharmacy Reprocessing
50.14.6 –Timeframes for Claims Filing
Appendix C – Part D Sponsor Guidance—Automated TrOOP Balance Transfer
(Rev.)
ACA-Affordable Care Act
ADAP-AIDS drug assistance program
AI/AN-American Indian/Alaskan Native
ATBT-automated TrOOP balance transfer
BAA-Business Associate Agreement
BIN-Bank Identification Number
BL-Black Lung
CHC-community health center
CMOP-consolidated mail outpatient pharmacy
COB-coordination of benefits
COBA-Coordination of Benefits Agreement
COBC-Coordination of Benefits contractor
CY-calendar year
DOB-date of birth
DSA-data sharing agreement
ECRS-Electronic Correspondence Referral System
EGHP-employer group health plan
EGWP-employer group waiver plan
EOB-Explanation of Benefits
FEHBP-Federal Employee Health Benefits Program
FFP-Federal Financial Participation
FIR-Financial Information Reporting
FPL-federal poverty level
FQHC-Federally Qualified Health Center
FSA-flexible savings accounts
GCDC-gross covered drug cost
HICN-health insurance claim number
HIPAA-Health Insurance Portability and Accountability Act
HPMS-Health Plan Management System
HRA-Health Reimbursement Accounts
HRSA-Health Resources and Services Administration
ICP- initial coverage period
IHS-Indian Health Service
I/T/U-Indian tribes and organizations and urban Indian organizations
LICS-low-income cost-sharing subsidy
LIS-low income subsidy
LTC-long-term care
MA-Medicare Advantage
MAPD- Medicare Advantage-Prescription Drug
MARx-Medicare Advantage-Prescription Drug system
MBD-Medicare Beneficiary Database
MMA-Medicare Modernization Act
MSA-Medicare Savings Accounts
MSP-Medicare Secondary Payer
MSPRC- Medicare as Secondary Payer Recovery Contractor
MTM-medication therapy management
NCPDP-National Council for Prescription Drug Programs
NCY-non-calendar year
NDC-National Drug Code
NDM-Network Data Mover
NET-newly eligible transition
Nx-reporting transaction
OIG-Office of the Inspector General
P2P-plan-to-plan
PACE-Program of All-Inclusive Care for the Elderly
PAP-patient assistance program
PBM-pharmacy benefit manager
PBP-plan benefit package
PCN-Processor Control Number
PCUG-Plan Communications User’s Guide
PDE-prescription drug event
PDP-prescription drug plan
PLRO-Patient Liability Reduction Due to Other Payer Amount
PO-PACE organization
POS-point of sale
PUF-Public Use File
RFQ-request for quote
RHC-rural health clinic
RxGRP-Group ID
RxID-Cardholder ID
SPAP-State Pharmaceutical Assistance Program
TBT-TrOOP balance transfer
TrOOP- true out-of-pocket
TRR-Transaction Reply Report
U&C-usual and customary
VAMC-VA Medical Center
VDSA-Voluntary Data Sharing Agreement
VHA-Veterans Health Administration
WCMSA-Workers’ Compensation Medicare Set-aside Arrangement
20 – Overview
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Part D sponsors are required to coordinate with State Pharmaceutical Assistance Programs (SPAPs) and other providers of prescription drug coverage with respect to the payment of premiums and coverage, as well as coverage supplementing the benefits available under Part D.1 The Medicare Modernization Act (MMA) specified that these coordination requirements must relate to the following elements: (1) enrollment file sharing; (2) claims processing and payment; (3) claims reconciliation reports; (4) application of the protection against high out-of-pocket expenditures by tracking true out-of-pocket (TrOOP) expenditures; and (5) other processes that CMS determines.
When a Medicare Part D enrollee has other prescription drug coverage, COB allows the plans that provide coverage for this same beneficiary to determine each of their payment responsibilities. This process is necessary in order to avoid duplication of payment and to prevent Medicare from paying primary when it is the secondary payer. While this is the principal purpose of COB within the contexts of Medicare Parts A and B, COB also serves an additional function within the Part D context: it provides the mechanism for support of the tracking and calculating of beneficiaries’ “true out-of-pocket” (TrOOP) expenditures, or “incurred costs” as defined in the MMA and CMS’ implementing regulations. Costs for covered Part D drugs are treated as “incurred” only if they were paid by the individual (or by another person, such as a family member, on behalf of the individual), paid by CMS on behalf of a low-income subsidy-eligible individual, or paid under a qualified SPAP as defined in CMS regulations. Costs do not count as “incurred” when: 1) no benefits are provided because of the application of either a formulary or the Medicare Secondary Payer (MSP) laws, or 2) when costs are reimbursed through insurance or otherwise, a group health plan, or similar third party arrangement. Therefore, only certain costs not paid for by the Part D sponsor count toward TrOOP. The Medicare Part D benefit parameters for the defined standard Part D benefit are updated annually and published in the Final Rate Announcement which is issued each April for the following year. The Part D benefit parameters are available in the Rate Announcements on the CMS Web site. See Appendix B for the specific Web address.
The MMA provided CMS with authority to impose user fees to defray the costs of Part D COB activities, as well as to retain a portion of those user fees to offset costs associated with the TrOOP facilitation (or Part D transaction) process. The MMA prohibits CMS from levying user fees on SPAPs, however. In CMS’ regulations, CMS clarifies that only Part D sponsors – not SPAPs or other payers – will be assessed user fees. However, although Part D sponsors may charge user fees to other payers for COB activities, these user fees must be reasonable and related to the Part D sponsors’ actual costs of COB with these entities. In addition, any user fees Part D sponsors charge other entities must specifically exclude those activities that are covered by the user fees CMS is collecting for COB. Thus, for example, Part D sponsors may not charge user fees for activities such as the costs of the claims transaction by supplemental payers (since Part D user fees funded by CMS are used in part for that purpose), but sponsors may charge for activities such as the exchange of claims data.
Section 1860D-23(a)(4) of the Social Security Act requires the Secretary, in establishing the requirements for coordination of benefits under Medicare Part D, to consult with State Pharmaceutical Assistance Programs, MA organizations, States, pharmaceutical benefit managers, employers, representatives of Part D eligible individuals, data processing experts, pharmacists, pharmaceutical manufacturers, and other experts. CMS has undertaken extensive consultation with these stakeholders actively participating with the National Council for Prescription Drug Programs (NCPDP) in developing with the industry Health Insurance Portability and Accountability Act of 1996 (HIPAA) standard processes for coordination of benefits.
Although this chapter provides guidance primarily for Part D sponsors, the various processes associated with COB involve interaction between multiple parties. For that reason, CMS provides detailed guidance regarding the COB requirements applicable to the various parties including beneficiaries, Part D sponsors, and other payers. In addition to the guidance contained in this chapter, NCPDP has created a white paper entitled, “Overview of the Medicare Part D Prescription Drug Coordination of Benefits (COB) Process.” This white paper provides an overview of the processes and entities associated with Part D COB, and includes recommendations for industry standard practices. Section 4 of the paper summarizes the COB requirements in the Social Security Act and Federal regulations and CMS’ implementing guidance. The guidance and recommendations in the subsequent sections of the white paper flow from CMS regulations and guidance. The document is available on the NCPDP Web site. See Appendix B for the specific Web address.
In Appendix A of this guidance, CMS provides an illustration of how the Part D transaction facilitation process works. Appendix B contains a list of Web sites relevant to COB and referenced in this chapter and Appendices C and D respectively include the automated TrOOP balance transfer guidance and the related addendum for PACE organizations. Appendix E provides detail on specific issues that may relate to (or be of particular interest to) other payers and entities with which Part D sponsors, per the requirements of 42 CFR 423.464(f), are required to coordinate, including SPAPs, Medicaid, VA, TRICARE, Indian Health Service and tribal health coverage, safety-net providers, patient assistance programs (PAPs), personal health savings vehicles, AIDS drug assistance programs (ADAPs), PACE plans, and Medicare Part B. Further guidance on systems requirements and technical details involved in the COB process has been issued in other communications and is included here by reference. In Appendix F, CMS addresses the applicability of COB to PACE requirements. Appendix G contains a copy of Section 4 of the NCPDP COB white paper referenced above, and Appendix H contains a glossary of terms.
30 – CMS Requirements
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
CMS leveraged its existing Medicare COB processes to facilitate COB under Part D. In addition, through the use of a Part D transaction facilitation process that uses an existing industry claims transactions set (described in further detail in section 30.4 of this chapter), CMS supports the tracking and calculation of enrollees’ TrOOP balances by Part D sponsors.
30.1 – Enrollment File Sharing
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Prior to the enactment of the mandatory insurer reporting provision of the Medicare, Medicaid, and SCHIP Extension Act of 2007 (Section 111 of P.L. 110-173), except for employers/union plans that are required by MSP-related law to report enrollment information on certain active employees, there was no requirement for other payers of health benefits to report their enrollment to CMS or the plans. The COB enrollment file sharing programs provide inherent incentives for other payers to coordinate drug benefits. Thus, many other payers voluntarily provide information regarding prescription drug coverage they offer that is either primary or supplemental to Part D.
The mandatory insurer reporting of MSP group health coverage requires the reporting of information about group health plan arrangements, and those provisions implemented July 1, 2010 require the reporting of information about liability insurance, no-fault insurance, and workers' compensation. Although these requirements are not specific to Part D, CMS encourages insurers providing prescription drug coverage to include this information in their mandatory reporting.
CMS coordinates benefits with other payers with respect to Part A and B coverage to reduce mistaken payments and administrative expenses that would otherwise be incurred by the Medicare program. The CMS COB contractor collects information on beneficiaries’ other coverage primarily through the use of data sharing agreements. Voluntary Data Sharing Agreements (VDSAs) and Coordination of Benefits Agreements (COBAs) that already existed were modified to include Part D information. CMS also created new types of agreements, such as those with SPAPs, ADAPs and PAPs, specifically to facilitate the exchange of Part D information. Collectively, VDSA, SPAP, ADAP, and PAP reporting programs are referred to as “data sharing agreement” – DSA – programs. To maintain consistency throughout all data sources and to expedite transactions, the DSA file submissions should include Rx Bank Identification Numbers (BINs) and Processor Control Numbers (PCNs) for payers whose payments count toward TrOOP (e.g., SPAPs and ADAPs) that are unique from the BINs and PCNs for payers whose payments do not apply to TrOOP (e.g., workers’ compensation and employer group health plans).
After the data sharing agreement is executed, the other payer sends the COB contractor a file of it enrollees. For Part D purposes, the COB contractor: 1) compares the list of the other payer’s enrollees to the current population of Medicare Part D enrollees; 2) captures and maintains the resulting matches and any information updates; and 3) transmits the matches/updates to the CMS Medicare Beneficiary Database (MBD). CMS sends this information as often as daily to the Part D transaction facilitator and the Part D sponsor for the sponsor’s enrollees. The data consist of a detail record for each enrollee whose other payer information is reported in the attachments to the detail record. Attachments to the detail record may include up to 20 primary records containing information on other payers that are primary to Part D, and up to 20 supplemental records containing information on payers that pay after Part D. The data elements that are included, if applicable, in the detail, primary, and supplemental records are reflected in tables below.
Table 30.1-1 COB File—Data Elements in Detail Record
-
Record Type
HICN/RRB Number
SSN
Date of Birth
Gender Code
Contract Number
Plan Benefit Package
Action Type
Table 30.1-2 COB File—Data Elements in Primary Record
Record Type
HICN/RRB Number
SSN
Date of Birth
Gender Code
RxID Number
RxGroup Number
RxBIN Number
RxPCN Number
Rx Plan Toll Free Number
Sequence Number
COB Source Code
MSP Reason (Entitlement Reason from COB)
Coverage Code
Insurer's Name
Insurer's Address-1
Insurer's Address-2
Insurer's City
Insurer's State
Insurer's ZIP Code
Insurer TIN
Individual Policy Number
Group Policy Number
Effective Date
Termination Date
Relationship Code
Payor ID
Person Code
Payer Order
Policy Holder's First Name
Policy Holder's Last Name
Policy Holder's SSN
Employee Information Code
Employer's Name
Employer's Address 1
Employer's Address 2
Employer's City
Employer's State
Employer's ZIP Code
Filler
Employer TIN
Filler
Claim Diagnosis Code 1
Claim Diagnosis Code 2
Claim Diagnosis Code 3
Claim Diagnosis Code 4
Claim Diagnosis Code 5
Attorney's Name
Attorney's Address 1
Attorney's Address 2
Attorney's City
Attorney's State
Attorney's ZIP
Lead Contractor
Class Action Type
Administrator Name
Administrator Address 1
Administrator Address 2
Administrator City
Administrator State
Administrator ZIP
WCSA Amount
WCSA Indicator
WCMSA Settlement Date
Administrator’s Telephone Number
Total Rx Settlement Amount
Rx Included in the WCMSA Settlement
Table 30.1-3 COB File—Data Elements in Supplemental Record
Record Type
HICN/RRB Number
SSN
Date of Birth
Gender Code
RxID Number
RxGroup Number
RxBIN Number
RxPCN Number
Rx Plan Toll Free Number
Sequence Number
COB Source Code
Supplemental Type Code
Coverage Code
Insurer's Name
Insurer's Address-1
Insurer's Address-2
Insurer's City
Insurer's State
Insurer's ZIP Code
Individual Policy Number
Group Policy Number
Effective Date
Termination Date
Relationship Code
Payor ID
Person Code
Payer Order
Further information about the format and business rules of the COB file to sponsors is contained in Section 11 of the Plan Communications User’s Guide (PCUG); the guide is available on the CMS Web site. For further information about current Medicare COB processes, see the Medicare Part D COB Web site. (See Appendix B for the specific Web addresses for these sites.)
The COB contractor will send as much information as is available. In some cases, CMS through the COB contractor may determine there is other prescription drug coverage, but may be unable to recognize the Rx identifiers. In such cases, CMS will supply the information so that the sponsors are at least aware of the other coverage.
30.2 – Validation of Information about Other Payers
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
When a Part D sponsor or a beneficiary provides information to the COB contractor about other coverage, the COB contractor validates the completeness of this information, then applies and maintains it in MBD. MBD transmits this information to both the TrOOP facilitator and Part D sponsors from the Medicare Advantage-Prescription Drug (MARx) system via the COB file.
The COB contractor’s role in Part D COB is to assist sponsors in identifying other coverage and in determining whether other payments count toward the beneficiary’s TrOOP by specifying the supplemental payer type.
The table below crosswalks the TrOOP eligibility of payments by other payers with the MSP reason codes and insurance or coverage type codes on the COB file.
Table 30.2-1-Other Payer Codes and TrOOP Eligibility
Other Payer |
MSP Reason Code |
Insurance or Coverage Type Code
|
Relationship of Coverage to Medicare
|
TrOOP Eligibility |
Employer Group Health Plan |
A (Working Aged) B (ESRD) G (Disabled) |
|
Primary |
N |
Non-Employer Group Health Plan |
D (Auto insurance; no fault) E (Workers’ Compensation (WC)) L (Liability) H (Black Lung (BL)) |
|
Primary |
N |
Secondary Insurance |
|
L (Supplemental insurance) M (Medigap) O (Other) |
Secondary |
N |
Federal Government Programs |
|
T (Federal Employees Health Benefit Program [FEHBP]), Veterans Administration (VA) coverage1, 2 (TRICARE)
|
Secondary |
N |
|
|
Indian Health Service (IHS)/Tribal coverage) |
Secondary |
Y (Effective 01/01/2011) |
Qualified State Pharmaceutical Assistance Program (SPAP)2 |
|
Q |
Secondary |
Y |
Non-qualified SPAP |
|
N |
Secondary |
N |
Medicaid |
|
1 |
Secondary |
N |
Manufacturer Patient Assistance Program (PAP)1 |
|
P |
Secondary |
N |
AIDS Drug Assistance Programs (ADAPs) |
|
S |
Secondary |
Y (Effective 01/01/2011) |
Charities |
|
R |
Secondary |
Y |
Health Reimbursement Accounts (HRAS)3 |
|
Z |
Secondary |
N |
1 Coverage is separate and distinct from Part D; see Appendix E for further discussion.
2 State-only funded SPAPs
3 For non-working, aged beneficiaries, payments are secondary to Medicare and non-TrOOP-eligible
30.3 – Establishing the Order of Payment for Part D Coordination of Benefits (COB)
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
In order to provide a consistent set of rules for the order of payment on Part D claims and establish a basis for the accurate calculation of the TrOOP balance, CMS establishes that Part D sponsors and all secondary payers on Part D claims should adhere to the following standards for order of payment: 1) All payers are legally required to adhere to MSP laws and any other federal and state laws establishing payers of last resort (e.g., TRICARE). 2) In all other situations, the Rules for Coordination of Benefits adopted in the most current National Association of Insurance Commissioners Coordination of Benefits Model Regulation should be followed.
The COB contractor includes payment order indicators on other payer records it sends to MBD. Sponsors use this data element to sort COB records for display in reply transactions to the pharmacy. The COB contractor calculates payer order based on MSP rules, relationship to policyholder, and type of supplemental insurance. Rules for using the payment order indicator are contained in the PCUG.
30.4 – Contracting with a Part D Transaction Facilitator
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
All Part D sponsors must correctly calculate the TrOOP amount in order to properly adjudicate beneficiary claims, as well as to communicate this information to plan enrollees. This process is logistically complex because there may be multiple payers (for example, SPAPs or employer or union plans). True COB, in which the order of payment among multiple payers with responsibility for paying prescription drug claims on behalf of an individual is established and programmed into the systems of the secondary payers, did not generally take place in pharmacy benefit management prior to Part D implementation.
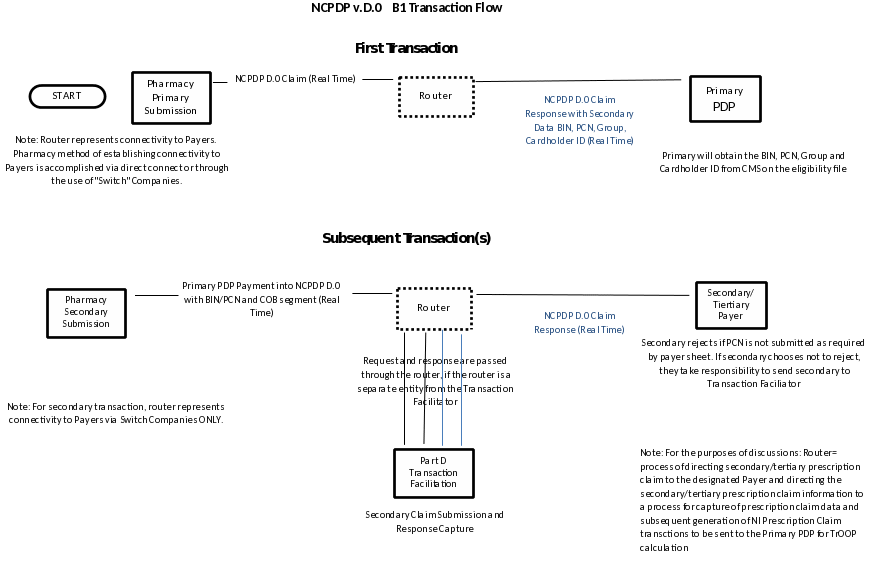
To reduce the need for Part D sponsors to separately create procedures to coordinate benefits with every other payer with responsibility for drug coverage for one of their Part D enrollees, CMS published a request for comment on the feasibility of an online real-time process. In response to this CMS request, representatives from pharmacies, pharmacy benefit manager (PBM) companies, pharmacy data processing and standard-setting organizations provided extensive input and comments to design an automated solution for COB and the facilitation of the TrOOP accounting process. The industry, working in collaboration with the NCPDP, developed a TrOOP facilitation process that allows the majority of pharmacy claims processing to take place “real time” at the pharmacy at point of sale (POS). To this end, supplemental payers are required to utilize the Health Insurance Portability and Accountability Act (HIPAA) coordination of benefits transaction standard, which requires the use of the NCPDP Telecommunication Standard to communicate secondary payer transactions back to the primary Part D sponsor for purposes of tracking TrOOP in real time. Version C.1 of the NCPDP Implementation Guide first detailed the processing requirements involved in the TrOOP facilitation process; the process continues to be defined in the NCPDP Telecommunication Standard Implementation Guide (Version D and above).
In 2005, CMS awarded a contract to NDC Health (d.b.a RelayHealth) to act as the TrOOP facilitator for Part D claims processing. In 2011, CMS re-competed a contract awarded to NDC Health to better describe the nature and scope of the contractor’s responsibilities and changed the name of the contractor’s role to the Part D transaction facilitator. The facilitator is responsible, in conjunction with CMS, for establishing procedures for facilitating eligibility queries (E1 transactions) at POS, identifying costs that are being reimbursed by other payers and alerting Part D sponsors about such transactions, and facilitating the transfer of TrOOP-related data when a beneficiary changes plan enrollment during the coverage year.
30.4.1 – Part D Transaction Facilitation Process
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
With the implementation of Medicare Part D, new electronic transaction capabilities became available to pharmacies. These capabilities allow pharmacies to submit eligibility inquiries without the need to fill a prescription and to bill payers supplemental to Medicare.
A pharmacy uses the eligibility inquiry process, known as an E1 transaction, to submit real-time transactions to the Part D transaction facilitator. Eligibility transactions are used to determine a Medicare beneficiary’s Part D coverage information. Pharmacies use this service when the beneficiary does not have their Medicare Part D Plan Card information to retrieve information needed to bill a claim to a patient’s insurance plan, or to determine billing order if the beneficiary has other drug coverage. Note that long term care (LTC) facilities should batch their end-of-year E1 requests for transmission to the facilitator.
Part D sponsors, supplemental payers, switches (claims routers), and the Part D transaction facilitator must interact to accurately track a patient’s true out-of-pocket expenses. Claims to supplemental payers, known as B transactions, are submitted by the pharmacy to their switch. The switch will forward to the transaction facilitator the B transactions that are not rejected by the supplemental payer and that contain an RxBIN/Processor Control Number (PCN) combination for a plan that covers Medicare Part D beneficiaries. This RxBIN/PCN combination is the flag that switches use to route the data to the facilitator.
The transaction facilitator uses the B transaction to trigger the creation of a reporting transaction (Nx) and delivers the N transaction to the Part D sponsor in real-time. All supplemental billing claims must be processed through a switch, which delivers the transactions to the transaction facilitator to enable accurate TrOOP reporting at the Part D sponsor.
30.4.2 – Enhancements to E1 Transactions
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Enhanced E1 capability, first introduced before the implementation of Medicare Part D, enables pharmacies to separately request verification of a beneficiary’s Medicare Part A/B eligibility— an essential step in the enrollment process for low-income newly eligible beneficiaries (described in section 50.10 of this chapter).
Further enhancements to the E1 inquiry added data elements and explicit messaging to the E1 response. Expanding the E1 response to include, for example, the Part D sponsor’s contract number, benefit ID, benefit effective date and benefit termination date, better informs pharmacies of beneficiaries’ enrollment in a Part D plan and assists pharmacists with processing beneficiary prescriptions. Because of the significant advantages associated with the enhanced E1, effective April 1, 2009, CMS discontinued support for the original E1 eligibility query and now supports only the enhanced E1.
Beginning December 14, 2010, the transaction facilitator, in accordance with the version D.0 implementation timeline outlined in the January 16, 2009 Final Rule (CMS-0009-F) regarding the adoption of updated HIPAA electronic transaction standards, began accepting NCPDP version D.0 transactions. As of that date, pharmacies were able to submit E1 requests for Medicare Part A/B as well as Part D eligibility information in either NCPDP version D.0 or version 5.1. However, effective July 1, 2012, all E1 requests must be in version D.0.
As experience with the Medicare Part D program has grown, CMS has continued to explore areas that offer opportunities for improvement. As part of this effort, effective January 1, 2011, the transaction facilitator implemented new matching logic for E1 queries. Under the new matching logic, pharmacies provide the following patient information on all E1 requests:
Cardholder ID;
Patient’s last name;
At least the first character of the patient’s first name; and
Patient’s date of birth.
Use of the enhanced matching logic enables the transaction facilitator to provide pharmacists and pharmacies with more accurate eligibility and enrollment information by decreasing the probability of false positive matches as well as the need for pharmacy reprocessing of the claims associated with the mismatches.
When a match is found, the industry has requested further enhancements to the E1 transaction response to include:
Specific low-income cost-sharing subsidy (LICS) level, rather than the general “Yes” or “No” that is currently included in the transaction;
Indicators to identify PACE plans and demonstration plans; and
A longer period of time (currently, the date of service must be 90 days before or after the submission date of the E1).
Two of these requested enhancements will be implemented on May 23, 2013. As of this date, when a match is found the E1 response will include the following:
The beneficiary’s LICS level;
The LICS effective date;
The LICS termination date; and
The Medicare plan type (for example, MAPD, PDP, employer group waiver plan (EGWP), PACE).
For more information about the E1 transactions, see the RelayHealth Web site. See Appendix B for the specific Web address.
30.4.3 – Real-time Versus Batch Processing
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
For instances in which Part D plan enrollees’ secondary coverage is identified in advance by CMS systems (as described in section 30.1 of this chapter), multiple-payer claims are automatically adjudicated at the POS. The transaction facilitator captures secondary payer claims transactions based on unique routing information collected previously at enrollment or through the COB contractor’s system. The transaction facilitator also has a batch process available for claims that it receives in a manner other than real time (for example, claims from programs such as the Indian Health Service (IHS) or those presented by the beneficiary to a secondary payer in hard copy). Other payers can then send their paid claims data directly to the transaction facilitator in batch form. Once the facilitator receives the batched paid claims data, it will follow the same online process, creating an NCPDP Nx transaction and sending it to the beneficiary’s Part D sponsor for accurate TrOOP recalculation.
30.4.4 – Enhancements to Nx Transactions
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
CMS, through the Part D transaction facilitator, continues to seek to enhance Nx transactions. One such enhancement involved the creation by the transaction facilitator of a unique Transaction Reference ID for each N1 transaction created and the inclusion of this ID in subsequent N transactions routed to the Part D sponsor. In handling adjustments and reversals, the transaction facilitator uses the following six fields to match the B transactions to prior N1 transactions: Service Provider ID, Date of Service, Rx/Service Reference Number, Product/Service ID, Cardholder ID, and Fill Number. When a B2 transaction is received without a Cardholder ID, the facilitator uses these fields to match the B2 transaction to the prior N1 transaction in order to retrieve the Cardholder ID for inclusion in the N2 transaction to the Part D sponsor. If an adjustment/reversal matches a prior B1 transaction on all six fields, the facilitator includes the Transaction Reference ID from the N1 transaction for the matched claim on the N2 and N3 transactions routed to the Part D sponsor. So, when the facilitator sends an N2, N3 and/or a final N1 transaction to a Part D sponsor, the transaction reference number is consistent among all transactions for the same prescription/service claim.
On December 14, 2010, the transaction facilitator, in accordance with the version D.0 implementation timeline outlined in the January 16, 2009 Final Rule (CMS-0009-F) regarding the adoption of updated HIPAA electronic transaction standards, began accepting NCPDP version D.0 transactions. As of that date, to ensure supplemental claims were appropriately captured and Nx transactions generated, the transaction facilitator accepted supplemental payer billing transactions (B1 and B2) in real time or batch mode in either NCPDP version D.0 or version 5.1 and created N1 and N2 transactions in real time or batch mode based on the version of the billing transaction received until July 1, 2012. The facilitator continued to accept supplemental payer B transactions in either Version 5.1 or D.0; however, beginning January 1, 2012, the facilitator converted supplemental payer claims received in either 5.1 or D.0 format to D.0 Nx transactions. As of July 1, 2012, the facilitator rejects supplemental payer B transactions that are not in Version D.0. As a result, as of July 1, 2012, TrOOP-eligible supplemental payers must use Version D.0; otherwise, their payments will not be credited toward TrOOP.
30.4.5 – TrOOP Accounting
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Part D sponsors are responsible for tracking, accumulating and reporting TrOOP. The role of the transaction facilitator and other parties is to collect and report information in real-time, but others do not do TrOOP accounting. See Appendix A for detail about the Part D transaction facilitation process. This process matters because Nx transactions can affect TrOOP. Part D sponsors should note the TrOOP eligibility status of other payers based on the information in the COB file to determine whether or not a payment should count toward TrOOP.
30.5 – Assessment of COB User Fees
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
The MMA provided CMS with the authority to impose user fees to facilitate the transfer of information necessary for benefit coordination. In conjunction with this authority, CMS uses the fees for activities such as covering the cost of Nx transactions, funding the COB contractor, and supporting CMS systems upgrades for transferring COB data to sponsors. Since this user fee reflects the costs associated with such COB-related activities, user fees may vary (increasing or decreasing) yearly to reflect those needs.
The annual COB user fee is announced in the Medicare Part C and D Call Letter which is an attachment to the Final Rate Announcement issued in April for the following year. Each year’s Part C and D Call Letters are available in the Rate Announcements on the CMS Web site. See Appendix B for the specific Web address.
40.1 – Providing Information to Sponsors on Other Coverage
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Beneficiaries must supply Part D sponsors with information about other prescription drug coverage they have. As provided in the MMA, beneficiaries are legally obligated to report this information, and any material misrepresentation of such information by a beneficiary may constitute grounds for termination of coverage from Part D. CMS guidance on material misrepresentation regarding third party reimbursement and disenrollments for this reason is provided in section 50.2.5 of Chapter 3 covering Part D Enrollment and Disenrollment Guidance available on the CMS Web site. See Appendix B for the specific Web address. Part D sponsors annually notify their enrollees of the other prescription drug coverage information on the COB file from CMS (as described in section 50.2 of this chapter) and report new and/or updated information reported by the beneficiary to the COB contractor for validation. Further information on coordination of benefits when a beneficiary has other prescription drug coverage is available in Medicare & You and Your Guide to Medicare Prescription Drug Coverage; both of these guides are released annually. These are available on the Medicare Web site; see Appendix B for the specific Web address to access Medicare beneficiary publications.
40.2 – Using On-line Processing
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
CMS expects beneficiaries to take advantage of automated real-time prescription drug claim processing whenever it is available so that the supplemental payer information can be utilized to coordinate benefits seamlessly at the point of sale. Paper claim (receipt) submission should be limited to those situations in which on-line claims processing is not available at the pharmacy in order to promote accurate TrOOP accounting (such as out-of-network pharmacies), and to minimize both administrative costs to the Part D sponsors and the Medicare program as well as opportunities for fraudulent, duplicative claim reimbursements. Further information on CMS rules for sponsor processing of paper claims is in section 50.4.3 of this chapter entitled, Direct Member Reimbursement.
40.3 – Submitting Documentation for Off-line Processing on a Timely Basis
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Beneficiaries are responsible for submitting documentation for purchases that are made off-line (i.e., when on-line claims processing is not available at the pharmacy). These would include out-of-network claims and other occasions when the beneficiary had to pay and submit a paper claim to the plan. It is the beneficiary’s responsibility to submit documentation to the Part D sponsor so that their TrOOP balance and other accumulators can be updated timely. However, not all of these claims may be reimbursable; further details are available in section 50.4.3 of this chapter.
50.1 – Providing 4Rx Data on Primary Coverage
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Beginning August 2007, all plan-submitted enrollment transactions to the MARx system must include 4Rx data. The 4Rx data, including the RxBIN, Processor Control Number (PCN), Group ID (RxGRP) and Cardholder ID (RxID), are identifying data required for claims routing. If CMS accepts the enrollment transaction, the enrollment information with the 4Rx data are sent by the MBD to the Part D transaction facilitation contractor to support eligibility (E1) transactions from pharmacies, which are needed anytime a beneficiary presents for the first time at a pharmacy and does not have a plan-issued card for drug benefits. For CMS-generated enrollment transactions, including auto-enrollments, facilitated enrollments, plan rollovers, reassignments, and user interface transactions, Part D sponsors are required to submit the 4Rx data via a Plan change (72) transaction to CMS within 72 hours of the sponsor’s receipt of the Transaction Reply Report (TRR), which reports these enrollments to the sponsor.
Two important developments result from this change in the enrollment process. CMS and the transaction facilitation contractor have a set of 4Rx data for all enrollees whose transactions have been processed successfully in CMS systems. In addition, most of the time lag between CMS accepting an enrollment and the transaction facilitator having 4Rx data has been eliminated.
Prior to April 2011, Part D enrollment and 4Rx data in the CMS MBD were linked. As a result, if a member’s 4Rx data changed (due, for example, to his or her sponsor’s change of claims processor necessitating an RxBIN change), the new 4Rx data for the member replaced the former data. Thus, any transactions that required processing by the former processor were inappropriately routed to the new processor. With the implementation of the changes in the April 2011 CMS systems release, multiple occurrences of 4Rx data within an enrollment period are permitted and transactions can be correctly routed based on the 4Rx effective dates.
In addition, in accordance with 42 CFR §423.120(c)(4),beginning January 1, 2012, sponsors must assign and exclusively use unique Part D 4Rx identifiers. These requirements will ensure beneficiary access to Part D negotiated prices and also ensure that proper concurrent drug utilization review (including safety checks) is performed. Further information on these requirements is provided in chapter 5 section 90.1, of this manual. This chapter is available on the CMS Web site. See Appendix B for the specific Web address.
50.2 – Notifying Beneficiaries Regarding Other Prescription Drug Coverage on File and Transmitting Updated Information to CMS
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
As provided in the MMA, and also mentioned in section 40.1 in this chapter, beneficiaries are legally obligated to report information about other prescription drug coverage or reimbursement for prescription drug costs that they have or expect to receive; any material misrepresentation of such information by a beneficiary may constitute grounds for termination of coverage from a Part D plan. Consequently, prior to 2009, Part D sponsors were required to regularly survey their enrollees regarding any other prescription drug coverage they may have had, and report the results of those surveys – including, if known, any Rx data (RxBIN, PCN, RxGRP, and RxID) – to the COB contractor so that it could be validated, captured, and maintained in MBD for COB purposes.
Since the implementation of Part D, the number of other payers participating in voluntary data sharing agreements with CMS has grown, improving the volume and quality of the other payer information available to Part D sponsors on the COB file. Additionally, the implementation of the new MSP reporting in 2009 for group health plan and non-group health plan insurers, including liability (including self-insurance), no-fault insurance, and workers’ compensation, will continue to expand the other payer information available for COB. Given these developments, CMS revised the Part D beneficiary COB survey requirements. Beginning in 2010, in lieu of a survey, Part D sponsors are required to notify each beneficiary of his/her other prescription drug coverage information as reflected in the COB file from CMS, and request that the beneficiary review the information and report back only updates (that is, corrections to existing information and new coverage information) to the sponsor.
This process is required annually for current enrollees and for new enrollees within 30 days of the date the sponsor processes a beneficiary’s enrollment. The annual and 30-day COB notifications will be sent only to enrollees with existing other coverage information on the COB file. If there is no coverage information on the COB file for the beneficiary, no annual or 30-day COB notification is required. However, if a new enrollee has no other drug coverage information on the COB file, but provided an affirmative response on the application regarding other drug coverage, the sponsor must follow up with the beneficiary to obtain sufficient credible information concerning their other coverage to report to the COB contractor via Electronic Correspondence Referral System (ECRS).
Absent a report of corrected or new information from the beneficiary, sponsors can assume the existing information is correct, and there will be no need for follow-up with non-responding beneficiaries. Likewise, if a sponsor receives no response to their initial follow-up with a new enrollee who responded affirmatively on the application regarding other prescription drug coverage, but has no other coverage on the COB file, sponsors can assume the application response was in error and no further sponsor action is required. CMS believes this new process, which provides for periodic review and correction of the CMS COB data, will further enhance the quality of the data available to Part D sponsors for COB.
Sponsors have the flexibility to design their COB notification process according to their own needs. Likewise, sponsors have the flexibility to design their COB notices and are not required to submit them to CMS for marketing material review. Sponsors may provide the COB notification by telephone, mail, email if available, or in-person. The notification process should not require that the beneficiary provide his or her SSN; instead, sponsors should use other identifiers, such as the Member ID. Also, if the COB notices are mailed, in addition to providing a self-addressed return envelope for beneficiaries to report updated or new coverage information, sponsors should include a mailing address and telephone number on the notice to be used in case the envelope is lost or damaged and the beneficiary has new or updated coverage information to report.
Anytime a Part D sponsor receives information concerning an addition or revision to an enrollee’s existing other coverage information, the new or revised information should be sent electronically via ECRS to the COB contractor within 30 days of receipt. Sponsors should not transmit information about other coverage that the COB contractor has already applied to MBD and that the sponsor has already received in the COB file, but rather only change transactions. In addition, updates to liability coverage, including liability insurance, no-fault insurance and workers’ compensation, cannot be processed through ECRS, but must be handled by the liability carrier. Therefore, sponsors should direct their members to contact the liability carrier directly if the liability coverage information requires correction.
Note that effective January 6, 2012, Part D sponsors are not permitted to update SPAP or ADAP records in ECRS. If an enrollee’s other coverage information includes an SPAP or an ADAP, Part D sponsors should not report either of these types of payers to ECRS as an “Other” payer. Doing so results in the SPAP’s or ADAP’s payment being counted as Patient Liability Reduction Due to Other Payer Amount (PLRO), which is a non-TrOOP-eligible amount, rather than being counted as other TrOOP. Instead, plan sponsors should contact the SPAP or ADAP to request that the program update the enrollee’s information in its next report of enrollment information to the COB contractor.
When an ECRS transaction is received from a Part D sponsor, that transaction’s information is automatically stored in the COB contractor system. The contractor edits the transaction to ensure the information furnished is valid, complete and consistent. Transactions failing these front-end edits are rejected back to the sponsor. Transactions that pass the front-end edits are moved through the COB contractor system for further processing. If the information on the transaction from the sponsor is determined insufficient to process the transaction to completion, the COB contractor will undertake development action to obtain additional information. Development action can take up to 100 days -- 45 days each for an initial development letter and a second development letter, and 5 days for mailing time per letter. If the COB contractor sent development letters but received no response, the contractor will attempt to take the requested action; however, if the contractor is unable to take action, the contractor will close the transaction and indicate on the response file to the sponsor that no development response was received.
50.3 – Connecting to Systems Supporting COB
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Data from CMS to sponsors
The COB contractor performs a daily update of information on other coverage to MBD. Part D sponsors must establish connectivity with CMS systems, which, among other things, allows them to have direct access to other payer status information as often as their business requirements indicate. Every Federal business day, the COB contractor pushes out updated information to MBD and then CMS sends the COB file to the Part D sponsors. For more information on receiving COB files, see the Plan Communications User’s Guide (PCUG) available on the CMS Web site. (Refer to Appendix B for the Web address.) It is incumbent upon Part D sponsors to identify any changes to existing other payer information available in CMS systems, and to send those changes to the COB contractor.
In March 2010, CMS initiated the process of annually creating and issuing to each Part D sponsor a full replacement COB file for all the sponsor’s enrollees. A full replacement file is created for each prescription drug plan based on the sponsor’s Part D enrollees as of the date the file is processed. Each plan’s file includes both the record updates that would normally be included in the daily COB notification files and the full replacement COB data for all enrollees with other coverage. As a result, during the days the combined daily update records and full replacement files are being issued, no separate daily COB notification files are sent. Due to file size constraints, sponsors with a large number of Part D enrollees with other coverage may receive multiple COB files over the period during which the replacement files are sent.
The combined daily update/full replacement COB files contain no special identifiers to distinguish them from the normal daily COB notification files, but they may be identifiable based on the date of receipt and the large size of the files. Each plan’s file(s) include only detail records for any beneficiaries whose other coverage information has been deleted; these records normally would be in the plan’s daily COB notification file. The plan’s file also includes the records for all its current Part D enrollees who have at least one occurrence of either primary or supplemental coverage. Not included in the file are records for any Part D enrollee without other coverage information. As a result, for the enrollees included in the file, the information is a full-record replacement that should be processed by the plan replacing its entire existing other coverage information for these enrollees with the daily update/full replacement file data. For its remaining Part D enrollees (that is, those members without other primary or supplemental coverage), the plan must retain the members’ existing detail records.
As with other COB notification files, the full replacement COB files include the last 27 months of other coverage information as of the date the file is processed. Thus, each year’s full replacement files are sent not only to the current plans of record, but also to any prior plans with enrollment periods for that beneficiary within the last 27 months.
Data from sponsors to the COB system
There is an electronic interface between Part D sponsors and the COB contractor known as the Electronic Correspondence Referral System (ECRS). ECRS allows Part D sponsors to submit post-enrollment transactions that change or add to currently known COB information. Part D sponsors may send ECRS transactions in any of three possible ways: 1) by using Network Data Mover (NDM) (a secure file transfer process) to connect to the ECRS Online Application; 2) by using NDM to send an ECRS flat file; or 3) by using a current SFTP connection to send an ECRS flat file. Part D sponsors are updated on the status of these transactions as they move through the COB systems and are informed of the determination made by the COB contractor on the transactions via a COB data report/file. Further information on ECRS is contained in the ECRS User Guide available on the CMS Web site; see Appendix B for the specific Web address.
The data provided by the COB contractor on supplemental payers and order of payment is generally the best available information for Part D sponsors and pharmacies to act upon. However, it is important to note that Part D sponsors must coordinate benefits with all other payers providing coverage for covered Part D drugs, even if the COB contractor is unaware of some payers who have submitted batched claims after the point-of-sale transaction at a network pharmacy. Although the COB contractor may be unaware of them, these other payers may submit claims directly to the Part D sponsor, thereby enabling benefit coordination by the Part D sponsor. Once a sponsor becomes aware of these other payers, it must submit this information via ECRS to the COB contractor.
In accordance with the regulatory requirements at 42 CFR 423.464(h), Part D sponsors must report credible new or changed supplemental prescription drug coverage information to the COB contractor according to CMS-specified processes and timeframes. By “credible,” we mean information that is consistent with conventions for how group health insurance coverage is identified, for instance, information that includes the name and address of the insurance company and the policy identification number. As noted in section 50.2 of this chapter, sponsors must report new or changes coverage information to the COB contractor within 30 days of receipt.
Sponsors should utilize the electronic interface established with CMS (via the MARx system) to handle plan enrollments, to transmit certain other payer data elements upon enrollment, and to receive daily transmissions of validated COB information. As new information about other prescription drug coverage is discovered, sponsors should use ECRS to send the information to CMS. Sponsors should not use the enrollment update transaction to communicate this subsequent information.
Beyond the electronic data transfers requirements described above, Part D sponsors must establish procedures for at least weekly COB file processing. Sponsors are required to not only receive information, but also to apply it to their systems.
50.4 – Processing Claims and Tracking TrOOP
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Part D sponsors must correctly calculate the TrOOP amount in order to properly adjudicate beneficiary claims, as well as to communicate this information to plan enrollees. In order to calculate TrOOP, Part D sponsors will have to determine if other entities have made payments on covered drugs, and whether such payments fall under the legal definition of incurred costs as described in 42 CFR §423.100. CMS assists in this process by providing a transaction facilitator (described in section 30.4 of this chapter). The transaction facilitation process requires that supplemental payers utilize the HIPAA coordination of benefits transaction standard, which necessitates the use of the NCPDP Telecommunication Standard to communicate other payer transactions back to the primary Part D sponsor for purposes of tracking TrOOP in real time. Part D sponsors are required to process claims and track TrOOP in real time, including providing known supplemental payer information to the pharmacy, and accepting and processing Nx transactions.
In accordance with the requirement at 42 CFR 423.120(c)(3), Part D sponsors must require their network pharmacies to submit claims to the Part D sponsor or its intermediary whenever the Part D member ID card is presented or is on file at the pharmacy unless the enrollee expressly requests that a particular claim not be submitted to the sponsor or its intermediary. Not only is requiring on-line adjudication of prescription drug claims the only way to ensure that enrollees have access to the plan’s negotiated price at the point of sale, it also ensures that proper concurrent drug utilization review (including safety checks) is performed.
Appropriately restricting the use of paper claims to those situations in which on-line claims processing is not available to the beneficiary at the point of sale will promote accurate TrOOP accounting, as well as minimize administrative costs to the Part D sponsors and the Medicare program, and limit opportunities for fraudulent duplicative claim reimbursements. When secondary payer information is not captured up front in CMS systems, however, Part D sponsors are required to retroactively adjust claims and TrOOP balances.
CMS establishes the payer order (see section 30.3) for the validated other payer data that is transmitted to both the transaction facilitator and the Part D sponsors from MARx via the COB file. This payer order assists sponsors in processing claims when there are multiple other payers on a beneficiary’s record. This is important, particularly for payers considered payers of last resort (e.g., SPAPs). Because Part D sponsors are ultimately responsible for accurately tracking TrOOP, they are required to retroactively adjust claims and TrOOP balances when errors in order of payment are made.
Special procedures for SPAPs and ADAPs
To ensure any payments made by SPAPs and ADAPs on behalf of their Part D eligible enrollees count toward TrOOP, these programs must have unique RxBINs and RxPCNs for their Part D-eligible beneficiaries. When a Part D sponsor receives an initial claim transaction and identifies the member as an SPAP or ADAP enrollee, the sponsor sends the member’s SPAP/ADAP 4Rx data back to the pharmacy in the claim response so that the pharmacy may appropriately bill the SPAP or ADAP for their portion of the enrollee’s cost sharing. The transaction facilitator uses the SPAP/ADAP claim request and response to create an Information Reporting (Nx) transaction report to the Part D sponsor. The sponsor uses the N transaction information to adjust the beneficiary’s TrOOP, calculating the amount of the SPAP/ADAP payment as the difference between the Part D cost-sharing and the beneficiary cost-sharing after the supplemental payment, and reporting the SPAP/ADAP payment as an “other TrOOP” amount.
SPAPs and ADAPs can only submit updates (i.e., changes, deletions and additions) to their eligibility files to the COB contractor once per month. Monthly file submission creates an inherent delay in the subsequent reporting of updated information to the MBD, the transaction facilitator and plan sponsors. To address this delay, CMS worked with the transaction facilitator and NCPDP to create a special list of SPAP/ADAP BIN/PCNs to which sponsors may refer if the information is not yet available on the COB file. The SPAP/ADAP BIN/PCN list is available on the NCPDP public Web site under the “Resources” tab. See Appendix B for the specific Web address.
As a result of recent systems changes, Part D sponsors can no longer make corrections to SPAPs’ and ADAPs’ eligibility file information using ECRS. Only SPAPs and ADAPs are now able to edit their data. However, as noted above, these programs can only submit eligibility files to the COB contractor once per month, which creates an inherent delay in reporting updated information to plan sponsors. Part D sponsors should not attempt to work around the delay by using ECRS to report SPAP or ADAP enrollment as “other” coverage. Doing so will result in incorrect coverage information on the COB file and the possible incorrect reporting of SPAP or ADAP payments as PLRO instead of other TrOOP on the PDE. The transaction facilitator has implemented a process to accommodate the eligibility reporting delay. This process involves continued attempts to create an N transaction reporting the SPAP/ADAP payments for up to 90 days to permit the eligibility information to be reported to the Part D sponsor.
Other sources of information on the facilitation process
While this document is not meant to capture the transaction facilitation process in exhaustive detail, other sources are available in:
Appendix A of this chapter, which contains more information in the form of a flow chart, about what the transaction facilitation process entails.
The transaction facilitation contractor Web site; see Appendix B for the specific Web address.
The NCPDP Telecommunication Standard Implementation Guide D.0, which provides the official guidelines for electronic prescription drug claim transaction processing.
The Prescription Drug Event (PDE) Data Guidance on the CMS Web site, which explains TrOOP and PDE data reporting; see Appendix B for the Web address.
Chapter 5 of this manual, which addresses benefits, beneficiary protections, and benefit design and contains information on incurred costs counting toward TrOOP.
50.4.1 – Receiving an Nx Transaction, Without Supplemental Payer on File
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Part D sponsors should accept Nx transactions even in those instances where they have no supplemental payer information on file to identify the payer. CMS encourages sponsors to subsequently follow up by contacting the beneficiary to identify the supplemental payer. Once the sponsor receives this information, except for SPAP/ADAP coverage, it should be transmitted to the COB contractor for verification of the secondary coverage.
Note that in the event that a Part D sponsor is a secondary payer in accordance with the application of MSP rules, the Part D sponsor is required to process claims in real time to support the TrOOP facilitation process.
Explanations of benefits (EOBs) provide enrollees with their year-to-date TrOOP balances and gross covered drug costs and information on the enrollees’ position in the Part D benefit. To ensure enrollees are appropriately informed, CMS requires that sponsors develop EOBs that provide information in a form understandable to all enrollees. Acceptable EOB formats are included in the Medicare Marketing Guidelines available on the Web site; see Appendix B for the specific Web address.
50.4.2 – Beneficiary Cash Purchases
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Previously, CMS permitted enrollees to purchase a covered Part D drug without using his or her Part D benefit or a supplemental card and have the cash price count toward the enrollees’ total drug spending and TrOOP. The policy applied if the enrollee could obtain a lower price at a network pharmacy than the plan’s negotiated price in any applicable deductible or coverage gap when the enrollee incurs 100 percent of the drug cost. The enrollee was required to submit the appropriate documentation to his or her plan for the incurred drug cost to be included in gross covered drug cost and TrOOP.
Since the beneficiary cash purchase policy was issued, the Part D benefit has undergone significant change. Beginning January 1, 2011, the changes created by the Affordable Care Act (the ACA) started closing the coverage gap for beneficiaries not receiving LIS. By establishing the Coverage Gap Discount Program, which makes manufacturer discounts available at point-of-sale to non-LIS beneficiaries in the coverage gap, and gradually increasing coverage in the coverage gap for both generic and brand name drugs and biologics, the ACA for the most part has eliminated the need for this policy.
Although beneficiaries can still purchase a covered Part D drug at a network pharmacy without using their Part D benefit or a supplemental card, CMS encourages beneficiaries to use their Part D benefit. Use of the benefit affords beneficiaries access not only to the plan’s negotiated prices, which in most cases are the lowest price available, but also to the plan’s drug utilization review and other safety edits that only can be provided when the plan adjudicates the claim. Beneficiaries who choose to make a cash purchase will continue to be responsible for submitting documentation to the plan for determination of whether they are eligible for reimbursement and for costs to be included in gross covered drug costs and TrOOP. Guidance included in section 50.4.3 below replaces CMS’ former cash purchase policy and clarifies plan processing of beneficiary-submitted claims for cash purchases as well as enrollee costs and amounts to be included in the enrollee’s gross covered drug costs and TrOOP.
50.4.3 – Direct Member Reimbursement
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10; Implementation Date: 02-01-14)
Since publication of the Part D Final Rule (70 FR 4194) in 2005, CMS guidance for out-of-network access to covered Part D drugs, as stated in the preamble, notes that enrollees will likely be required to pay more for a covered Part D drug purchased out-of-network than one purchased at a network pharmacy, but explains that any out-of-network differential (that is, the difference between the out-of-network pharmacy’s usual and customary (U&C) price and the plan allowance) that an enrollee is required to pay for purchases made consistent with the Part D sponsor’s out-of-network access policy will count toward his or her TrOOP balance. For LIS-eligible individuals, the guidance states that CMS will pay the out-of-network differential, as applicable, for appropriate out-of-network purchases. The guidance was silent regarding the handling of the out-of-network differential for non-LIS-eligible individuals. As a result, the policy was ambiguous and sponsors have chosen to handle the differential in different ways. For example, some sponsors include only the negotiated price for the drug in the enrollee’s total gross covered drug cost accumulator (Prescription Drug Event (PDE) record field 45,) but include the differential in TrOOP.
Additionally, aside from the beneficiary cash purchase policy explained in section 50.4.2 of this chapter, no clear guidance has been available to sponsors concerning the reimbursement of beneficiary paper claims for covered Part D drugs from network pharmacies. To ensure consistent handling of out-of-network claims for both LIS and non-LIS eligible beneficiaries as well as paper claims for drugs accessed from network pharmacies, effective beginning in 2013 CMS is providing consistent guidance on direct member reimbursement in this section.
Section 1860D-4(b)(1)(C)(iii) of the Social Security Act required CMS to establish pharmacy access standards that include rules for adequate emergency access to covered Part D drugs by Part D enrollees. The special rules for out-of-network access to covered Part D drugs at pharmacies are specified in regulation (42 CFR 423.124) and discussed in chapter 5, section 60.1 of this manual. For out-of-network claims to meet the conditions for emergency access requires that the enrollee cannot be reasonably expected to obtain the covered Part D drugs at an in-network pharmacy and such access cannot be routine.
CMS regulations and guidance specifically address the requirement for Part D sponsors to issue standardized cards that may be used by an enrollee to ensure access to negotiated prices under section 1860D-2(d) of the Act. The only way that an enrollee can be assured access to the negotiated price at the point of sale is through online adjudication of the prescription drug claim. Therefore, to ensure access to these negotiated prices, the billing information on the standardized cards issued by the Part D sponsor must be used by the pharmacies at which beneficiaries fill their prescriptions to submit claims to an enrollee’s plan sponsor (or its intermediary). Thus, another price available to the beneficiary at the point of sale, for instance, the pharmacy’s “cash price,” would not be the negotiated price because it is not accessed by the use of the standardized card.
CMS encourages beneficiaries to use the Part D benefit, because generally it believes it is in the best interest of Part D enrollees to have their claims consistently processed through the Part D sponsor (or its intermediary). Not only does processing claims through the Part D sponsor ensure access to Part D negotiated prices, but it also ensures that proper concurrent drug utilization review (including safety checks) is performed (as required under 1860D-4(c) of the Act). Only the plan can prevent payment to excluded providers or conduct accurate concurrent drug utilization review when a beneficiary uses multiple pharmacies. Online, real-time processing also facilitates accurate accounting for enrollees' true out-of-pocket (TrOOP) and total drug costs by the Part D sponsor so that each claim is processed in the appropriate phase of the benefit and accurate cost sharing assessed.
Guidance in section 50.4 of this chapter instructs plan sponsors to process all claims online and in real time. The requirements of accurate TrOOP accumulations, Part D benefit administration of multiple coverage intervals, and coordination of benefits with other payers all necessitate online, real-time adjudication of individual pharmacy claims. This guidance states further that CMS expects Part D sponsors will establish policies and procedures appropriately restricting the use of beneficiary-submitted paper claims to those situations in which online claims processing is not available to the beneficiary at point-of-sale (such as out-of-network pharmacies) in order to promote accurate TrOOP accounting as well as to minimize administrative costs to the Part D sponsors and the Medicare program and reduce opportunities for fraudulent duplicative claim reimbursements.
Having been made aware of an increasing number of instances in which network pharmacies were not submitting on-line pharmacy claims to Part D on behalf of Part D enrollees, CMS codified this guidance in regulation at §423.120(c)(3.) The pharmacies were discouraging beneficiaries from using their Part D benefit when going outside the benefit would have resulted in the same cost to the beneficiary because the pharmacies wanted to avoid incurring the claims transaction costs. As a result, the enrollee paid cash for the drug and submitted a paper claim to Part D for reimbursement. The regulation requires Part D sponsors to contractually mandate that their network pharmacies submit claims electronically to the Part D sponsor or its intermediary on behalf of the beneficiary whenever feasible unless the enrollee expressly requests that a particular claim not be submitted to the Part D sponsor or its intermediary.
Requirements for Direct Member Reimbursement: To ensure uniformity, the following table clarifies what these regulations require in terms of direct member reimbursement. The table specifies the requirements for direct member reimbursement involving out-of-network and in-network pharmacies and applies to all LIS beneficiaries and all others.
Table 50.4.3-1-Direct Member Reimbursement Requirements
Direct Member Reimbursement Situation |
Part D Processing and Plan Paid Amount |
Enrollee Costs |
PDE Reporting of Total Gross Covered Drug Cost Accumulator and TrOOP |
Out-of-network pharmacy claim and requirements of 423.124 are met |
Reimburse the plan allowance based on the U&C price |
Enrollee pays the cost-sharing under the plan based on the plan allowance plus the difference between the cash price and the plan allowance if the cash price is higher (i.e., the out-of-network differential)
For LIS beneficiaries, the out-of-network differential is paid by CMS |
Total Gross Covered Drug Cost= Cash price of drug
TrOOP=Cost-sharing under the plan plus the difference between the cash price and the plan allowance if the cash price is higher |
Out-of-network pharmacy claim and requirements of 423.124 are not met |
Drug does not meet requirements for coverage |
Enrollee is responsible for the total cash price |
No Total Gross Covered Drug Cost or TrOOP are reportable |
Enrollee voluntarily pays out-of-pocket at an in-network pharmacy and doesn’t submit a claim for reimbursement |
No action required |
Enrollee is responsible for the total cash price |
No claim; therefore, no PDE and TrOOP is not reported |
Enrollee voluntarily pays out-of-pocket at an in-network pharmacy and submits a claim for reimbursement |
Reimburse the plan allowance based on the negotiated price for the drug |
Enrollee pays the cost-sharing under the plan plus the difference between the cash price and the plan’s negotiated price if the cash price is higher |
Total Gross Covered Drug Cost= negotiated price for the drug
TrOOP= Only the cost-sharing under the plan (calculated based on the plan allowance) |
For out-of-network situations, CMS policy reflects the statutory protection for provision of adequate emergency access for Part D enrollees to covered Part D drugs. In out-of-network situations when the requirements of §423.124 are not met, the drug is not covered. Part D sponsors should employ their out-of-network policy to evaluate out-of-network claims and make payment determinations.
For cash purchases made at in-network pharmacies, CMS expects the enrollee to be responsible for the difference between the cash price and the plan’s negotiated price. As noted previously, under section1860D-2(d), Part D sponsors must provide enrollees with access to negotiated prices used for payment of covered Part D drugs. This requirement limits sponsor reimbursement to the negotiated price for the drug. Under §423.100, incurred costs are defined to include only costs incurred by the beneficiary for the annual deductible, or other cost-sharing prior to satisfying the out-of-pocket threshold, including the out-of-network price differential for which the individual is responsible when the requirements of §423.124 are met. Because in this instance the requirements of §423.124 are not met, the price differential incurred for cash purchases at an in-network pharmacy are not included in either the member’s gross covered drug costs or TrOOP.
Although CMS recognizes there may be circumstances when a cash purchase is reasonable—such as when the pharmacy’s or payer’s system is down—these would be extremely rare and, in the case of a systems outage, of brief duration. There may also be instances when a family member or other person who is filling a prescription on the enrollee’s behalf doesn’t have the enrollee’s card and the enrollee is not in the pharmacy’s system. However, at this point, CMS expects enrollees to use their Part D plan’s card or the family member or other person to identify the patient to the pharmacy as a Medicare beneficiary for the pharmacy to submit an E1 eligibility query to the Part D transaction facilitator. As noted above, because in these instances the differential between the cash price and the negotiated price would exceed the negotiated price, but would not meet the regulatory definition of incurred costs, the differential would not be reimbursed and would not count toward the enrollee’s gross drug costs or TrOOP.
CMS expects sponsors to implement this policy as soon as possible, but no later than February 1, 2014.
50.5 – Use of Standardized Technology
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
In the CMS-4085 Final Rule (75 FR 19678) published on April 15, 2010, CMS added a new paragraph (c)(2) to §423.120 which codified existing guidance that Part D sponsors use standard electronic transactions for processing Part D claims in compliance with CMS guidance on the use of optional or conditional fields in the HIPAA standard transactions when so instructed through Call Letter and Prescription Drug Benefit Manual instructions. The prior guidance in this section of the manual, previously entitled, “Standardized Claims Messaging,” was superseded by the new regulatory provision requiring Part D sponsors to utilize standardized electronic transactions established by 45 CFR 162.1102 for processing Part D claims. The preamble of the above-referenced regulation notes that CMS routinely works with NCPDP and industry representatives to arrive at recommendations for standardized use of optional or conditional fields when necessary to improve the administration of the Part D benefit and will issue guidance on the use of these fields within such standards. An example of such guidance would include section 50.4 of this chapter on “Processing Claims and Tracking TrOOP.” Such instructions are consistent with the rules governing use of HIPAA transactions whereby use of optional and conditional fields is governed by contractual terms between trading partners.
50.5.1 – Primary Payer Use of Fields to Support COB
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
CMS recognizes version D.0 (and any future version) of the NCPDP Telecommunication Standard Implementation Guide as the official vehicle for establishing the special electronic processing rules to be used in coordinating benefits and generating the N1 transaction. Unlike earlier versions, D.0 requires that primary payers provide certain fields in the response pricing segment of the telecommunication standard that support COB. These fields include “Amount Applied to Periodic Deductible” [517-FH] and “Amount of Copay,” [518-FI] if the amount reported in “Patient Pay Amount” [505-F5] includes a deductible and/or copay amount, and “Benefit Stage Amount” [394-MW] and “Benefit Stage Qualifier” [393-MV]. These fields assist secondary payers in administering their benefit and when provided by the primary payer, can be transmitted by the pharmacy to the secondary payer.
50.6 – Accepting Payment of Premiums from Other Payers
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
As provided by the MMA, supplemental payers may wish to pay premiums on behalf of Part D enrollees instead of (or in addition to) providing wrap-around coverage. Part D sponsors are required to facilitate the billing and collection of such premiums. While Part D sponsors must accept premium payments by supplemental payers on behalf of their Part D enrollees, the details of such arrangements are strictly between Part D sponsors and those payers. Part D sponsors should ensure that in accordance with the uniform premium requirement, the total premium payment for a beneficiary does not vary among plan enrollees, except in the case of employer group plans for which this requirement has been waived in part.
A beneficiary must not be disenrolled from a Part D sponsor if it has been notified that the premiums are being paid by an SPAP or other payer and the sponsor has not yet coordinated receipt of the premium payments with the SPAP or other payer. In these cases, Part D sponsors are required to work directly with the SPAPs or other payers to systematically coordinate and accept premium payments in accordance with the Federal regulations at 42 CFR 423.464(a)(1). That is, sponsors must bill the SPAP or other payers directly for the beneficiary’s premium and not bill the beneficiary. Until the sponsor can bill the SPAP or other payers directly, sponsors will not be in compliance with the coordination of benefit requirements. Sponsors must not take any action, including sending disenrollment notices directly to the beneficiary, to disenroll the beneficiary for failure to pay premiums when the sponsor has failed to coordinate the collection of premiums from other payers.
Sponsors currently receive data from CMS in the COB file indicating which beneficiaries are covered under SPAPs. The Supplemental Type Code data field of the COB file (see the PCUG, Appendix F.5.4) indicates the type of supplemental coverage a beneficiary has. An indicator of 'Q' identifies a beneficiary with qualified SPAP coverage. (Refer to Appendix B for the PCUG Web site.) Sponsors could use this data to withhold systematic release of disenrollment notices to these enrollees when an SPAP is paying on behalf of the enrollee.
In addition to accepting payment of premiums from other payers, Part D sponsors may wish to consider providing advance notice to such payers when an enrollee is at risk of losing coverage due to failure to pay their portion of a premium.
50.7 – Coordinating Payment of a Lump Sum for Supplemental Coverage
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
The MMA specifies that CMS’ COB requirements must include a method for the application by a Part D sponsor of specified funding amounts (i.e., a lump sum per capita method) from an SPAP for supplemental prescription drug benefits. These COB requirements also apply to other entities providing supplemental drug coverage. Consequently, Part D sponsors are required to coordinate the receipt and management of lump sum arrangements with other payers. It is important to note, however, that the cost sharing funded by lump sum amounts will generally only apply toward TrOOP if made by a qualified SPAP, an ADAP, the IHS/Tribal coverage, or a bona fide charity, and if made for expenditures on covered Part D drugs before the beneficiary reaches the annual out-of-pocket threshold.
SPAPs (and other prescription drug plans) may choose to provide their wrap-around benefits to Part D beneficiaries using four basic approaches:
Pay premiums for basic and/or supplemental benefits offered by Part D sponsors.
Wrap-around benefits at the point-of-sale; i.e., the pharmacy files a secondary claim to the SPAP (or its processor) for payment.
Contract with Part D sponsors on a risk or non-risk-based lump sum per capita method; i.e., solicit lump sum per capita bids from Part D sponsors in exchange for the provision of wrap-around benefits.
Provide some combination of these approaches.
Regardless of the approach adopted, SPAPs and other prescription drug plans:
In accordance with §1860D-23(b)(2) of the Social Security Act, must not discriminate in determining either eligibility or the amount of assistance to Part D enrollees based on the Part D plan in which the SPAP beneficiary enrolls. The non-discriminatory standards also apply to education and enrollment of beneficiaries by the SPAP and to co-branding with Part D sponsors. Therefore, the State must ensure that its beneficiaries receive equal access to enrollment in, and comparable information on, all the Part D sponsors participating in the chosen approach, without any steering to particular plans.
Cannot request Part D sponsors violate Part D rules.
May offer a benefit package to eligible beneficiaries that is more than Part D, but cannot be less.
Guidance concerning the requirements on SPAPs with respect to non-discriminatory beneficiary education, enrollment and co-branding activities exists on CMS’ Web site; for example, guidance on co-branding with SPAPs is included in the Medicare Marketing Guidelines available on the CMS Web site; see Appendix B for the specific Web address.
50.7.1 – Lump Sum Per Capita Approach
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
States that elect to adopt a lump sum per capita approach must issue a request for quote (RFQ) inviting all Part D sponsors in the region to submit a quote (note – the quote is for the increment above basic benefits) and must work with all sponsors that respond. As part of the State’s RFQ and contract, any Part D sponsor that submits a quote would be required to accept the lump sum per capita payments made by the State under its chosen approach.
Part D sponsors that do not opt to participate in this market are not required to submit quotes. However, if a sponsor is not participating in the State’s lump sum approach, the State should still explain that beneficiaries may enroll in that sponsor’s plan, but the beneficiaries will get only basic coverage – without the SPAP additional defined benefit – if they do so. Also, States are not obligated to provide wrap-around benefits to any beneficiaries choosing to enroll in non-participating Part D plans, or to promote these Part D plans, but a State electing to do so may provide wrap-around coverage on behalf of SPAP beneficiaries choosing to enroll non-participating Part D plans. In fact, if the SPAP also elects to pay the premium for all basic benefits, this approach does not permit the SPAP to exclude payment of premium for any Part D sponsors not participating in the lump sum approach.
The regulation at 42 CFR 423.464(a) requires that Part D sponsors must coordinate with SPAPs and other entities providing other prescription drug coverage. This includes scenarios when the SPAP or other payer is adopting a lump sum per capita approach when supplementing Part D benefits in accordance with section 42 CFR 423.464(a)(2). Therefore, CMS requires all Part D sponsors to have the capacity to participate in non-risk based arrangements, if offered by the State, SPAPs or other payers so that their enrollees can receive coordinated, wrap-around coverage at the point-of-sale. If a sponsor is out of compliance with this regulatory requirement, CMS will not disqualify a state program from its qualified SPAP status. CMS will not view SPAPs as discriminating, in violation of section 1860D-23(b)(2) of the Act, due to a Part D sponsor’s failure to adhere to this COB requirement.
50.8 – Transferring TrOOP Balance When a Beneficiary Changes Part D Sponsors
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Part D rules require sponsors to track the beneficiary’s TrOOP and correctly apply these costs to the TrOOP limit in order to provide enrollees the catastrophic level of coverage at the appropriate time. The TrOOP threshold is calculated on an annual basis and must be transferred between Part D sponsors if a beneficiary disenrolls and re-enrolls at any time before the end of a coverage year or whenever a PartD plan other than the plan of record has paid. Sponsor collection, and transfer if appropriate, of the TrOOP and gross covered drug spending balances are essential for sponsors to correctly manage the Part D benefit.
50.8.1 – Automated TrOOP Balance Transfer Process
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
In 2009, Part D sponsors began using the NCPDP Financial Information Reporting (FIR) standard to transfer TrOOP balances and gross covered drug costs whenever a beneficiary makes an enrollment change at the contract-level during the coverage year. The transfer process begins with the transaction facilitator's identification of a change in enrollment at the contract level. Upon identification of the change, the facilitator generates a FIR transaction to each prior sponsor with which the beneficiary was enrolled or that paid covered part D drug claims for the beneficiary during the coverage year. Transactions begin with a FIR Inquiry to the earliest sponsor on record in the coverage year; that sponsor's Inquiry response is returned to the facilitator. Each sponsor responds with their monthly gross covered drug costs and TrOOP amounts. If there are multiple plans prior to the current plan of record, the accumulator values from the response just received are placed in a FIR Exchange transaction and forwarded to the next sponsor. The facilitator receives that next sponsor’s transaction response and continues the process of receiving and forwarding the prior accumulators until each subsequent sponsor in consecutive order has received and responded to a FIR Exchange transaction. The final Exchange transaction response contains the year-to-date monthly TrOOP-related data for all plans prior to the current plan of record; these accumulated monthly amounts are then forwarded by the facilitator via a FIR Update transaction to the current plan of record.
An updated version of the CMS automated TrOOP balance transfer implementation guidance issued by CMS on October 20, 2008, is included in Appendix C. Detail on the FIR transaction standard is provided in the NCPDP Financial Information Reporting Standard Implementation Guide v1.0 and is available to NCPDP members on their Web site; see Appendix B for the specific Web address.
Plan sponsors are notified of unsuccessful transactions via daily beneficiary-level exception reports from the transaction facilitator to both the sponsor’s processor’s and the sponsor’s automated Troop Balance Transfer (TBT) contacts (as entered in the Health Plan Management System (HPMS)). CMS expects that when issues arise, they are resolved expeditiously to achieve successful transfer of the beneficiary data in a timely manner 100 percent of the time. Sponsors should not delegate problem-solving to their claims processor alone; certain issues leading to transaction failure are beyond the scope of the processor’s responsibility. For example, a significant number of problems that have occurred to-date appear to stem from inconsistencies between the 4Rx data reported to CMS and used by the transaction facilitator for the TBT transactions, and the 4Rx data in the sponsor’s processor system. It is the responsibility of the Part D sponsor to ensure that consistent beneficiary-identifying data are reported to CMS and its Part D claims processor, and that any inconsistencies are corrected. Part D sponsors must ensure not only that they or their delegated enrollment-processing vendors submit accurate 4Rx data on behalf of all enrollees upon enrollment in accordance with section 50.1 of this chapter, but also that previously submitted 4Rx data are updated within CMS whenever processing arrangements cause this data to change for existing enrollees.
NOTE: If a sponsor changes its FIR processor, the sponsor must ensure that FIR transactions are routed to the appropriate processor and that transactions related to the prior year can continue to be processed for the 36-month period required by CMS (42 CFR 423.466(b)). This may require making arrangements either with the former processor to continue processing prior year FIRs or with the new processor to assume that responsibility. However, since the automated FIR process for a year continues only until the end of the subsequent June, any TrOOP balance transfers after that point would require manual processing. See section 50.14 of this chapter for more information on the 36-month time period and Appendix C for more information on the FIR process.
50.8.2 – TrOOP Balance Transfer When CMS Terminates a Part D Sponsor Contract
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
In 2011, CMS added §423.509 (e) to Part D regulations, to articulate the requirement to provide the timely transfer of beneficiary data and files, including TrOOP-related accumulators, from those Part D sponsor contracts terminated by CMS to a beneficiary’s new Part D sponsor. When CMS terminates a contract it must have assurances that the sponsor will maintain sufficient staff and operations to make a smooth transition of the sponsor’s enrollees to their new Part D plan, while facilitating continuity of care and fiscal responsibility.
This new regulation informs Part D sponsors that they are required by Federal regulation to maintain and provide access to all requested data and files to CMS or its designee for the required time as specified under §423.505 (d) and (e). Plans that fail to comply with this requirement may be subject to a Civil Monetary Penalty as defined in § 422.752 (c) and § 423.753 (c).
50.9 – Special Transition Period for Retroactive Enrollment Situations
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
In 2007, CMS implemented a special transition period, which is available to all Medicare beneficiaries, with important COB implications that require Part D sponsors to provide limited reimbursement for covered Part D drugs for a time immediately preceding the minimum 30- or 90-day transition period. This requirement applies to those situations involving:
Enrollees whose enrollment request was not processed to completion until after the effective date of coverage; and
Enrollees who have been retroactively enrolled in a Part D sponsor by CMS. These latter situations almost exclusively involve enrollees who are full-benefit dual eligible beneficiaries.
Although CMS works with the States to identify beneficiaries in advance of the date they will become dually eligible in order to minimize issues involving retroactivity, there are some situations when CMS will not be able to identify a dual eligible beneficiary in advance. Because eligibility for Medicaid may be retroactive for up to 3 months prior to the month in which the Medicaid application was filed, and Medicaid applications frequently require significant time for the State to process, periods of retroactivity can continue to be several months in duration. CMS expects that this problem will usually be mitigated by the fact that, as a Medicare beneficiary, the individual will have had an opportunity to enroll in a Part D sponsor’s plan and apply for the low-income subsidy. For those who do enroll in a Part D plan, and then are determined retroactively eligible for Medicaid, the effective date of their Part D plan enrollment will be adjusted to the later of the first of the month the beneficiary is dually eligible, or January 2006.
In 2006, with respect to claims incurred during a period covered under actual Part D enrollment, Part D sponsors were responsible for paying or reimbursing the costs of an enrollee’s Part D covered drugs to the extent that the sponsor would have paid as a primary payer. If the enrollee’s existing drug regimen required prior authorization or included non-formulary drugs, and the retroactive period preceded the sponsor’s transition period, this may have resulted in gaps in coverage. Coverage gaps may also have resulted from out-of-network pharmacy status or pricing in excess of the sponsor’s negotiated rates that were paid by the enrollee or another payer on the enrollee’s behalf.
In the years 2007 through 2009, CMS required sponsors to provide a special transition period to accommodate claims incurred during a 7 month (or less) period of retroactive eligibility. As mentioned at the beginning of this section, this special transition period is available to all Medicare beneficiaries. During the special transition period, standard transition rules apply, but sponsors are responsible for the allowable charges paid by other third party payers for all Part D drugs, including non-formulary drugs provided outside the transition period and formulary drugs with prior authorization requirements. The enrollee, or CMS in the case of low-income subsidy individuals, is responsible for any out-of-network or pricing differentials.
In January 2010, CMS implemented a new demonstration project, known as the limited income newly eligible transition (NET) program, to handle situations involving retroactive Part D enrollment. Under the demonstration, a single, competitively procured Part D sponsor will cover all Part D prescription drug claims for all periods of retroactive Part D coverage for full benefit dual eligible and SSI-eligible enrollees as well as POS coverage at the pharmacy for certain LIS individuals who are not yet enrolled in a Part D plan. Beneficiaries who are retroactively auto/facilitated enrolled by CMS and LIS beneficiaries confirmed eligible for the demonstration will be temporarily enrolled in the demonstration contractor’s plan. These enrollees will subsequently be randomly prospectively auto/facilitated enrolled in a qualified PDP. The low-income NET demonstration eliminates the routine need for sponsors to reimburse claims incurred by individuals eligible for the program during periods of retroactive Part D enrollment.
Sponsors, however, retain responsibility for making retroactive claims adjustments for beneficiaries enrolled in a sponsor’s Part D plan who become retroactively eligible for Medicaid during the period of Part D enrollment. For example, a beneficiary has been enrolled in a Part D plan since January 1, 2009 and in December 2009 receives a notice of Medicaid eligibility effective March 1, 2009. The sponsor is responsible for retroactively adjusting the enrollee’s cost-sharing for claims incurred beginning March 1, 2009 forward, in accordance with the guidance in section 50.14.3.
50.10 – Sharing Formulary Information with Other Payers
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Although Part D sponsors may share detailed information about their formularies (in electronic format) with other payers upon request, there is no specific requirement that they do so. CMS has made the Medicare Prescription Drug plan information available for purchase in Public Use Files (PUFs). These files contain all plan and formulary data for all of the plans with the exception of the pricing data, which is considered proprietary. This is the only data set that is publicly available. Further information is available on the CMS Web site; see Appendix B for the specific Web address.
In addition, as required by 42 CFR 423.120(b)(5)(i), sponsors will be required to inform other payers of formulary changes (i.e., formulary deletions or changes in the tiering status of a drug) at least 60 days in advance of the change. This may be accomplished by posting the new or changed information on Part D sponsor Web sites.
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
CMS does not have the authority to require data exchanges between Part D sponsors and States except as required for COB purposes. While the MMA requires Part D sponsors to allow SPAPs and other entities providing prescription drug coverage to “coordinate” with them, this language does not support requirements on the coordination of anything but payment. However, CMS strongly encourages Part D sponsors to independently share historical and ongoing data on any shared enrollees with other payers – particularly with States – provided such disclosure is consistent with the requirements of the HIPAA Privacy Rule. CMS encourages Part D sponsors to discuss reciprocal arrangements with State Medicaid Plans under which Part D sponsors would provide Part D drug claims data in exchange for both historical prescription drug claims data and ongoing medical claims (particularly diagnoses) on the dual eligible population to assist with medication therapy management (MTM) and other quality assurance programs. CMS also encourages sponsors to provide this reciprocal data exchange without charging any user fees.
Part D sponsors and States may negotiate details regarding the development of a Standard File Format for Patient Drug History and Standard Data Sharing Agreement. NCPDP, which is the national standards organization for pharmacy claims, has adopted the Post Adjudication Standard. Section 10 of the “Post Adjudication Standard Implementation Guide, Version 4.2” contains the “Post Adjudication Utilization Record,” which is the recommended standard record States and Medicare Part D sponsors could use to exchange drug history information. In order to access the NCPDP documentation and use the Post Adjudication Utilization Record, States and/or their contractors must be members of NCPDP.
If the States and Medicare Part D sponsors agree to exchange enrollees’ drug history information, then States and sponsors are new business associates. Thus, it is necessary that the exchange of data complies with HIPAA requirements. To adhere to HIPAA requirements, a Patient Drug History Data Sharing Agreement signed by the Medicare Part D sponsor and the State must be in place prior to executing file transfers between these entities.
CMS believes States have the authority under 42 USC §1396a(a)(25) to request information to coordinate benefits they may have paid under the State Medicaid program. CMS encourages Part D sponsors to review 42 USC §1396a(a)(25) as well as the related CMS guidance.
50.12 – Applying Medicare Secondary Payer (MSP) Requirements
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
The MMA (§1860D-2(a)(4)) extended MSP requirements that are applicable to MA organizations to include Part D sponsors. Accordingly, Part D sponsors will have the same responsibilities under MSP requirements as MA plans, including the collection of mistaken primary payment from insurers, group health plans, employer sponsors, enrollees, and other entities; and the relationship between MSP rules and State laws. Part D sponsors must properly apply MSP requirements and regulations to their payments (e.g., working aged, worker’s compensation (WC)).
Part D sponsors are responsible for adjudicating enrollees’ claims in accordance with the following MSP requirements. Also, sponsors are responsible for identifying and recovering any MSP-related mistaken payments and submitting associated adjustments to CMS.
According to statute, Medicare is the secondary payer in the following situations:
1. Employer group health plans (EGHP) MSP
a. Working Aged GHP – The beneficiary is actively working and is covered under the employer's GHP or the beneficiary's spouse is actively working and the beneficiary is covered under the spouse's employer GHP (≥20 employees; or another employer in GHP> 20 employees.) (42 U.S.C. §1395(y)(b)).
b. Disability with GHP – The beneficiary is actively working for a large employer and is covered under the employer's GHP, or a beneficiary’s family member is actively working for a large employer and the beneficiary is covered under the family member’s employer GHP (LGHP, ≥100 employees).
c. End Stage Renal Disease (ESRD) GHP – GHP (any size) is primary for the first 30 months when an individual also becomes eligible for Medicare Part A due to ESRD status. After 30 months of Part A eligibility, Medicare becomes primary.
2. Non-GHP MSP
a. WC – Beneficiary covered under WC due to job-related illness or injury.
b. Black Lung (BL) – The beneficiary has black lung disease and is covered under the Federal Black Lung Program.
c. No-Fault/Liability – The beneficiary is covered by no-fault or liability insurance due to an accident.
However, Part D sponsors should not immediately pay only as a secondary payer. The action required of the Part D sponsor is dependent on the type of other primary payer as follows:
1. For the types of Employer Group Health Plans (EGHP) listed above, the Part D sponsor will always deny primary claims that fall within the EGHP’s applicable coverage dates and default to MSP. The types, as listed above, include: working aged GHP, disability GHP, and ESRD GHP for first 30 months of Medicare Part A eligibility.
2. For WC, BL, and No-Fault or Liability coverage, the sponsor will always make conditional primary payment unless the sponsor is aware that the enrollee has WC/BL/No-Fault/Liability coverage and has previously established that a certain drug is being used exclusively to treat a related injury. For example, when a beneficiary refills a prescription previously paid for by WC, the Part D sponsor may deny primary payment and default to MSP.
In all other instances, the Part D sponsor is required to make conditional primary payment then recover any mistaken payments where it should have only paid secondary to WC/BL/No-Fault/Liability coverage. For example, if a sponsor does not know whether a given drug for which it is billed is related to the covered injury, the sponsor must pay for the drug (if it is a covered Part D drug) and later recoup any amounts that the other insurance should have covered.
If the sponsor has established it should pay only secondary for a Part D enrollee and receives a primary claim, the sponsor should not pay the primary claim. Rather, receipt of the primary claim should prompt the sponsor to question whether MSP requirements continue to apply. If MSP is no longer applicable, this information should be reported to the COB contractor via ECRS to update CMS.
Similarly, absent information on the COB file that Part D is secondary for a Part D enrollee, should the sponsor receive a secondary claim, the claim cannot be paid. Instead, the sponsor should determine if the enrollee has coverage that is primary to Medicare and, if so, report this information to the COB contractor via ECRS to update CMS.
The following sections provide clarification regarding a limited number of MSP situations; however, Part D sponsors are required to apply all MSP requirements, whether or not they are specifically mentioned here.
50.12.1 – Workers’ Compensation
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Medicare may not pay for any item or service when payment has been made, or can reasonably be expected to be made, for such item or service under a WC law or plan of the United States or any State. CMS recognizes that diagnostic information is not collected at the point of sale, however, Part D sponsors are expected to make good faith efforts to identify claims associated with WC.
It is imperative that Medicare’s interests be protected when parties enter into WC settlements. One method of protecting Medicare’s interest in a WC situation is a Workers’ Compensation Medicare Set-aside Arrangement (WCMSA), which allocates a portion of the WC settlement for future medicals and future prescription drug expenses. “Future medicals and future prescription drugs” are those services and items provided after the final WC settlement. CMS recommends Medicare beneficiaries (and individuals who expect to become entitled to Medicare within 30 months of receiving a WC settlement) who are parties to WC settlements, judgments or awards submit WCMSA proposals to CMS for review prior to settlement to ensure Medicare’s interests are considered. CMS reviews WCMSA proposals for Medicare beneficiaries with WC settlements greater than $25,000 and for individuals who are within 30 months of Medicare entitlement and possess a WC settlement greater than $250,000. Based on this review, CMS will either concur with the proposal or determine a different amount deemed adequate in order to protect Medicare’s interest. Additional information regarding CMS’ WCMSA policies, procedures and guidelines is available on the CMS Web site; refer to Appendix B for the specific Web address.
WCMSA funds are administered by the claimant or a professional administrator employed by the workers’ compensation employer, carrier or the claimant. CMS keeps a record of the WCMSA amount determined by CMS to be adequate to protect Medicare’s interests with regard to the claimant’s future medical treatment and/or prescription drug expenses. The claimant/professional administrator is responsible for submitting an annual attestation form or professional accounting to the Medicare contractor. This document attests that the claimant has appropriately expended the WCMSA funds for that year.
In order to assist the Part D sponsors in making proper payments to WCMSAs, at the end of 2009, CMS began including costs related to prescription drugs in its settlements and reporting WCMSAs under a distinct non-GHP MSP cost on the COB file. The WCMSA amount reported on the COB file is the combined amount for future medicals and future prescription drug costs related to the WC injury. In addition, the file will include the administrator’s name, address and telephone number, the WCMSA settlement date, the total prescription drug settlement amount, and an indicator specifying whether prescription drug costs are included in the WCMSA amount.
Beginning in 2010, if the COB file record received from CMS indicated prescription drugs are included in the WCMSA, Part D sponsors continued to make conditional primary payment under Part D and promptly contact the administrator to determine which claims should not be paid for under Part D. Once the Part D sponsor established that a certain drug was included in the set-aside, the sponsor set appropriate point-of-sale edits, denied payment and rejected the claim for billing to the primary payer.
Exhaustion of the combined WCMSA amount includes both services (i.e., future prescription drug treatment and future medicals). For example, if the total WCMSA amount provided to the Part D sponsors is $10,000, this amount can include $7,000 for future prescription drug treatment and $3,000 for future medical expenses. However, Part D sponsors must understand that although the total WCMSA amount is $10,000, the final actual expenditures could be $6,000 for future prescription drug treatment and $4,000 for the future medical expenses, which will still appropriately exhaust the WCMSA.
The Part D sponsors do not have the ability, via ECRS, to report the exhaustion of a WCMSA fund. The beneficiary is provided paperwork, in the WCMSA approval package, to complete and mail to the MSPRC when WCMSA funds have been exhausted. Once the documents are received, the MSPRC will then take the steps necessary to notify the COBC of this development. The CMS Regional Offices also have the ability, via ECRS, to report the exhaustion of WCMSA funds. Once the entire CMS-approved WCMSA has been properly exhausted, the Medicare Part D plan sponsor resumes responsibility for paying claims for covered Part D drugs.
50.12.2 – Flexible Savings Accounts (FSAs), Health Savings Accounts (HSAs), Archer Medicare Savings Accounts (MSAs), and Health Reimbursement Accounts (HRAs)
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
HSAs, FSAs, and MSAs
Part D sponsors should not require enrollees to use the funds in their FSAs, HSAs, or MSAs before making payments when the group health plans attached to those accounts are primary under the MSP laws. However, note that an enrollee would only have an FSA or HSA when these accounts are carried over from an employee health plan. An enrollee may have an MSA at any time; it is similar to the plan attached to an HSA, but is offered exclusively to Medicare beneficiaries.
HRAs
However, under the MSP group health plan laws (e.g., when an enrollee with current employment status has an HRA through his employer), sponsors should make secondary payments after HRA funds are used.
When an enrollee is non-working, an HRA is secondary to Medicare, but drug costs paid or reimbursed from the HRA are not TrOOP-eligible.
50.13 – Executing Business Associate Agreement (BAA) with Part D Transaction Facilitator
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Consistent with the HIPAA Privacy Rule (45 CFR Parts 160 and 164), the transaction facilitator will be a business associate of Part D sponsors for the purpose of performing TrOOP facilitation and COB functions when it receives data directly from the sponsor. Currently, the facilitator receives data from a sponsor whenever a beneficiary makes a contract-level enrollment change during the coverage year, and the automated TrOOP balance transfer process is triggered. In that process, the transaction facilitator receives data from the disenrolling Part D plan as well as from any prior Part D plan in which the beneficiary was enrolled during that coverage year. Note that the BAA requirement is applicable not only to sponsors directly reporting the TrOOP accumulators to the transaction facilitator, but also to sponsors using a processor for the automated TrOOP balance transfer process. Therefore, it is critical that each Part D sponsor has a signed agreement with the Transaction Facilitator. However, the requirement for a BAA can be met by either the sponsor having a signed agreement with the transaction facilitator or the sponsor’s processor entering into a BAA with the facilitator on behalf of the plan sponsor. A BAA executed between the sponsor and their processor is not sufficient to meet this requirement because, in the automated TrOOP balance transfer process, the facilitator is doing work on behalf of the plan sponsor. Therefore, there must be an executed BAA between either the sponsor or the sponsor’s subcontractor (that is, the processor) and the transaction facilitator. If the sponsor has a BAA with the facilitator, the sponsor’s PBM/processor is not required to enter into separate BAA with the transaction facilitator, since data at the PBM/processor will be protected through the BAA between the Part D sponsor and the PBM. To facilitate the execution of these agreements between the transaction facilitator and the Part D sponsors, CMS has developed a standard language business associate agreement and sponsors are strongly encouraged to sign this agreement without modification.
50.14 – Payment Reconciliation
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Because of program start-up issues in 2006, lags in the information available to pharmacies at the point-of-sale regarding which Part D sponsor to bill for a prescription may have resulted in the pharmacies’ having access to outdated or incomplete information. Because pharmacies generally relied in good faith on this information, in some cases the wrong payer paid for a prescription. Given the volume of drug claims that pharmacies would need to re-adjudicate as a result of incorrect Part D enrollment information available at the point-of-sale, re-adjudication would have imposed a significant administrative and financial burden on pharmacies. Therefore, payer-to-payer reconciliation procedures were developed to mitigate the administrative and financial burden involved with re-adjudication of claims.
Although this payer-to-payer process was designed initially to be a temporary measure during Part D’s start-up phase, CMS requires that sponsors continue to use the payer-to-payer process. In addition, unforeseeable future events may necessitate processes to reconcile payments when a payer other than the correct Part D sponsor of record pays as primary for a covered Part D drug for an enrollee. These other reconciliation processes may be developed by CMS to accomplish payment reconciliation without involving pharmacy reversal and re-adjudication of claims or the public release of a payer’s proprietary information, such as negotiated rates.
50.14.1 – Plan-to-Plan Reconciliation During Transition Periods
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Beneficiaries have the opportunity to change their Part D plan enrollment during the coverage year, which consequently creates situations in which, due to lags associated with the enrollment process and information systems updates, the sponsor from which a beneficiary has transferred makes payment for covered prescription drug costs incurred after the effective date of the beneficiary's enrollment in the new sponsor of record. In 2006, CMS developed a plan-to-plan (P2P) reconciliation process with sponsor participation. This process, implemented in three phases, enables CMS to process prescription drug event (PDE) data in these P2P transition situations and allow for financial reconciliation between the affected Part D sponsors. The process's design reflects the consensus of sponsor participants to prevent disclosure of proprietary pricing information by masking the NDC coding. Furthermore, to protect sponsors from exposure to costs outside the initial formulary transition period, CMS established a 30-day P2P transition period. Therefore, the P2P transition period includes claims with dates of service less than or equal to the later of:
30 days after the effective date of the new plan enrollment, or
30 days after the date CMS processes the new contract of record enrollment.
To address the payment reconciliations needed to resolve these enrollment transition issues, CMS requires the ongoing use of the P2P reconciliation and reimbursement process. Therefore, throughout each coverage year, Part D sponsors will continue to receive monthly P2P reports showing the payables and receivables for which financial settlement is required. PDE guidance describing the process is available on the CMS Web site; see Appendix B for the specific Web address.
50.14.2 – Other CMS-Defined Reconciliation Processes
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Unforeseeable future events may create the need for processes requiring Part D sponsors to coordinate benefits on a timely basis with other third parties and use CMS-developed reconciliation processes, when established, in situations in which a payer other than the correct Part D sponsor of record pays for covered Part D drug costs as a primary payer. This includes, for example, the scenario in 2006, with the State-to-Plan Reconciliation Project in which some States made drug payments for dual eligible beneficiaries and low-income subsidy entitled beneficiaries enrolled in Part D and were subsequently reimbursed by CMS through a special demonstration authority. Processes similar to the State -to-Plan Reconciliation process employed in 2006 may need to be developed by CMS in lieu of requesting pharmacy claims reversals and re-adjudications or the public release of a payer’s proprietary information (e.g., negotiated prices).
50.14.3 – Retroactive Claims Adjustments
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Part D sponsors must coordinate benefits with SPAPs and other providers of prescription drug coverage and appropriately adjudicate claims. Compliance with this requirement entails that the sponsor not only coordinate benefits with other payers at POS, but also work with beneficiaries and other payers to resolve post-adjudicative payment issues arising from retroactive claims changes.
Retroactive claims adjustments can be necessitated by beneficiary changes (such as those resulting from retroactive LIS eligibility determinations, LIS status changes, or midyear Part D enrollment changes), sponsor receipt of other payer information, or errors in payer order. Some of these changes, i.e., those occurring within the payers’ timely filing window (which must be a minimum of 90 days for Part D, but may be as short as 30 days, for other (non-Part D) payers) may be addressed through pharmacy-initiated reverse and rebill transactions. However, as specified in section 50.15.5 of this chapter, sponsors generally should limit requests for pharmacy reprocessing to those situations involving a payment error. All retroactive claims adjustments that cannot be addressed through pharmacy reverse and rebilling must be handled by the Part D sponsor through other means.
Post-adjudicative changes, such as those that are due to enrollment changes, are changes that affect beneficiary cost-sharing, premiums and/or plan benefit phase. Part D sponsors must make the retroactive adjustments timely and promptly issue refunds or initiate recovery once complete information regarding the claims adjustment is received. Federal regulations at §423.466(a) require sponsors to process the adjustment and issue refunds or recovery notices within 45 days of recipt of information necessitating the claims adjustment. Federal regulations at §423.800(e) apply this same timeframe to retroactive adjustments to cost-sharing for low-income subsidy eligible individuals.
Federal regulations at §423.800(c) require plan sponsors to reimburse beneficiaries amounts owed due to changes in LIS status. However, as specified in chapter 13, section 70.3.1 of this manual, sponsors must make reasonable efforts to determine which party should be reimbursed; i.e., the beneficiary or other party who paid on the beneficiary’s behalf, for the excess cost sharing paid during a period of LIS retroactive coverage. Sponsors should develop procedures for making these reimbursement determinations and not adopt a “one size fits all” approach. Specifically, sponsors should not automatically reimburse beneficiaries residing in LTC facilities, since it is unlikely that the LTC pharmacy has collected the applicable cost-sharing due to the expectation that the sponsor would eventually reimburse the pharmacy retroactively for these amounts. Rather, sponsors should work with their network pharmacies to provide direct reimbursement for any cost-sharing amounts not collected from LIS-eligible enrollees. Chapter 13 is available on the CMS Web site; see Appendix B for the specific Web address.
The instability of LIS data and Part D enrollments creates a significant volume of retroactive adjustments, and it has become evident that sponsors are facing more claims adjustments than current pharmacy claim reversal and rebilling approaches can adequately address. In the case of a claims adjustment, if the beneficiary is no longer at the counter and a supplemental payer's claim filing window is closed, the pharmacy can no longer effectively coordinate benefits between payers. In addition, payers cannot effectively coordinate among themselves, both because of the absence of electronic standards for post-adjudication claim adjustments among payers (as opposed to between pharmacies and payers), and the presence of contractual prohibitions between payers and pharmacies on the disclosure of proprietary pricing information. Therefore, CMS continues to work with the industry to determine how best to handle retroactive claims adjustments whenever the adjustment cannot be resolved simply between the sponsor and the pharmacy.
Regardless of the cause of the retroactive claims adjustment, sponsors have two choices for determining the change to beneficiary TrOOP. The sponsor may adjust each claim that was affected by the retroactive change, or they may process the adjustment as they administer the benefit, provided that:
TrOOP accumulators are updated immediately;
Monies owed beneficiaries are refunded promptly;
Claims are restacked and adjustments are processed at least quarterly; and
An exceptions process exists for more frequent processing to meet beneficiary needs, such as at disenrollment during the coverage year.
The methodologies for handling retroactive changes in TrOOP are described in Section 9 of the Prescription Drug Event (PDE) guidance available on the CMS Web site. See Appendix B for the specific Web address. For further detail on reconciling payments, see section 50.14 of this chapter.
Part D sponsors also must determine whether or not any amount paid by any other payers was TrOOP-eligible and must adjust, as necessary, the affected beneficiaries’ TrOOP balances.
50.14.4 – Resolution Directly with Other Payers
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
The plan-to-plan reconciliation process resolves those situations in which a Part D sponsor other than the sponsor of record paid claims for a beneficiary during the initial transition period. However, situations will continue to arise outside the plan-to-plan process in which other payers that are not Part D sponsors either pay when they should not have paid at all, or pay more than they should have, because they paid out of the correct payer order. In these situations, Part D sponsors are required to work with these providers of other prescription drug coverage to resolve these types of payment issues. Other payers, as well as beneficiaries, are entitled to seek compensation from the Part D sponsor once the Part D enrollment is confirmed.
Therefore, sponsors should implement processes to handle payment resolution directly with other payers, beneficiaries, and others who are holding receivables on the beneficiaries’ behalf. Sponsors may not restrict the payment resolution process by imposing timely filing requirements on these other parties that are more restrictive than the timeframe required in Federal regulations at 42 CFR 423.466(b). This provision requires Part D sponsors to coordinate benefits with SPAPs and other entities providing prescription drug coverage, beneficiaries and others paying on the beneficiaries’ behalf for a period not to exceed 3 years from the date on which the prescription for a covered Part D drug was filled.
In instances when Medicaid has paid for a covered Part D drug and then seeks reimbursement from Part D, the sponsor should handle the Medicaid subrogation as follows:
Refund Medicaid the lesser of the sponsor’s out-of-network pharmacy allowed amount or the amount sought by Medicaid.
Apply no beneficiary cost-sharing or low-income subsidy to the claim.
The pharmacy-initiated reverse and rebill approach supports only a portion of the retroactive claims adjustments a Part D sponsor must handle. Therefore, sponsors must work directly with other payers to resolve reimbursements and recoveries for the majority of retroactive claims adjustments. Resolution of these latter adjustment actions becomes more complex by the absence of the other payers’ amount paid on the N transaction to the Part D plan. In order to ensure the confidentiality of pharmacy pricing information, coordination of benefits on initial claims is accomplished by reporting to the Part D sponsor only the amount of the beneficiary’s payment after the supplemental payment. As a result, a Part D sponsor attempting to determine refund or recovery amounts without having the pharmacy reverse and rebill the original claim must calculate the amount of any supplemental payment made by another payer by determining the difference between the Part D cost-sharing and the beneficiary amount paid after the supplemental payment. While CMS acknowledges that electronic transaction standards are not yet available to support timely, reliable, and precise coordination on adjusted claims when multiple payers are involved, it continues to hold sponsors accountable for making best efforts to coordinate benefits generated by claim adjustments.
50.14.5 – Re-adjudication Versus Pharmacy Reprocessing
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
If the total payment to the pharmacy for a claim was correct, but the sponsor subsequently determines that an adjustment is required that does not affect the total payment and does alter the sponsor-beneficiary liability split, the sponsor must re-adjudicate the claim within its own system without involving the pharmacy. This is most likely to occur when the sponsor corrects low-income beneficiary cost-sharing subsidy levels.
Part D sponsors are encouraged to avoid pharmacy reprocessing, but CMS recognizes that reversals may be appropriate under certain circumstances. Sponsor requests for pharmacy reprocessing should generally be limited to those situations where the total payment to the pharmacy changes (e.g., when there is a pricing error). Sponsors are responsible for reimbursing or collecting amounts from beneficiaries that result from the reprocessing of these claims and should not transfer this responsibility to pharmacies.
50.14.6 – Timeframes for Claims Filing
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
A number of issues associated with Part D, such as incidents involving multiple payers, payer order, and retroactive eligibility, create challenges for coordinating benefits among Part D sponsors and other providers of prescription drug coverage. When all payer information is available at the point-of-sale, pharmacies typically serve as the intermediary, facilitating coordination between Part D sponsors and other payers. However, when the information necessary to identify the correct primary payer for Part D drugs provided to Medicare beneficiaries enrolled in Part D sponsors is incomplete or lacking, pharmacies may, through no fault of their own, bill the State and other payers instead of a beneficiary’s Part D sponsor.
CMS may address many of these situations through a special, one-time reconciliation process. However, some of these situations may require resolution through claims reversal and rebilling. In their role of facilitating coordination between Part D sponsors and payers, some pharmacies agree to reverse incorrect claims and bill the proper Part D sponsor. CMS believes that in those circumstances in which the pharmacy is not at fault, it would be inappropriate for Part D sponsors to impose the conventional 30-90 day timely filing limits rather than a less restrictive timeframe, since this industry standard generally applies only when the pharmacy is in a position to correctly bill but fails to do so. CMS believes that this process is also appropriate for use in the Point of Sale Facilitated Enrollment process when incorrect health insurance claim numbers (HICNs) were used.
Beginning in 2007, in lieu of a requirement for a 180-day timeframe, which was implemented to accommodate the identification and resolution of coordination of benefits issues requiring claims reversal and rebilling to appropriate payers when Medicare Part D was introduced in 2006, CMS requires sponsors to establish at least a 90-day claims filing timeframe and to make appropriate allowances for COB claims on a case-by-case basis. It is important to note also that plans may be liable for claims from the prior year that are received after March 31st. While in these instances contractual provisions regarding timely claims filing may limit claims from network pharmacies, non-network pharmacies and beneficiaries must still have the opportunity to submit claims for reimbursement.
With the inclusion in the claim segment of the transaction standard for retail pharmacy drug claims of field number 357-NV, Part D sponsors may use certain delay reason codes in the external code list, to specify the reason for the delay in claims submission, in order to differentiate COB-related delays from other types of delays.
60.1 – Reporting the Existence of Prescription Drug Coverage Provided to Enrollees
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
As discussed in section 30.1 of this chapter, CMS expects that other payers will provide information regarding any other prescription drug coverage that their Medicare enrollees may have. As noted in section 40.1 of this chapter, Medicare beneficiaries are required to disclose this information to Part D sponsors; consequently, other payers responsible for payment or reimbursement of Part D claim cost sharing should assist their enrollees in releasing this obligation. Certain legal requirements exist to inform CMS when another payer provides coverage that is primary to Medicare under the MSP laws (e.g., employers sent the Data Match questionnaire described later in this chapter, the 42 CFR 411.25 notice requirement). MSP reporting entails that affected entities use the MSP-specific reporting methods CMS requires (e.g., Data Match forms) or provides (e.g, VDSA in lieu of Data Match forms). However, for seamless benefit coordination and accurate TrOOP accounting, CMS strongly encourages payers to report their coverage information even when it is not legally required.
To do this, CMS makes available a direct and easy data exchange process through a vendor, the COB contractor. A data exchange with CMS allows other payers:
To assist beneficiaries in fulfilling their statutory obligation to disclose third party reimbursement for Part D drug costs.
To avoid the cost of paying as primary when the payment should be secondary to Part D.
As a sponsor of record, to be notified if a paid claim is reversed or adjusted outside an on-line adjudication process.
If TrOOP-eligible, to cease payments for beneficiaries receiving the full low-income subsidy who reach the catastrophic phase of the benefit, since at that point, Medicare fully subsidizes the beneficiary's incurred costs for covered Part D drugs.
The data exchange agreements require payers to periodically submit an input file containing certain enrollee populations. In return, the payer will receive a response file from the COB contractor indicating which of its enrollees are Medicare Part D beneficiaries. More information about the COB process offered by CMS is available on the Medicare COB Web site. See Appendix B for the specific Web address.
60.2 – Obtaining and Reporting Rx Identifiers
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Payers supplemental to Medicare should obtain a unique RxBIN and/or PCN combination for Medicare eligible beneficiaries that will identify their paid claim requests and responses for TrOOP tracking purposes when Part D is the primary payer. CMS recommends that payers obtain an RxBIN and/or PCN combination unique to each separate plan they offer in order to distinguish each of their plans from one another. This allows each benefit plan to fulfill its obligation as a supplemental payer if it is identified on the COB file as secondary coverage.
In order for Rx identifier information to be available at point-of-sale through the transaction facilitator and Part D sponsors, payers must report these unique identifiers to CMS through the COB reporting process described in section 30 of this chapter. Payers primary to Medicare will continue to use their existing BIN and/or PCN.
NOTE: Not all other prescription drug coverage will have Rx identifiers. For instance, incident-driven coverage, such as Worker’s Compensation, does not usually provide electronic, point-of-sale benefits and thus does not need such identifiers; also, SPAPs that only offer premium assistance will not have Rx identifiers.
60.3 – Supplying Claims Information When a Supplemental Payment Is Made
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
In order for the COB and TrOOP tracking processes to function effectively, other payers should supply paid claims information to the Part D sponsor after making a payment that is supplemental to a Medicare payment. This will happen automatically if the other payer reports their coverage information to CMS in accordance with the processes described in section 60.1 of this chapter with the appropriate Rx BIN and/or PCN combination to enable the transaction facilitator to identify the supplemental payer’s status.
However, if the other payer is aware that the transaction facilitation process was not used, or if the other payer does not have electronic claims capability, the payer may alternatively submit a batch file of supplemental claims information or make arrangements to submit information in another format to the transaction facilitator. The supplemental claims data submitted to the facilitator will then be supplied to Part D sponsors for TrOOP calculation. If a payer uses the batch process, it must still establish a unique RxBIN and/or PCN combination and participate in the data sharing exchange with CMS' COB contractor. Further information on the batched claims process is available on the transaction facilitator’s Web site; see Appendix B for the facilitator's Web address. If a payer does not either support the on-line or batch process, no Nx transaction will be created and Part D sponsors will not be required to coordinate benefits if post-adjudicative claims adjustments are made.
60.4 – Coordinating with Part D Sponsors for Payment of Premiums
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
If one of the “other payers” listed in 42 CFR 423.464 chooses to pay Part D premiums on behalf of its members who are enrolled in Part D sponsors’ plans, that payer should coordinate directly with the appropriate Part D sponsor. Part D sponsors are required to allow and facilitate premium payment coordination with other payers. If the sponsor fails to comply with this requirement, it cannot disenroll a beneficiary for failure to pay premiums. Further discussion on coordination of premiums is contained in section 50.6 of this chapter.
60.5 – Following MSP Laws and Order of Payment Standards
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
MSP laws apply to all payers, including those providing prescription drug coverage, and all payers are legally required to make themselves aware of and follow these laws. This chapter provides clarification regarding the limited number of MSP situations described below; however, payers are required to know and apply all MSP laws whether or not they are mentioned in this chapter.
Appendix A – Transaction Facilitation Process
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
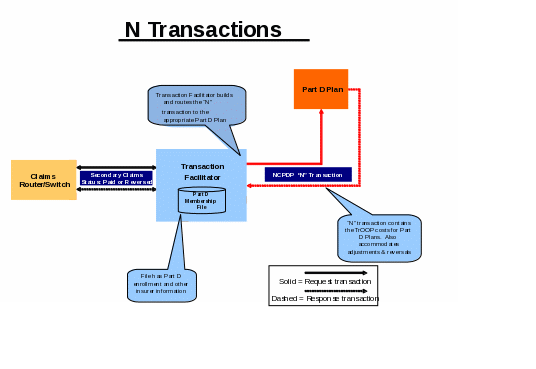
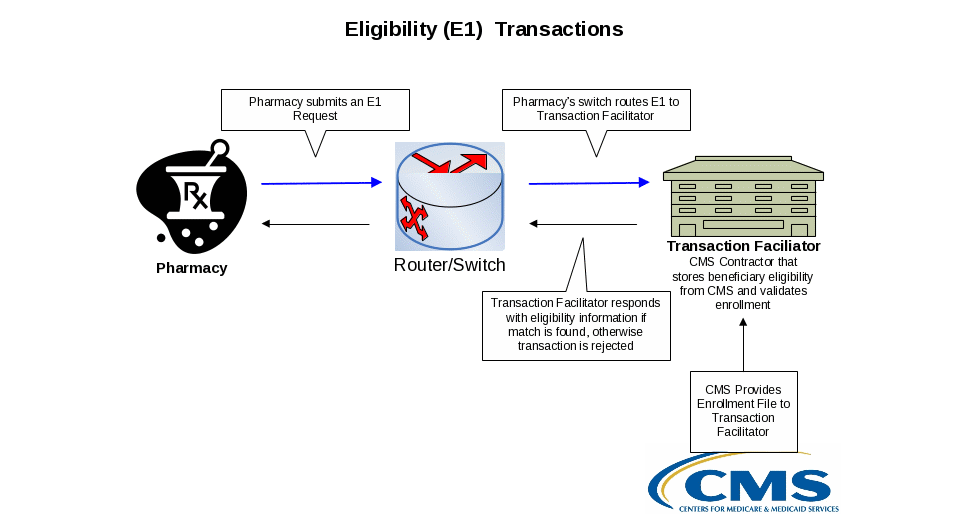
Appendix B – COB-related Web Sites
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
CMS Medicare Prescription Drug Coverage Contracting
http://www.cms.hhs.gov/PrescriptionDrugCovContra/
CMS WCMSA Policy
http://www.cms.hhs.gov/WorkersCompAgencyServices
Dual Eligible PACE Plan Beneficiary Accumulated True Out-of-Pocket Cost Calculator
http://www.cms.hhs.gov/apps/troopcalculator/
ECRS User Guide
http://www.cms.hhs.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/msp105c05_att1.pdf
Medicare Beneficiary Publications
Medicare COB
http://www.cms.hhs.gov/COBGeneralInformation/
Medicare Marketing Guidelines
Medicare Part C and D Call Letters and Part D Benefit Parameters
http://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Announcements-and-Documents.html
National Council for Prescription Drug Programs
National Institute of Standards and Technology
http://csrc.nist.gov/groups/STM/cmvp/documents/140-1/1401vend.htm
OIG Guidance on Part D and PAP
http://www.oig.hhs.gov/fraud/docs/advisoryopinions/2006/AdvOpn06-03F.pdf
PAP Data Sharing Agreements
PDE Guidance
PDE Participant Guide
Part D Eligibility, Enrollment and Disenrollment Guidance
Plan Communications User’s Guide (PCUG)
http://www.cms.gov/Research-Statistics-Data-and-Systems/CMS-Information-Technology/mapdhelpdesk/Plan_Communications_User_Guide.html
Public Use Files (PUFs)
Part D Transaction Facilitator (NDCHealth d/b/a RelayHealth)
http://medifacd.relayhealth.com/
Appendix C – Part D Sponsor Guidance—Automated TrOOP Balance Transfer
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
Part D Plan Sponsor Guidance on the Financial Information Reporting (FIR) Transactions for Transferring True Out-of-Pocket Balances
Table of Contents
Background on TrOOP Balance Transfers
Testing and Certification Requirements
Compliance
Procedures for TrOOP Balance Transfer Using FIR Transactions
Role of theTransaction Facilitator
Inclusion of non-plans of record
Evaluation of transaction responses
Multiple enrollments within a contract
Contract-level enrollment changes involving a single processor
Receipt of Inquiry when a prior plan is known
Sponsor requested FIR transactions
Other automatically generated FIR transactions
Correction of unacceptable responses
Other FIR-related sponsor activity
Timing of the FIR Transaction Sequences
Exceptions from Automated Processing
Reports to Sponsors
Background on TrOOP Balance Transfers
Part D rules require sponsors to track the beneficiary’s true out-of-pocket (TrOOP) costs and gross covered drug spending and properly apply these costs to the TrOOP and benefit limits in order to correctly place the beneficiary in the benefit and provide the catastrophic level of coverage at the appropriate time. The TrOOP threshold and gross covered drug spending are calculated on an annual basis and must be transferred between Part D plans if a beneficiary disenrolls and re-enrolls at any time before the end of the coverage year.
The TrOOP-related data must also be transferred between Part D plans in those circumstances in which a Part D plan other than the plan of record paid for covered Part D drug costs as a primary payer and subsequently becomes aware, for example, through a CMS enrollment reconciliation process, that the beneficiary is enrolled in another Part D plan.
Prior to the implementation of the FIR transaction standard that supports the automated plan-to-plan transfer of TrOOP-related data, CMS required the use of a manual process to transfer these data between plans. Once the NCPDP approved the FIR transaction standard, the “transaction facilitation process,” established by CMS to capture TrOOP-relevant data from Part D sponsors on-line and send these data to the appropriate Part D Plan for TrOOP calculation, uses the FIR to electronically transfer any TrOOP-related data between plans.
With the January 1, 2009, implementation of the new FIR transactions to electronically transfer TrOOP and gross covered drug costs, further routine need for the manual data transfer process was eliminated.
Testing and Certification Requirements
The Part D transaction facilitator in collaboration with CMS, NCPDP and industry representatives developed a set of testing scenarios and a FIR testing certification process. Guidance describing this process is available on the transaction facilitator’s Web site; see Appendix B for the specific Web address. Each coverage year, new Part D sponsors (with the exception of PACE organizations that opt not to use the automated process) must ensure that their PBM or other processors are certified. Therefore, new Part D sponsors should require their PBM/processor to cooperate fully with and respond timely to all contacts from the transaction facilitator, to participate in the testing process and achieve certification. During certification testing, the facilitator will monitor the process and notify CMS of any new contract sponsors that have not met the requirements. CMS will initiate appropriate compliance action.
Additionally, as new versions of the FIR transaction standard are approved by NCPDP and scheduled for implementation, sponsors must ensure their PBMs/processors undergo certification testing for the new version of the transaction. Thus, prior to the September 1, 2012, implementation of the Contract/Plan Benefit Package fields in new Version 1.2 of the FIR transaction, certification testing was conducted by the transaction facilitator. The certification test cases for the FIR version 1.2 are available on the facilitator’s website under the heading “Certification Test Cases Description- Version 1.2”
Compliance
CMS reminds sponsors that under the regulations at 42 CFR 423.464, Part D sponsors are required to coordinate benefits with other Part D plans, which includes the transfer of TrOOP and gross covered drug costs when a beneficiary changes enrollment during the coverage year to enable the new plan of record to properly position the beneficiary in the benefit. According to this regulation, sponsors must also comply with CMS established processes to ensure coordination between plans. If the procedures and timelines outlined in the FIR testing and certification guidance are not adhered to by Part D sponsors and any applicable plan contractors, CMS has the authority to consider the sponsor out of compliance with the Part D requirements and to take appropriate action.
In 2010, CMS indicated that it expected Part D sponsors to successfully transfer TrOOP accumulator data for beneficiaries making contract-level enrollment changes during the coverage year in a timely manner 100% of the time. To measure compliance, CMS established a 30-day timeframe for successful transfer of the data beginning with the effective date of the enrollment change or, if later, the first automated TrOOP balance transfer (ATBT) transaction. Sponsors failing to meet this timeframe were considered to be out of compliance and subject to compliance actions. Beginning in January 2013, sponsors are required to successfully transfer TrOOP accumulator data within 15 days of the effective date of the new enrollment or, if later, the date of the initial ATBT transaction. For example, if the effective date of the enrollment change is March 1, 2013, the beneficiary’s TrOOP accumulators must be successfully transferred on or before March 15. However, if the Part D transaction facilitator did not receive notice of the enrollment change until March 6, the initial ATBT transaction would be sent on March 6 and the TrOOP data would need to be successfully transferred on or before March 20.
For purposes of the automated TrOOP balance transfer process:
A “plan of record” is a Part D sponsor with a valid, effective enrollment in the CMS system for a Medicare beneficiary for whom the sponsor receives final monthly payment. A sponsor may be the beneficiary’s initial plan of record for the coverage year, a subsequent plan of record with a closed period of enrollment, and/or the current plan of record.
A “non-plan of record” is a Part D sponsor that paid covered Part D drug claims for a Medicare beneficiary for whom the sponsor did not have a valid and effective enrollment in the CMS system and for whom the sponsor did not receive final monthly payment. This may occur in situations when the sponsor submitted an enrollment transaction that was processed, but then audited it off due to CMS’ receipt of a subsequent valid enrollment transaction for the same effective date, or if the sponsor’s enrollment transaction was not accepted by CMS and, therefore, is not in the CMS system. There might be multiple non-plans of record for a beneficiary during a coverage year, even for the same month.
Procedures for TrOOP Balance Transfer Using FIR Transactions
Role of the Transaction Facilitator
Using the information the CMS transaction facilitator receives nightly from the CMS MBD, the facilitator identifies when a change in enrollment at the contract-level has occurred and generates a FIR transaction to each prior sponsor with which the beneficiary was enrolled or that paid covered part D drug claims for the beneficiary during the coverage year. Transactions begin with a FIR Inquiry to the earliest sponsor on record in the coverage year; that sponsor's Inquiry response is returned to the facilitator. Each sponsor responds with their monthly gross covered drug costs and TrOOP amounts. If there are multiple plans prior to the current plan of record, the accumulator values from the last response received are placed in a FIR Exchange transaction and forwarded to the next sponsor. The facilitator receives that next sponsor’s transaction response and continues the process of receiving and forwarding the prior accumulators until each subsequent sponsor in consecutive order has received and responded to a FIR Exchange transaction. The final Exchange transaction response contains the year-to-date monthly TrOOP-related data for all plans prior to the current plan of record; these accumulated monthly amounts are forwarded by the facilitator via a FIR Update transaction to the current plan of record. The FIR transaction process flows, involving a single prior plan and multiple prior plans, are detailed in section 4 of the NCPDP Financial Information Reporting Standard Implementation Guide v1.2.
Inclusion of non-plans of record
As noted previously, TrOOP-related data must also be transferred between Part D plans when a Part D plan other than the plan of record (i.e., a non-plan of record) paid for covered Part D drug costs as a primary payer and subsequently becomes aware that the beneficiary is enrolled in another Part D plan. This may occur if the other plan’s enrollment was processed and then audited off due to CMS’ receipt of a subsequent valid enrollment transaction for the same effective date, or if the enrollment in the other plan was not accepted by CMS and, therefore is not in the CMS system. Most audited enrollments will be identifiable by the facilitator, unless more than one record was audited off on the same day; in this case, only the latest audited record will be reflected on the TrOOP file.
In situations when the facilitator is unable to identify the existence of a non-plan of record (e.g., when the enrollment was never accepted by CMS, but the plan paid claims), in order for the TrOOP data to be transferred, the non-plan of record sponsor must contact the facilitator and request inclusion in the FIR reporting. To include these non-plan-of-record sponsors in the FIR process, the facilitator must create a “proxy” enrollment record identifying the sponsor, rather than CMS, as the source of the information, including the contact person providing the information and the date of contact. The facilitator will include the non-plan of record in the FIR transaction sequence preceding the actual plan of record for the month(s) the non-plan of record paid Part D claims.
Evaluation of transaction responses
The transaction facilitator uses a set of business rules to evaluate the acceptability of the sponsor’s FIR response; these rules are limited to edits to verify that there are no missing/invalid data elements in the response that are required by the facilitator to generate the next FIR transaction in the sequence. If any of these business rules are violated, the facilitator will suspend the transaction sequence and notify the sponsor of the rejected transaction on the daily report. The transaction facilitator will re-initiate the transactions with the next regularly scheduled FIR sequence.
Part D sponsors must track TrOOP-related data for their months of coverage for beneficiaries who disenroll during the coverage year and report these data, even if the accumulator values are zeros (see NCPDP FIR Standard Implementation Guide for reporting no claim activity), to the facilitator in response to FIR transaction requests. FIR accumulators should be based on the month of service, not the month the claim was processed.
Sponsors must also receive FIR transactions reporting TrOOP-related data reported by prior plan sponsors through the facilitator, update their systems to incorporate these data, examine their claims history and any previously reported amounts from prior plan sponsors to determine the impact of any changes in reported data on the beneficiary’s position in the benefit and re-calculate, as necessary, any prior claims affected by changes in the TrOOP accumulators.
It is CMS’ expectation that FIR transactions are processed in real-time. This includes not only reporting data, but also receiving the data reported by prior plans and using this information in benefit administration.
NOTE: A change at the contract level will trigger the FIR transaction process. If the beneficiary changes plan benefit packages (PBPs) within a contract and the BIN/PCN is unchanged, the sponsor is responsible for ensuring that the TrOOP balance and gross covered drug costs for all months of the first PBP’s coverage are available to the subsequent if the PBPs within the contract use the same processor.
Further, some sponsors use different contractors for eligibility/enrollment functions and claims processing. It is the sponsor’s responsibility to ensure that the contractor responsible for TrOOP balance transfer has all eligibility and enrollment information to properly administer the TrOOP balance transfer process, consistent with this guidance and the NCPDP Financial Information Reporting Standard Implementation Guide. This includes having information to identify the beneficiary (e.g., the CMS date of birth), and his or her eligibility and enrollment periods consistent with CMS requirements.
Multiple enrollments within a contract
When a beneficiary has multiple enrollments within a contract prior to a contract-level enrollment change, whether the BIN/PCN for the multiple enrollments within the contract are the same or different, the facilitator will send the transactions in the usual manner. When the BIN/PCN for multiple enrollments within the contract are the same, the processor, however, has two options for reporting for their months of coverage:
Report all periods of coverage on all transactions received for the member; or
Report each period sequentially as the transactions are received.
The following scenarios describe the FIR reporting requirements under both options for situations when a beneficiary has multiple plan enrollments within a contract during the coverage year, involving the same BIN/PCN combinations.
Scenario 1- Option 1 Reporting
Beneficiary Enrollment History
Months of Coverage |
Contract/PBP Number |
Plan |
BIN/PCN |
FIR Transaction |
Processor Response |
Jan. – Mar. |
S0001-001 |
A |
611220/ 1234567890
|
FIR Inquiry |
Reports Jan. – May data |
Apr.- May |
S0001-002 |
B |
611220/ 1234567890
|
FIR Exchange |
Reports Jan. – May data |
Effective June |
S0002-001 |
C |
121212/ 23232323bb |
FIR Update |
|
When the facilitator identifies the contract-level enrollment change to Plan C, a FIR Inquiry transaction will be sent to the BIN/PCN for Contract S0001. Since the BIN/PCN combination is the same for both contract S0001 PBPs, the processor responds with the January through May accumulators, reporting all months of enrollment in Plans A and B. The Plan B sponsor processor will then receive a FIR Exchange transaction and responds by reporting the January through May accumulators. The processor must exercise care to avoid duplicating their accumulators when using this reporting option. The monthly accumulators for January through May will be forwarded by the facilitator to the Plan C sponsor in a FIR Update transaction.
Scenario 1- Option 2 Reporting
Beneficiary Enrollment History
Months of Coverage |
Contract/PBP Number |
Plan |
BIN/PCN |
FIR Transaction |
Processor Response |
Jan. – Mar. |
S0001-001 |
A |
611220/ 1234567890
|
FIR Inquiry |
Reports Jan. – Mar. data |
Apr.- May |
S0001-002 |
B |
611220/ 1234567890
|
FIR Exchange |
Reports Apr. – May data |
Effective June |
S0002-001 |
C |
121212/ 23232323bb |
FIR Update |
|
When the facilitator identifies the contract-level enrollment change to Plan C, a FIR Inquiry transaction will be sent to the BIN/PCN for Contract S0001. Although the BIN/PCN combination is the same for both contract S0001 PBPs, the processor responds with the January through March accumulators, reporting the months of enrollment in Plan A only. The Plan B sponsor processor will then receive a FIR Exchange transaction and responds by reporting the April through May accumulators. The monthly accumulators for January through May will be forwarded by the facilitator to the Plan C sponsor in a FIR Update transaction.
Scenario 2- Option 1 Reporting
Beneficiary Enrollment History
Months of Coverage |
Contract/PBP Number |
Plan |
BIN/PCN |
FIR Transaction |
Processor Response |
Jan. – Mar. |
S0001-001 |
A |
611220/ 1234567890
|
FIR Inquiry |
Reports Jan. – Mar. & June – Aug. data |
Apr.- May |
S0002-001 |
B |
121212/ 23232323bb |
FIR Exchange |
Reports Apr. – May data |
June – Aug. |
S0001-001 |
C |
611220/ 1234567890 |
FIR Exchange |
Initially reports Jan.- Mar data & June - Aug. data (Subsequently reports any changes to June – Aug. data resulting from Apr.- May data) |
Effective Sept. |
S0003-001 |
D |
999991/ 1552bbbbbb |
FIR Update |
|
When the facilitator identifies the contract-level enrollment change to Plan D, a FIR Inquiry transaction will be sent to the BIN/PCN for Contract S0001. Since the BIN/PCN is the same for both Plans A and C, the processor responds with the January through March and June through August accumulators, reporting all months of enrollment in Plans A and C. The Plan B sponsor will then receive a FIR Exchange transaction and must respond by adding the April through May accumulators. Next, although Plan C has already reported the June through August accumulators, the processor will receive a FIR Exchange transaction from the facilitator to provide Plan B data from April to May. Plan C will respond with their January through March and June through August accumulators pending any necessary adjustments resulting from reprocessing based on their receipt and review of the April through May data from Plan B. Plan C will report adjusted amounts in the next/later response to the facilitator; these adjustments must be made within 45 days of the plan’s receipt of the data from Plan B. The accumulators for all months January through August will be forwarded by the facilitator to the Plan D sponsor in a FIR Update transaction.
Scenario 2- Option 2 Reporting
Beneficiary Enrollment History
Months of Coverage |
Contract/PBP Number |
Plan |
BIN/PCN |
FIR Transaction |
Processor Response |
Jan. – Mar. |
S0001-001 |
A |
611220/ 1234567890
|
FIR Inquiry |
Reports Jan. – Mar. data |
Apr.- May |
S0002-001 |
B |
121212/ 23232323bb |
FIR Exchange |
Reports Apr. – May data |
June – Aug. |
S0001-001 |
C |
611220/ 1234567890 |
FIR Exchange |
Initially reports June - Aug. data (Subsequently reports any changes to June – Aug. data resulting from Apr.- May data) |
Effective Sept. |
S0003-001 |
D |
999991/ 1552bbbbbb |
FIR Update |
|
When the facilitator identifies the contract-level enrollment change to Plan D, a FIR Inquiry transaction will be sent to the BIN/PCN for Contract S0001. Although the BIN/PCN is the same for both Plans A and C, the processor responds with the January through March accumulators, reporting only months of enrollment in Plan A. The Plan B sponsor will then receive a FIR Exchange transaction and must respond by adding the April through May accumulators. Next, the Plan C processor will receive a FIR Exchange transaction from the facilitator to provide Plan B data from April to May. Plan C will be required to make any necessary adjustments resulting from reprocessing based on their receipt and review of the April through May data from Plan B and will report adjusted amounts in the next/later response to the facilitator. The accumulators for all months January through August will be forwarded by the facilitator to the Plan D sponsor in a FIR Update transaction.
The following scenario describes the FIR reporting requirements for situations when a beneficiary has multiple plan enrollments within a contract during the coverage year involving different BIN/PCN combinations:
Scenario 3
Beneficiary Enrollment History
Months of Coverage |
Contract/PBP Number |
Plan |
BIN/PCN |
FIR Transaction |
Processor Response |
Jan. – Mar. |
S0001-001 |
A |
611220/ 1234567890
|
FIR Inquiry |
Reports Jan. – Mar. data |
Apr.- May |
S0002-001 |
B |
121212/ 23232323bb
|
FIR Exchange |
Reports Apr. – May data |
June – Aug. |
S0002-002 |
C |
166666/ 88Abbbbbbb
|
FIR Exchange |
Reports June – Aug. data |
Effective Sept. |
S0003-001 |
D |
999991/ 1552bbbbbb |
FIR Update |
|
When the facilitator identifies the contract-level enrollment change to Plan D, a FIR Inquiry transaction will be sent to the BIN/PCN for Contract S0001. The processor will respond with the January through March accumulators. Although Plan B and C are within the same contract, the PBPs have different BIN/PCNs. Therefore, the facilitator will send a FIR Exchange transaction to the Plan B BIN/PCN and the processor will respond by providing the April through May accumulators. A subsequent FIR Exchange transaction will be sent to the Plan C BIN/PCN for that processor to report the data for the months of Plan C enrollment; this is the June through August accumulator data. The accumulators for all months January through August will be forwarded to the Plan D sponsor in a FIR Update transaction.
While these scenarios do not depict every possible situation involving multiple plan enrollments within a contract, they are illustrative of the application of the NCPDP FIR transaction flow to these situations and the potential need for sponsors to respond to sequential FIR transaction requests.
Any time a plan sponsor has paid Part D drug claims for a beneficiary who is later determined to be enrolled in another plan but has not received a FIR transaction to report the beneficiary’s TrOOP-related data, the sponsor must contact the transaction facilitator to initiate the FIR process and include the additional sponsor in the transaction flow.
Contract-level enrollment changes involving a single processor
When a beneficiary has had prior contract-level enrollment changes involving a single processor with the same BIN/PCN, the processor may report accumulator data for all months of enrollment in both contracts or may report only data related to the first contract enrollment when responding to a FIR Inquiry transaction. In either case, the processor will also subsequently receive a FIR Exchange transaction. Because the processor will not know whether there was an intervening enrollment in another contract, the Exchange transaction will have to be examined to determine if the accumulators have changed and adjustments are necessary.
Beneficiary Enrollment History
Months of Coverage |
Contract/PBP Number |
Plan |
BIN/PCN |
FIR Transaction |
Processor Response |
Jan. – Mar. |
S0001-001 |
A |
611220/ 1234567890
|
FIR Inquiry |
Reports Jan. - May |
Apr.- May |
S0002-002 |
B |
611220/ 1234567890
|
FIR Exchange |
Reports Jan. - May |
Effective June |
S0003-001 |
C |
121212/ 23232323bb |
FIR Update |
|
When the facilitator identifies the contract-level enrollment change to Plan C, a FIR Inquiry transaction will be sent to the BIN/PCN for Contract S0001. Since the BIN/PCN is the same for both Plans A and B, the processor may respond with the January through May accumulators, reporting all months of enrollment in Plans A and B, or may report only the Contract S0001 accumulators for January through March. Then the same processor will receive a FIR Exchange transaction, determine that the accumulators are as previously reported and respond with the previously reported information. If the processor reported all months on the FIR Inquiry, the response to the FIR Exchange will include the previously reported information. If the processor previously reported only the Contract S0001 accumulators, the processor must include the Contract S0002 accumulators in their response to the FIR Exchange. The accumulators for all months January through May will be forwarded by the facilitator to the Plan C sponsor in a FIR Update transaction.
Regardless of whether a sponsor is a plan of record or a non-plan of record, the sponsor must receive FIR transactions with TrOOP-related data reported by prior plans (both prior plans of record and non-plans of record), update their systems to incorporate these data, examine their claims history and previously reported amounts from the prior plans to determine the impact of these data on the beneficiary’s position in the benefit, and recalculate as necessary any prior claims affected by the new TrOOP accumulator data. The recalculation of prior claims by both non-plans of record and plans of record based on the receipt of new TrOOP-related data reported to them is necessary to ensure that beneficiary adjustments resulting from the recalculation are appropriately handled by the sponsor that adjudicated the affected claim(s).
In addition, for any month in which a plan other than the actual plan of record for the month (whether a prior plan of record or non-plan of record) has paid claims, the other plan will precede the actual plan of record for the month in the FIR transaction sequence. The other plan’s accumulator data also will precede the actual plan of record’s claims data for that month.
The following scenario describes FIR reporting in situations involving multiple enrollment types.
Beneficiary Enrollment History
Months of Coverage |
Contract/PBP Number |
Plan |
BIN/PCN |
FIR Transaction |
Processor Response |
Jan. – Feb., but paid claims for Mar. |
S0001-001 |
A (plan of record) |
611220/ 1234567890
|
FIR Inquiry |
Reports Jan. – Mar. data |
Mar. - June |
S0002-001 |
B (plan of record) |
121212/ 23232323bb
|
FIR Exchange |
Initially, reports Mar– June data (Subsequently, reports any changes to Mar.- June date resulting from Plan A’s Jan. – Mar. data) |
July – Aug. |
S0003-001 |
C (non-plan of record)
|
999991/ 1552bbbbbb
|
FIR Exchange |
Reports July – Aug. data |
Effective July |
S0004-001 |
D (plan of record) |
166666/ 88Abbbbbbb |
FIR Update |
|
In August, the facilitator identifies a contract-level enrollment change involving the auditing off of the Plan C enrollment and the new enrollment in Plan D effective July. A FIR Inquiry will be sent to the BIN/PCN for Contract S0001. The processor will respond with the accumulator data for their months of enrollment, January and February. In addition, because the Plan A paid claims in early March prior to receiving the TRR from CMS reporting the beneficiary’s change in enrollment, the processor will include their accumulator data for March as well.
The facilitator will send a FIR Exchange transaction to the BIN/PNC for Contract S0002. Initially, the processor responds with the accumulator data for their months of enrollment March through June. However, Plan B must incorporate the Plan A data into their system, including applying the March data from Plan A prior to the Plan B claims for March. After examining the amounts previously reported and their own claims history and recalculating any prior claims as necessary, the sponsor will respond to future transactions from the facilitator with their revised March through June accumulators.
A subsequent FIR Exchange transaction will be sent to the BIN/PCN for the non-plan of record Plan C. Initially, this sponsor will respond with July through August accumulators. However, the sponsor must incorporate the Plan A and B data into their system. After examining the amounts previously reported and their own claims history and recalculating any prior claims, as necessary, the sponsor will respond to future transactions from the facilitator with their revised July through August accumulators.
The monthly accumulators for January through August will be forwarded to the Plan D sponsor in a FIR Update transaction. With the retroactive enrollment of the beneficiary in Plan D back to July, the Plan D sponsor must apply the July and August accumulators reported by Plan C to each of those months prior to any claims Plan D adjudicated in July and August.
Receipt of Inquiry when a prior plan is known
If a plan receives an Inquiry transaction from the facilitator, but is aware there was a prior plan, the plan should process the FIR Inquiry transaction. The identity of the prior sponsor must be known and may be determined by the sponsor’s previous receipt of a P2P Plan Payable Report (Report 43) from CMS requiring payment to another Part D sponsor, or the beneficiary’s presentation to the current plan of a paper Explanation of Benefits (EOB) from a prior Part D payer.
In the Inquiry response, the sponsor will report the financial accumulators for their months of enrollment only. The sponsor should contact the TBT contact listed in HPMS for the prior plan to alert that sponsor of the need to request that the facilitator initiate a new transaction series.
Sponsor requested FIR transactions
Sponsors receiving a P2P Plan Receivable Report (Report 41) from CMS indicating the amount due from the beneficiary’s plan or record should request that the facilitator create a proxy enrollment record and add the sponsor to the FIR process flow. This request will cause a new FIR sequence to be initiated and permit the plan to report their accumulators to the subsequent plan(s) into which the beneficiary was enrolled during the coverage year.
Also, if a change in a beneficiary’s TrOOP-related data occurs outside the scheduled timing and is of such a magnitude that the sponsor believes it is important to transfer the updated data without waiting for the next scheduled transaction, the sponsor should complete the form to request a sequence be initiated and email the completed form to the transaction facilitator. Details regarding these procedures are available on the RelayHealth Web site. See Appendix B for the specific Web address.
Other automatically generated FIR transactions
In addition to the FIR transaction series initiated as a result of an enrollment change (i.e., a contract ID change or PBP ID change with a BIN and/or PCN change) or in response to a sponsor request, if a series is already underway and the facilitator receives either a change to the beneficiary’s 4Rx data (without PBP/Contract ID change) or a change to the beneficiary’s date of birth (DOB), a one-time FIR sequence will be initiated if one is not already scheduled on the day of receipt of the change. A transaction sequence will also be initiated if a FIR series has completed as long the 4Rx or DOB change is received prior to March 31st following the plan year.
Correction of unacceptable responses
When the facilitator rejects a FIR response transaction as unacceptable, (e.g., if the accumulated TrOOP reported for a month is a negative number) the sponsor must make the necessary changes to ensure the transaction is successful when the facilitator triggers the next regularly scheduled FIR sequence. Each sponsor must identify in the HPMS a TBT contact at the entity responsible for processing the sponsor’s FIR transactions. The facilitator will contact this person as necessary to explore any significant problems/issues identified with the transaction flow and notify CMS.
Previously, if a transaction was suspended, the facilitator continued the transaction sequence with the next payer. This permitted the new/current plan of record to receive the accumulators from all the other prior plans to position the beneficiary in the benefit. However, unintended consequences associated with this procedure were identified in 2012. As a result, effective July 1, 2012, the facilitator terminated the procedure. Whenever a transaction is rejected, the FIR sequence is ended and is re-initiated with the next regularly scheduled sequence.
Sponsors should not routinely question balances reported on the FIR transactions, including accumulated TrOOP reported in excess of the maximum. A sponsor may initially report accumulated TrOOP amounts that exceed the maximum for the coverage year, but must reduce reported TrOOP to the maximum in a subsequent transaction sequence. The resolution of an amount reported in excess of the TrOOP limit will require that the sponsor examine claims-level data to determine which claims will require reprocessing.
Part D sponsors may reject FIR transactions for missing or invalid data (e.g., a missing/invalid BIN number). However, under current CMS rules, X2 (Accumulated Gross Covered Drug Cost exceeds maximum) will not be used.
The FIR transaction standard requires a patient date of birth, if known, in the patient segment. If the date of birth is reported, the date reported in this field must match the CMS date of birth to avoid a reject for a missing/invalid date of birth.
Other FIR-related sponsor activity
Special requests for an automated FIR sequence
Once the regularly scheduled FIR sequences in the series have concluded, sponsors needing to report updated TrOOP accumulator data may do so by submitting a special request to the transaction facilitator to initiate a FIR sequence. A form for requesting a special sequence is available on the RelayHealth Web site. See Appendix B for the specific Web address.
Adjustment actions
When there is a change in a FIR accumulator due to a claims reversal or other change, such as a retroactive LIS adjustment, that affects the beneficiary’s TrOOP and/or gross covered drug costs, the change is accumulated in the month of service for FIR reporting purposes. When a change alters the benefit phase of a subsequent claim(s), CMS expects the plan to take this change into account, either by processing future claim(s) in a different benefit phase or by adjusting existing claims. If the change was identified after the year has ended, the plan has only one option; it must adjust the affected claim(s) because no future claims are expected. This is consistent with CMS’ 2008 PDE Regional Training Participant Guide (sections 4.5 and 5.8), which explains that plans “pay back the benefit” by adjusting claims when a reversal occurs after the end of the benefit year or following disenrollment. The PDE Participant Guide is available online; see Appendix B for the specific Web address.
Whenever the data reported on a FIR transaction causes a sponsor to recalculate a claim and recoup payments from the beneficiary in order to “pay back the benefit,” the sponsor need not actually recover the payment before updating the beneficiary’s accumulators. Recovery should be sought, accumulators updated, and PDE data adjusted promptly. The maximum time for a “true up;” i.e., adjustments to accumulators due to claims reversals, etc. that occur during the coverage year, is 45 days.
Record retention
FIR transactions are subject to the 10-year record retention requirements specified in the Federal regulations at 42 CFR 423.505(d). For imaged or electronically stored records, follow the Federal guidelines outlined by CMS in Pub. 100-01, chapter 7, section 30.30.1.4.
Negative accumulator values
If a sponsor currently has negative value for the beneficiary’s TrOOP, the sponsor may respond to a FIR transaction with a forced zero amount. However, the current frequency of the FIR sequence must be considered in these cases. If the next sequence will be soon (i.e., the sequences are in the first month) and correct accumulator data can be reported at that time, the forced zero is permissible. However, if the next scheduled sequence will not occur within a week, as soon as correct data are available, a request for the initiation of a sequence should be made to the facilitator.
FIR processor changes
If a Part D sponsor changes its FIR processor, the sponsor must ensure that FIR transactions are routed to the appropriate processor and that transactions related to the prior year can continue to be processed for the period required by CMS. This may require making arrangements either with the former processor to continue processing prior year FIRs or with the new processor to assume that responsibility. The transaction facilitator and CMS in conjunction with the NCPDP Work Group 1 Financial Information Reporting Task Group developed a white paper outlining the scenarios relevant to Medicare Part D Plans changing processors and the tasks to ensure that coordination of benefits occurs for the plan years originally contracted with the prior processor. The white paper, entitled “Medicare Part D Plans Moving Processors,” is available on the NCPDP Web site; see Appendix B for the specific Web address.
The TrOOP facilitator will be unable to distinguish non-calendar year (NCY) from calendar year (CY) EGWPs. Therefore, all EGWPs must be treated the same for automated TBT purposes.
For EGWPs, the following principles apply:
NCY plans, like CY plans, will report the FIR transactions on a CY basis.
CMS will assume there is no need to transfer TrOOP accumulators for any end-of-CY enrollment changes.
CMS has taken the position that if a beneficiary changes enrollment during the CY to a NCY plan, the Part D benefit will start anew if the effective date of the NCY enrollment is the same as the beginning of the NCY plan’s coverage year. Data reported on the FIR transactions will be ignored by the NCY plan. However, the FIR transaction itself should be accepted, despite the fact that the dollar values and data reported in the FIR will not be applied.
If a beneficiary changes enrollment during the CY to a NCY plan and the effective date of the NCY enrollment does not correspond to the beginning of the NCY plan’s coverage year, the plan will receive and add the accumulators reported from the prior plan(s) for the months from the beginning of the plan year to the month of the enrollment change to position the beneficiary in the NCY plan’s benefit.
The scenarios below demonstrate the application of these principles.
Non-Calendar Year Employer Group Waiver Plans
|
Scenario |
Effective Date of Transfer |
Automated FIR Triggered? |
Plan Action |
1 |
A beneficiary in a CY Plan A moves to a NCY Plan B with benefit year 7/1/11-6/30/12 |
9/1/2011 based on a special enrollment period (SEP) |
Yes |
Plan A reports Jan-Aug 2011 data. Plan B uses accumulators from July-Aug. to position the beneficiary in the Plan B benefit.
|
2 |
A beneficiary in a CY Plan A moves to NCY Plan B with benefit year 7/1/11-6/30/12
|
1/1/2012 |
No, end of year changes do not trigger FIR transactions |
Plan B begins beneficiary in the benefit with $0 accumulators. |
3 |
A beneficiary in NCY Plan A with benefit year 7/1/11-6/30/12 moves to another NCY Plan B with a benefit year 3/1/12-2/28/13 |
3/1/2012 |
Yes |
Plan A reports Jan-Feb 2012 data. Plan B ignores the reported data as the Plan benefit year begins with the effective date of enrollment. The receiving plan determines which months apply to its benefit.
|
4 |
A beneficiary has been enrolled in a NCY Plan A with a benefit year 10/1-9/30 since 2010 and moves to another NCY Plan B with a benefit year 7/1/11-6/30/12
|
11/1/2009 |
Yes |
Plan A reports Jan-Oct 2011 data. Plan B ignores Jan-June data, but uses the July-Oct accumulators to position the beneficiary in the Plan B benefit. |
5 |
A beneficiary in a NCY Plan A with benefit year 7/1/11-6/30/12 moves to CY Plan B |
1/1/2012 |
No, end of year changes do not trigger FIR transactions |
Plan B begins beneficiary in the benefit with $0 accumulators at the beginning of the new plan year.
|
6 |
A beneficiary in a NCY Plan A with benefit year 7/1/11-6/30/12 moves to CY Plan B |
3/1/2012 based on a SEP |
Yes |
Plan A reports Jan-Feb 2012 data. Plan B uses the Jan-Feb data to position the beneficiary in the Plan B benefit. |
7 |
A beneficiary in a CY Plan A is retroactively enrolled on 1/15/2010 into a NCY Plan B with a benefit year 7/1/11-6/30/2012 |
12/1/2011
|
Yes, but, although the FIR will be triggered 1/15/2012, the transaction will be for 2011 data. |
Plan A reports Jan-Dec 2011 data. Plan B uses the July-Dec accumulators. The TrOOP facilitator should identify a change in the Jan 2012 plan of record, add Plan A as a proxy plan and initiate a FIR inquiry to Plan A for Jan 2012 accumulators. If this does not occur, Plan A will need to request the facilitator initiate a FIR inquiry.
|
7b |
A beneficiary in a CY Plan A is retroactively enrolled on 1/15/2012 into a NCY Plan B with a benefit year 7/1/11-6/30/12
|
1/1/2012
|
No, end of year changes, even when processed in January, will not trigger FIR transactions.
|
If Plan A paid claims in January prior to receiving the TRR with the disenrollment, the plan should request the facilitator initiate a FIR inquiry. If this does not occur, once Plan A submits the PDEs for the January claims, Plan B will be aware via plan-to-plan recon of the need for a FIR transaction stream and can request it. |
If, for example in scenario 3, the beneficiary remains enrolled in Plan B into the subsequent calendar year (i.e., 2013), the TrOOP accumulators will be rolled over and used to determine the beneficiary’s position in the benefit until the end of the benefit year or disenrollment from the plan, whichever is earlier.
In scenario 7b, because the beneficiary changed enrollment in a subsequent CY, accumulators from the prior CY cannot be transferred and used by the new plan, even if the transfer is done outside the FIR process. Transfer-out balances are accumulated and forwarded only on a CY basis.
Timing of the FIR Transaction Sequences
Given the new 15-day compliance period specified in the Compliance section of this guidance, CMS worked with the NCPDP Work Group 1 Financial Information Reporting Task Group to revise the timing of the FIR transactions to increase the number of the transactions sent during the 15-day compliance period. Thus, beginning April 1, 2013, for enrollment changes occurring in 2013 or later FIR transactions are sent as follows:
The first FIR transaction sequence is sent one day prior to the effective date of the change or on the day the facilitator receives notice of the enrollment change, if later.
The second sequence is sent on the day following the initial sequence.
During the first month, subsequent transaction sequences are sent on days 8, 10, 12, 14, 21 and 28 following the second sequence.
The next transaction sequence is sent on day 73; i.e., 45 days (or a month and half) after the sequence sent on day 28.
The next sequence is sent on day 118; i.e., 45 days (or a month and half) later.
Additionally, to enable sponsors to report data changes that occur late in the current year or in the early months of the subsequent year, a change was made to the timing of the last FIR transaction sequences in the series. As a result, the last 3 FIR sequences are sent on December 1, of the current year and January 15 and February 28 of the subsequent year.
Under this schedule, the last regularly scheduled FIR transaction sequences are sent on the last day of February instead of March 31st, which was the previous ending data. Sponsors needing to update beneficiaries’ TrOOP accumulator data may submit a special request to the transaction facilitator during the period March 1 through May 31st for a FIR transaction series to be initiated for those beneficiaries.
The revised timing provides substantial additional opportunity to transfer updated beneficiaries’ TrOOP accumulator data and for subsequent plans to consider these changes in administering the Part D benefit. As a result, CMS expects that changes that would have previously required manual transfer will be transferred under the revised automated process and, absent extraordinary circumstances, it will no longer be necessary for sponsors to transfer updated accumulator data manually after June 15 of the subsequent year. However, sponsors must continue to receive and act on information that necessitates a claim adjustment for the 36-month COB timeframe and to submit PDEs reflecting the changes.
The facilitator will time out transactions without a response in 15 seconds. If a transaction is timed-out, the facilitator will retry the transaction every 15 minutes for 48 hours. If after the 48-hour period the plan never responds, the facilitator will suspend the sequence and initiate another at the next regularly scheduled time. The suspended sequence will be reported on the next daily report after the 48 hour period has expired.
Exceptions from Automated Processing
Part D sponsors should accept FIR data as reported unless a problem is identified. Problems may be identified through conflicting information, such as paper EOBs presented by, or on behalf of, the beneficiary, that suggests reported data are wrong. Also, there will be rare situations in which a discrepancy exists between the CMS and sponsor’s enrollment information for a beneficiary, which affects the FIR-reported data. These situations, or those in which the beneficiary complains that his/her TrOOP accumulators are materially incorrect, must be removed from automated processing. In these instances, the sponsor should contact the facilitator’s help desk to request the facilitator suspend the FIR transactions until the discrepancy is resolved or, if necessary, for the remainder of the coverage year. Once the error is resolved, the facilitator will remove the suspension and re-initiate the FIR process.
Reports to Sponsors
The Transaction Facilitator produces a number of reports to permit sponsors and their FIR processors to identify and resolve problems. These include the new Daily Cumulative FIR Aging Report which identifies for each sponsor all beneficiaries for whom balances have not successfully transferred and provides additional information to assist sponsors in complying with CMS’ ATBT requirements. This report replaced the prior daily reports of rejected TBT transactions as well as the bi-monthly report of unresolved TBT exceptions. The Daily Cumulative FIR Aging Report layout and a report guide, including a sample report, are available on the Transaction Facilitator Web site; see Appendix B for the specific Web address. In addition to the daily report, sponsors receive a weekly statistical report of the numbers of transactions successfully processed and rejected by type of reject.
Scenario One: The beneficiary was enrolled in Plan A in January, 2008, in Plan B in February, 2008 and in Plan C for the remainder of the year. Both Plan A and B had claim activity as reflected below.
Month |
Plan A |
|
|
Plan B |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
200.00 |
275.00 |
|
|
|
February |
|
|
|
50.00 |
200.00 |
March |
New plan C begins coverage |
|
|
|
|
Plan C began adjudicating claims with the $475 drug spend and $250 TrOOP amounts received from Plan B. In April, Plan A received a reversal on a $100 claim and in response to the next FIR Inquiry reported the following updated information to Plan B.
Month |
Plan A |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
150.00 |
175.00 |
Plan B compared the previous transaction from Plan A and determined that the drug spend accumulator decreased by $100. Plan B administers the defined standard benefit. The plan reviewed its claims history and determined that the $100 decrease moved Plan B’s first $100 claim from the Initial Coverage Period (ICP) back to the Deductible. Because Plan B needed to recalculate this claim to change it from $75 plan pay, $25 patient pay to $100 patient pay, the plan passed on the new Plan A accumulators and its existing February amounts to Plan C. In order to “pay back the benefit” Plan B was responsible for recouping the $75 differential from the beneficiary. In response to the next FIR Exchange transaction received, Plan B reported its updated amounts to Plan C as shown below.
Month |
Plan A |
|
|
Plan B |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
150.00 |
175.00 |
|
|
|
February |
|
|
|
125.00 |
200.00 |
March |
New plan C begins coverage |
|
|
|
|
Scenario Two: The circumstances are the same as those described in Scenario One, except Plan B administers a Basic Alternative benefit with no deductible; for the first $2500 the plan pays 75% and the beneficiary pays 25%. Plan B reviewed its claims history and determined that the $100 decrease in Plan A gross covered drug cost had no claims impact, because no claims were repositioned in different benefit phases. Plan B forwarded to Plan C the updated Plan A amounts for January and the existing Plan B accumulators for February.
Scenario Three: The beneficiary was enrolled in Plan A in January, 2008, in Plan B in February, 2008 and in Plan C for the remainder of the year. Both Plan A and B had claim activity as reflected below.
Month |
Plan A |
|
|
Plan B |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
175.00 |
175.00 |
|
|
|
February |
|
|
|
125.00 |
200.00 |
March |
New plan C begins coverage |
|
|
|
|
Plan C began adjudicating claims with the $375 drug spend and $300 TrOOP amounts received from Plan B. In April, Plan A received documentation from the beneficiary showing a $100 out-of-network prescription drug purchase. Plan A adjudicated the paper claim and in response to the next FIR Inquiry reported the following updated information to Plan B.
Month |
Plan A |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
275.00 |
275.00 |
Plan B compared the previous transaction from Plan A and determined that the drug spend accumulator increased by $100. The plan reviewed its claims history and determined that the $100 increase moved Plan B’s first $100 claim from the Deductible into the ICP. Because Plan B needed to recalculate this claim to change it from $100 patient pay to $75 plan pay, $25 patient pay, the plan responded to the next FIR Exchange transaction by passing on to Plan C the updated Plan A amounts for January and Plan B’s existing February amounts. Plan B was responsible for reimbursing $75 to the beneficiary.
In response to the next FIR Exchange transaction received, Plan B forwarded its updated TrOOP accumulator to Plan C.
Month |
Plan A |
|
|
Plan B |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
275.00 |
275.00 |
|
|
|
February |
|
|
|
50.00 |
200.00 |
March |
New plan C begins coverage |
|
|
|
|
Scenario Four: The beneficiary was enrolled in Plan A in January, 2008, in Plan B in February, 2008 and in Plan C for the remainder of the year. Both Plan A and B had claim activity as reflected below.
Month |
Plan A |
|
|
Plan B |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
275.00 |
275.00 |
|
|
|
February |
|
|
|
50.00 |
200.00 |
March |
Plan C begins |
|
|
|
|
Plan C began adjudicating claims with the $475 drug spend accumulator it received from Plan B. In April, Plan A received documentation from the beneficiary showing a $100 out-of-network prescription drug purchase. Plan A adjudicated the paper claim and in response to the next FIR Inquiry reported the following updated information to Plan B.
Month |
Plan A |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
300.00 |
375.00 |
Plan B compared the previously reported amounts from Plan A and determined that the gross covered drug cost had increased. Plan B administers the defined standard benefit. Based on a review of its claims history, Plan B determined that the $100 increase had no claims impact, because no claims were repositioned in different benefit phases.
Therefore, Plan B responded to the FIR Exchange transaction by reporting the following amounts to Plan C.
Month |
Plan A |
|
|
Plan B |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
300.00 |
375.00 |
|
|
|
February |
|
|
|
50.00 |
200.00 |
March |
New plan C begins coverage |
|
|
|
|
Scenario Five: The beneficiary was enrolled in Plan A in January and February, 2008 and in Plan B for March, 2008 and forward. Plan B administers the defined standard benefit. Because Plan A had no claim activity, it reported zero accumulators to Plan B on the initial Inquiry transaction and Plan B adjudicated a $100 claim in the Deductible on March 1.
Later on March 1, Plan B received a FIR Update transaction reporting the following amounts from Plan A.
Month |
Plan A |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
200.00 |
225.00 |
February |
100.00 |
250.00 |
Upon receipt of this transaction, Plan B reviewed its claims history and determined that the $475 increase moved Plan B’s first $100 claim from the Deductible into the ICP. Plan B recalculated this claim to change it from $100 patient pay to $75 plan pay, $25 patient pay. Plan B was also responsible for reimbursing $75 to the beneficiary.
Scenario Six: The beneficiary initially enrolled in Plan A during the AEP in December 2007. On December 31, 2007, the beneficiary sends an application to Plan B for enrollment effective January 2008. Both plans administer the defined standard benefit, and both issue a member ID card to the beneficiary. In February, the beneficiary changed enrollment to Plan C.
During the month of January, the beneficiary used the ID cards from both Plan A and B. Prior to receiving the transaction reply report (TRR) reflecting the enrollment change, Plan A paid claims in January totaling $100 all patient pay in the Deductible. Plan B then paid a $50 claim in January, also all patient pay in the Deductible. Because the Plan A enrollment was processed for January, the transaction facilitator was able to identify the change of enrollment to Plan B and sent a FIR Inquiry to Plan A. Upon the subsequent enrollment change to Plan C, the Plan A and B amounts are reported as follows:
Month |
Plan A |
|
|
Plan B |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
100.00 |
100.00 |
|
50.00 Plan B
100.00 (Plan A) + 50.00 (Plan B) = 150.00(to new plan) |
50.00 Plan B
100.00 (Plan A) + 50.00 (Plan B) = 150.00(to new plan) |
February |
New plan C begins coverage |
|
|
|
|
In March, one of Plan A’s paid claims from January was reversed by the pharmacy decreasing the beneficiary’s gross covered drug cost and TrOOP amounts to $50. Plan A reported the new accumulators to Plan B on the next FIR Inquiry transaction and submitted a deletion PDE for the reversed claim.
Plan B reviewed its claims history and determined that the $50 decrease had no claims impact, because no claims were repositioned in different benefit phases. Plan B sent the updated amounts to Plan C as follows:
Month |
Plan A |
|
|
Plan B |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
50.00 |
50.00 |
|
50.00 Plan B
50.00 (Plan A) + 50.00 (Plan B) = 100.00(to new plan) |
50.00 Plan B
50.00 (Plan A) + 50.00 (Plan B) = 100.00(to new plan) |
February |
Plan C begins |
|
|
|
|
Scenario Seven: The beneficiary was in Plan A January-March 2008, in Plan B in April and May 2008, and in Plan C for the remainder of the year. Both Plan A and B had claim activity as reflected below.
Month |
Plan A |
|
|
Plan B |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
150.00 |
150.00 |
|
|
|
February |
125.00 |
125.00 |
|
|
|
March |
31.25 |
125.00 |
|
|
|
April |
|
|
|
187.50 |
750.00 |
May |
|
|
|
62.50 |
250.00 |
June |
New plan C begins coverage |
|
|
|
|
Plan C began adjudicating claims with the $1400 in gross covered drug cost it received from Plan B.
Plan A responded to the next FIR Inquiry transaction by reporting its existing accumulators of $400 in gross covered drug costs and $306.25 in TrOOP to Plan B, but Plan B was unable to respond before the Exchange transaction was timed out. The transaction facilitator retried Plan B as specified in their FIR protocol. Once Plan B responded, a FIR Inquiry was again sent to Plan A, and on their Exchange transaction, Plan B responded with their current balances. The transaction facilitator then sent a FIR Update transaction to Plan C reporting Plan A and B balances.
Scenario Eight: The beneficiary was in Plan A January-March 2008. During these months, Plan A had claims activity. On March 12, the beneficiary elected enrollment in Plan B for April, but subsequently, on March 29, elected enrollment for April in Plan C. Because the Plan B enrollment was processed prior to the April cut-off, Plan B received a TRR reporting the enrollment and issued a member ID card to the beneficiary. During April, the Plan C enrollment was processed and Plan B enrollment was audited. The beneficiary remained in Plan C through May and enrolled in Plan D effective June 2008. With the transaction facilitator’s identification of the Plan B enrollment, Plan A received a FIR Inquiry transaction on March 31st and reported accumulators to Plan B.
Month |
Plan A |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
100.00 |
100.00 |
February |
175.00 |
175.00 |
March |
31.25 |
125.00 |
Plan B began adjudicating claims in April with the $400 drug spend accumulator. The Plan C enrollment was processed in April with a retroactive enrollment data of April 1. Both Plan B and Plan C received TRRs reporting the Plan C enrollment, however prior to receipt of this TRR, Plan B paid $100 in claims.
With the transaction facilitator’s notification of the Plan C enrollment, Plan A again received a FIR Inquiry transaction and reported their accumulators to Plan B. Plan B compared this with the previous FIR transaction from Plan A, determined there had been no change, and forwarded the following accumulators to Plan C.
Month |
Plan A |
|
|
Plan B |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
100.00 |
100.00 |
|
|
|
February |
175.00 |
175.00 |
|
|
|
March |
31.25 |
125.00 |
|
|
|
April |
|
|
|
25.00 |
100.00 |
Plan C began adjudicating claims with the $500 drug spend accumulator it received from Plan B, and had claims activity. With the transaction facilitator’s identification of the Plan D enrollment, Plan A again received a FIR Inquiry transaction and reported their accumulators to Plan B. Plan B again compared this with the previously reported amounts from Plan A, determined there had been no change, and forwarded the balances to Plan C. Plan C compared this with the previous FIR Exchange transaction from Plan B, determined there had been no change, and forwarded the balances to Plan D.
Month |
Plan A |
|
Plan B |
|
Plan C |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
100.00 |
100.00 |
|
|
|
|
February |
175.00 |
175.00 |
|
|
|
|
March |
31.25 |
125.00 |
|
|
|
|
April |
|
|
25.00 |
100.00 |
37.50 Plan C
25.00 (Plan B) + 37.50 (Plan C) = 62.50(to new plan) |
150.00 Plan C
100.00 (Plan B) + 150.00 (Plan C) = 250.00(to new plan) |
May |
|
|
|
|
125.00 |
500.00 |
June |
New Plan D |
|
|
|
|
|
Plan D began adjudicating claims with the $1150 drug spend accumulator it received from Plan C.
Scenario Nine: The beneficiary was enrolled in Plan A effective January 1, 2008 and the plan had claims activity. On January 30, the beneficiary elected enrollment in Plan B effective February 1. Because the Plan B enrollment was processed after the February cut-off, Plan A continued processing claims until mid-February when the Plan B enrollment was processed and Plan A received a TRR reporting the audited enrollment. On March 10, the beneficiary’s enrollment request for Plan C was processed with an effective date of April 1. In February, when the transaction facilitator identified the Plan B enrollment, Plan A received a FIR Inquiry transaction and reported the beneficiary’s accumulators to Plan B.
Month |
Plan A |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
175.00 |
175.00 |
February |
112.50 |
150.00 |
Plan B began adjudicating claims with the $325 drug spend accumulator. In March, the pharmacy reversed a $75 February claim to Plan A changing the plan’s accumulators for February. When the Plan C enrollment was processed in March, the transaction facilitator identified the enrollment change and sent a FIR Inquiry transaction to Plan A which reported the following updated accumulators to Plan B.
Month |
Plan A |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
175.00 |
175.00 |
February |
75.00 |
75.00 |
Plan B reviewed its claims history and determined that the $75 decrease moved Plan B’s first February claim from wholly in the ICP to straddling the Deductible and ICP. Because Plan B needed to recalculate this claim, the plan reported to Plan C the updated Plan A January accumulators, the combined Plan A and B February drug costs, and the total of the updated Plan A February TrOOP amount with the previous Plan B February TrOOP balance.
Month |
Plan A |
|
|
Plan B |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
175.00 |
175.00 |
|
|
|
February |
75.00 |
75.00 |
|
25.00 Plan B
75.00 (Plan A) + 25.00 (Plan B) = 100.00(to new plan) |
100.00 Plan B
75.00 (Plan A) + 100.00 (Plan B) = 175.00(to new plan) |
With the next FIR Inquiry transaction, Plan A reported unchanged accumulators for January and February to Plan B. Plan B reported the accumulators as previously sent to Plan C, except the plan was also able to send an updated TrOOP balance for February reflecting the re-adjudication of the straddle claim.
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
175.00 |
175.00 |
|
|
|
February |
75.00 |
75.00 |
|
43.75 Plan B
75.00 (Plan A) + 43.75 (Plan B) = 118.75 (to new plan) |
100.00 Plan B
75.00 (Plan A) + 100.00 (Plan B) = 175.00(to new plan) |
After re-adjudicating the first February claim that had previously been processed in the ICP as $75 plan pay and $25 patient pay, Plan B was responsible for recovering the additional amount owed by the beneficiary.
Scenario Ten: The beneficiary was in Plan A January-February 2008, then Plan B during March through June. Both plans had claims activity during the months of the beneficiary’s enrollment in their plan. Effective July, the beneficiary chooses to re-enroll in Plan A.
With the transaction facilitator’s identification of the Plan B enrollment, Plan A received a FIR Inquiry transaction and reported accumulators to Plan B as follows:
Month |
Plan A |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
75.00 |
75.00 |
February |
75.00 |
75.00 |
Subsequent FIR Inquiry transactions were sent to Plan A according to the established schedule and the accumulators reported to Plan B. Then, with the transaction facilitator’s identification in late June of prospective Plan A re-enrollment effective July 1st, Plan A received a FIR Inquiry transaction and reported the accumulators to Plan B. Plan B received and responded to a FIR Exchange transaction with the combined accumulators. The following data were sent to Plan A in a FIR Update transaction and Plan A began to adjudicate claims in July using $450 in gross covered drug costs.
Month |
Plan A |
|
Plan B |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
75.00 |
75.00 |
|
|
February |
75.00 |
75.00 |
|
|
March |
|
|
25.00 |
25.00 |
April |
|
|
100.00 |
100.00 |
May |
|
|
14.25 |
75.00 |
June |
|
|
25.00 |
100.00 |
July |
Re-enrollment Plan A |
|
|
|
Subsequently in early July, Plans A and B received TRRs indicating that the Plan A re-enrollment was audited due to the beneficiary’s election to remain enrolled in Plan B. However, because the Plan A re-enrollment was processed, Plan A paid claims in July prior to receipt of the TRR. With the transaction facilitator’s identification of the audited Plan A re-enrollment and the continuation of Plan B enrollment, Plan A received a FIR Inquiry transaction and reported their January, February and July accumulators to Plan B.
Month |
Plan A |
|
Plan B |
|
|
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
Accumulated TrOOP |
Accumulated Gross Covered Drug Cost |
January |
75.00 |
75.00 |
|
|
February |
75.00 |
75.00 |
|
|
March |
|
|
25.00 |
25.00 |
April |
|
|
100.00 |
100.00 |
May |
|
|
14.25 |
75.00 |
June |
|
|
25.00 |
100.00 |
July |
23.75 |
95.00 |
|
|
Plan B compared these data with the January and February accumulators previously reported by Plan A to determine if there had been a change that would affect Plan B’s adjudication of the claims processed during the period March through June. Plan B then began processing claims in July with $545 in gross covered drug costs.
Appendix D - Automated TrOOP Balance Transfer Implementation Guidance-- PACE Addendum
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
POLICY:
Part D sponsors must report TrOOP-related data for their months of coverage for beneficiaries who disenroll during the coverage year to the beneficiary’s subsequent Part D plan sponsor. Plan sponsors, with the exception of PACE organizations, will report these data to the transaction facilitator in response to FIR transaction requests. Because PACE organizations have been exempted from this automated TrOOP transfer process, TrOOP related data will be transferred as follows:
For beneficiaries enrolling into a PACE plan after disenrolling from another Part D plan, the PACE plan will request from the beneficiary the most recent explanation of benefits (EOB) provided by the prior Part D sponsor.
For beneficiaries disenrolling from a PACE plan to enroll in another Part D plan, the PACE plan will report the data to the beneficiary to communicate to his or her subsequent plan sponsor.
PACE plans may elect to participate in the automated TrOOP balance transfer process. Plans electing to participate should contact the transaction facilitator and request inclusion.
AUTHORITY:
42 CFR §423.104 – Requirements related to qualified prescription drug coverage.
42 CFR §423.464 – Coordination of benefits with other providers of prescription drug coverage.
APPLICABLE GUIDANCE:
Pub. 100-18, Medicare Prescription Drug Manual- Chapter 14 Coordination of Benefits, Section 50.9.2- Automated TrOOP Balance Transfer Process.
HPMS Memo, Medicare Drug Benefit Group, Change in Implementation Schedule for Automated TrOOP Balance Transfer (March 18, 2008).
HPMS Memo, Updated Part D Sponsor Automated TrOOP Balance Transfer Operational Guidance (October 21, 2008)
BACKGROUND:
Part D rules require sponsors to track the beneficiary’s TrOOP costs and gross covered drug costs and properly apply these costs to the TrOOP and benefit limits in order to correctly place the beneficiary in the benefit and provide the catastrophic level of coverage at the appropriate time. The TrOOP threshold and gross covered drug costs are calculated on an annual basis and must be transferred between Part D plans if a beneficiary disenrolls and re-enrolls at any time before the end of the coverage year.
FACILITATOR PROCEDURE:
The facilitator will access the monthly list of active Part D contracts available on the CMS Web site at: http://www.cms.hhs.gov/MCRAdvPartDEnrolData/01_Overview.asp#TopOfPage to identify the contract numbers for all PACE organizations with a Part D contract. The list is in a zip file under the “Monthly Enrollment by Contract” link, and provides all active contracts and their organization type.
When the facilitator identifies a beneficiary plan enrollment change at the contract level, the facilitator must determine if a PACE contract is the disenrolling sponsor. If so, the facilitator should follow the TrOOP balance transfer sequence outlined below.
TrOOP balance transfer sequence involving PACE plan enrollment
Once the facilitator determines the disenrolling plan is a PACE contract, it must determine if there was Part D plan enrollment during the coverage year.
If there was prior Part D enrollment, the facilitator will exempt the beneficiary from all automated TrOOP balance transfers and refer the case to CMS for coordination of the transfer of the TrOOP-related data.
If there was no prior Part D enrollment, the new plan will receive the accumulator data from the beneficiary and use these data to correctly position the beneficiary in the benefit.
If the beneficiary makes subsequent plan enrollment changes during the coverage year, the facilitator will initiate the FIR sequence without regard to the PACE plan enrollment with an Inquiry transaction to the first non-PACE plan, Exchange transactions to any subsequent plans, and an Update transaction to the newly enrolling plan.
The first non-PACE plan will respond to the Inquiry transaction, reporting the PACE accumulators in the month prior to the first month of enrollment in the non-PACE plan and the plan’s own accumulator data.
PACE ORGANIZATION PROCEDURES:
Enrollment into a PACE plan from another Part D plan
For these beneficiaries, the PACE plan will request the beneficiary’s most recent prior Part D plan EOB to determine the member’s gross covered drug costs and TrOOP.
Disenrollment from a PACE plan into another Part D plan
For these beneficiaries, the PACE plan will report the TrOOP-related data to the beneficiary and direct the beneficiary to communicate the information to his or her subsequent plan sponsor. The data reported will depend upon whether the PACE is a Medicare-only or dual eligible plan.
Medicare-only PACE Plans
If a member disenrolls from a Medicare-only PACE plan and re-enrolls with a new Part D plan sponsor, the PACE organization must take the following steps:
Compute the member’s PACE-only gross covered drug costs. TrOOP amounts for these beneficiaries will always be zero.
Provide these data as well as the months during the coverage year the member had incurred costs for prescription drugs and the coverage year being reported to the member in writing with instructions to communicate the information to his or her new plan. This report must be provided to the beneficiary within 7 days of the date of the TRR notifying the PACE organization of the member’s disenrollment.
If the PACE plan’s GCDC for the beneficiary change for any reason during the current calendar year through March of the subsequent year, the PACE plan must notify the beneficiary of the changed data in writing with instructions to communicate the updated information to his or her new plan. This notification to the beneficiary must be sent by the 15th of the month following the month in which the GCDC change occurred.
Dual Eligible PACE Plans
If a member disenrolls from a dual-eligible PACE plan and re-enrolls with a new Part D plan sponsor, the PACE organization must take the following steps:
1. Compute the member’s PACE-only GCDC.
2. Enter the PACE-only GCDC (i.e., (total gross covered drug costs) into the Dual Eligible PACE TrOOP Calculator to determine the PACE TrOOP amount.
3. Provide these data as well as the months during the coverage year the member had incurred costs for prescription drugs and the coverage year being reported to the member in writing with instructions to communicate the information to his or her new plan. This report must be provided to the beneficiary within 7 days of the date of the TRR notifying the PACE organization of the member’s disenrollment.
4. If the PACE organization’s accumulators for the beneficiary change for any reason during the current calendar year through March of the subsequent year, the PACE must notify the beneficiary of the changed data in writing with instructions to communicate the updated information to his or her new plan. This notification to the beneficiary must be sent by the 15th of the month following the month in which the change in the accumulators occurred.
Sample format for the beneficiary notice:
Notice of Benefit Information for Your New Medicare Prescription Drug (Part D) Plan
THIS IS NOT A BILL. Report this information to your new prescription drug plan and keep this notice for your records.
<Member Name> <Date>
<Street Address> Member ID Number: <Member ID>
<City, State ZIP Code> <Rx PCN or Rx Group Number>:
This notice includes:
TrOOP and Gross Drug Costs balances from the PACE plan during <coverage year>.
Any adjustments to your out-of-pocket costs or total drug costs due to new claims, reversed claims, or any other adjustments.
Totals
(Insert total of all covered drug costs paid, including by the plan, the enrollee, the LIS subsidy, and all others who paid on the enrollee’s behalf, i.e., Gross Covered Drug Costs) Total PACE Drug Costs from <date> to <date>:
(Insert total of all covered drug costs paid by the enrollee, the LIS subsidy, and all others whose payments count toward the enrollee’s TrOOP costs, i.e., TrOOP amount) Out-of-Pocket costs during PACE plan enrollment:
Out-of-Pocket Costs - Includes payments that you and/or certain others on your behalf paid for covered drugs during the coverage year. This includes payments made in the [deductible,] [and/or] initial coverage period [and/or coverage gap] this coverage year. Payments made by certain others that count toward your out-of-pocket costs include those made by family members, [(applicable to LIS only) Medicare’s extra help,] State Pharmaceutical Assistance Programs (SPAPs), and most charities. This amount does not include amounts paid by <plan name> or certain others making payments on your behalf. Payments made by certain others that don’t count toward your out-of-pocket costs include those made by group health plans (like from a current or former employer or union), other insurance, or Government-funded health programs. Once your out-of-pocket costs reach <$xx>, you move into the catastrophic coverage period.
Total Drug Costs - This is the total amount spent on your covered drugs this coverage year by <Plan Name>, you, and/or all others making payments on your behalf during all coverage periods. [(Applicable to LIS only.) This amount also includes any extra help you got from Medicare this year.]
Example:
A beneficiary disenrolled from a dual eligible PACE plan effective August 2009. The beneficiary had prescription drug claims in all months January -July totaling $2660.74. Using the dual eligible PACE plan beneficiary accumulated TrOOP calculator, the plan determines the member’s accumulated TrOOP equaled $931.28.
This notice includes:
TrOOP and Gross Covered Drug Cost balances from the PACE plan during 2009.
Any adjustments to your out-of-pocket costs or total covered drug costs due to new claims, reversed claims, or any other adjustments.
Totals
Total PACE Covered Drug Costs from January 1, 2009 to July 31, 2009: $2660.74
Out-of-Pocket costs during PACE plan enrollment: $931.28
Appendix E – Issues for Other Entities Providing Prescription Drug Coverage
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
As provided in 42 CFR 423.464(f), Part D plans must permit SPAPs and entities providing other prescription drug coverage to coordinate benefits with them. Examples of entities providing other prescription drug coverage include SPAPs, Medicaid programs, group health plans, Federal Employees Health Benefits Program (FEHBP) plans, military coverage, IHS coverage, charities, manufacturer PAPs, Federally qualified health centers (FQHCs), and rural health centers (RHCs). In this appendix, CMS discusses COB issues applicable to some of these entities.
State Pharmaceutical Assistance Programs
Qualified SPAPs are unique among other payers because any payments they make that supplement the benefits available under Part D coverage before reaching the plan’s annual out-of-pocket limit count toward TrOOP. CMS expects that qualified SPAPs will share enrollment files with CMS through the data sharing arrangements outlined in section 30.1. Although SPAP wrap-around coverage automatically counts toward TrOOP – and some programs have questioned the need for SPAPs to participate in CMS’ COB and TrOOP facilitation processes – there are benefits to participation in the COB process as other payers. For example, as part of the enrollment file sharing with SPAPs, CMS provides SPAPs with certain information fields (for example, low-income subsidy status and details) that they will need to effectively wrap-around Part D coverage on behalf of their Part D enrollees. In addition, as noted above, by making their claim payments a matter of record with the Part D plans, SPAPs provide the means for Part D sponsors to execute reimbursement of erroneous payments, such as those that may occur in reimbursing cost sharing incurred by low-income subsidy eligible enrollees between the date of their eligibility and the time the subsidy has been programmed by the Part D sponsor. Most importantly, participation in the TrOOP facilitation process allows the beneficiary’s multiple benefits to process seamlessly at the point of sale, even if they do not present all of their ID cards.
Exchanging Historical and Ongoing Claims Data
As mentioned in section 50.11 of this chapter regarding the sharing of claims data, CMS cannot require data exchanges between Part D sponsors and the States, except as required for COB purposes. However, CMS strongly encourages sponsors to independently share historical and ongoing data on these shared enrollees with SPAPs, provided such disclosure is consistent with the requirements of the HIPAA Privacy Rule. Drug history exchanges between States and sponsors are discussed further in section 50.11 of this chapter.
Coordinating Payment
As provided in these guidelines, SPAPs may choose to coordinate their benefits with Part D sponsors using a variety of approaches. With the exception of the risk-based approach, all Part D sponsors are required to coordinate with the SPAP. As indicated in the prior section discussing the non-risk approach, CMS will take compliance action against all sponsors that do not comply with the non-risk requirement. If a sponsor is out of compliance with this requirement, CMS will not disqualify a state program from its qualified SPAP status. SPAPs will not be viewed as discriminating based on a Part D sponsor’s non-compliance because CMS believes the sponsor, by failing to adhere to this COB requirement, has effectuated the discrimination. CMS will require states to collect an attestation from the sponsor that it does not want to participate in the non-risk approach. States will submit this attestation to CMS so that CMS may work with the sponsors to comply with this COB requirement. A sponsor will also be required to communicate to its beneficiaries that it is not participating in the State’s program.
In addition to the lump sum scenarios mentioned in section 50.7 of this chapter, SPAPs may provide their own wrap-around benefit at the point-of-sale, or solicit a sponsor or processor who agrees to administer their wrap-around benefit for them. The sponsor or processor (who may or may not be a Part D sponsor) will administer their SPAP wrap-around benefit. This organization will agree to administer the SPAP benefit to all Part D beneficiaries that qualify for the SPAP benefit regardless of the Part D sponsor in which the beneficiary is enrolled. As the administrator of the benefit, SPAPs will most likely require these organizations to:
Process secondary claims using the NCPDP V. 5.1 electronic claims format.
Require COB segment on the secondary claim.
Provide coverage of drugs on the State’s formulary.
Provide coverage of drugs at SPAP network pharmacies.
Administer rebates applicable to the SPAP wrap benefit.
Enrollment
Certain SPAPs may have the authority to enroll their members directly into Part D sponsors if using an enrollment methodology expressly approved by CMS, and have expressed a desire to be allowed to use a standard electronic file format to complete the enrollment process. While Part D sponsors will not be required to accept a standard electronic file directly from an SPAP, CMS encourages Part D sponsors to negotiate with SPAPs on this point so as to facilitate a streamlined enrollment process.
Medicaid
Medicaid cannot receive Federal Financial Participation (FFP) for drugs covered under Part D that are provided to full benefit dual eligibles. State Medicaid programs may provide Medicaid coverage of drugs listed under section 1927(d)(2) of the Social Security Act, which the MMA excludes from the definition of coverage under Part D drugs. To the extent that Medicaid covers those excluded drugs, the state can receive FFP for that coverage. However, coverage of non-Part D drugs by State Medicaid programs will not count toward a beneficiary’s TrOOP balance.
Drug coverage - CMS understands that many Medicaid programs may wish to provide coverage for non-Part D drugs to provide continuity of coverage to dual eligible Part D enrollees. To that end, Part D sponsors may wish to develop a process whereby the pharmacy is informed that Medicaid is a payer only if a claim is denied as a non-Part D drug and there are no other secondary/tertiary payers that may pay the claim. Part D sponsors are required to implement reject messaging that will allow pharmacies to identify claims for excluded Part D drugs that can be billed to the State.
Data exchange - As discussed previously in section 50.11 of this chapter, CMS does not have the authority to require data exchanges between Part D sponsors and the States, except as required for COB purposes. However, CMS strongly encourages Part D sponsors to independently share historical and ongoing data on these shared enrollees with State Medicaid agencies, provided such disclosure is consistent with the requirements of the HIPAA Privacy Rule. CMS believes claims data exchanges will be mutually beneficial to States and Part D sponsors as they structure their benefits.
Veterans Administration Coverage
VA benefits – including prescription drug coverage – are separate and distinct from benefits provided under Part D. By law, VA cannot bill Medicare. In other words, coordination of benefits between Part D and VA benefits is not possible. While a beneficiary may be eligible to receive VA prescription drug benefits and enroll in a Part D sponsor, he or she cannot use both benefits for a single prescription. VA prescriptions generally must be written by a VA physician and can only be filled in a VA facility or through VA’s Consolidated Mail Outpatient Pharmacy (CMOP) operations. VA does not fill prescriptions for Part D sponsors. Since VA and Part D benefits are separate and distinct, a veteran’s payment of a VA medication copayment does not count toward his or her gross covered drug costs or TrOOP expenditures under his or her Part D benefit.
Because VA prescription drug coverage is creditable coverage, beneficiaries will not face a penalty if they delay enrollment in a Part D plan. However, some beneficiaries who receive less than full VA prescription drug benefits may benefit from enrollment in a Part D plan, particularly if they are eligible for the low-income subsidy.
TRICARE
TRICARE for Life pays secondary to Medicare to the extent that a benefit is payable by both Medicare and TRICARE. TRICARE for Life’s pharmacy benefit wraps around Medicare Part D and will pay any beneficiary cost-sharing remaining, up through the cost-sharing that beneficiary would have had otherwise paid under TRICARE. However, this applies only if a beneficiary is enrolled in a Part D plan, the drug is a covered Part D drug, the covered Part D drug is also covered by TRICARE, and the drug is obtained at a pharmacy participating in both the Part D plan’s and TRICARE’s network.
Because TRICARE for Life is creditable coverage, beneficiaries will not face a penalty if they delay enrollment in a Part D plan. However, some beneficiaries who receive TRICARE for Life benefits may benefit from enrollment in a Part D plan – particularly if they are eligible for the low-income subsidy. To the extent that a beneficiary is enrolled in both TRICARE for Life and a Part D plan, information about that beneficiary’s TRICARE coverage should be captured and maintained by the COB contractor, and available to Part D sponsors as part of the COB process, through the MARx system. Any wrap-around payments made by TRICARE for covered Part D drugs will count toward a Part D enrollee’s gross covered drug costs but not toward TrOOP since TRICARE is a government-funded health program and, as such, a TrOOP-excluded payer.
Indian Health Service (IHS)/Tribal Health Coverage
The Indian health care system, consisting of tribal, urban, and federally operated IHS programs, delivers a spectrum of clinical and preventive health services to its beneficiaries, via a network of hospitals, clinics, and other entities. Section 42 CFR 423.464(f) implementing the Part D COB requirements requires sponsors to coordinate benefits with the IHS and providers of other prescription drug coverage. Tribal health coverage is recognized by CMS as a provider of other prescription drug coverage.
Initially, supplemental coverage by IHS, Indian tribes and organizations, and urban Indian organizations (collectively I/T/U) facilities were not TrOOP eligible because these entities fall under CMS’ definition of “government-funded health program,” in 42 CFR 423.100. However, in certain cases tribes, when providing other prescription drug coverage were independent entities that used only non-government funding to pay secondary coverage for all medical services, including Part D drugs. In those cases, the secondary coverage could have been TrOOP-eligible.
Effective January 1, 2011, section 1860D-2(b)(4)(C) of the Social Security Act was amended to permit assistance with Part D cost-sharing by I/T/U pharmacies to count as incurred costs toward meeting the out-of-pocket threshold at which catastrophic coverage under the Part D benefit begins. CMS regulations continue to require all Part D sponsors to offer network contracts to all I/T/U pharmacies operating in their service area and, in addition, will have to demonstrate to CMS that they provide convenient access to I/T/U pharmacies for American Indians/Alaskan Natives (AI/AN). Thus, COB with the IHS and tribes is inextricably tied to pharmacy network contracting with I/T/U pharmacies. I/T/U pharmacies may submit claims to Part D sponsors electronically (or via paper claims, since some of the more remote I/T/U sites lack electronic capability).
If a tribal member new to the Part D benefit is initially unable to receive Part D benefits through his/her Part D plan, the tribe may have stepped in to pay for the AI/AN Medicare eligible’s Part D prescription drugs in lieu of a Part D plan’s primary coverage. In such cases, tribes are entitled to seek compensation from the Part D plan once enrollment is confirmed. Consistent with CMS COB requirements, plans will be required to reimburse tribes when the tribe has paid primary, just like any other provider of prescription drug coverage.
Safety-Net Providers
A majority of Medicare beneficiaries served by safety-net provider organizations have limited incomes. These safety-net providers typically include Federal, State, and locally supported community health centers (CHCs) or clinics, many of which are deemed FQHCs, public hospital systems, and local health departments. In some communities, they also include mission-driven teaching hospitals, community hospitals and ambulatory care clinics (which are often located in central city areas or serve as the sole provider of health care in the community). RHCs, small rural hospitals, critical access hospitals, clinics that receive Ryan White HIV/AIDS grant funding, and nurse-managed clinics also constitute key components of the safety net.
An estimated 12,000 safety-net providers participate in the Health Resources and Services Administration’s (HRSA) 340B Drug Pricing Program, which allows them to buy their prescription drugs at significantly discounted prices. Participation in the 340B Program can enable pharmacies to provide prescriptions to their patients at lower-than-market price. Because many safety-net providers acquire their prescription drugs through Federal purchasing programs such as the 340B Drug Pricing Program, access to prescription drugs and pharmacy services may be limited to their own patients and not to the public at large. Such “closed pharmacies” may therefore not be open to the general public. For this reason, safety-net pharmacies are typically smaller and less visible to the public than retail pharmacies.
Part D sponsors are not required to contract with safety-net providers. However, CMS created an incentive for Part D sponsors to contract with certain safety-net providers – FQHCs and RHCs – by allowing them to count these pharmacies toward their retail pharmacy networks.
COB between Part D sponsors and safety-net providers is inextricably tied to pharmacy network contracting with safety net pharmacies because the assistance with cost-sharing provided by safety-net pharmacies consists of waived or reduced Part D cost-sharing amounts for beneficiaries enrolled in plans with which the pharmacies contract. The MMA added a new exception to the anti-kickback statute under which pharmacies are permitted to waive or reduce Part D cost-sharing amounts under certain circumstances. For more information about this exception to the anti-kickback statute and the potential impact on TrOOP of Part D cost-sharing waived or reduced by safety-net pharmacies, refer to Pub. 100-18, Medicare Prescription Drug Benefit Manual, chapter 5, section 30.4.
Charities
Regardless of whether a charity is a bona fide charity – and unless the charity is a group health plan, insurance or otherwise, or other third party payment arrangement – any assistance with Part D cost-sharing a charity provides on behalf of a Part D enrollee will count toward a beneficiary’s TrOOP balance. However, any charity is TrOOP eligible only if it’s a legitimate charity. Additionally, to the extent that a charity provides assistance in the form of in-kind donations, CMS generally considers that entity to be a manufacturer patient assistance program (PAP) operating outside Part D, and the value of that assistance does not count toward a beneficiary’s TrOOP balance (refer to the section below on manufacturer PAPs for more detail).
Manufacturer Patient Assistance Programs
Pharmaceutical manufacturers sponsor a number of PAPs that provide free product (through in kind product donations) to low income patients – particularly those with incomes below 200 percent of the federal poverty level (FPL) – with insufficient or no prescription drug coverage. Part D sponsors are required to coordinate with manufacturer PAPs (hereinafter referred to simply as “PAPs”), as detailed below.
Although sponsors are required to coordinate with PAPs, because PAPs operate entirely outside the Part D benefit (unlike charities offering cost-sharing assistance), this coordination is different in nature than coordination of benefits with supplemental payers operating within the benefit. This is because any assistance a PAP provides to a Part D enrollee for drugs that would have been covered under his/her Part D plan cannot count as an incurred cost that would be applied toward the enrollee’s TrOOP balance or total drug spend. In other words, beginning when a beneficiary’s assistance under a PAP is effective (and for as long as the beneficiary remains eligible for PAP assistance), a claim for a drug for which a PAP has provided assistance will never be submitted to a beneficiary’s Part D plan.
The most effective – and, ultimately, for the beneficiary, the safest – way for PAPs to operate outside the Part D benefit involves front-end data exchanges with CMS through the use of PAP data sharing agreements (DSAs). General information about eligibility file exchange with supplemental payers and other entities is provided in section 30.1 of this chapter. Specific information about PAP DSAs is available on the CMS Web site; see Appendix B for the Web address.
To address safety concerns associated with prescription drugs provided outside the Part D benefits, the front-end data exchange process will enable sponsors to follow-up with PAPs to identify those Part D drugs an enrollee is receiving outside the Part D benefit. This will facilitate sponsors' provision of required drug utilization review and, if applicable, medication therapy management program activities. Alternatively, a PAP that does not participate in CMS's DSA process may provide its enrollees with a notice indicating that they are receiving one or more drug products from that PAP. Sponsors should follow up with PAPs regardless of how they receive information about the possibility of PAP-provided assistance for any of their enrollees.
When a PAP exchanges an eligibility file with CMS, it is identified on the COB data file as Coverage Type “P,” which is not TrOOP-eligible. When a sponsor receives a COB data file for an individual indicating a Coverage Type of “P,” it must follow up with the PAP to obtain the drug-specific information it needs in order to: (1) set its systems to recognize that drug as part of a patient’s profile for purposes of drug utilization review; and (2) set its systems edits to prevent any payment for that prescription. This will be a manual follow-up process because the COB file does not provide sponsors with information about the specific drugs being provided to enrollees by the PAP. Although CMS provides PAP sponsors with a list of COB contacts for each sponsor on the CMS Web site to facilitate this communication, it remains a sponsor requirement to coordinate the exchange of information with PAPs operating outside the Part D benefit.
Contact information for PAPs will be available in the COB data file, and sponsors should use this number to initiate this manual follow-up and data exchange process. The PAP’s phone number will appear in the PDP COB data file, Appendix E.6.4 Supplemental Record: Subordinate to DTL (Unlimited Occurrences), which can be found in the PCUG. The phone number will be located in the data field labeled “Rx Plan Toll Free Number” when the “Supplemental Code Type” is “P=Patient Assistance Program.”
CMS has encouraged PAPs operating outside the Part D benefit to enter into DSAs with CMS similar to those entered into by supplemental payers coordinating benefit administration with Medicare. Manufacturers sponsoring PAPs continue to express interest in entering into DSAs with CMS, and CMS expects that sponsors will see an increase in “P” (PAP) coverage type indicators on their COB data files as more PAPs enter into DSAs and enroll Part D enrollees.
Sponsors may provide information on or even facilitate enrollment in PAPs for financially needy enrollees, particularly as they reach the coverage gap. To the extent that they do so, however, their bids will need to account for the potential decrease in utilization resulting from enrollees’ receipt of free assistance.
Operating outside the Part D benefit does not preclude a PAP from requiring its enrollees – including those enrolled in a Part D plan – from paying a nominal copayment when they fill a prescription for a covered Part D drug for which they provide assistance. CMS believes that any copayments assessed by PAPs operating outside the Part D benefit should be nominal, since only nominal beneficiary cost-sharing is consistent with the concept of operating outside Part D. Moreover, given that copayments are typically assessed for purposes of minimizing drug overutilization, the assessment of anything but nominal cost-sharing by PAPs is seemingly inconsistent with the mission of a charitable organization structured to provide assistance with prescription drug costs to low-income patients.
Although PAP payments made for those covered Part D drugs outside the benefit may never count toward enrollees’ TrOOP or total drug spend balances, CMS clarifies that any nominal PAP copayment amounts paid by Part D enrollees will be aggregated to their TrOOP and total drug spend balances, provided the enrollees take responsibility for submitting the appropriate documentation to their plan. It will not be permissible, however, for beneficiary payments structured as administrative fees or premiums to be aggregated to Part D TrOOP and total drug spend balances, as these types of beneficiary out-of-pocket expenditures do not meet the definition of “incurred costs” at 42 CFR 423.100 and in Pub. 100-18, Medicare Prescription Drug Benefit Manual, chapter 5, section 30.
Enrollee submission of this documentation is necessary because a PAP operating outside the Part D benefit should never submit a claim for assistance provided for a covered Part D drug to an enrollee’s Part D plan. Consistent with CMS guidance on claims processing, plans should process these enrollee-submitted claims in the order in which they are received, not based on date of service.
Organizations or entities offering PAPs must comply with all relevant fraud and abuse laws, including, when applicable, the Federal anti-kickback statute and the civil monetary penalty prohibiting inducements to beneficiaries. Liability under the anti-kickback statute requires a case-by-case analysis of the particular facts and circumstances, including the intent of the parties. The HHS Office of the Inspector General (OIG) enforces Federal fraud and abuse statutes, and all questions regarding the compliance of specific arrangements with these statutes should be referred to the OIG. General OIG guidance regarding Part D and PAPs is available on the OIG Web site; see Appendix B for the specific Web address.
Personal Health Savings Vehicles
HSAs, FSAs, and MSAs
In the final Part D regulations, CMS indicated that HSAs, FSAs, and MSAs are not group health plans for TrOOP purposes, and that distributions from these personal health savings vehicles will count as incurred costs for the purposes of TrOOP accounting. Thus, information about these accounts need not be reported to CMS. However, if any of these accounts is set up to pay benefits at the point-of-sale, and wishes to be included in the automated payer data exchange provided by the transaction facilitator, the administrators of such accounts would need to exchange eligibility files with CMS and be included in the COB files provided by CMS. Alternatively, account administrators may require beneficiaries to submit paper claims after the POS transaction so they can then submit those claims to the TrOOP facilitation contractor in batch form. The transaction facilitator will create an NCPDP Nx transaction based on that batched claims data and will send it back to the beneficiary’s Part D sponsor for accurate TrOOP recalculation.
HRAs
HRAs, however, generally are considered group health plans for purposes of Part D, and distributions from these accounts will not count toward TrOOP. HRAs are therefore group health plans subject to all the requirements that apply to other payers providing prescription drug coverage. HRA administrators will have the option of entering into data sharing agreements offered by CMS, or they can submit batched claims data to the transaction facilitator after the POS transaction. This will help supplement the information about other payers that beneficiaries must relay to their Part D sponsors and aid in the accurate calculation of TrOOP.
AIDS Drug Assistance Programs (ADAP)
AIDS Drug Assistance Programs (ADAPs), which are funded under the Ryan White CARE Act, are an integral component of the safety-net for HIV/AIDS patients because they fill coverage gaps in public and private insurance for critical HIV/AIDS drug treatments. Although initially assistance with Part D cost-sharing by ADAPs did not count as incurred costs toward meeting the out-of-pocket threshold at which catastrophic coverage under the Part D benefit begins, effective January 1, 2011, section 1860D-2(b)(4)(C) of the Social Security Act was amended to permit costs borne or paid for by an ADAP to count toward a beneficiary’s TrOOP.
To the extent that ADAPs want to be set up to pay benefits at the point-of-sale and wish to be included in the automated payer data exchange provided by the COB contractor, they will need to exchange eligibility files with CMS and be included in the COB files provided by CMS. The advantage to this approach is that claims will be automatically adjudicated at point-of-sale (POS) and routed to the Transaction Facilitator.
Alternatively, ADAPs may require beneficiaries to submit paper claims after the POS transaction so they can then submit those claims to the transaction facilitator in batch electronic format per the NCPDP standard. The transaction facilitator will create an NCPDP Nx transaction based on that batched claims data and will send it back to the beneficiary’s Part D sponsor for accurate TrOOP recalculation.
CMS and the transaction facilitator have developed a process to increase the likelihood that these SPAP and ADAP claims are appropriately applied to the member’s TrOOP and that the appropriate entity is refunded in the event of a copay/coinsurance adjustment. Guidance describing this process is available on the NCPDP Web site under the “Resources” tab. See Appendix B for the specific Web address.
Appendix F – Part D Requirements Waived for PACE Organizations
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
PACE is a comprehensive, coordinated model of care designed to meet the needs of frail elders. There are several key differences between the way in which PACE organizations (POs) provide the Part D benefit and how it is provided by other Part D sponsors.
Tracking of TrOOP
Dual Eligible Beneficiaries:
CMS fully subsidizes dual eligible individuals’ Part D coverage in PACE organizations. Therefore, consistent with PACE rules, there is no beneficiary out-of-pocket expense, which eliminates the applicability of TrOOP for these beneficiaries.
Beneficiaries Eligible for Only Medicare:
PACE beneficiaries who are only Medicare eligible pay a supplemental premium based on the anticipated cost-sharing covered by the PACE plan. As a result, for these beneficiaries TrOOP does not apply.
Accessing Covered Part D Drugs
For the most part, POs fully coordinate their participants’ access to covered Part D drugs, providing prescriptions directly to the participant. As a result, most POs are not set up for real-time, on-line prescription drug claims processing and neither have nor report 4Rx data to CMS.
Transferring Data When a Beneficiary Changes Sponsors
When a beneficiary disenrolls from a PO and re-enrolls in another Part D sponsor at any time during the coverage year, the PO is required to transfer the TrOOP balance (if any) and the gross covered drug costs to the new sponsor of record to permit the correct placement of the beneficiary in the benefit.
Prior to the January 1, 2009, implementation of the automated TBT process, POs must send the beneficiary’s year-to-date TrOOP and gross covered drug costs, including amounts accumulated during the beneficiary’s period of enrollment in the PO plus amounts previously reported to the PO by a prior plan sponsor for months of enrollment during the same coverage year. For beneficiaries who are dually eligible, POs should use the Dual Eligible PACE Plan Beneficiary Accumulated True Out-of-Pocket Cost Calculator to calculate the amount of TrOOP to be reported to the new plan sponsor. The calculator is available on the CMS Web site; see Appendix B for the specific Web address.
POs are exempt from the automated TBT process implemented January 2009; however, POs are not precluded from using the automated process and may elect to do so. Guidance outlining the requirements for the transfer of TrOOP balances involving PO enrollees was issued as an addendum to the automated TrOOP balance transfer guidance and is in Appendix D.
Waiver for Veterans Administration (VA)-eligible PACE Enrollees
In 2010, the Veterans Health Administration (VHA) awarded funds to VA Medical Centers (VAMC) to pursue and implement service agreements with PACE organizations to coordinate prescription drug coverage between the VA and VA-eligible PACE enrollees. Seven VAMCS contracted with 11 PACE organizations to provide prescription drug coverage for veterans enrolled in PACE.
Existing PACE regulations at 42 CFR 460.92 require that the PACE provide all Medicare-covered services, including Part D prescription drug coverage. In addition, Part D regulations at 42 CFR 423.30(c) require PACE enrollees to obtain their prescription drug benefits from their PACE organization. As a result, PACE enrollees who are also eligible for the VA benefit must receive prescription drug coverage through their PACE organization and Medicare-only PACE veterans must pay a significant Medicare Part D premium to the PACE organization to obtain the drug coverage.
The arrangements between the VA and PACE organizations, by facilitating coordination of prescription drug benefits between the VA and Medicare, permitted CMS to waive the requirements in §423.30(c) thus permitting PACE veterans to receive prescription drug coverage through the VA and avoid paying the Part D premium. PACE organizations with a service agreement with a VAMC received notification from CMS of the waiver of §423.30(c), which was issued under the authority of §423.458(d) which permits waivers of requirements as necessary to improve coordination between Part D and PACE. The notice also stated that CMS was granting the PACE organizations a conditional, organization-wide waiver of §460.92 of the PACE regulation under the authority of section 903 of the Benefits Improvement and Protection Act of 2000, permitting PACE enrollees eligible for VA drug coverage to choose to receive drug coverage through the VA.
It is expected that these waivers will continue as long as the service agreements are in place and the VHA continues to provide funding.
CMS will continue to develop guidance to further clarify the applicability of the COB requirements to the POs.
Appendix G – NCPDP White Paper- Overview of the Medicare Part D Prescription Drug Coordination of Benefits (COB) Process
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
4. OVERVIEW OF MEDICARE COB REQUIREMENTS FOR PART D ENROLLEES
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA) (P.L.108-173) was enacted in December 2003 and amended Title XVIII of the Social Security Act by establishing a new Part D: the Voluntary Prescription Drug Program effective January 1, 2006. Under the prescription drug benefit program, eligible Medicare beneficiaries are provided access to coverage options, including options with enhanced benefits, and additional beneficiary protections and assistance, such as access to negotiated prices, catastrophic coverage limits, and premium and cost-sharing subsidies for certain low-income beneficiaries. The requirements and recommendations in the following sections of this white paper flow from CMS regulations and policy guidance that are updated periodically and reflect the cooperation between CMS and the industry working in collaboration with NCPDP as required under 1860D-23(a)(4) of the Social Security Act.
Part D sponsors are required to coordinate with State Pharmaceutical Assistance Programs (SPAPs) and other providers of prescription drug coverage with respect to the payment of premiums and coverage, as well as coverage supplementing the benefits available under Part D. Entities that provide other prescription drug coverage with which Part D sponsors must coordinate include: Medicaid programs; group health plans; the Federal Employee Health Benefit Program; military coverage; the Indian Health Service (IHS); Federally qualified health centers, rural health clinics; other Part D plans; and other prescription drug coverage as CMS may specify. The MMA specified that these coordination requirements must relate to the following elements:
Enrollment file sharing;
Claims processing and payment;
Claims reconciliation reports;
Application of the protection against high out-of-pocket expenditures by tracking true out-of-pocket (TrOOP) expenditures; and
Other processes that CMS determines.
When a Medicare Part D enrollee has other prescription drug coverage, coordination of benefits allows the plans that provide coverage for this same beneficiary to determine each of their payment responsibilities. This process is necessary in order to avoid duplication of payment and to prevent Medicare from paying primary when it is the secondary payer. As required by the MMA, Medicare secondary payer procedures apply to Part D sponsors in the same way as they apply to Medicare Advantage organizations under Part C. Regulations require Part D sponsors to report credible new or changed primary payer and supplemental prescription drug coverage information to the CMS COB Contractor; CMS guidance specifies that this reporting should be accomplished electronically via the Electronic Correspondence Referral System (ECRS) within 30 days of the sponsor’s receipt of the information. Updated primary and supplemental coverage information reported to the COB Contractor is entered into CMS systems and CMS forwards the information as often as daily to the Part D Transaction Facilitator and Part D sponsors for their enrollees.
Under Part D, COB also provides the mechanism for support of the tracking and calculating of beneficiaries’ “true out-of-pocket” (TrOOP) expenditures, or “incurred costs” as defined in the MMA and CMS’ implementing regulations. Incurred costs under Part D include only costs incurred by the beneficiary for the annual deductible, or other cost-sharing prior to satisfying the out-of-pocket threshold, including the out-of-network price differential for which the individual is responsible when the emergency access requirements are met. Incurred costs are costs paid by the beneficiary, by another person on the beneficiary’s behalf, by CMS on behalf of a low-income subsidy (LIS) eligible individual, or by a qualified SPAP, the IHS or an AIDS Drug Assistance Program (ADAP) that are not reimbursed through or paid under insurance or otherwise, a group health plan, or other third party arrangement. Incurred costs must be incurred for a covered Part D drug which is a Part D drug included in the individual’s Part D plan’s formulary, or treated as being included as a result of a coverage determination or appeal, and obtained at a network pharmacy, unless emergency access provisions have been met. Part D sponsors must exclude costs that do not meet these requirements from a beneficiary’s TrOOP.
Section 1860D-2(b)(4)(D) of the Act authorizes CMS to establish procedures for the exchange of information for determining whether costs reimbursed by third parties for Part D enrollees may be included in their TrOOP and for alerting Part D sponsors about such reimbursements. The TrOOP facilitation process developed by CMS and the industry in collaboration with NCPDP allows the majority of pharmacy claims processing and benefit coordination to take place in real-time at the pharmacy point of sale. CMS’ Transaction Facilitator contractor, in conjunction with CMS, is responsible for establishing procedures for facilitating eligibility queries, identifying costs being reimbursed by other payers and reporting such transactions to Part D sponsors, and facilitating the transfer of TrOOP-related data when a beneficiary changes plans during the coverage year.
The CMS COB Contractor consolidates the activities that support the collection, management, and reporting of other coverage for Medicare beneficiaries. Through the data exchange processes, many payers voluntarily report information regarding prescription drug coverage they offer which is either primary or supplemental to Part D. In addition, many other insurers providing group health coverage, liability insurance, no-fault insurance, and workers’ compensation, include prescription drug coverage in conjunction with their mandatory reporting under section 111 of the Medicare, Medicaid, and SCHIP Extension Act of 2007 (P.L. 110-173). A data exchange with CMS allows other payers:
To assist beneficiaries in fulfilling their statutory obligation to disclose third party reimbursement for Part D drug costs.
To avoid the cost of paying as primary when the payment should be secondary to Part D.
As a sponsor of record, to be notified if a paid claim is reversed or adjusted outside an on-line adjudication process.
If TrOOP-eligible, to cease payments for beneficiaries receiving the full low-income subsidy who reach the catastrophic phase of the benefit, since at that point, Medicare fully subsidizes the beneficiary's incurred costs for covered Part D drugs.
For this process to work, payers supplemental to Part D should obtain a unique RxBIN and/or RxPCN combination that will identify their paid claim responses for TrOOP tracking purposes when Part D is the primary payer. CMS also recommends that supplemental payers obtain an RxBIN and/or RxPCN combination unique to each separate plan they offer in order to distinguish each of their plans from one another. This allows each benefit plan to fulfill its obligation as a supplemental payer if it is identified on the COB file as secondary coverage. CMS guidance notes that for the COB and TrOOP tracking processes to function effectively, other payers should supply paid claims information to the Part D sponsor after making a payment that is supplemental to a Medicare payment. This will happen automatically only if the other payer reports their coverage information with the appropriate RxBIN and/or RxPCN combination to CMS thereby enabling the Transaction Facilitator to identify the supplemental payer’s status. Therefore, it is critical that the RxBIN/RxPCN and Rx Cardholder ID (RxID) reported by the supplemental payer to the CMS COB contractor, entered into CMS systems and reported to the Transaction Facilitator matches the RxBIN/RxPCN and RxID on the supplemental claim request transaction. A match is required for the Transaction Facilitator to create the Information Reporting (N) transaction.
CMS requires that Part D sponsors coordinate benefits with supplemental payers that adhere to the CMS Data Sharing Agreement and transmit their eligibility data to CMS. Those supplemental payers that use the established on-line or batch COB process will derive the benefits associated with the creation of N transactions and their transmission to the beneficiary’s Part D sponsor. Other supplemental payers that do not comply with the on-line or batch COB process forfeit COB and the benefits associated with it.
CMS regulations specify the requirements for plans sponsors to coordinate benefits with both other Part D plans when a Part D sponsor other than the sponsor of record paid claims for a beneficiary during the initial transition period and with other entities providing prescription drug coverage when that entity incorrectly paid as primary. Sponsors must follow CMS-established processes for plan-to-plan reconciliation in the former instances, and in the latter instances work directly with the other entities to achieve timely reconciliation.
Responsibility for Part D sponsors to account for other providers of prescription drug coverage when a retroactive claims adjustment creates an overpayment or underpayment is addressed in the Part D regulations. Part D sponsors must coordinate benefits with SPAPs and other providers of prescription drug coverage and appropriately adjudicate claims. Compliance with this requirement entails the sponsor not only coordinate benefits with other payers at POS, but also work with beneficiaries and other payers to resolve post-adjudicative payment issues arising from retroactive claims changes.
Retroactive claims adjustments can be necessitated by beneficiary changes (such as those resulting from retroactive LIS eligibility determinations, LIS status changes, or midyear Part D enrollment changes), sponsor receipt of other payer information, or errors in payer order. Some of these changes, those occurring within the payers’ timely filing window, may be addressed through pharmacy-initiated reverse and rebill transactions. However, CMS guidance states that sponsors generally should limit requests for pharmacy reprocessing to those situations involving a payment error. All retroactive claims adjustments that cannot be addressed through pharmacy reverse and rebilling must be handled by the Part D sponsor through other means. Part D sponsors must determine whether or not any amount paid by any other payers was TrOOP-eligible and must adjust, as necessary, the affected beneficiaries’ TrOOP balances.
CMS has established timeframes for Part D COB. Plan sponsors must coordinate benefits with SPAPs, other entities providing prescription drug coverage, beneficiaries, and others paying on the beneficiaries’ behalf for a period not to exceed 3 years from the date the prescription for a covered Part D drug was filled. CMS also requires that whenever a sponsor receives information that necessitates a retroactive claims adjustment, the sponsor must process the adjustment and issue refunds or recovery notices within 45 days of the sponsor’s receipt of complete information regarding the adjustment.
Requirements for Part D COB are specified in statute and codified in Federal regulations. CMS Part D COB guidance is provided in Chapter 14 of the Medicare Prescription Drug Benefit Manual available on the CMS Web site at: http://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Chapter14.pdf . This section does not supersede official CMS guidance, but is intended to convey a very general understanding of Part D COB requirements.
(Rev. 17, Issued: 08-23-13, Effective Date: 06-07-10, Implementation Date: 01-01-11)
(NOTE: These definitions are for purposes of this manual chapter only.)
4RX Information: Identifying data used for the electronic routing of pharmacy claims. The information includes:
Rx Bank Identification Number (BIN)
Rx Processor Control Number (PCN)
Rx Group
Rx Member ID
AIDS Drug Assistance Program (ADAP): A State-administered program authorized under Title II of the CARE Act that provides FDA-approved medications to low-income individuals with HIV disease who have limited or no coverage from private insurance or Medicaid.
Coordination of Benefits (COB): Effective exchange of information and coordination between a Part D plan and other entities providing other prescription drug coverage for—
Payment of premiums and coverage;
Payment for supplemental prescription drug benefits as described in §423.104(f)(1)(ii)(including payment to a Part D plan on a lump sum per capita basis) for Part D eligible individuals enrolled in the Part D plan and the SPAP or entity providing other prescription drug coverage; and
Retroactive claims adjustments, underpayment reimbursements, and overpayment recoveries as described in paragraph (g) of this section and § 423.466(a) of this subpart.
Financial Information Reporting (FIR): When a Part D enrollee has changed from one benefit plan to another during the plan year, Financial Information Reporting is the NCPDP standard process whereby point-in-time financial information (accumulated TrOOP and Gross Covered Drug Cost) is moved from the previous plan processor to the new processor. This information is necessary for the new plan to accurately process claims and position the enrollee in the correct stage of the Part D benefit.
Gross Covered Drug Cost: On a claim level, this is the amount (Ingredient Cost Paid + Dispensing Fee Paid + Total Amount Attributed to Sales Tax) paid to the pharmacy for a covered drug. Accumulated gross covered drug costs represent the year-to-date sum of the beneficiary’s covered drug costs and determine what phase of the benefit the beneficiary is in.
Other Health Insurance (OHI): Other insurance that can be primary or supplemental to Part D.
Other TrOOP: Qualified third party payments that contribute to a beneficiary's TrOOP, except for LICS and Patient Pay Amount. Examples include payments made on behalf of a beneficiary by qualified SPAPs, ADAPs and charities.
Part D Transaction Facilitator: The CMS contractor responsible for receiving and responding to eligibility queries (E1 transactions) from the pharmacy at point-of-sale, identifying costs that are reimbursed by other payers and reporting supplemental claims information to Part D sponsors (N transactions), identifying beneficiary enrollment changes requiring TrOOP balance transfers and sending and receiving the FIR transactions. (Formerly the TrOOP facilitator.)
Patient Liability Reduction Due to Other Payer (PLRO): Amounts by which patient liability is reduced due to payments by other payers that do not participate in Part D and are not TrOOP-eligible. Examples include payments made on behalf of a beneficiary by Workers’ Compensation, group health plans and liability insurance.
Qualified status: The status assigned to supplemental third parties whose payments made on a beneficiary’s behalf count towards TrOOP.
State Pharmacy Assistance Programs (SPAP): A State program is considered to be a State Pharmaceutical Assistance Program if it-
Provides financial assistance for the purchase or provision of supplemental prescription drug coverage or benefits on behalf of Part D eligible individuals;
Provides assistance to Part D eligible individuals in all Part D plans without discriminating based upon the Part D plan in which an individual enrolls;
Meets the benefit coordination requirements specified in Federal regulations at 42 CFR 423.464;and
Does not follow or adopt rules that change or affect the primary payer status of a Part D plan.
Switch: A pharmacy claims router. Pharmacies use switches to route claims to the appropriate processor and, if applicable, to the Part D transaction facilitator.
Transactions:
B Transaction: A pharmacy claim or service billing.
N Transaction: Information reporting transaction containing information on a paid supplemental claim and sent by the transaction facilitator to the enrollee’s Part D plan.
E1 Transaction: Eligibility query, used by a pharmacy to verify an individual’s Medicare A/B eligibility or Part D enrollment information.
True Out-of-Pocket (TrOOP): Incurred allowable costs that are paid by the beneficiary or by specified third parties on their behalf within the limits of the standard benefit, up to a legislatively specified out-of-pocket threshold.
1 Under 42 CFR 423.458(d), Part D requirements may be waived for Programs of All-Inclusive Care for the Elderly (PACE) organizations if the requirements are determined to be duplicative of, or in conflict with, provisions that would otherwise be applicable to these organizations. Appendix F provides additional guidance on the applicability of the COB requirements to PACE organizations.
| File Type | application/msword |
| File Title | 10 – Introduction |
| Author | CMS |
| Last Modified By | Deborah Larwood |
| File Modified | 2013-08-20 |
| File Created | 2013-08-20 |
© 2026 OMB.report | Privacy Policy