Self-test Completion
Att 3h Reporting of Self-Test completion.docx
Evaluation of Free Rapid HIV at-home Testing
Self-test Completion
OMB: 0920-1018
Form Approved
OMB No. 0920-XXXX
Expiration Date: XX/XX/XXXX
Evaluation of Rapid HIV Home-Testing among MSM Trial
Attachment 3h
Reporting of Self-Test Results at the completion of the study
Public reporting burden of this collection of information is estimated to average 5 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; Attn: OMB-PRA (0920-XXXX)
Reporting of Self-Test Results
____________________________________________________
AUTO1. Date of Reporting: __ __ / __ __ / __ __ __ __
(M M / D D / Y Y Y Y)
AUTO2. Time Began Reporting __ __:__ __:__ __ [24 Hour time HH:MM:SS]
Thank you for participating in our study! This survey includes personal questions about your rapid HIV home test results. Questions marked with a red asterisk (*) are required questions that you must answer to move forward. You may choose to not answer any questions that make you feel uncomfortable.
If you have any questions, problems with using the tests, or if you test positive, you can call this toll-free study support number 24 hours a day, 7 days a week: 1-866-728-1885.
____________________________________________________
Section A. Kit usage information
The package we sent you contained 3 items: 1 oral fluid HIV test (OraQuick), 1 dried blood spot (DBS) specimen collection kit, and 1 finger-stick blood HIV test (Sure Check).
KU1. Which items in the package did you use? Check all that apply.
Oral fluid HIV test (OraQuick)
DBS specimen collection kit
Finger-stick blood HIV test (Sure Check)
If KU1 = “Oral fluid HIV test (OraQuick)”, ask questions in Section B.
If KU1 = “Finger-stick blood HIV test (Sure Check)”, ask questions in Section C.
If KU1 = “DBS specimen collection kit”, ask questions in Section D.
Section B. Kit verification and results reporting for OraQuick
OQ1. What is the Kit Number printed on your oral fluid HIV test (OraQuick)?*
____________________
If you don’t know where the Kit Number is located, please click here [link to image displaying where the number is located; use image below].

Please hit the ‘Next’ button to submit your answer.
If OQ1 is not answered, display “Please enter the Kit Number printed on your oral fluid HIV test to proceed.” and loop back to enter the number.
If OQ1 ≠ Valid number, display “Sorry. The Kit Number you entered does not match our records. Please carefully re-enter the number printed on your kit to proceed.” and loop back to enter the number.
Allow participant 3 attempts to enter the number.
Please tell us the result of your oral fluid HIV test (OraQuick), and then select an image that most looks like your test device.
OQ2.
What was the result from your OraQuick test? (Please check one of the answers):
Positive
Negative
Test is not working (results do not look like example OR there are no lines on the stick)
OQ3. Please select the image that most looks like your test stick:
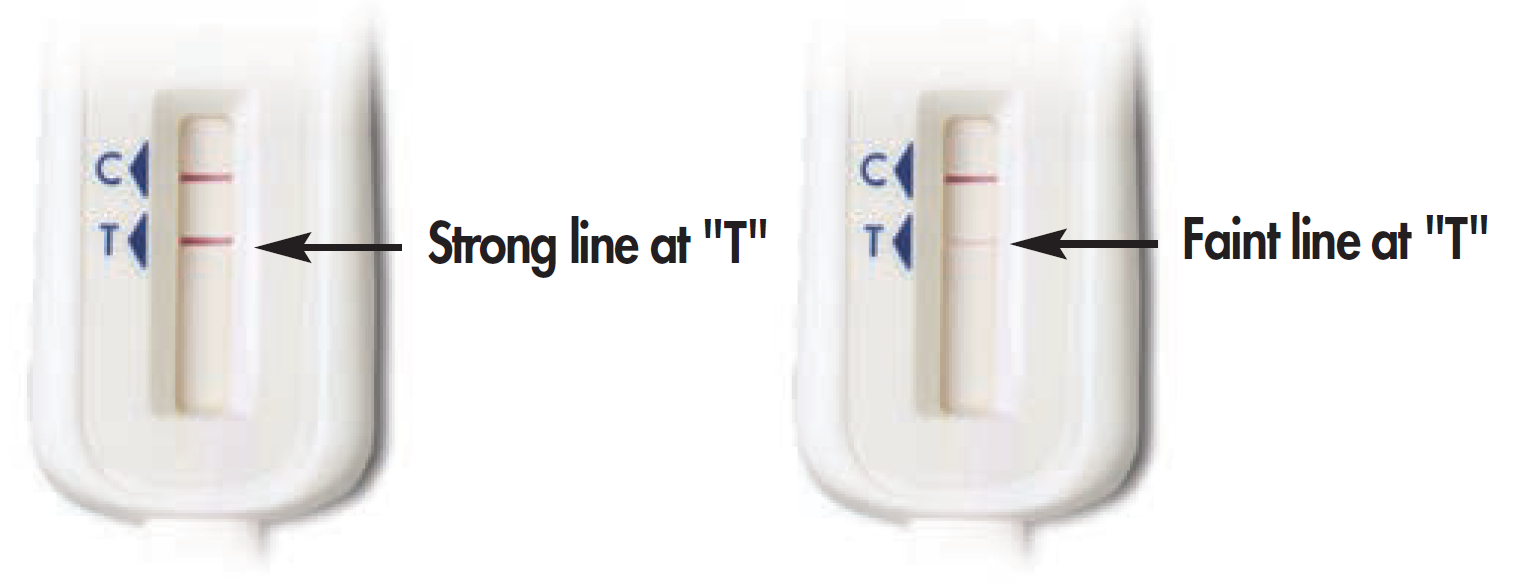
Some lines, but my results do not look like the above examples
No lines on the test stick
If OQ2 = “Positive” or “Negative”, skip OQ4.
OQ4. You mentioned that your oral fluid HIV test (OraQuick) test is “not working.” What happened when you tried to run the test? Check all that apply.
I did not understand the instructions
I spilled the liquid from the test tube
The test stick got dirty before I was able to swipe my gums
I could not swipe the test stick on my gums properly
I did not put the test stick into the test tube
I did not time the test correctly
I did not follow steps in the order described in the instructions
There were no lines on the test stick
I could not see the lines on the test stick clearly
I did not understand what the lines on the test stick meant
I think a part of the test kit was missing
Other (Specify___________)
Section C. Kit verification and results reporting for Sure Check
SC1. What is the Kit Number printed on your finger-stick blood HIV test (Sure Check)?* ____________________
If you don’t know where the Kit Number is located, please click here [link to image displaying where the number is located; use image below].

Please hit the ‘Next’ button to submit your answer.
If SC1 is not answered, display “Please enter the Kit Number printed on your finger-stick blood HIV test (Sure Check) to proceed.” and loop back to enter the number.
If SC1 ≠ Valid number, display “Sorry. The Kit Number you entered does not match our records. Please carefully re-enter the number printed on your kit to proceed.” and loop back to enter the number.
Allow participant 3 attempts to enter the number.
Please tell us the result of your finger-stick blood HIV test (Sure Check), and then select an image that most looks like your test device.
SC2. What was the result from your Sure Check test?
Positive
Negative
Invalid
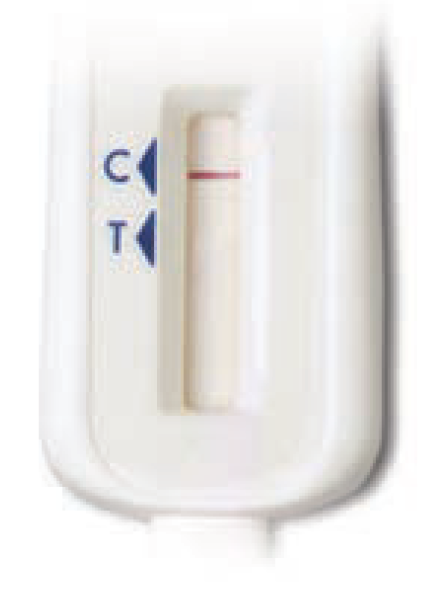
SC3. Please select the image that most looks like your test device:
Some lines but my results do not look like the above examples
If SC2 = “Positive” or “Negative”, skip SC4.
SC4. You mentioned that you got an “invalid” result after using the finger-stick blood HIV test (Sure Check). What happened when you tried to run the test? Check all that apply.
I did not understand the instructions
The test device got dirty before I was able to collect a blood sample
I did not stick my finger to collect a blood sample
I could not collect a blood sample properly
I did not push the test device into the test holder
I did not time the test correctly
I did not follow steps in the order described in the instructions
There were no lines on the test device
I could not see the lines on the test device clearly
The lines on the test device did not look like the examples in the instruction booklet
I did not understand what the lines on the test device meant
I think a part of the test kit was missing
Other (Specify___________)
Section D. Kit verification and specimen return for DBS
DB1. What is the Kit Number printed on your DBS specimen collection kit?*
____________________
If you don’t know where the Kit Number is located, please click here [link to image displaying where the number is located; use image below].

Please hit the ‘Next’ button to submit your answer.
If DB1 is not answered, display “Please enter the Kit Number printed on your DBS specimen collection kit to proceed.” and loop back to enter the number.
If DB2 ≠ Valid number, display “Sorry. The Kit Number you entered does not match our records. Please carefully re-enter the number printed on your kit to proceed.” and loop back to enter the number.
Allow participant 3 attempts to enter the number.
DB2. Have you mailed the filter card with your blood sample in the shipping envelope we provided?
No
Yes
If DB2 = “No” display “Please remember to package the filter card with your blood sample in the shipping envelope we provided and put it in the mail. After we receive your DBS specimen, we will send you an email with a link to the study website where you can specify how you want to receive an additional $20 token of appreciation or gift card.”
If DB2 = “Yes” display “Thank you for mailing the filter card with your blood sample. After we receive your DBS specimen, we will send you an email with a link to the study website where you can specify how you want to receive an additional $20 token of appreciation or gift card.”
___________________________________________________
Thank you for reporting your rapid HIV home test results!
Please read the following important information about the results you reported. After that, you will be able to go to the token of appreciation information section and specify how you would like to receive your $10 token of appreciation.
If either of the reported results is positive, the participant will receive the following message:
You have reported a positive result using at least one of the rapid tests. If this is the first time you have received a positive HIV result, it is important that you understand that this is only a preliminary result. You will need to go to a health care provider or an HIV counseling and testing center to be tested again. You might be feeling scared or intimidated, and might not know what all this really means. If you haven’t done so already, we encourage you to call our study’s toll-free number 1-(866) 728-1885 to talk to someone that will be able to help you sort things out and provide information on where to get tested.
You do not need to wait for the results from further HIV testing that will be conducted on your dried blood spot specimen. Please click on this link to get information on where to get counseling and additional testing at a location near you: www.aidsvu.org
If you prefer going to your own personal health care provider, you can print the following page that explains the study and the rapid tests you have used, and take it to your next appointment [link to a pdf page].
If the participant reports both negative test results, the participant will receive the following message:
The rapid test results you entered were negative. It is important to know that people who are very recently infected can get a negative test result. This is because there is a period of several weeks when a person may be infected, but they have not produced enough antibodies to the virus for the test to detect. If you think you are at risk or that you may have been exposed to HIV, it will be important for you to test again in three months to be certain of a negative result. You will also receive the results from the HIV testing that will be conducted on your dried blood spot specimen. Meanwhile please click on this link to get more information on how soon after infection these HIV tests detect infection: [link to website]. If you desire, please click on this link to get information on where to get counseling and additional testing at a location near you: www.aidsvu.org
If the participant reports an invalid or “not working” test result and no positive result, the participant will receive the following message:
The rapid test results you entered were “invalid" or “not working.” Please wait for the results from further HIV testing that will be conducted on your dried blood spot specimen. Please click on this link to get information on where to get counseling and additional testing at a location near you: www.aidsvu.org
___________________________________________________
Section E. Token of appreciation information
We will now ask you some questions about how you would like to receive $10 for completing this survey.
PY1. How would you like to receive your $10 token of appreciation? Choose only one method.
Token of appreciation through PayPal (requires a bank account)
Amazon.com electronic gift card (will be sent by email)
I do not wish to claim my token of appreciation
If PY1 = “Token of appreciation through PayPal”, then go to PY2.
If PY1= “Amazon.com electronic gift card”, then skip to PY4.
If PY1 = “I do not wish to claim my token of appreciation” then skip to End.
Receiving your token of appreciation by PayPal requires that you have
a bank account. You will NOT be required to provide information
about your bank account to this survey, only to PayPal. If you
do not have bank account, please return to the previous question and
select another option for your token of appreciation.
We
will send your PayPal token of appreciation to the email address you
provided during registration, unless you prefer for us to use another
email address.
PY2. Do you want us to use the email address [insert email address from QS1] to send your PayPal token of appreciation?
Yes
No
If PY2= “No”, then go to PY3 else skip to End.
PY3. Please enter the new email address where you would like us to send your PayPal token of appreciation. ____________________
We will send your electronic gift card to the email address you provided during registration, unless you prefer for us to use another email address.
PY4. Do you want us to use the email address [insert email address from QS1] to send your electronic gift card?
Yes
No
If PY4 = “No”, then go to PY6 else skip to End.
PY6. Please enter the new email address where you would like us to send your electronic gift card. ____________________
___________________________________________________
End
You can now take a follow-up survey to tell us about your testing experience. If you complete the follow-up survey you can receive an additional $10 as a token of appreciation.
Click here to take the follow-up survey [link to follow-up survey].
Otherwise, you can close your browser window. Thank you for your time. ____________________________________________________
AUTO3. Time Ended Reporting: __ __:__ __: __ __ [24 Hour time HH:MM:SS]
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Freeman, Arin (CDC/OID/NCHHSTP) |
| File Modified | 0000-00-00 |
| File Created | 2021-01-27 |
© 2026 OMB.report | Privacy Policy