Supporting Statement A 0368
Supporting Statement A 0368.docx
Health Center Patient Survey
OMB: 0915-0368
Supporting Statement A
Health Center Patient Survey
OMB Control No. 0915-0368
Terms of Clearance: None.
This submission requests Office of Management and Budget (OMB) clearance to conduct the Health Center Patient Survey (herein referred to as the HCPS; OMB No. 0915-0368). The survey is being conducted by RTI International for the U.S. Department of Health and Human Services (DHHS) Bureau of Primary Health Care (BPHC) within the Health Resources and Services Administration (HRSA). The HCPS is authorized under Sections 330 and 331 of the Public Health Service Act (USC 254 b,d)
HRSA’s Bureau of Primary Health Care administers the Health Center Program. Health Centers (HCs) improve the health of the Nation’s underserved communities and vulnerable populations by assuring access to comprehensive, culturally competent, quality primary health care services. The Health Center Program administered by BPHC supports the provision of community-based preventive and primary health care services to the medically underserved and to vulnerable populations with special needs, through Section 330 grants to HCs and community organizations. The populations include, but are not limited to: poor and near poor, homeless, public housing residents, migrant and seasonal farm workers, at-risk women, minorities, persons with HIV/AIDS, uninsured and underinsured, and non-English speakers.
The HCPS will collect in-depth information about HC patients such as their health status, the reasons they seek care at HCs, their diagnoses, the services they use at HCs and elsewhere, the quality of those services, and their satisfaction with the care they receive. The HCPS will be conducted in two stages: (1) a pilot survey or pretest of the survey instrument, survey sampling methodologies and procedures, which was conducted during fall 2013, and (2) the main survey, consisting of a personal interview of a stratified random sample of patients of the HC program. Both the pretest and main study will sample patients of Section 330-funded HCs. The HCPS builds on previous periodic User-Visit Surveys that were conducted to learn about the process and outcomes of care in Community Health Center (CHC), Health Care for the Homeless (HCH), Migrant Health Center (MHC), and Public Housing Primary Care (PHPC) projects. In addition, the HCPS will include patients from different racial/ethnic backgrounds, including Chinese, Koreans, and Vietnamese, subgroups not included in the previous surveys. The original questionnaires were derived from the National Health Interview Survey (NHIS) and the National Ambulatory Medical Care Survey (NAMCS) conducted by the National Center for Health Statistics (NCHS). Conformance with the NHIS and NHAMCS allowed comparisons between these NCHS surveys and the previous CHC and HCH User/Visit Surveys. The present HCPS instrument (Attachment 2) was developed using a similar questionnaire methodology to that used in the past, and will allow comparisons for HC projects with the previous User/Visit survey data, including monitoring of process outcomes over time.
Prior to the conduct of the main HCPS, a small-scale pretest was conducted to test main study implementation materials and procedures and identify potential questionnaire problems. The pretest included 69 in-person cognitive interviews conducted in English, Spanish, Chinese (Mandarin and Cantonese), Vietnamese, and Korean. Along with testing operational materials and procedures, the interviews focused specifically on identifying problems with instrument question wording, instructions, and assumptions as well as evaluating timing and the flow of the questions. Pretest results (Attachment 3) led to procedural and questionnaire improvements for the main study.
The data gathered from the pretest ensured proper administration of the main survey. The data from the main HCPS will facilitate the Bureau’s mission to improve the health of the nation’s underserved communities and ensure access to high-quality primary health care services. The data generated from these surveys will assist BPHC to assess how well HRSA-supported health care sites are able to meet health care needs, guide planning decisions, and determine the extent to which HCs meet the needs of underserved populations, as well as how patients perceive the quality of their care. Additionally, the data resulting from the survey will serve as a complementary source of data that are not routinely collected from other BPHC data sources.
Furthermore, data collected from this round of the Patient Survey will serve as a baseline to assess the impact of Affordable Care Act implementation on HC patients. The Patient Survey incorporates all measurement constructs from the Consumer Assessment of Health-care Providers and Systems (CAHPS) explicitly mentioned in H.R. 3590 (Affordable Care Act) for evaluating quality of care and patient satisfaction.
The Patient Survey results are essential to compare HC procedural, health care, and health outcome data with other national surveys (e.g., NHIS) and to conduct analyses that will enable comparisons of HC patient characteristics, health-related behaviors, health service utilization, health conditions, patient satisfaction, and patient access to care with patients in other health care settings. In addition, this iteration of the Patient Survey will be aligned with Section 4302 of the Affordable Care Act1 (ACA) and the 2011 race and ethnicity data collection standards specified by the Secretary of the U.S. Department of Health and Human Services.2 This alignment will provide unprecedented detailed information on the diversity of HC patients, their health insurance, and their health service use and experience than any other Patient Survey. To meet the HHS standards for data collection will require the Patient Survey to select a sample of patients from the 50 states (and the District of Columbia), increase its historical analytical sample size from 4,500 to 6,600, use respondent oversampling methods, and conduct the survey in four languages other than English.
The HCPS will include interviews of patients of Section 330-funded HCs. The study will specifically encompass 6,600 in-person interviews at no more than 520 sites within a sample of 165 HC grantees. Details regarding grantee and site sampling procedures are included in Supporting Statement B. Materials for recruitment, training site staff, and data collection are included in Attachments 4 and 5. Specifically, introductory letters and/or materials for the grantees, sites, and patients are included in Attachment 4 while informed consent forms and other procedural forms are included in Attachment 5.
A BPHC HC grantee often has several sites. Once the grantee is recruited, sampled HC sites will be contacted for participation in the survey. Patients seen at the HC in the past 12 months will be eligible for selection. All interviews will be conducted in-person by a field interviewer via computer-assisted personal interview (CAPI) in English, Spanish, Chinese (Mandarin or Cantonese), Korean, or Vietnamese at participating sites. (Some rescheduled interviews may take place outside the site. Details are included in Statement B 3d.) The interviews will take approximately 1.25 hours (75 minutes). To complete 6,660 interviews, there will be at least one field interviewer per sampled grantee. An estimated 130 local interviewers will be needed. For grantees that have substantial non-English speaking populations, a certified bilingual interviewer will be used.
Patients will enter the site and register with the receptionist for services. The receptionist will select patients on a flow basis according to the detailed study sample selection protocols to ensure the selection of a random sample of patients. Specifically, the receptionist will select the first patient registered after the RTI staff member informs the receptionist that he or she is ready for the next interview. The receptionist will read a brief recruitment script to the patient (or his or her parent or guardian, for selected children) and give him or her a study brochure that will provide patients with information about the study, how long the interview takes, who to contact for more information on the study, and of the $25 incentive (or noncash equivalent) they will receive for their participation. If the selected patient is interested in participating or has questions about the study, she or he will approach the RTI team member. The RTI staffer will work to gain cooperation and then take interested participants to the designated private location at the site to begin the screening, informed consent, and interviewing process. The RTI staff will ask the participant some initial screening questions from the Participant Screening Form (see Attachment 4) to determine the patient’s eligibility for the study (which includes determining patient type), and if he or she is eligible, administer the appropriate informed consent and continue with the interview.
RTI staff will employ one of the following informed consent procedures depending on (1) the age of the respondent and (2) if the respondent is 13 to 17, whether he or she is accompanied by an adult. The respondent’s answers to the questions included on the Participant Screening Form will inform which procedures the RTI staff member will follow.
Self-consent for adult respondents aged 18 and older—RTI staff will present the subject with a copy of the Adult Survey Participation Consent Form and read it aloud. Afterward, the subject will be invited to ask any questions about the study. Respondents who agree to participate will then be asked to sign the appropriate consent form; if respondents cannot sign their names, they will be asked to make a mark for their names.
Parental/guardian consent for child respondents aged 12 and younger (proxy interviews)—RTI staff will present the subject with a copy of the Parent/Guardian Participation in Proxy Interview for Accompanied Children Consent Form and read it aloud. Afterward, the subject will be invited to ask any questions about the study. Respondents who agree to participate will be asked to sign the consent form; if respondents cannot sign their names, they will be asked to make a mark for their names.
Parental/guardian consent and adolescent assent for respondents aged 13 to 17 who are accompanied by a parent/guardian—RTI staff will first approach the parent/guardian and obtain parental consent on the Parent/Guardian Permission Form for Adolescent Participation with the adolescent present. RTI staff will next request the parent/guardian go to a waiting area, therefore allowing the interviewer to privately obtain youth assent on the Minor Participant Assent Form.
The consent/assent forms will be read both to the parents and the adolescent. Both parents and adolescents will be invited to ask any questions. Parents/respondents who agree to participate will be asked to sign the consent form; if parents/respondents cannot sign their names, they will be asked to make a mark for their names.
All participants will be provided with an unsigned copy of the consent form to take with them after completion of the interview. In the parental/guardian consent and adolescent assent for respondents aged 13 to 17 protocol, a copy of the parent/guardian consent form will be given to the parent/guardian and a copy of the assent form will be given to the participant. After the administration of the informed consent, the interview will begin.
If at any time the privacy of the interview setting is compromised, the interviewer will pause the interview until privacy can be reestablished, rescheduling as necessary. After the interview is completed each respondent will be given a $25 incentive payment (or noncash equivalent) and asked to initial a receipt.
All data collection materials that are shown or read to respondents will be available in English, Spanish, Chinese (Mandarin and Cantonese), Vietnamese, and Korean.
Purpose and Use of Information Collection
The HCPS is unique in its effort to capture national, person-level data from patients of all types of HC Program grantees. The data collected from the HCPS will be used to:
Gather nationally representative data about the patients of the CHC, MHC, HCH, and PHPC programs and the services they obtain;
Enable comparisons of care received by HC patients with care received by the general population, as measured by NHIS and other national surveys; and
Gather information which will assist policymakers and BPHC staff to:
assess how well HRSA-supported HCs are currently able to meet health care needs,
identify areas for improvement and guide planning decisions, and
complement data that are not routinely collected from other BPHC data sources.
The specific BPHC priorities for analysis will be comparisons of HC patients with patients served in other primary care settings with respect to:
1. Access to care
2. Health disparities
3. Health conditions
4. Quality of care
5. Care coordination
6. Patient experience
Comparisons will be made with results from national surveys and with results from the 2009 Patient Survey. The data elements included in the HCPS instrument are collected through the following 16 modules administered to patients:
A. Introduction
B. Access to Care
C. Routine Care
D. Conditions
E. Conditions Follow-up
F. Cancer Screening
G. Health Center Services
H. Health Insurance
I. Prescription Medications
J. Dental
K. Mental Health
L. Substance Abuse
M. Prenatal Care / Family Planning (Females aged 15-49)
N. HIV Testing
O. Living Arrangements
Q. Income and Assets
R. Demographics
Use of Improved Information Technology and Burden Reduction
RTI will conduct the interviews using computer-assisted personal interviewing (CAPI). The CAPI approach offers several advantages that keep participant burden at a minimum while ensuring collection of high-quality data. First, the questionnaire is somewhat complex and involves numerous skip patterns and screening questions. These are easily and quickly performed by the computer upon completion of CAPI programming. In addition, the CAPI instrument detects erroneous and inconsistent responses, increasing data accuracy and validity. The use of CAPI, therefore, will enable the interview to be completed in less time and with more accuracy than the paper-and-pencil interview.
Efforts to Identify Duplication and Use of Similar Information
BPHC does not routinely collect this type of information from HC sites, and these data are not available from the Uniform Data System or any other source. The information to be collected through the HCPS is unique to this survey and cannot be obtained elsewhere.
Prior and Concurrent Related Studies
The HCPS builds on the Community Health Center Patient Survey conducted in 2009 (OMB No. 0915-0326), the Health Care for the Homeless User/Visit Surveys conducted in 2003 (OMB No. 0915-0274), the 2002 Community Health Center and National Health Service Corps Site User/Visit Survey (OMB No. 0915-0186), and the 1995 Community Health Center User/Visit Survey (OMB No. 0915‑0185). A pretest for the HCPS was conducted in 2013 and this is a request for the 2014 full scale data collection.
Impact on Small Businesses or Other Small Entities
This project will not have a significant impact on small businesses or small entities. Information regarding the best time to interview clients, policies regarding minors’ participation, and preferences regarding respondent remuneration type will be discussed with site staff to accommodate grantees and sites.
Consequences of Collecting the Information Less Frequently
BPHC conducted the Community Health Center Patient Survey in 2009, the Health Care for the Homeless User/Visit Surveys conducted in 2003, the 2002 Community Health Center and National Health Service Corps Site User/Visit Survey, and the 1995 Community Health Center User/Visit Survey. Collecting nationally representative data from patients of the CHC, MHC, HCH and PHPC programs 5 years after the previous CHCPS in 2009 will provide BPHC timely data, and enable BPHC to compare the HRSA-supported HCs patients with the general population, as measured by NHIS in meeting health care needs, identifying areas for improvement and guide planning decisions etc. Collecting the information less frequently or not at all could impact the measures BPHC is trying to assess and delay improvements to patient care.
Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This information collection fully complies with 5 CFR 1320.5(d)(2).
Comments in Response to the Federal Register Notice/Outside Consultation
Section 8A:
A 60-day Federal Register Notice was published in the Federal Register on January 11, 2013, Vol. 78, No. 8; pp. 2411-12. A copy of the notice is included in Attachment 6. One public comment was received on January 12, 2013 requesting for a copy of the data collection plan and the proposed instrument. The documents were sent to the commenter within one week from the request.
Section 8B:
In recognition of the significance of the HCPS pretest and main survey data collection efforts, several strategies have been incorporated into the project work plan that allow for the critical review and acquisition of comments relating to project activities, interim and final products, and projected and actual outcomes. These strategies include consultations with persons and organizations both internal and external to the BPHC, the U.S. Department of Health and Human Services, and the Federal government.
Specifically, a Technical Advisory Committee (TAC) was assembled for the HCPS. A list of the TAC participants for the project is provided in Attachment 7. Membership represents a broad spectrum of grantee staff members, representatives from coalitions/associations, nationally recognized research experts, and Federal government employees. Committee members serve as expert reviewers on the instrument design and analysis plans. Individual members will be consulted throughout the survey development process by telephone and participate (if available) in in-person meetings. The TAC reviewed the draft pretest data elements during an initial in-person meeting in January 2013. Written and/or verbal responses were received from all of those consulted, and the recommendations were incorporated into the survey and instrument design to the extent possible.
RTI International staff have been extensively involved in the statistical design of the survey and in the development of the data collection forms. RTI conducted the pretest and will conduct the full-scale survey implementation for the HCPS.
Explanation of any Payment/Gift to Respondents
Participants will be offered a $25 incentive (in the form of cash or a noncash equivalent) for taking part in the interview. This is a standard incentive amount provided to respondents for the burden placed on them. Sites will be given the option of requesting that RTI supply a cash or noncash incentive to the participants. If a site prefers noncash incentives, RTI will assist in determining an appropriate alternative. Examples of noncash options include gift cards and food vouchers. Interviewers will be required to complete a receipt for all incentives (whether they are cash or cash equivalent) and have the respondent sign the receipt. A large body of empirical survey research literature has been devoted to examining the role of incentives in survey outcomes. This research has typically examined how incentives affect survey response rates, sample composition, item nonresponse, and measurement error. Don Dillman (2000) states that offering an incentive is a critical element needed to predict a respondent’s reaction to the survey request. He explains that this is based on the social exchange theory and includes three components: rewards, cost, and trust. Furthermore, incentives have repeatedly been found to increase co-operation rates among certain groups: low-income and low-education groups, larger households and households with dependent children, minority ethnic groups and younger respondents (Dodd, 1998).
Please see the below breakdown:
Number of Survey Participants (Patients) |
Amount of Incentive |
Total |
6,600 patients |
$25 |
$165,000 |
Dillman, D. A. (2000). Mail and Internet surveys: The tailored design method (2nd ed). New York, NY: John Wiley & Sons.
Dodd, T. (1998) “Incentive Payments on Social Surveys: A Summary of Recent Research”. Survey Methodology Bulletin, 43: 23-27.
Assurance of Confidentiality Provided to Respondents
Participating individuals and institutions will be informed that the information provided in the HCPS will be kept secure and will be protected. Data collected will be in total conformity with HRSA’s standards for protecting personally identifiable information on individuals. Consistent with the Privacy Act of 1974, BPHC’s contractor will not provide participant names or information about participants to persons who are not part of the survey team.
The plan for maintaining privacy is outlined in a Data Security Plan (Attachment 8) created by BPHC’s contractor. Some highlights from the Data Security Plan include (1) maintaining safeguards to minimize the possibility of inadvertent disclosure, (2) conducting extensive training regarding privacy, and (3) obtaining privacy pledges from all personnel who will have access to individual identifiers.
Minimizing Inadvertent Disclosure
To avoid someone obtaining the information provided to RTI during the interview, RTI will conduct the interview in a private location where answers cannot be overheard. In addition, RTI will create an identification (ID) number that will be used instead of the respondent’s name, which will prevent anyone from identifying who supplied the answers. Finally, the patients will be selected for the study using onsite recruitment procedures that will protect the patients’ identity before they consent to participate in the study. The patient selection procedures are also designed to address Health Insurance Portability and Accountability Act (HIPAA) privacy concerns. Namely, the interviewer will not be allowed to approach any of the site’s patients nor obtain any information about the patients unless the selected patient initiates contact with the interviewer. The consent form accompanying the questionnaire will serve to inform respondents that that their participation is voluntary and will reiterate the protection of survey information.
All information collected will be kept private. Some respondent information will be collected and stored on paper. RTI staff will carry paper forms for recording respondent consent, receipt of incentives, and the actions which took place with each case including the current case status (via a contact summary report form). BPHC and RTI designed the data collection protocol to minimize the amount of identifying information (i.e., information that could identify the respondent as a study participant) that is stored on paper forms. Only the consent form and the contact summary report form will include the respondent’s personal information. The consent form will include the respondent’s signature while the contact summary report form will include the respondent’s case ID and, only if an appointment is set, the respondent’s first name, contact number, appointment location, and parent or guardian’s name (if applicable). RTI has specified physical safeguarding and shipping procedures for paper forms, and protocols for training RTI staff in the use of these procedures. Additional details regarding the procedures are included below.
Safeguarding Physical Materials in the Field
RTI staff will be trained on the importance of maintaining the privacy of all study materials containing case-specific information. Specific procedures designed to ensure that physical security of these items is not compromised. When interviewers are working in the field, case materials must be kept with the interviewer or kept in a locked trunk. However, materials may not be left in the trunk overnight. No materials may be left visible in an unoccupied vehicle.
To prevent opportunities for data loss after an interview is completed, RTI staff will be held accountable for adhering to the following specified procedures for shipping materials to back to RTI.
Completed consent forms, incentive receipts, and contact summary report form will be shipped to RTI periodically.
RTI staff will prepare a transmittal sheet and place it in the Federal Express package. The transmittal sheet contains the air bill number, name of the person who will receive the package, date the package is sent, and the case identification numbers of the case folders. RTI staff will keep a copy of the transmittal sheet. If a package is lost, an inventory of missing items is readily available.
Field staff will be instructed to send Federal Express tracking information via e‑mail each time they ship study materials to RTI. This e‑mail must include a list of the items in the Federal Express package, the shipment date, the expected delivery date, the delivery address, and the tracking number. No respondent personally identifying information is included in these e‑mails.
Packages cannot include the name or acronym of the study anywhere on the package or on the shipping bill.
Paper forms will be received, logged, and stored at RTI in a controlled-access location.
Project staff will train receipting staff on procedures for receipt and storage of materials.
All documentation (e.g., consent forms, incentive receipts, and contact summary report forms) will be kept in a locked RTI cabinet.
RTI Staff Training/Privacy Pledges
Comprehensive training on all data privacy and security protocols will be provided to RTI interviewing staff with particular emphasis on challenges to protecting privacy when conducting research in HC. The training will include detailed information on all data privacy and security protocols, including requirements for safeguarding private information and shipment of private materials. Also included will be a description of RTI’s disciplinary procedures for failure to follow project protocols, including those related to data privacy and security. RTI staff who violate protocols for protecting privacy and data security will be subject to RTI’s disciplinary procedures including verbal warnings, written warnings, probation, and termination. All staff working on the project will be required to sign a Privacy Pledge.
Additional Safeguards
RTI maintains a standing Committee on Human Subjects to ensure that all RTI surveys of human populations comply with applicable regulations concerning informed consent, confidentiality, and protection of privacy. This group serves as RTI’s Institutional Review Board (IRB) as required by law (45 CFR #46). RTI policy requires that the IRB independently review and approve the study design, instruments, and procedures, and monitor the study annually to ensure that respondents’ rights are fully protected.
Study notification materials provided to patients will describe the voluntary nature of the HCPS and convey the extent to which respondent identifiers and all responses will be kept private. Similarly, the scripts to be read by interviewing staff will be very specific in the assurances made to respondents and contacts.
Exceptions to the Assurance of Privacy
It is possible a respondent may become uncomfortable or upset during the interview or experience some psychological stress. To ensure that patients understand their participation is voluntary and that they can take a break, skip any questions, and/or refuse to participate at any time, this information is stated in the consent form and is reviewed with the participant prior to questionnaire administration. However, it is important to note that one exception to the assurance of privacy exists and is stated clearly during the informed consent process: “There is one important exception to this promise of privacy. If I learn during our talk that your life or health, or another person’s life or health could be in danger, I am required to inform the clinic staff or the proper authorities.” If a respondent becomes extremely distressed, expresses that he or she is considering suicide, expresses that he or she is considering harming another person, or discloses that he or she has been the victim of child abuse or neglect, interviewers will be trained to handle these types of critical incidents by following the critical incident protocol (Attachment 5).
In addition, RTI will create a resource list that will be supplied to the respondents at the end of each interview with helpline numbers to be used as needed.
Justification for Sensitive Questions
The HCPS instrument contains several items that may be viewed as “sensitive.”
Federal regulations governing the administration of these questions, which might be viewed as sensitive due to personal or private information, require (a) clear documentation of the need for such information as it relates to the primary purpose of the study, (b) provisions to respondents that clearly inform them of the voluntary nature of participation in the study, and (c) assurances of private treatment of responses. The following areas have been identified as potentially sensitive:
Questions on substance use and mental health status, and perceived need for and use of mental health and substance abuse services may be perceived as sensitive by some respondents. However, such information is important for understanding of the degree of unmet need for mental health and substance abuse services.
Questions on HIV testing status and HIV infection status may be perceived as sensitive by some respondents. However, such information is important for understanding the experiences of HC patients.
Questions on race/ethnicity can be viewed as sensitive. However, we are specifically over-sampling certain racial groups such as Asian, Latino, Pacific Islanders, etc.. We will need to have accurate data on race/ethnicity to ensure that we have accurate coverage of these sub-populations.
There is a question inquiring about the respondent’s annual earnings and public assistance. The question is designed to obtain the most accurate response to annual income. However, respondents can elect to respond using an income range if they feel more comfortable.
We also have general questions about health status such as questions on cancer, hepatitis, high blood pressure, tuberculosis, among others. We understand that patients may find it difficult to discuss these health conditions with an interviewer. However, we are interviewing patients within the health center so there is an expectation that these types of questions will be asked.
As noted in the informed consent procedures detailed previously, prior to conducting the interview all respondents are informed about the voluntary nature of their participation and the private treatment of their survey responses. Additionally, when a series of sensitive questions appear in the survey, they will be preceded by a statement read to the respondent that reiterates the points discussed during the informed consent administration. Respondents will understand that they have the right to refuse any question that they do not want to answer. They will also understand that refusing any question will not impact the care they currently receive from the HC.
Estimates of Annualized Hour and Cost Burden
Burden estimates for data collection activities are provided in Table 1, and estimated costs to respondents are presented in Table 2.
Table 1. Maximum estimated burden on respondents for the HCPS
MAIN SURVEY |
|
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Total Responses |
Average Burden per Response (in hours) |
Total Burden Hours |
Patient Selected and Approached FI |
6,996 |
1
|
6,996 |
.17 |
1,189 |
Patient Survey
|
6,600* |
1 |
6,600 |
1.25 |
8,250 |
Total National Study |
6,996 |
1 |
13,596 |
1.42 |
9,439
|
*This rate is based off of the 2009 Patient Survey where approximately 94% of the patients who were referred and approached the FIs completed the interview.
Table 2. Maximum estimated costs to respondents for the HCPS
MAIN SURVEY |
|
|
|
||||||
Type of Respondent; Activity Involved |
Total Hour Burden
|
Rate per Hour ($) |
Total Cost ($)
|
|
|
|
|
|
|
Patient Screening |
1,189 |
7.25* |
8,620 |
|
|
|
|
|
|
Patient Survey |
8,250 |
7.25* |
59,813 |
|
|
|
|
|
|
Total - Survey |
9,439 |
|
68,433 |
|
|
|
|
|
|
*This rate was calculated using the poverty guidelines for 2012 for families with 2 persons. The poverty level is set at $15,130, this was divided by 52, then 40 hours per week which is a rate of $7.25 hour.
Estimates of Other Total Annual Cost Burden to Respondents or Recordkeepers/Capital Costs
There are no capital, startup, or operating costs to respondents for participation in the project. No equipment, printing, or postage charges will be incurred to respondents.
Annualized Cost to Federal Government
Table 3 presents a summary of estimated annual costs to the Federal government for the HCPS. Included in the contract estimates are all staff time, reproduction, postage, and telephone costs for the management, data collection, analysis, and reporting for which clearance is requested. An annual breakdown of the contract costs is provided in Table 4.
Table 3. Individual and total costs to BPHC for HCPS field test and full-scale implementations
Annual Costs to BPHC |
Amount (in $) |
HRSA Salaries and Expenses, GS-13-01 Contract Costs
|
19,000 2,626,396 |
Annual Total |
2,656,396 |
|
|
Table 4. Annual contract costs for the HCPS implementation
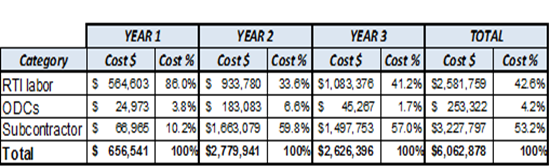
RTI labor covers labor for technical staff. ODCs include materials, telecommunications, shipping expenses, consultants, and domestic travel. Subcontractor includes labor and expenses for field data collectors.
Explanation for Program Changes or Adjustments
This is a new data collection.
Plans for Tabulation, Publication, and Project Time Schedule
The operational schedule for the survey is shown in Table 5. This will not be a public use data file. The results of the survey will be used in internal and external presentations regarding the Health Center Program, and for analyses, including analyses that may be published in peer-reviewed journals. In addition, conducting the patient survey is one of the items on BPHC’s Program Assessment Rating Tool (PART) Improvement Plan. An analysis plan with illustrative table shells is provided in Attachment 9.
Table 5. Operational schedule for the HCPS
Activity |
Projected Completion (# of weeks post OMB approval) |
Interviewer Training |
Week 12 |
Data Collection |
Week 31 |
Final Report and Products Delivery |
Week 69 |
Reason(s) Display of OMB Expiration Date is Inappropriate
The expiration date for OMB approval of the information collection will be displayed on data collection instruments and materials. No special exception to this request is requested.
Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification statement.
1 http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlid=208
2 http://www.hhs.gov/news/press/2011pres/10/20111031b.html
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Instructions for writing Supporting Statement A |
| Author | Jodi.Duckhorn |
| File Modified | 0000-00-00 |
| File Created | 2021-01-27 |
© 2026 OMB.report | Privacy Policy