Reporting Self Test
Att 3e Reporting of Self-Test Results.docx
Evaluation of Free Rapid HIV at-home Testing
Reporting Self Test
OMB: 0920-1018
Form Approved
OMB No. 0920-XXXX
Expiration Date: XX/XX/XXXX
Evaluation of Rapid HIV Home-Testing among MSM Trial
Attachment 3e
Reporting of Home-Test Results
Public reporting burden of this collection of information is estimated to average 5 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; Attn: OMB-PRA (0920-XXXX)
Reporting of Home-test Results
How did you get the home rapid HIV test kit?*
I was mailed my test as part of KNOWatHOME (I am a KNOWatHOME participant) (link to page for participant login)
I was given my test by someone I know (I am not a KNOWatHOME participant) (link to page to enter kit number)
Enter the kit number on the test kit to enter website. [link to image displaying where the number is located; use image below].

Enter code here: _____________
If code is valid, display “Activate My Kit” button
If OQ1 is not answered, display “Please enter the Kit Number printed on your oral fluid HIV test to proceed.” and loop back to enter the number.
If OQ1 ≠ Valid number, display “Sorry. The Kit Number you entered does not match our records. Please carefully re-enter the number printed on your kit to proceed.” and loop back to enter the number.
Allow participant 3 attempts to enter the number.
____________________________________________________
AUTO1. Date of Reporting: __ __ / __ __ / __ __ __ __
(M M / D D / Y Y Y Y)
AUTO2. Time Began Reporting __ __:__ __:__ __ [24 Hour time HH:MM:SS]
Thank you for reporting your home HIV test results. Questions marked with a red asterisk (*) must be answered to move forward. You may choose to not answer other questions.
____________________________________________________
If KNOWatHOME Participant, go to Reporting of Results for Study Participants
If Not a KNOWatHOME Participant, go to Reporting of Results for Friends and Sex Partners
Reporting of Results for Study Participants
Emory University and MANILA Consulting Group, Inc. are conducting the study, which is funded by the Centers for Disease Control and Prevention (CDC).
Select button below to watch video on home HIV testing
OraQuick Video English OraQuick Video Spanish
Sure Check Video Sure Check Video Spanish
OraQuick Results Reporting
(Ask only if a participant has “activated” an OraQuick test kit)
ST1a. When did you test yourself with OraQuick?*
__ __ / __ __ / __ __ __ __ [MM/DD/YYYY]
ST2a. What was the result from your OraQuick test?* (Please check one of the answers):
Positive
Negative
Test is not working (results do not look like example OR there are no lines on the stick)
ST3a. Please select the image that most looks like your Test Stick:*
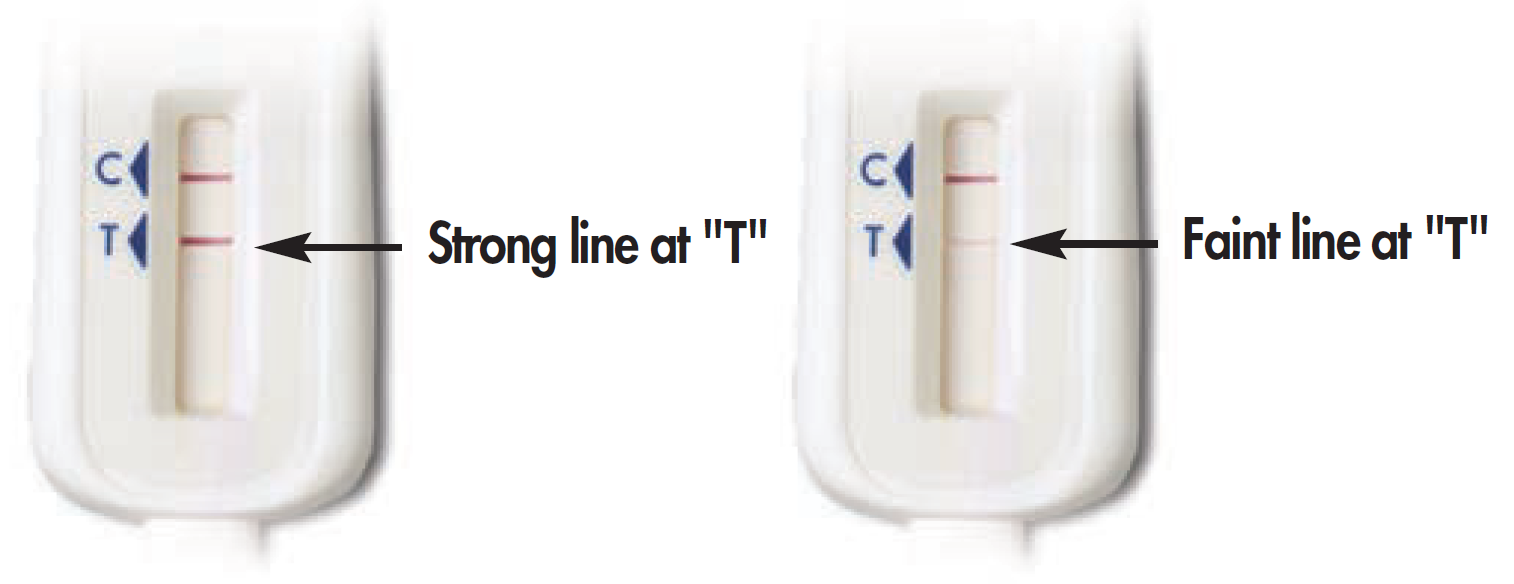
Some lines but my results do not look like the above examples
No lines on the test stick
ST4a. You mentioned that your oral fluid HIV test (OraQuick) test is “not working.” What happened when you tried to run the test? Check all that apply.*
I did not understand the instructions
I spilled the liquid from the test tube
The test stick got dirty before I was able to swipe my gums
I could not swipe the test stick on my gums properly
I did not put the test stick into the test tube
I did not time the test correctly
I did not follow steps in the order described in the instructions
There were no lines on the test stick
I could not see the lines on the test stick clearly
I did not understand what the lines on the test stick meant
I think a part of the test kit was missing
Other (Specify___________)
ST5a. OraQuick should be stored at 36◦-80◦F (2◦-27◦C). Did you keep the test kit within this temperature range?
Yes
No, I left it in a hot place (such as the trunk of my car)
No, I left it in a cold place (such as my refrigerator)
Not sure
ST6a. Where did you test yourself?
At home
At work
At a friend’s place
In a car
Other place (Specify___________)
I prefer not to answer
Depending upon test result (positive, negative or test is not working), display appropriate messages directing participants to the study referral support system for access to care and supplemental HIV testing. (Appendix J)
Sure Check Results Reporting
(Ask only if after a participant has “activated” a Sure Check test kit)
ST1b. When did you test yourself with Sure Check?*
__ __ / __ __ / __ __ __ __ [MM/DD/YYYY]
ST2b. What was the result from your Sure Check test?*
Positive
Negative
Invalid
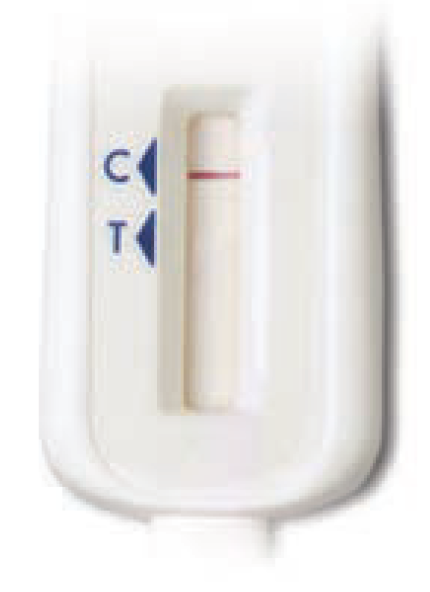
ST3b. Please select the image that most looks like your test device:*
Some lines but my results do not look like the above examples
ST4b. You mentioned that you got an “invalid” test result after using the finger-stick blood HIV test (Sure Check). What happened when you tried to run the test? Check all that apply.
I did not understand the instructions
The test device got dirty before I was able to collect a blood sample
I did not stick my finger to collect a blood sample
I could not collect a blood sample properly
I did not push the test device into the test holder
I did not time the test correctly
I did not follow steps in the order described in the instructions
There were no lines on the test device
I could not see the lines on the test device clearly
The lines on the test device did not look like the examples in the instruction booklet
I did not understand what the lines on the test device meant
I think a part of the test kit was missing
Other (Specify___________)
ST5b. Sure Check should be stored at 46◦-86◦F (8◦-30◦C). Did you keep the test kit within this temperature range?
Yes
No, I left it in a hot place (such as the trunk of my car)
No, I left it in a cold place (such as my refrigerator)
Not sure
ST6b. Where did you test yourself?
At home
At work
At a friend’s place
In a car
Other place (Specify___________)
I prefer not to answer
____________________________________________________
Depending upon test result (positive, negative, invalid, not working), display appropriate messages directing participants to the study referral support system for access to care and supplemental HIV testing. (Appendix J)
End Survey.
AUTO3. Time Ended Reporting: __ __:__ __: __ __ [24 Hour time HH:MM:SS]
Reporting of Results for Friends and Sex Partners
This home HIV test kit has been distributed as part of a research study funded by the Centers for Disease Control and Prevention (CDC). To learn more about the study, click here. On the following web pages, we will provide you with some help on how to use the HIV test kit, and ask you to provide some anonymous information as part of the study.
If participant clicks for more information, display:
The Emory University Rollins School of Public Health is doing a research study of men who use the Internet. This study is to find out if men will test for HIV at home and if they will give test kits to others. What we learn will help create better HIV prevention programs for our community.
Thank you for taking the time to complete this survey! By continuing and using this website to answer questions about yourself and your previous testing experience, you are agreeing to be part of a research study.
Questions marked with a red asterisk (*) are required questions that you must answer to move forward. You may choose to not answer any questions that make you feel uncomfortable. Your answers are private: the information you provide us will be kept secure and known only to study staff. This information will not be shared or used for any other research purposes.
If you have any questions or feel you have been harmed in this study please contact a member of the research team:
Patrick Sullivan, DVM PhD
Emory University
Rollins School of Public Health
1518 Clifton Road NE
Room 464
Atlanta, GA 30322
(404) 727-2038
If you have any questions about your rights as someone receiving a study home HIV test kit from a study participant or feel you have been harmed, you can contact the institutional review board at Emory University.
For Emory University contact:
Emory IRB
1599 Clifton Road
5th Floor East
Atlanta, GA 30322 USA
Tel: 404 712 0720
Toll free: 877 503 9797
Email:[email protected]
You may keep a copy of this form for your records if you like.
____________________________________________________
We would like to ask you a few questions to help you best use the kit. Your answers are private and anonymous.
SP1. How old are you?* __ __
If SP1 <18 display:
The home test kits provided by this study are for persons age 18 and older. To get information on where to get HIV testing at a place near you, click on this link: http://www.aidsvu.org/hiv-testing-site-locator
skip to End
SP2. Do you consider yourself Hispanic or Latino?
No
Yes
I prefer not to answer
SP3. Which racial group or groups do you consider yourself to be in? Check all that apply.
American Indian or Alaska Native
Asian
Black or African American
Native Hawaiian or Other Pacific Islander
White
Does not apply
I prefer not to answer
SP4. Do you consider yourself to be male, female, or transgender?
[Check only one]
Male
Female
Transgender
I prefer not to answer
SP5. Where did you get the rapid HIV home test kit(s)?
Friend
Sex partner
Family member/relative
Other (Specify___________)
I prefer not to answer
SP6. Before using the study home HIV test kit(s), when was your last HIV test?
__ /____ [MM/YYYY]
I have never been tested for HIV
I don’t know
I prefer not to answer
OraQuick Results Reporting
(Ask only if a participant has “activated” an OraQuick test kit)
ST1a. What was the result from your OraQuick test? (Please check one of the answers)*
Positive
Negative
Test is not working (results do not look like example OR there are no lines on the stick)
ST2a. Please select the image that most looks like your test stick: *


Some lines, but my results do not look like the above examples
No lines on the test stick
If ST1a = “Positive” or “Negative”, skip ST3a.
ST3a. You mentioned that your oral fluid HIV test (OraQuick) test is “not working.” What happened when you tried to run the test? Check all that apply. *
I did not understand the instructions
I spilled the liquid from the test tube
The test stick got dirty before I was able to swipe my gums
I could not swipe the test stick on my gums properly
I did not put the test stick into the test tube
I did not time the test correctly
I did not follow steps in the order described in the instructions
There were no lines on the test stick
I could not see the lines on the test stick clearly
I did not understand what the lines on the test stick meant
I think a part of the test kit was missing
Other (Specify___________)
Depending upon test result (positive, negative or not working), display appropriate messages directing participants to the study referral support system for access to care and supplemental HIV testing. (Appendix J)
Sure Check Results Reporting
(Ask only if after a participant has “activated” a Sure Check test kit)
ST4b. What was the result from your Sure Check test? *
Positive
Negative
Invalid
ST5b. Please select the image that most looks like your test device: *

Some lines but my results do not look like the above examples
If ST4b = “Positive” or “Negative”, skip ST6b.
ST6b. You mentioned that you got an “invalid” test result. What happened when you tried to run the test? Check all that apply.
I did not understand the instructions
The test device got dirty before I was able to collect a blood sample
I did not stick my finger to collect a blood sample
I could not collect a blood sample properly
I did not push the test device into the holder
I did not time the test correctly
I did not follow steps in the order described in the instructions
There were no lines on the test device
I could not see the lines on the test device clearly
The lines on the test device did not look like the examples in the instruction booklet
I did not understand what the lines on the test device meant
I think a part of the test kit was missing
Other (Specify___________)
____________________________________________________
Depending upon test result (positive, negative or invalid), display appropriate messages directing participants to the study referral support system for access to care and supplemental HIV testing. (Appendix J)
End Survey.
AUTO3. Time Ended Reporting: __ __:__ __: __ __ [24 Hour time HH:MM:SS]
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Freeman, Arin (CDC/OID/NCHHSTP) |
| File Modified | 0000-00-00 |
| File Created | 2021-01-27 |
© 2026 OMB.report | Privacy Policy