HospiceDevelop_For_OMB_Appdx_A_
HospiceDevelop_For_OMB_Appdx_A_10-24-14.docx
National Implementation of the Hospice Experience of Care Survey (CAHPs Hospice Survey)
HospiceDevelop_For_OMB_Appdx_A_
OMB: 0938-1257
Final Report
Appendix A
Hospice Experience of Care Survey Development and Field Test
Rebecca Anhang Price, Denise D. Quigley, Melissa Bradley, Joan Teno, Layla Parast, Marc N. Elliott, Ann Haas, Brian Stucky, Brianne Mingura, Karl Lorenz
RAND Health
PR-1222-DHHS
June 2014
Prepared for Centers for Medicare & Medicaid Services

Preface
In September 2012, the Centers for Medicare & Medicaid Services (CMS) entered into a contract with RAND to design and field test a future Consumer Assessment of Healthcare Providers and Systems (CAHPS®) survey to measure the experiences of patients and their caregivers with hospice care. The survey was developed to provide a source of information from which selected measures could be publicly reported to beneficiaries and their family members as a decision aid for selection of a hospice program; aid hospices with their internal quality improvement efforts and external benchmarking with other facilities; and provide CMS with information for monitoring the care provided. CMS intends to implement the survey nationally in 2015. Eligible hospices will be required to administer the survey for a dry run for at least one month in the first quarter of 2015. Beginning in the second quarter of 2015, hospices will be required to participate on a monthly basis in order to receive the full Annual Payment Update.
This work was sponsored by CMS under contract number HHSM-500-2012-00126G, for which Lori Teichman serves as project officer. The research was conducted in RAND Health, a division of the RAND Corporation. A profile of RAND Health, abstracts of its publications, and ordering information can be found at www.rand.org/health.
Table of Contents
Preface ii
Figures v
Tables vi
Executive Summary viii
Survey Instrument Development viii
Call for Topic Areas ix
Literature Review and Environmental Scan ix
Technical Expert Panel x
Cognitive Interviews x
Field Test Design and Procedures xi
Eligibility Criteria xi
Sampling Hospices xi
Sampling Deaths within Hospices xii
Survey Administration Procedures xiii
Survey Instruments xiii
Field Test Results xiii
Characteristics of Field Test Hospices, Decedents and Caregiver Respondents xiii
Response Rates xiv
Item Nonresponse and Ceiling Effects xvi
Psychometric Analyses / Development of Composites xvii
Case Mix Adjustment xix
Association between Hospice, Decedent and Caregiver Characteristics and HECS Outcomes xxi
Open-Ended Responses xxii
Final Survey Instrument xxii
Recommendations for National Implementation xxiii
Survey eligibility criteria xxiii
Timing of Survey Administration xxiv
Sampling Procedures and Methods of Sampling xxiv
Mode of Survey Administration xxv
Data Requirements xxv
Abbreviations xxvii
Chapter One. Introduction 1
Chapter Two. Survey Instrument Development 3
Call for Topic Areas 4
Literature Review and Environmental Scan 5
Technical Expert Panel 7
Cognitive Testing 8
General Findings 9
General Findings: Spanish Only 10
Chapter Three. Field Test Design and Results 12
Field Test Procedures 12
Eligibility Criteria 12
Sampling Hospices 12
Hospice Recruitment 15
Sampling Deaths Within Hospices 15
Survey Administration Protocol 16
Timing of Administration 17
Survey Instruments 17
Characteristics of Field Test Hospices, Decedents and Caregiver Respondents 17
Response Rates 21
Predictors of Unit Response 22
Analysis of Unit Response 23
Analysis of Response Mode 23
Item Nonresponse and Ceiling Effects 39
Floor and Ceiling Effects 51
Psychometric Analyses and Development of Composites 63
Item and scale-level correlations 65
Setting-specific composites 66
Case Mix Adjustment 70
Association between Hospice, Decedent and Caregiver Characteristics and Hospice Experience of Care Survey Outcomes 94
Open-Ended Responses 100
Chapter Four: Final Survey Instrument 104
Chapter Five: Recommendations for National Implementation 105
Survey eligibility criteria 105
Timing of Survey Administration 105
Sampling Procedures and Methods of Sampling 106
Mode of Survey Administration 106
Data Requirements 107
Appendix A: Members of the Technical Expert Panel 108
Appendix B: Hospice Experience Survey – Home Version 109
Appendix C: Hospice Experience Survey – Nursing Home Version 127
Appendix D: Hospice Experience Survey – Inpatient Version 143
Appendix E: Item Response Rates Among Unit Respondents 160
Appendix F: Summary of Changes to Field Test Survey 166
References 176
Figures
Figure 3.1. Plot of Percentage Inappropriate Skips by Item Mean Position, all items 50
Figure 3.2. Plot of Percentage Inappropriate Skips by Item Mean Position, omitting Latino origin items 52
Tables
Table 2.1. Cognitive-Interview Location, Patient Care Setting, and Income 9
Table 3.1. Targets for Hospices in Each of Three Sampling Strata 13
Table 3.2. Targeted Sample Sizes, by Hospice Care Setting and Size 14
Table 3.3. Characteristics of hospices participating in the HECS field test and nationwide using hospices “currently active” in 2012 Provider of Service file 18
Table 3.4. Characteristics of Decedents Whose Caregivers Completed the Field Test Survey and Medicare Hospice Decedents Ages 65 and Older 19
Table 3.5. Characteristics of Field Test Respondents and Respondents in the 2013 Family Evaluation of Hospice Care survey repository 21
Table 3.6. Response Rates by Setting and Survey Versiona 25
Table 3.7. Composition of Eligible Sample and Response Rates, by Hospice Characteristic 27
Table 3.8. Composition of Eligible Sample and Response Rates, by Caregiver Characteristic 29
Table 3.9. Composition of Eligible Sample and Response Rates, by Decedent Characteristic 29
Table 3.10. Mixed Linear Regression Model of Unit Response 32
Table 3.11. Unit Response Rates, by Final Setting of Care (%) 34
Table 3.12. Comparison of Eligibles, Respondents by Mail, and All Respondents, by Caregiver Characteristic 35
Table 3.13. Comparison of Eligibles, Respondents by Mail, and All Respondents, by Decedent Characteristic 36
Table 3.14. Item Nonresponse Rates by Mode and by Final Setting of Care 43
Table 3.15. Probability of item nonresponse: Logistic regression of non-legitimate missingness among all unit respondersa 45
Table 3.16. Inappropriate Skips and Item Nonresponse Rates 49
Table 3.17. Ceiling Effects: Percentage of respondents in the lowest and highest possible category and ICC 52
Table 3.18. Survey items comprising multi- and single-item composites and item-total correlations 67
Table 3.19. Correlations among composites and single-item indicators. 69
Table 3.20. Potential Case-Mix Adjustors 72
Table 3.21. Hospice-Level Intraclass Correlation Coefficients of Potential Case-Mix Adjustors to Be Included in the Models 74
Table 3.22. Summary of Models and Impact Analysis for Potential CMA and Overall Rating 77
Table 3.23. Summary of Models and Impact Analysis for Potential CMA and Willingness to Recommend 78
Table 3.24. Summary of Models and Impact Analysis for Potential CMA and the Hospice Team Communication Scale 80
Table 3.25. Summary of Models and Impact Analysis for Potential CMA and the Treating your Family Member with Respect Scale 82
Table 3.26. Summary of Models and Impact Analysis for Potential CMA and the Providing Emotional Support Scale 84
Table 3.27. Summary of Models and Impact Analysis for Potential CMA and the Getting Help for Symptoms Scale 86
Table 3.28. Parameterization of Ordinal CMA: p-values of test of hull hypothesis that categorical variables does not improve prediction beyond inclusion of the linear form. 91
Table 3.29. Overall Unadjusted Mean Scores for Overall Rating, Willingness to Recommend, and Composites 96
Table 3.30. Adjusted mean response for overall rating of hospice by hospice characteristics 97
Table 3.31. Adjusted mean response for overall rating of hospice by patient and respondent characteristics 98
Table 3.32. Adjusted Mean Response for Each Developed Composite, Overall Rating, and Willingness to Recommend, by Final Setting of Care 100
Table 3.33. Themes Identified in Open Ended Responses 101
Table E.1. Item Response Rates Among Unit Respondents 160
Table F.1. Summary of Changes to Field Test Survey 166
Executive Summary
The Centers for Medicare & Medicaid Services (CMS) has implemented experience of care surveys in a number of settings including traditional Medicare, Medicare Advantage and Part D Prescription Drug Plans, hospitals, and home health agencies. While CMS and/or the Agency for Healthcare Research and Quality (AHRQ) have developed additional Consumer Assessment of Healthcare Providers and Systems (CAHPS®) surveys for in-center hemodialysis facilities, nursing homes and clinician and group practices, none of these surveys address experiences with hospice care.
In September 2012, CMS entered into a contract with RAND to design and field test a future CAHPS survey to measure the experiences of patients and their caregivers with hospice care. The survey was developed to (1) provide a source of information from which selected measures could be publicly reported to beneficiaries and their family members as a decision aid for selection of a hospice program; (2) aid hospices with their internal quality improvement efforts and external benchmarking with other facilities; and (3) provide CMS with information for monitoring the care provided. National implementation of the survey will begin in 2015. Eligible hospices will be required to administer the survey for a dry run for at least one month in the first quarter of 2015. Beginning in the second quarter of 2015, hospices will be required to participate on a monthly basis in order to receive the full Annual Payment Update.
In this report, we briefly summarize the work that RAND conducted to develop and field test the new survey, referred to throughout the report as the Hospice Experience of Care Survey (HECS). We provide an overview of the survey development process; describe the field test design and procedures; present analytic methods and findings from the field test; present the final survey instrument for national implementation; and make recommendations for national implementation.
Survey Instrument Development
Content and design of the HECS were informed by the following inputs:
a Call for Topic Areas in the Federal Register;
a review of the literature and environmental scan of existing tools for measuring end-of-life care;
input and feedback from survey and hospice care quality experts at a technical expert panel (TEP); and
cognitive testing with primary caregivers of hospice patients.
Call for Topic Areas
In response to a Call for Topic Areas published in the Federal Register in January 2013, stakeholder groups provided suggestions for survey content, including the following:
perceptions of the adequacy and frequency of provider visits;
measures of physical, psychosocial and economic distress of patients receiving hospice care in the nursing home;
level of support by the nursing home in obtaining a hospice referral;
adequacy and redundancy of services by the hospice care team and the residential facility;
information about experiences with medication changes;
regular use of comprehensive symptom management instruments in the hospice setting;
speed and degree of symptom management as well as flexibility in meeting patient needs;
availability of information to support informed decision making by patients and their caregivers;
degree to which hospice providers discussed, understood, respected and met patient and care giver preferences regarding the extent and intensity of life-prolonging care; and
specific items to address patient provider communication, care coordination, shared decision making, symptom management including pain and anxiety, access to care, understanding hospice, respect and dignity, care planning process, confidence of caregiver to perform care tasks, emotional and spiritual support, caregiver circumstances, and recommendation of the hospice to others.
Literature Review and Environmental Scan
A systematic review of the peer-reviewed literature on experiences with end-of-life care identified 87 articles containing 50 unique survey tools. The most common categories of survey content were as follows:
information / care planning / communication (Number of survey questions=632)
symptoms (303)
provider care (223)
spiritual / religious / existential (187)
overall assessment (134)
psychosocial care (131)
personal care (80)
veteran care (72)
responsiveness / timing (71)
caregiver support (59)
quality of death / last days (51)
bereavement care (33)
environment (28)
patient-centered care (20)
financial (14)
Technical Expert Panel
In December 2012, we convened a Technical Expert Panel (TEP), including experts on hospice care quality, survey research and performance measurement and improvement as well as individuals representing organizations that could have a major influence on the adoption of a standardized hospice care survey and promotion of its use in public reporting and quality improvement. TEP members agreed with the main survey content domains proposed: Access to care / responsiveness; Communication; Shared decision making; Care coordination; Symptom management / palliation; Information / skills for caregivers; Emotional / spiritual support; Environment; Overall rating of care.
TEP members agreed that the field test should exclude from sampling those cases in which the hospice patient died within 48 hours of admission, there was no caregiver listed in hospice records, or the primary caregiver in hospice records is a non-familial/friend (i.e., legal) guardian. TEP members recommended that the survey be administered no sooner than 1 month after death, and no later than 6 months after death, but noted that the logistics of sampling (i.e., receipt of data from hospices, data processing and mailing) would likely preclude sampling before 6 weeks after death.
Cognitive Interviews
Based on input from the Call for Topic Areas, literature review, qualitative interviews, and TEP, we drafted and refined three setting-specific survey instruments for cognitive testing, one for the home setting, one for the nursing home setting, and one for the inpatient setting, including both freestanding hospice inpatient units (IPUs) and acute care hospitals.
The team conducted three rounds of cognitive interviews to test interpretation and comprehension of survey content, revising survey instruments and protocols revised between each round of interviews. Interviews resulted in refinements to the carrier phrase (“while your family member was in hospice care”); re-organization of the survey to separate items inquiring about the respondents own experience with hospice from items inquiring about the patient’s experience; and removal of an item regarding pain treatment decisions in favor of an item regarding side effects of pain medicine.
Field Test Design and Procedures
From November 12 through December 23, 2013, we conducted a field test of the three setting-specific versions of the HECS. The survey was administered between 2 and 5 months following the death of the hospice patient, corresponding to deaths that had occurred between July 26, 2013 and September 11, 2013.
The field test was designed to assess survey administration procedures and to develop composite measures of hospice performance, while enabling comparisons of response rates and response patterns for larger and smaller hospices, and for the four settings of hospice care:
home, which includes home and assisted living facilities
nursing home, which includes skilled and regular nursing facilities
two sub-settings of inpatient care
acute care hospitals
free-standing hospice IPUs.
Eligibility Criteria
The following groups of hospice patients and the primary caregivers noted in their hospice’s administrative records were eligible for inclusion in the sampling universe:
Patients over the age of 18
Patients with death at least 48 hours following admission to their final setting of hospice care
Patients for whom a caregiver is listed or available and for whom caregiver contact information is known
Patients whose primary caregiver are people other than nonfamilial legal guardians
Patients for whom primary caregivers have U.S. or U.S. Territory home addresses.
Patients or caregivers of patients who requested that they not be contacted (those who sign no publicity requests while under the care of hospice or otherwise directly request not to be contacted) were excluded. Identification of patients and caregivers for exclusion was based on hospice administrative data.
Sampling Hospices
We used 2012 CMS Provider of Service and hospice claims files to characterize a sample frame of all hospices in the United States. We excluded hospices that were not eligible for, or had terminated, their participation in Medicare, those that had closed or had no claims for care services, and those that cared for fewer than 10 decedents per month, as these smaller hospices did not have enough volume to produce a sufficient sample size during the field test. We aimed to sample 30 hospice programs, 20 midsize-to-large (“larger”) hospice organizations (targeting completed surveys for 30 decedents per larger organization) and 10 smaller hospice organizations (targeting completed surveys for 10 patients per smaller organization). To increase the number of Spanish-speaking respondents, we sought to include at least one Puerto Rican hospice and one high-Hispanic mainland hospice.
In addition, we aimed to include a targeted number of hospices with the following characteristics in the final participating field test sample: a natural mix of hospices across 4 geographic regions in the U.S.; at least 1 hospice belonging to a national chain; 10 to 15 for-profit hospices; 1 government hospice; and at least 3 rural hospices, so as to establish feasibility of survey implementation and identify potential challenges (e.g., variation in response rates or rates of missingness) related to hospice characteristics.
To satisfy these targets, we randomly selected hospices proportionately with respect to region, and disproportionately with respect to hospice size, chain status, profit status, government ownership, and rural location. Because the design was not fully factorial, a simulation-based sampling approach was employed to derive a sample draw that was within a small pre-specified tolerance. Our sample target was 2,430 across hospice care settings and hospice size. We assumed 25 percent of deaths would be deemed ineligible, and a 40 percent response rate from caregivers.
Sampling Deaths within Hospices
Representatives from each hospice that agreed to participate in the field test submitted data files to support survey administration and analyses, including data on characteristics and care patterns of decedents, and contact information for primary caregivers. For each hospice, we identified and removed cases that were ineligible to participate.
To ensure a sufficient number of responses to compare experiences across settings of hospice care, we selected all eligible cases in the less common settings of care: nursing home, acute care hospital, and hospice inpatient unit. We subsampled cases in the largest setting, home care, with a higher sampling rate of 50% in hospices with higher proportions of black or Hispanic decedents (defined as 10% or more in either category). Across all hospices, we sampled 729 cases in the home setting, 639 in nursing homes, 198 in acute care hospitals, and 701 in hospice inpatient units, for a total of 2,267 cases.
Survey Administration Procedures
We used a mixed mode survey administration protocol, including one survey mailing, one prompt letter, and telephone as the secondary or nonresponse mode. In keeping with HCAHPS guidelines, the entirety of the field period from initial survey mailing to cessation of calling was no longer than 42 days (six weeks).
Survey Instruments
There were three setting-specific versions of the survey instrument, corresponding to the final setting in which the decedent received hospice care: home (including assisted living facility), nursing home, and inpatient (including acute care hospital and hospice inpatient unit).
Several survey sections were identical across the three versions: The Hospice Patient (3 items); Your Role (2 items); Starting Hospice Care (2 items); Your Own Experience with Hospice (7 items); Overall Rating of Care (3 items); About Your Family Member (4 items); and About You (7 items). The section on Your Family Member’s Hospice Care had 41 items on the home version, 37 items on the nursing home version, and 36 items on the inpatient version; 33 of these items were the same across all three versions. The home version had an additional section on Special Medical Equipment (3 items) and the inpatient version had an additional section on The Hospice Environment (3 items). The home version had a total of 72 items, the nursing home version had 65 items, and the inpatient version had 67 items; 61 items were the same across all versions.
Field Test Results
Characteristics of Field Test Hospices, Decedents and Caregiver Respondents
Thirty-three hospice programs from 29 hospice organizations agreed to participate in the field test. In keeping with our aim to include hospices with a range of size, ownership, geographic region, urbanicity, and chain status, 75.6% of hospices participating in the field test were small (10 to 29 deaths per month in the non-flu months of April through October), 39.4% were non-profit, 12.1% were located in rural areas, and 15.2% were members of national chains (Table 5). Compared to hospices nationwide, hospices participating in the field test were significantly more likely to be not-for profit (p=0.03) and had lower rates of live discharge (p=0.07). Hospices with fewer than 10 deaths per month in non-flu months were not eligible to participate in the field test, and therefore are not represented in the field test sample; such small hospices represent more than half (56.5%) of all hospices nationwide.
In all, 1,136 respondents completed the field test survey, reporting care experiences for 1,136 hospice decedents. The mean age of decedents was 79.8; 5.6% were black, and 4.3% were Hispanic. For more than one-third (34.7%) of decedents, the last setting of hospice care was a home or assisted living facility; last location was a nursing home for 27.9% of decedents, a hospice freestanding IPU for 29.7%, and an acute care hospital for 7.8%. The age, sex and race distributions of field test decedents were generally similar to the population of Medicare beneficiaries receiving hospice care. Hospice patients who died after less than 48 hours on hospice service were excluded from the field test; hence, the field test sample underrepresents those with short lengths of stay when compared to the national data.
Nearly three-quarters (72.6%) of respondents were female, 44.8% were age 65 or older, and 5.8% were black. Nearly half (46.6%) were children of the hospice patient, while one-third were the spouse or partner.
Response Rates
Unit nonresponse occurs when an eligible sampled individual does not respond to any of the items in a survey. We describe rates of unit nonresponse/response and assess hospice-, caregiver- and decedent-level characteristics associated with unit nonresponse.
The overall response rate among eligible members of the sample was 53.6%. The response rate in the home setting was slightly higher (56.5%) than in the other three care settings (51.3-52.9%). Multivariate regression analyses showed that the relationship between the survey caregiver and the decedent, previous receipt of the FEHC survey, decedent age at death, decedent race/ethnicity and length of final episode of hospice care are all significantly associated with the probability of response. In particular, spouses and parents were more likely to respond than children, those who were mailed the FEHC survey were less likely to respond, caregivers of older decedents were more likely to respond than those of younger decedents, and caregivers of Hispanic decedents were less likely to respond compared to other race/ethnicity categories. In addition, caregivers of decedents who had a longer length of final episode of hospice care were more likely to respond than those with a shorter length. Given the anticipated suspension of the FEHC during national implementation of the HECS, we may expect improved response rates in national implementation. Specifically, FEHC mailing was associated with an 8.8% lower response rate compared with those who were not mailed the FEHC in this field test and about 90% of eligible caregivers were mailed the FEHC; given our observed overall response rate of 53.6%, in the absence of the FEHC we would expect a response rate of about 61.4% given the same administration procedures and field period.
Non-responding cases include refusals, the majority of which were identified during telephone data collection and directly from the sampled caregiver rather than an informant on the caregiver’s behalf. Approximately 19% of caregivers who refused did not provide a specific reason for refusal, either simply hanging up or indicating they were not interested. Telephone interviewers could code more than one reason for refusal. Where reasons were provided, the most frequently cited were that the caregiver was too busy (cited by 34.4% of refusals) and/or not emotionally ready to discuss the patient’s care (cited by 31.3% of refusals). Some caregivers indicated that they had previously provided information, perhaps thinking about the FEHC, and would not do so again (cited by 14.4% of refusals). It seems likely that at least a portion of these refusals would have completed if they had not previously received the FEHC. A smaller proportion of refusing caregivers (11.3%) declined to participate citing that they did not know enough about the patient’s care; just over half of this group noted that the time the decedent spent in care was too short to properly comment. This follows along with the finding that caregivers of decedents with shorter lengths of stay were less likely to respond.
Caregivers with a longer time between decedent death and the beginning of mailing of the HECS, caregivers of younger decedents and caregivers of black and Asian/Pacific Islander decedents were less likely to respond by mail compared to phone. Given that a longer time between the decedent’s date of death and the date of first mailing tended to result in a lower probability of response by mail and thus a higher probability of response by phone and that mail mode is generally less costly than phone mode, this might suggest a recommendation that mailings go out more quickly than what we implemented in this field test. For example, these results suggests that delays between death and mailing that were in the highest quartile, a delay of 98 days or more, should be avoided in national implementation.
In addition, one-fifth of eligible non-responding cases were un-locatable during the field test. As caregivers may move or change contact information after patient death, this further underscores the need for fielding the survey in a timely manner after patient death. The number of un-locatable cases also highlights the need for hospices to give attention to verification of caregiver contact information, and to consider collecting and maintaining multiple sources of contact information for caregivers.
These response analyses also show that while caregivers of black and Hispanic decedents are less likely to respond to the survey in general compared to caregivers of white decedents, caregivers of black and Asian decedents that do respond are more likely to respond by phone rather than mail. With such small minority representation in the field test and likely across hospices in general, this highlights the importance of telephone follow-up to ensure that such groups are represented. Use of the telephone mode in addition to the mail mode yielded a group of respondents that were more similar to the eligible sample in terms of race/ethnicity of the decedent and in terms of other characteristics including relationship to decedent, age of decedent, and payer for hospice care, although differences still persist between all respondents and the eligible sampled group.
Item Nonresponse and Ceiling Effects
Item nonresponse occurs when a unit respondent inappropriately skips an item. We describe rates of item nonresponse and assess hospice-, caregiver-, and decedent-level characteristics associated with item nonresponse. In addition, we investigate floor and ceiling effects by examining both the number of respondents validating extreme response categories expressed as a proportion of valid responses obtained and the intraclass correlation coefficients (ICC). ICCs measure the amount of variability in response among hospices. Low ICCs indicate highly similar mean scores across hospices relative to variability within hospices and may indicate that an item was poorly understood and require modifications. However, a low ICC in combination with a very high or very low mean score may indicate a ceiling or floor effect (i.e. where most hospices score near the maximum or minimum limiting the ability of that question to distinguish performance between hospices).
Item nonresponse analyses showed that overall item missingness among eligible items was 5.5% with a lower item missingness rate observed in the home care setting even though the survey instrument for this setting is longer (62.9 eligible items compared to 56.0-58.4 for the other care settings). Higher non-response in the non-home care settings was not restricted to setting-specific items asked only in the nursing home and inpatient survey instruments. This pattern may be due to caregivers of decedents in the home care setting being more familiar with their family member’s care than caregivers of patients in other settings. Item missingness tended to be higher with an increased number of applicable items and for those items that appeared later in the survey instrument. While there was a slightly higher item non-response rate among respondents by phone compared to mail, it is common in CAHPS settings to see much higher item nonresponse by phone due to break-off (i.e., respondent hanging up before call is completed) than what was observed in this field test. This may indicate that break-off is less likely in the hospice survey due to the emotional content of the survey. Among unit respondents, several characteristics were associated with higher item missingness, including caregivers who were spouse/partners and non-family members (i.e., friends) of the decedent, caregivers of decedents covered by Medicaid or Medicaid/private insurance, caregivers of decedents in nursing home and inpatient care settings, and caregivers of decedents with a primary diagnosis of dementia/neurological disease or cardiovascular disease. Among unit respondents, several characteristics were associated with lower item missingness including caregivers of younger decedents, caregivers of Asian/Pacific Islander decedents, caregivers of decedents with longer final episodes of hospice care and caregivers who reported they ‘usually’ or ‘always’ took part in care of the decedent. This observed pattern in item nonresponse by caregiver relationship and decedent age may be largely driven by the fact that these caregivers may be older themselves and older age is often associated with higher item nonresponse in CAHPS. In addition, the observed lower rates of inappropriate missingness were observed among caregivers who reported ‘usually’ or ‘always’ taking part in care for family member compared with those who ‘sometimes’ took part in care is not surprising, as these respondents likely know more about the care that was received.
The analysis of floor and ceiling effects showed that 12 items had a high proportion of responses in the highest category and 11 of these 12 also had very small ICC estimates indicating a ceiling effect for these 11 items. For these 11 items, the ability to distinguish performance between hospices based on responses to these items is very limited. Given the anticipated larger number of respondents per hospice and larger number of hospices in national implementation, ICC estimates may be better estimated in national implementation.
Psychometric Analyses / Development of Composites
Composites are collections of items on the survey that assess similar content domains. When a set of items measure a given content domain, combining those items into a composite allows for a more precise estimate of a respondent’s experience of care than would be possible from any single item and allows fewer measures to be presented to consumers, reducing cognitive burden. We constructed factor analytic models to establish domains of interest (i.e., composites), and calculated item- and scale-level correlations to ensure the domains measure distinct content.
The analytic process resulted in the development of multi-item composites and single-item measures of key HECS domains, as follows. (Alpha is shown for multi-item composites, and refers to Cronbach’s alpha, a 0 to 1 index that increases with the number of items in a domain and their average correlation with one another. Higher values indicate better measurement of the underlying construct that the composite is intended to measure.) Survey items in each of the multi-item composites and single-item measures are:
Hospice team communication (alpha = .89) |
|
|
|
|
|
Getting timely care (alpha =.72) |
|
|
Treating your family member with respect (alpha =.69) |
|
|
Providing emotional support (alpha = .68) |
|
Providing Support for Religious and Spiritual Beliefs
|
Getting help for symptoms (alpha = .80) |
|
|
|
|
Information continuity |
|
Understanding the side effects of pain medication
|
|
Getting hospice care training (Home setting only; alpha = .87) |
|
|
|
|
The scales are generally moderately intercorrelated. There is a slight tendency for the inter-correlations between composites and measures to be highest for the Hospice team communication (r = .38 to .66). This is due in part to the survey generally assessing the communication between the hospice team and the family, but is also reflective of the high internal consistency of this composite. The inter-correlations are somewhat lower for the Information continuity (r = .23 to .38) and Providing emotional support (r = .16 to .53) composites, indicating that these domains measure content that is distinct on the survey.
Case Mix Adjustment
Previous research, both within and outside of CAHPS, has identified respondent characteristics that are not under the control of the entities being assessed but tend to be related to survey responses. For example, individuals who are older, those with less education and those in better overall and mental health generally tend to give more positive ratings and reports of care in Medicare CAHPS. Hence entities with disproportionate numbers of patients with such characteristics (favorable case mix) are advantaged relative to those with less favorable case mix. To ensure that comparisons between hospices reflect differences in performance rather than differences in case mix, responses must be adjusted for such characteristics.
We make recommendations for case-mix adjustment (CMA) of hospices participating in the field test, examine adjusted scores, and describe the impact of adjustment. Note that these are preliminary recommendations based solely on the field test and may be further informed by information obtained from national implementation. In general, only respondent characteristics that are determined not to be endogenous (i.e., not to be related to satisfaction or quality of care) should be considered as potential case-mix adjustors. Given this particular setting and available information, we considered both respondent and decedent characteristics as potential case-mix adjustors. Outcomes examined were: overall rating of hospice care, willingness to recommend the hospice, and the multi-item composites Hospice team communication, Treating your family member with respect, Providing emotional support, and Getting help for symptoms.
Overall, little-to-moderate variation in the following respondent and decedent characteristics was observed among hospices in the field test: language of completed survey, payer type, language spoken at home, prior receipt of the FEHC, decedent age, decedent education, primary diagnosis of dementia/neurological vs. other, and respondent education. A small number of characteristics were significantly associated with at least one of six outcomes examined in either a univariate or multivariate model: respondent sex, primary diagnosis of dementia/neurological vs. other, payer type, language spoken at home, primary diagnosis of cardiovascular disease vs. other, and language of completed survey. Only prior receipt of the FEHC demonstrated substantial marginal impact on adjustment of hospice-level scores.
Though decedent age, decedent sex, decedent education, respondent age, and respondent education neither were significantly associated with any examined outcomes nor had moderate or large (standardized regression coefficient greater than 0.20 SD) nonsignificant effects, one might consider retaining them in the survey for case-mix adjustment or other purposes. First, other CAHPS surveys including MCAHPS and CAHPS for Accountable Care Organizations (ACOs) observe substantial variation in respondent age and respondent education among entities being evaluated and significant associations with ratings and reports of care and thus adjust for such respondent characteristics. Our potentially limited power in the field test to observe such effects leads us to recommend retaining these items in the survey for further evaluation in national implementation. Second, while improved power in national implementation will also allow further evaluation of decedent age, sex and education as case-mix adjustors, one would also be interested in retaining these items in the survey regardless of adjustment potential to allow for description and reporting of observed true differences in quality of care by these characteristics at a national level. Similarly, this reasoning also supports the retention of survey items related to decedent race/ethnicity. While this decedent characteristic was ruled out for case-mix adjustment consideration, it should be retained in the survey so that potential disparities in quality of care can be examined moving forward. Respondent race/ethnicity, on the other hand, was not considered for adjustment and would likely not be needed for future analyses. Furthermore, among respondents who answered survey items relating to the respondent’s race/ethnicity and the decedent’s race/ethnicity, race/ethnicity matched in 94.8% of cases.
Payer type demonstrated substantial variation among hospices and was significantly associated with multiple outcomes. Therefore, we recommend including this variable in the final CMA model. Note that this is similar to the inclusion of Medicaid dual eligibility in the CMA models for MCAHPS and CAHPS for ACOs.
While the characteristic indicating whether a respondent was located in the same state as the hospice was included in our initial list of candidate adjustors and examined in these analyses, further discussion of this variable, along with potential inclusion of a variable indicating whether the respondent was located in the same city as the hospice, has led us to recommend that both variables be excluded from CMA consideration due to the fact that they seem to be proxies for census region. In general, stakeholders do not tend to support adjustment for region in CAHPS and to maintain consistency with other CAHPS survey initiatives we recommend not including variables that directly or indirectly measure region. Finally, while respondent’s relationship to the decedent was not significantly associated with any examined outcomes and varied very little among hospices, we recommend including this characteristic provisionally in the CMA model for the field test and recommend further examination in national implementation.
For the purposes of providing hospice level scores for hospices participating in the field test, we recommend a CMA model that includes the following:
language of completed survey
decedent age
decedent education
decedent sex
payer type (all categories)
primary diagnosis (all categories)
respondent age
respondent education
respondent sex
language spoken at home (all categories)
relationship to decedent (all categories)
prior receipt of FEHC Survey
This recommended case-mix adjustment model should be further examined and evaluated in national implementation. Prior receipt of the FEHC is unlikely to be relevant in the context of national implementation. Future considerations could include discussion about whether one should categorize primary diagnosis as dementia/neurological vs. cardiovascular disease vs. other, categorize payer type as Medicare only vs. Medicare and Medicaid vs. Medicaid only/Medicaid and private, categorize language spoken at home as English only vs. other and categorize relationship to decedent as spouse/partner vs. other.
Association between Hospice, Decedent and Caregiver Characteristics and HECS Outcomes
We explore a range of hospice, patient, and caregiver characteristics that may be associated with differences in care experiences, particularly geographic region, hospice size, chain status and profit status at the hospice level, and setting of care at the decedent level.
Overall, across hospice, decedent and caregiver characteristics, the mean overall rating of hospice care was 93.0 out of 100. Mean scores for each composite were generally high, ranging from 81.0 for Understanding the side of effects of pain medication and 85.2 for Getting hospice care training to 94.9 for Information continuity and 95.7 for Treating your family member with respect.
Adjusted means varied greatly by hospice region with lower adjusted means for overall rating and willingness to recommend for hospices in the Northeast and Puerto Rico. Regional results should be interpreted with caution given that field test hospices may not be representative of hospices within their regions, and that Puerto Rico results reflect only one hospice. Chain hospices also tended to have lower adjusted mean scores compared to non-chain hospices. Differences in adjusted mean scores by hospice size were not observed for any outcomes examined.
In keeping with prior analyses reported by the Medicare Payment Advisory Commission (MedPAC) regarding important concerns with provision of hospice care in nursing homes, we find that reported experiences of care are typically worse in the nursing home setting, particularly with regard to Understanding the side effects of pain medication, Getting help for symptoms, Getting timely care, and Hospice team communication. Such differences may be associated with different visit patterns in the nursing home setting (i.e., fewer visits from skilled nursing staff). The field test findings support that experiences of care in freestanding hospice IPUs are rated best by caregivers. There were few significant associations between patient and respondent characteristics and outcomes.
Open-Ended Responses
All versions of the field test instrument included an open-ended survey item meant to elicit detailed comments from respondents on both exemplars and problems related to the care the patient received from the hospice. One purpose of including the open-ended question was to determine if any domains not represented by the field test questions should be considered for inclusion in the final survey.
The open-ended text responses were analyzed to identify general themes. Text responses were first coded as positive or negative. Positive and negative comments were furthered coded into 14 themes; themes were identified based on the survey content and some emerged from the text itself. The most prevalent themes identified in the text included concern and respect, communication, emotional support, access, staff/team care, medication, knowledge imparted to caregiver, and religious support. The open-ended questions elicited rich and detailed responses regarding these themes, but for the most part addressed issues for which survey questions already existed.
Final Survey Instrument
We identified items to maintain for the final survey instrument using several general guidelines. First, we removed items that were included on the field test instrument solely to facilitate tests of construct validity (e.g., “Did your family member begin getting hospice care too early, at the right time, or too late?”), and those that exhibited little variation or ceiling effects. Some items with limited variation were maintained due to the importance of the measured constructs to hospice stakeholders or consumers (e.g., an item regarding spiritual/religious support). For parallel items regarding caregivers’ and decedents’ experiences (e.g., “How often did the hospice team listen carefully to you?” and …”to your family member?”), we generally included the item directed to the caregiver respondent rather than the decedent on the grounds that respondents’ answers regarding their own experiences have greater face validity than proxy answers on behalf of family members. Finally, we retained items, such as respondent and decedent race and education, that may be used for case-mix adjustment or other analytic purposes.
Because few setting-specific items were maintained for the final version of the survey instrument, and because it is simpler and less expensive to administer one survey instrument in national implementation, rather than multiple setting-specific versions, the three setting-specific survey instruments administered during the field test were consolidated into one instrument designed to measure experiences with care in all care settings in which the patient received care. Items specific to the nursing home setting are presented under the heading “Hospice Care Received in a Nursing Home,” and tailored nonapplicable responses are offered for items specific to the home setting. No inpatient-specific items were maintained for the final survey. The final survey instrument is 47 items.
Recommendations for National Implementation
Based on the experiences in the field test, and the input of a subsequent TEP convened for the National Implementation of the HECS contract, we recommend the following procedures for national implementation.
Survey eligibility criteria
The following groups of patients discharged from hospice are eligible for inclusion in the sampling universe:
decedents over the age of 18
decedents with death at least 48 hours following last admission to hospice care
decedents for whom there is a caregiver of record
decedents whose caregiver is someone other than a non-familial legal guardian; and
decedents for whom the caregiver has a U.S. or U.S. Territory home address.
Decedents or caregivers of decedents who request that they not be contacted (those who sign “no publicity” requests while under the care of hospice or otherwise directly request not to be contacted) will be excluded. Patients whose last admission to hospice resulted in a live discharge will be excluded.
These eligibility criteria closely match those of the field test with the notable exception that the required length of stay of 48 hours is not restricted to the final setting of hospice care as it was in during the field test. This recommendation follows from the decision to implement one consolidated survey, rather than setting-specific versions, in national implementation. During the field test we needed to ensure that patients had a minimum of 48 hours in the last setting of care, to ensure that caregivers had enough experience to respond to the setting-specific questions. With the one consolidated survey, all caregiver respondents, even those whose family member experienced a transition in care setting, should be able to respond to all questions. Approximately 99% of transitions in care setting occur within the same hospice organization (analysis of 2012 CMS hospice claims data); therefore, respondents reporting on care experiences across settings are highly likely to be reporting about the hospice named on the survey cover.
Timing of Survey Administration
We recommend that the 42-day data collection period begin 2 to 3 months following patient death. This will result in caregivers being surveyed between 2 and 4.5 months after their family member’s death. This recommendation is in keeping with the field test, but modified to reflect monthly data submission by hospices to vendors during national implementation. Survey administration should begin two calendar months following the completion of the data submission month (e.g., on April 1 for deaths occurring anytime between January 1 and January 31). The time lag is designed to be respectful of caregiver grief while allowing for adequate recall of hospice care experiences, and keeping to a minimum the proportion of the sample frame that will have changed contact information in the period following the death.
Sampling Procedures and Methods of Sampling
The field test did not examine alternative methods of sampling; however, given that many hospices participating in national implementation will have a small patient volume, we make the following recommendation:
Hospices with fewer than 50 decedents during the prior calendar year should be exempt from the survey data collection and reporting requirements. Hospices with 50 to 699 decedents in the prior year (n = 2,326 in 2012) should be required to survey all cases. Large hospices with 700 or more decedents in the prior year (n = 274 in 2012) should be required to survey a minimum sample of 700 using an equal-probability design. Prior to the introduction of the HECS, most hospices sponsoring the FEHC survey administered it to all cases (a census). While we do not recommend requiring census administration, this option should be available to hospices that wish to continue it.
Our sampling recommendations are derived from the assumptions, based on the HECS field test, that approximately 85% of cases will be eligible, and that approximately 50% of those in the sample frame will respond. These rates will result in an estimated 300 completed questionnaires for each large hospice and between 21 and 300 completed questionnaires for hospices with at least 50 decedents during the calendar year. Assuming a total of 300 completes within each hospice and an intraclass correlation coefficient (ICC) of 0.01, which measures the amount of variability between hospices, we would achieve an interunit reliability of 0.75. Note that in Medicare CAHPS (MCAHPS) a reliability of 0.75 is regarded as a minimal acceptable standard.
Mode of Survey Administration
The HECS field test did not examine the effects of survey mode on patterns or rates of response. As such, we recommend that hospices be allowed to administer the survey using one of the three mode protocols currently in use for other CMS CAHPS data collection efforts, such as HCAHPS. Specifically, the three recommended modes are: mail only (one mailed survey followed by an additional mailed survey to non-responders 21 days later); telephone only (up to five telephone attempts); and mixed mode (one mailed survey followed by telephone follow-up to non-responders 21 days later with up to five telephone attempts). During the first year of national implementation, a mode experiment will be conducted to assess the degree to which results from the three modes of survey administration are comparable, and to develop analytic adjustments to compensate for any differences across modes if needed.
Data Requirements
We recommend that hospices be required to supply monthly data files to their vendors containing the following types of data elements for hospice patients who died within a calendar month while under the care of the hospice program (first day of month through last day of month).
Information about the hospice patient
patient name (first, middle (if available), last) and prefix/suffix
date of birth
date of death
sex
race/ethnicity
primary diagnosis
admission date for final episode of hospice care
payers (primary, secondary, other)
last location / setting of care (i.e., home, assisted living facility, nursing home, acute care hospital, freestanding hospice inpatient unit)
Information about the primary caregiver
caregiver name (first, middle (if available), last) and prefix/suffix
contact information, including mailing address, telephone numbers, email address (if available)
relationship to hospice patient (i.e., spouse/partner, child, sibling, etc.)
Survey vendors should conduct all sampling activities. Hospices should be required to document the complete list of all patients/caregivers for whom information has been withheld from the survey vendor for any reason, and to provide counts of patients by each of the ineligible categories to allow for tracking. Ineligible categories are:
patient was discharged alive
decedent was over the age of 18
decedent’s death was less than 48 hours following last admission to hospice care
decedent has no caregiver of record
decedent’s caregiver is a non-familial legal guardian
decedent’s caregiver has an address outside the U.S. or U.S. Territories; and
decedent or caregiver requested not to be contacted (i.e., signed “no publicity” requests or otherwise directly requested not to be contacted).
Abbreviations
ACO |
Accountable Care Organization |
AHRQ |
Agency for Healthcare Research and Quality |
CAHPS |
Consumer Assessment of Healthcare Providers and Systems |
CFA |
confirmatory factor analysis |
CI |
confidence interval |
CMA |
case-mix adjustment |
CMS |
Centers for Medicare & Medicaid Services |
COPD |
Chronic Obstructive Pulmonary Disease |
COR |
Contracting Officer’s Representative |
EFA |
Exploratory Factor Analysis |
FEHC |
Family Evaluation of Hospice Care |
HCAHPS |
Consumer Assessment of Healthcare Providers and Systems Hospital Survey |
HECS |
Hospice Experience of Care Survey |
HRQOL |
Health-Related Quality of Life |
HSAG |
Health Services Advisory Group |
ICC |
intraclass correlation coefficient |
IPU |
inpatient unit |
MCAHPS |
Medicare Consumer Assessment of Healthcare Providers and Systems Survey |
MedPAC |
Medicare Payment Advisory Commission |
SD |
standard deviation |
SES |
socioeconomic status |
TEP |
technical expert panel |
Chapter One. Introduction
The Institute of Medicine has identified patient-centeredness as a cardinal feature of health care quality, alongside safety, effectiveness, timeliness, efficiency, and equity (Institute of Medicine, 2001). Surveys of care experience directly evaluate the degree to which care is patient-centered and therefore assess an intrinsically important dimension of care quality. Care experience measures derived from surveys complement other measures of care quality (Berenson, Pronovost, and Krumholz, 2013), facilitate providers’ efforts to improve patients’ experiences of care (Goldstein et al., 2001; Friedberg et al., 2011), and provide patients with valuable information for selecting health care providers and plans (Kolstad and Chernew, 2009).
The Centers for Medicare and Medicaid Services (CMS) has implemented experience-of-care surveys in a variety of settings, including traditional Medicare, Medicare Advantage, Medicare Part D Prescription Drug Plans, hospitals, and home health agencies. Although CMS and the Agency for Healthcare Research and Quality (AHRQ) have developed additional Consumer Assessment of Healthcare Providers and Systems (CAHPS®) surveys for in-center hemodialysis facilities, nursing homes, and clinician and group practices, none of these surveys addresses experiences with hospice care.
In September 2012, CMS entered into a contract with RAND to design and field test a future CAHPS survey to measure the experiences that patients and their caregivers have had with hospice care. The survey was developed to (1) provide a source of information from which selected measures could be publicly reported to beneficiaries and their family members as a decision aid for selection of a hospice program, (2) aid hospices with their internal quality improvement efforts and external benchmarking with other facilities, and (3) provide CMS with information for monitoring the care provided. CMS intends to implement the survey nationally in 2015. Eligible hospices will be required to administer the survey for a “dry run” for at least one month in the first quarter of 2015. Beginning in the second quarter of 2015, hospices will be required to participate on a monthly basis in order to receive the full annual payment update.
This report provides a summary of the work that we have conducted to develop and field test the new survey, the Hospice Experience of Care Survey (HECS). The report is divided into three parts. Chapter Two briefly describes each of the steps of survey development, including a public request for information about publicly available measures and important topics to measure; a review of the existing literature on tools that measure experiences with end-of-life care; exploratory interviews with caregivers of hospice patients; a technical expert panel (TEP) attended by survey development and hospice care quality experts; and cognitive interviews to test draft survey content. Chapter Three describes the field test design and procedures and presents analytic methods and findings, including unit response rates; item nonresponse and ceiling effects; composite development; case-mix adjustment (CMA) modeling; variation in performance by hospice, patient, and caregiver characteristics; and key drivers of overall performance ratings. Chapter Four presents the final survey instrument for national implementation. Chapter Five describes recommendations for national implementation. We also include six appendices: Appendix A lists participants in the TEP, Appendices B through D contain the three setting-specific field test survey instruments, Appendix E presents item response rates, and Appendix F summarizes changes to the field test survey.
Chapter Two. Survey Instrument Development
Rigorous development and testing are needed to develop an experience-of-care survey that can be used for a variety of purposes, including informing consumers, monitoring performance, identifying quality improvement targets, and promoting accountability (Darby, Hays, and Kletke, 2005; Crofton, Lubalin, and Darby, 1999). Survey development must take into account prior literature regarding experiences of care in the setting under consideration, perspectives of the consumers who may use reported care experience measures for decisionmaking, and stakeholders who will administer the survey and apply its results for quality improvement and accountability. Accordingly, the following steps were pursued to develop the content and design of the HECS:
call for topic areas in the Federal Register
review of the literature and environmental scan for existing tools for measuring end-of-life care
input and feedback from survey and hospice care quality experts at a TEP
cognitive testing with primary caregivers of hospice patients.
Throughout the development process, the project team incorporated input from each of these sources in an incremental process of revision and refinement to allow for more-precise measurement and to produce survey data that would better meet the information needs of consumer and stakeholder audiences.
CMS and RAND agreed on two critical design features before undertaking survey development, verifying these choices with the TEP. First, HECS respondents are informal caregivers (i.e., family members and close friends) of hospice patients, not hospice patients themselves. Direct reports from patients usually are not feasible because of the acuity of illness and speed of decline they experience. However, caregivers are critical informants for understanding hospice performance because the majority of hospice support is provided at home with the caregiver playing a constant, essential role in daily care. Surveys of family caregivers have been shown to be acceptable, given moderate agreement between patient and proxy responders (Kutner et al., 2006; Jones et al., 2011).
Second, because experiences of hospice care vary substantially by care setting, separate survey versions were developed to allow for exploration of setting-specific issues. The most common settings for hospice care are the patient’s home (including assisted living facilities) and nursing homes, while smaller proportions of patients receive hospice care in freestanding hospice inpatient units (IPUs) and acute care hospitals. Across settings, patients differ in care trajectories, acuity of illness, and cost of service provision (Nicosia et al., 2009). Familial caregivers play important—if different—roles in each of the settings, generally providing hands-on care in home settings and advocating for care quality in nursing home settings.
Call for Topic Areas
CMS published a Federal Register notice, “Request for Information to Aid in the Design and Development of a Survey Regarding Patient and Family Member/Friend Experiences with Hospice Care” on January 25, 2013 (U.S. Department of Health and Human Services, 2013). This call was designed to elicit suggestions for potential survey items and topics from organizations and stakeholder groups. The stakeholder groups provided suggestions and concerns about the following:
survey administration, including coordinating or deduplicating within and across surveys of the same or similar populations as the proposed hospice experience survey to minimize survey burden; combining survey administration modes, including Internet, to increase response rate and reduce costs; and consideration of survey vendor costs
timing of the survey, including recommendations that the timing of the survey administration begin one to three months after death, not sooner, and that surveys received several months or more after received be included in data analysis
length of the survey, including considerations regarding survey burden
survey population, including the suggestion that surveys be administered to hospice patients in addition to their caregivers, and targeting specific patient groups, such as those with and without cancer diagnoses
value of including an open-ended comment question for provision of valuable quality improvement feedback for hospices
importance of testing the survey among a diverse population so as to explore the cultural competence of care, sensitivity to preferences, beliefs, goals, and those who have experience with hospice
specific survey content, including questions on the following suggested topics:
perceptions of the adequacy and frequency of provider visits
measures of physical, psychosocial, and economic distress of patients receiving hospice care in the nursing home
level of support from the nursing home in obtaining a hospice referral
adequacy and redundancy of services from the hospice care team and the residential facility
information about experiences with medication changes
regular use of comprehensive symptom management instruments in the hospice setting
speed and degree of symptom management, as well as flexibility in meeting patient needs
availability of information to support informed decisionmaking by patients and their caregivers
degree to which hospice providers discussed, understood, respected, and met patient and caregiver preferences regarding the extent and intensity of life-prolonging care
specific items to address patient–provider communication; care coordination; shared decisionmaking; symptom management, including pain and anxiety; access to care; understanding hospice; respect and dignity; the care planning process, the caregiver’s confidence to perform care tasks; emotional and spiritual support; caregiver circumstances; and recommendation of the hospice to others.
Literature Review and Environmental Scan
We systematically reviewed the peer-reviewed literature on experiences with hospice and palliative care to identify survey content and any related data-collection methods and reporting and quality improvement issues. We also conducted a search of the gray literature (e.g., New York Academy of Medicine Grey Literature Report, Google, and the National Quality Measures Clearinghouse).
References captured in the searches were screened first by title and abstract and finally by review of the entire article for articles deemed relevant to the topic. The primary inclusion criteria were that the article (1) measured domains of patient or caregiver satisfaction and experience with hospice or palliative care and (2) included survey questions or instruments regarding patient or caregiver satisfaction or experience with hospice or palliative care. This included surveys developed by individual organizations and by researchers. Our primary exclusion criteria related to studies of pediatric populations and studies of health care provider satisfaction with hospice care. Researchers then reviewed each article, survey, or measure identified and abstracted information regarding the research study design and population, survey type, and content of survey questions and measures. For the most commonly used surveys, we also abstracted information about the identification of proxy respondents for after-death surveys, the timing and method of survey administration, and the health care setting assessed.
Our search of PubMed and PsycINFO identified 2,094 unique articles. After screening the titles using the inclusion and exclusion criteria described in the previous paragraph, reviewing abstracts, and reviewing full articles or reports, we identified 87 articles. These articles contained 50 unique surveys with available content. We characterized 14 content areas variably present across the 50 surveys. We reviewed an additional 39 surveys, measures, websites, and reports obtained from a search of the gray literature. Final review of other sources resulted in four other articles that were added to the full literature review and nine new surveys not identified in the literature review. Two tool kits (Hospice Assessment, Intervention, Measurement [AIM] Toolkit by the Prepare, Embrace, Attend, Communicate, and Empower [PEACE] Project and the Toolkit of Instruments to Measure End-of-Life Care [TIME]) were also identified. Through the review of articles and other sources, we identified 2,180 survey items (not unique).
To develop categories for potential domains, we used an iterative process whereby multiple researchers reviewed different survey questions and attempted to describe the key focus of the questions. After this exercise, one of the researchers finalized categories that were developed. This process resulted in 14 unique categories. The resulting most common categories were
information, care planning, and communication (number of survey questions = 632)
symptoms (303)
provider care (223)
spiritual, religious, and existential (187)
overall assessment (134)
psychosocial care (131)
personal care (80)
veteran care (72)
responsiveness and timing (71)
caregiver support (59)
quality of death and last days (51)
bereavement care (33)
environment (28)
patient-centered care (20)
financial (14).
Many of the survey questions identified through this literature search were found in multiple studies and had been tested and validated in one or more settings.
We also examined the survey administration procedures, including mode and timing. The primary mode of administration was a mailed survey. Other modes of survey administration included computer-assisted telephone, in person, mixed mode (in person and telephone, in person and mail), paper completed at site, and telephone. There was considerable variation in timing of survey administration across articles and among the same surveys, indicating that there is little consensus about when each survey should be administered. In four articles, surveys were administered to patients before death (i.e., two to seven days after a do-not-resuscitate order), and, in 37 articles, surveys were administered to caregivers after the patient’s death. The shortest reported time frame after death for survey administration was three to six weeks, and the longest time frame reported was up to 372 days after death. The majority of articles (n = 21) reported that surveys were administered within approximately one to six months after death.
Technical Expert Panel
In December 2012, we convened a TEP, including experts on hospice care quality, survey research, and performance measurement and improvement, as well as people representing organizations that could have a major influence on the adoption of a standardized hospice care survey and promotion of its use in public reporting and quality improvement. The TEP provided feedback regarding field test survey methods, survey design principles, and domains for the field test. Two main themes emerged from the TEP discussion. First, from the perspective of both CMS and the community of hospice providers, the unit of care for the survey is the patient and the family. Second, given plans to publicly report survey results, TEP participants noted the importance of developing survey content that is useful to consumers for prospective decisionmaking (i.e., to select a hospice). Survey content needs to allow for retrospective evaluation of hospice services because that would be useful for both quality improvement and CMS monitoring of care quality.
The TEP agreed that it was important to include in the field test hospices that varied according to size (i.e., number of deaths), geographic region, and chain status. Panel members recommended also considering including hospices that vary with regard to affiliation with a health system, ownership, and urbanicity.
TEP members agreed with the proposed exclusion criteria for sampling informal caregivers (i.e., family members or friends) within hospices: patient died within 48 hours of admission, no caregiver is listed in hospice records, or primary caregiver in records is a nonfamilial or friend (i.e., legal) guardian. TEP members also agreed that the survey should be administered no sooner than one month after death and no later than six months after death but noted that the logistics of sampling (i.e., receipt of data from hospices, data processing and mailing) likely preclude sampling before six weeks after death. It was agreed that the aim would be a median time of between one and three months, based on feasibility considerations.
TEP members agreed with the main survey content domains proposed: access to care and responsiveness, communication, shared decisionmaking, care coordination, symptom management and palliation, information and skills for caregivers, emotional and spiritual support, environment, and overall rating of care. They also made recommendations for consideration of supplemental content specific to veterans because this group of patients is more common than any other individual cultural group for which CMS might consider developing specific survey content. TEP members also emphasized the value of an open-ended question for quality improvement purposes.
TEP members also suggested that the following concepts potentially be explored in the survey:
degree to which the respondent is the family member or friend most knowledgeable about the patient’s hospice care
coordination between hospice and nonhospice personnel
degree to which the hospice team listened carefully to the family member
communication with caregivers who live far away geographically
assessment of whether the caregiver or patient needed help to manage communication across providers
management of bowel symptoms
Spanish-language version only: degree to which caregivers received the language services they needed
availability of a hospice care team member who spoke the patient’s or family’s language (if not English)
degree to which the patient and family’s wishes were respected regarding where and how the patient died (recognizing that not all patients can die where and how they might like)
care planning and goal setting for care
services for which hospice is responsible (beyond medical equipment, which was already covered in the draft survey)
making volunteers available to patients and caregivers
pharmacy services (e.g., getting medicines in a timely manner).
Cognitive Testing
Informed by input from the call for topic areas, literature review, qualitative interviews, and TEP, we drafted and refined three setting-specific survey instruments for cognitive testing: one for the home setting, one for the nursing home setting, and one for the inpatient setting, including both freestanding hospice IPUs and acute care hospitals.
The team completed three rounds of cognitive interviews to test interpretation and comprehension of survey content, including 11 English interviews (six in round 1, three in round 2, and two in round 3) and four Spanish cognitive interviews. Six of the interviews were completed in person, and nine were completed by telephone. The participants all had recent experience acting as caregivers for family members in hospice care. Participation targets were designed to ensure variation in SES of respondents (i.e., low versus high income) and final setting in which hospice care was delivered (i.e., home, nursing home, IPU). Participants were also recruited to ensure participation by African American and Hispanic caregivers. Details of participant location and patient care setting are provided in Table 2.2.
Table 2.1. Cognitive-Interview Location, Patient Care Setting, and Income
Interview |
Location |
Setting |
Income |
Round 1 |
Delaware Los Angeles Los Angeles Los Angeles North Carolina Kentucky |
Nursing home Nursing home Home Home IPU IPU |
Low High Low High Low High |
Round 2 |
Delaware Los Angeles Kentucky |
Nursing home IPU Home |
Low High Low |
Round 3 |
Los Angeles Los Angeles |
Nursing home Home |
High Low |
Spanish |
Los Angeles |
Home |
Low |
|
Los Angeles |
Home |
Low |
|
Los Angeles Florida |
Nursing home IPU |
High Low |
We recruited cognitive-interview respondents, and trained interviewers conducted the interviews using interview protocols specific to the patient’s care setting at time of death. Interviews were conducted at RAND, in participants’ homes, and by phone. A bilingual, bicultural interviewer conducted the Spanish interviews. Respondents taking part in the interview by phone were sent a survey via FedEx to complete during the call. Each interview began with the interviewer obtaining oral informed consent and describing the overall goals of the session. Interviews were audiotaped. Each respondent was paid $125 for participating.
Following each cognitive interview, project staff drafted summaries from the audio recordings and paper notes taken during each interview. After the first six interviews, the team participated in a debriefing meeting to discuss the instrument, identify common problem areas, and come to consensus about ways to change items to address the problems identified with the instrument during round 2 of cognitive testing. A similar meeting took place after round 2 interviews. Two additional interviews were conducted to test the final revisions to the instrument. Key findings are summarized below.
General Findings
Respondents whose family members were in more than one care setting had difficulty including only the final care setting in their responses. In round 2, we tested the carrier phrase, “While your family member was in his or her last location of hospice care” to determine whether the mention of “last location” would focus respondents on the final setting. However, the wording was confusing and irritating to nearly all of the respondents. After review of data showing that the proportion of patients who change care settings is quite small, the team chose to revert to the original wording, “While your family member was in hospice care.”
Round 1 interviews revealed that participants had difficulty distinguishing between questions about their own experience with hospice and the patient’s experience (e.g., “While your family member was in hospice care, how often did the hospice team spend enough time with you?” versus “While your family member was in hospice care, how often did the hospice team spend enough time with your family member?”). The ordering of the items was rearranged to present separate sections for “Your Family Member’s Hospice Care” and “Your Own Experience with Hospice.” Respondents were better able to attend to the distinction between these two types of questions in rounds 2 and 3.
Questions regarding the patient’s condition were met with confusion because condition was interpreted to mean the patient’s medical diagnosis. The phrase condition and care was tested in rounds 2 and 3 and determined to work better.
When responding to a draft question regarding shared decisionmaking for pain management, respondents reported very little involvement in such decisions, other than the hospice staff asking them whether their loved ones were in pain. Although nearly all respondents mentioned not wanting the patient to be in pain, several also noted that they were unaware of how drastically the pain medication would alter the patient’s consciousness. This concern was brought up several times by respondents in various sections of the cognitive interview but was not being tapped in any particular question. To ensure that this concept was addressed in the instrument, a question was added about the side effects of pain medication.
General Findings: Spanish Only
The term for hospice as originally tested (centro para enfermos terminales) (center for care of terminally ill patients) and hospicio (hospice, as used in other studies) presented a challenge for respondents. For example, one mentioned that, when she initially heard the term hospicio from a social worker, she was rather insulted because, in her home country, hospicio means “center or home where people who are homeless can live.”
Recommendation: Use the term hospicio because that is the term commonly used by hospice staff. However, a clear definition should be provided at onset in the Spanish survey in case a respondent is not familiar with this term in Spanish or staff used the English word hospice when referring to the hospice program.
The proposed Spanish translation for the term nursing home, casa de retiro, did not resonate with any of the respondents. Other terms tested were institución de cuidados de ancianos, centro de convalescencia, centro de recuperación, and the current CAHPS Nursing Home Survey version of the wording, hogar de ancianos y recuperación.
Recommendation: Poll nursing facilities and assisted living facilities to ascertain terms they use with patients when referring to their facilities. Alternatively, use the term employed by the CAHPS Nursing Home Survey instruments: hogar de ancianos y recuperación. Potentially also include a definition or description of a nursing home as part of the question.
Complex or double-barreled questions (e.g., question 32, “did your family member ever have trouble breathing or receive treatment for trouble breathing?”) took extra time and consideration to answer. However, responses were in keeping with the goals of the questions.
Recommendation: We do not recommend any changes to these questions.
Chapter Three. Field Test Design and Results
Field Test Procedures
From November 12 through December 23, 2013, we conducted a field test of three setting-specific versions of the HECS, reflecting three settings in which hospice care is delivered: home, nursing home, and inpatient (including both acute care hospitals and freestanding hospice IPUs). The field test was designed to assess survey administration procedures and to develop composite measures of hospice performance while enabling comparisons of response rates and response patterns for larger and smaller hospices, and the four settings of hospice care:
home, including home and assisted living facilities
nursing home, including skilled and regular nursing facilities
two subsettings of inpatient care:
acute care hospitals
freestanding hospice IPUs.
Eligibility Criteria
Eligibility criteria for hospice patients and their primary caregivers were determined in consultation with CMS and with input from the TEP and closely parallel Hospital CAHPS (HCAHPS) and Family Evaluation of Hospice Care (FEHC) survey eligibility criteria. The following groups of hospice patients and the primary caregivers noted in their hospices’ administrative records were eligible for inclusion in the sampling universe:
any patient over the age of 18
any patient with death at least 48 hours following admission to his or her final setting of hospice care
any patient for whom a caregiver is listed or available and for whom caregiver contact information is known
any patient whose primary caregiver is someone other than a nonfamilial legal guardian
any patient for whom the primary caregiver has a U.S. or U.S. territory home address.
Patients or caregivers of patients who requested that they not be contacted (those who sign no-publicity requests while under the care of hospice or otherwise directly request not to be contacted) were excluded. Identification of patients and caregivers for exclusion was based on hospice administrative data.
Sampling Hospices
We used 2012 CMS Provider of Services and hospice claim files to characterize a sample frame of all hospices in the United States. We excluded hospices that were not eligible for or had terminated their participation in Medicare, those that had closed or had no claims for care services, and those that cared for fewer than ten decedents per month because these smaller hospices did not have enough volume to produce a large enough sample during the field test. We aimed to sample 30 hospice programs: 20 midsize to large (“larger”) hospice organizations (with a target of completed surveys for 30 decedents per larger organization) and ten smaller hospice organizations (with a target of completed surveys for ten patients per smaller organization). To increase the number of Spanish-speaking respondents, we sought to include at least one Puerto Rican hospice and one high-Hispanic mainland hospice.
In addition, to establish feasibility of survey implementation and identify potential challenges (e.g., variation in response rates or rates of missingness) related to hospice characteristics, we aimed to include a targeted number of hospices with the following characteristics in the final participating field test sample: a natural mix of hospices across four geographic regions in the United States, at least one hospice belonging to a national chain, ten to 15 for-profit hospices, one government hospice, and at least three rural hospices.
To satisfy these targets, we randomly selected hospices proportionately with respect to region and disproportionately with respect to hospice size, chain status, profit status, government ownership, and rural location. Because the design was not fully factorial, a simulation-based sampling approach was employed to derive a sample draw that was within a small prespecified tolerance. Specifically, we divided hospices into three strata based on hospice-level criteria: high-proportion Hispanic, midsize or large, and small. We expected each of these strata to have a different proportion of hospices that was willing and able to participate in the study; therefore, we oversampled by different factors for each stratum (Table 3.1), establishing targeted numbers of hospices to approach for recruitment into the field test for each stratum.
Table 3.1. Targets for Hospices in Each of Three Sampling Strata
Stratum |
Hospice Characteristic |
Target for Final Sample |
Oversample Factor |
Target Number of Hospices to Approach for Recruitment |
A |
High-proportion Hispanic, regardless of size |
1 |
6 |
6 |
B |
Midsize or large, not high-proportion Hispanic |
19 or 20 |
3.75 |
73 |
C |
Small, not high-proportion Hispanic |
10 or 9 |
5.25 |
50 |
|
Total |
30 |
|
129 |
Additionally, we set targets for cases sampled within those hospices based on care setting and hospice size. To allow for empirical comparisons between any two of the four settings in which hospice care is delivered (i.e., home, nursing home, freestanding IPU, and acute care hospital), we aimed to sample cases in each of these settings. The acute care hospital is much less common than the other settings, comprising only 7.8 percent of all hospice deaths occurring in 2009 (analysis of CMS claim data). To ensure robust empirical comparisons between each of the more common settings of care, we aimed to evenly split 90 percent of our sample across caregivers whose family members or friends received hospice care at home (30 percent), in a nursing home (30 percent), and in freestanding units (30 percent). We aimed for the remaining 10 percent of the sample to consist of caregivers of those who received hospice care in acute care hospitals.
Table 3.2 describes how we aimed to distribute the sample target of 2,430 across hospice care settings and hospice size. We assumed that 25 percent of deaths would be deemed ineligible, resulting in 1,823 eligible deaths—68 per larger hospice and 23 per smaller hospice. The assumption of 25-percent ineligibility was designed to be conservative to ensure a sufficient number of completed surveys for analysis. Assuming a 40-percent response rate from caregivers, an estimate that reflects prior experience with the FEHC, we estimated that this sample target would result in approximately 730 completes—630 from larger hospices and 100 from smaller hospices—and approximately 219 from each of the three more common settings of care (home, nursing home, and freestanding unit) and 73 from the least common setting of care, acute care hospitals.
Table 3.2. Targeted Sample Sizes, by Hospice Care Setting and Size
Hospice Characteristic |
Target Sample Size |
Eligible (assumes 25% ineligibility rate) |
Completes (assumes 40% response rate) |
Hospice setting |
|
|
|
Home |
729 |
547 |
219 |
Nursing home |
729 |
547 |
219 |
Freestanding unit |
729 |
547 |
219 |
Acute care hospital |
243 |
182 |
73 |
Hospice size |
|
|
|
Midsize or large |
2,100 |
1,575 |
630 |
Smaller |
330 |
248 |
100 |
Total |
2,430 |
1,823 |
730 |
The simulation model first drew sets of hospices designed to conform to the hospice- and beneficiary-level targets in Table 3.2. Within these sets, hospices were then drawn to meet the additional criteria described above with regard to geographic region, chain status, profit status, government ownership, and urbanicity. The resulting final set consisted of a list of 127 hospices from which to conduct recruitment for the field test, two fewer than the original target.
Hospice Recruitment
The list of hospices for recruitment was divided into nine mutually exclusive queues of similar hospices, as follows:
high-proportion Hispanic hospices in Puerto Rico
high-proportion Hispanic hospices not in Puerto Rico
government hospices
rural hospices
hospices in a national chain
small for-profit hospices
small not-for-profit hospices
medium or large for-profit hospices
medium or large not-for-profit hospices.
Hospices were randomly sorted within each queue. Recruitment proceeded from top to bottom in each queue until the number of successfully recruited hospices reached the target number or the end of the queue was reached.
Health Services Advisory Group (HSAG) conducted outreach to hospices to secure an initial agreement of participation. We followed up with interested hospices to discuss details of data-transmission requirements and to secure fully executed business associate agreements and data-use agreements. Hospice recruitment occurred from June through September 2013, and all data-use agreements were in place in advance of the first data delivery in October 2013.
Sampling Deaths Within Hospices
Representatives from each hospice that agreed to participate in the field test submitted data files to support survey administration and analyses, including data on characteristics and care patterns of decedents, and contact information for primary caregivers. For each hospice, we identified and removed cases that were ineligible to participate. The most common reasons for ineligibility were length of stay less than 48 hours in the last setting of care (4.45 percent), insufficient contact information for the caregiver (3.31 percent), and patient death date not within the required time frame (1.92 percent). Caregivers identified as nonfamilial legal guardians were very rare (0.50 percent). Although some hospices excluded no-publicity cases, the number of exclusions for this reason was not reported.
To ensure a sufficient number of responses to compare experiences across settings of hospice care, we selected all eligible cases in the less common settings of care: nursing home, acute care hospital, and hospice IPU. We subsampled cases in the largest setting, home care, with a higher sampling rate of 50 percent in hospices with higher proportions of African American or Hispanic decedents (defined as 10 percent or more in either category). Across all hospices, we sampled 729 cases in the home setting, 639 in nursing homes, 198 in acute care hospitals, and 701 in hospice IPUs, for a total of 2,267 cases.
Survey Administration Protocol
We used a mixed-mode survey administration protocol, including one survey mailing, one prompt letter, and telephone as the secondary or nonresponse mode. Initial mailings included a personalized cover letter, a survey, and business reply envelope. The cover letters were two-sided, with English on one side and Spanish on the other. Cover letters were personalized with the patient name and the hospice name. The letter also provided a toll-free number for respondents to call if they had questions. The cover of the survey included a label indicating the name of the hospice and, if applicable, the specific hospice or nursing home facility in which the family member resided. English-language surveys were mailed to the sampled caregivers in the continental United States, and Spanish-language surveys were mailed to sampled caregivers in Puerto Rico.
Reminder letters were mailed to all sampled caregivers one week after the initial survey mailing. Telephone follow-up to nonresponders began three weeks after the initial mailing, and five telephone attempts were made for each nonresponding case. Phone attempts were made at varying times of day and days of the week to maximize the likelihood of reaching respondents. Respondents with incorrect or disconnected phone numbers were tracked by calling directory assistance and via LexisNexis. Telephone follow-up was available in both English and Spanish. Telephone staff strongly encouraged caregivers to complete the survey over the phone but also offered the option of having the caregivers return the survey by mail.
In keeping with HCAHPS guidelines, the entirety of the field period, from initial survey mailing to cessation of calling, was no longer than 42 days (six weeks).
Virtually all hospices routinely collect survey data, via the FEHC or an alternative instrument, because hospices are required by their conditions of participation in Medicare to administer such surveys. We strongly encouraged field test hospices to suspend data collection of other surveys during the field test period. However, many were unable or unwilling to do so for all or part of the field test period. To reduce the confusion and suppressed response rates that may result from administration of two surveys within a limited time period, we included text in the survey cover letter indicating why the respondent might have received a prior survey and encouraged completion of both surveys. We assessed the effects of prior receipt of the FEHC on response rates in the analyses of field test data.
Timing of Administration
The timing of survey administration and duration of the field period were informed by the literature review and cognitive interviews. The survey was administered between two and five months after the death of the hospice patient, corresponding to deaths that had occurred between July 26, 2013, and September 11, 2013.
Survey Instruments
There were three setting-specific versions of the survey instrument, corresponding to the final setting in which the decedent received hospice care: home (including assisted living facilities), nursing home, and inpatient (including acute care hospitals and hospice IPUs). Field test survey instruments are attached in Appendices A through C.
Several survey sections were identical across the three versions: “The Hospice Patient” (three items), “Your Role” (two items), “Starting Hospice Care” (two items), “Your Own Experience with Hospice” (seven items), “Overall Rating of Care” (three items), “About Your Family Member” (four items), and “About You” (seven items). The section “Your Family Member’s Hospice Care” had 41 items on the home version, 37 items on the nursing home version, and 36 items on the inpatient version, and 33 of these items were the same across all three versions. The home version had an additional section, “Special Medical Equipment” (three items), and the inpatient version had an additional section, “The Hospice Environment” (three items). The home version had a total of 72 items, the nursing home version had 65 items, and the inpatient version had 67 items; 61 items were the same across all versions.
Many items on each survey version were applicable to only a subset of respondents (e.g., only to those respondents whose family members experienced pain or shortness of breath). Screening questions were used to determine applicability for these dependent questions; respondents for whom the dependent items were not relevant were asked to skip them, and phone interviewers automatically skipped those items.
Characteristics of Field Test Hospices, Decedents and Caregiver Respondents
Thirty-three hospice programs from 29 hospice organizations agreed to participate in the field test. In keeping with our aim to include hospices with a range of size, ownership, geographic region, urbanicity, and chain status, 75.6% of hospices participating in the field test were small (10 to 29 deaths per month in the non-flu months of April through October), 39.4% were non-profit, 12.1% were located in rural areas, and 15.2% were members of national chains (Table 3.3). Compared to hospices nationwide, hospices participating in the field test were significantly more likely to be not-for profit (p=0.03) and had lower rates of live discharge (p=0.07). Hospices with fewer than 10 deaths per month in non-flu months were not eligible to participate in the field test, and therefore are not represented in the field test sample; such small hospices represent more than half (56.5%) of all hospices nationwide.
Table 3.3. Characteristics of hospices participating in the HECS field test and nationwide using hospices “currently active” in 2012 Provider of Service file
Hospice Characteristic |
Field Test Sample
|
2012 Hospice Providers “currently active”
|
P value (tests difference in distribution between in sample v. not in sample) |
N |
33 |
3,743 |
|
Hospice Characteristics |
|
|
|
Ownership |
|
|
0.0252 |
Non-profit |
39.39 |
29.66 |
|
For-profit |
36.36 |
56.80 |
|
Government |
3.03 |
4.62 |
|
Other |
21.21 |
8.92 |
|
Region |
|
|
0.2830 |
Northeast |
18.18 |
12.42 |
|
South |
30.30 |
41.86 |
|
Midwest |
33.33 |
23.46 |
|
West |
15.15 |
21.16 |
|
Puerto Rico |
3.03 |
1.10 |
|
Rural/Urban |
|
|
0.2085 |
Urban |
87.88 |
79.00 |
|
Rural |
12.12 |
21.00 |
|
Chain |
|
|
0.2311 |
No |
84.85 |
90.84 |
|
Yes |
15.15 |
9.16 |
|
Size (# deaths per month, nonflu season) |
|
|
<.0001 |
Fewer than 10 |
0.00 |
56.48 |
|
10 to fewer than 30 |
75.76 |
16.43 |
|
30 or more |
24.24 |
27.09 |
|
Rate of live discharge, from 2012 hospice Medicare claims |
|
|
0.0711 |
Less than 10% |
12.12 |
6.67 |
|
10% to less than 20% |
45.45 |
32.61 |
|
20% to less than 30% |
27.27 |
24.53 |
|
30% to less than 40% |
12.12 |
15.05 |
|
40% or higher |
3.03 |
21.14 |
|
Mean length of stay, day, from 2012 hospice Medicare claims |
|
|
0.2231 |
Less than 20 |
0.00 |
1.63 |
|
20-39 |
27.27 |
25.96 |
|
40-59 |
57.58 |
42.20 |
|
60-79 |
6.06 |
19.96 |
|
80+ |
9.09 |
10.25 |
|
Source: CMS Provider of Services and hospice claims files, 2012.
In all, 1,136 respondents completed the field test survey, reporting care experiences for 1,136 hospice decedents. The mean age of decedents was 79.8 (Table 3.4); 5.6% were black, and 4.3% were Hispanic. For more than one-third (34.7%) of decedents, the last setting of hospice care was a home or assisted living facility; last location was a nursing home for 27.9% of decedents, a hospice freestanding IPU for 29.7%, and an acute care hospital for 7.8%. To allow for comparison of the field test sample to hospice decedents nationwide, Table 3.4 characterizes the subset of field test respondents who were age 65 and older and had Medicare as a payer with claims data on Medicare decedents who received hospice services in 2012. The age, sex and race distributions of field test decedents were generally similar to the population of Medicare beneficiaries receiving hospice care. Hospice patients who died after less than 48 hours on hospice service were excluded from the field test; hence, the field test sample underrepresents those with short lengths of stay when compared to the national data.
Table 3.4. Characteristics of Decedents Whose Caregivers Completed the Field Test Survey and Medicare Hospice Decedents Ages 65 and Older
Patient Characteristic |
Field Test Sample
|
Field Test Sample 65 and older with Medicare |
Medicare Decedents 65 and older, FY2012 |
N |
1,136 |
950 |
858,207 |
Gender |
|
|
|
Male (%) |
46.83 |
44.63 |
42.09 |
Age (mean, SD) |
79.8 (13.1) |
84.0 (8.6) |
83.7 (8.6) |
Race |
|
|
|
White |
85.75 |
87.57 |
89.43 |
Black |
5.62 |
4.59 |
7.05 |
Hispanic |
4.31 |
4.37 |
1.76 |
Asian or Pacific Islander |
1.59 |
1.01 |
0.94 |
Multiracial or other |
2.72 |
2.46 |
0.83 |
Final Setting of Care |
|
|
|
Home |
34.68 |
34.32 |
51.51 |
Nursing Home |
27.90 |
31.37 |
23.33 |
Acute Care Hospital |
7.75 |
7.26 |
7.58 |
Hospice Inpatient Unit |
29.67 |
27.05 |
17.58 |
|
|
|
|
Length of final episode of hospice care |
|
|
|
Less than 1 week |
28.24 |
28.32 |
36.89 |
1 to less than 2 weeks |
18.49 |
17.81 |
15.93 |
2 to less than 4 weeks |
14.26 |
13.83 |
14.31 |
1 to less than 2 months |
11.78 |
11.95 |
12.85 |
2 to less than 4 months |
10.86 |
10.51 |
10.53 |
4 to less than 6 months |
5.06 |
5.31 |
4.63 |
6 or more months |
11.32 |
12.28 |
4.87 |
|
|
|
|
|
|
|
|
Primary Diagnosis |
|
|
|
Cancer |
37.10 |
31.22 |
31.89 |
Dementia/Neurological |
19.22 |
21.71 |
17.11 |
Cardiovascular diseases |
21.27 |
24.27 |
18.05 |
Renal failure |
2.67 |
2.80 |
3.04 |
Liver failure |
2.06 |
1.22 |
1.02 |
COPD |
4.83 |
5.49 |
5.18 |
Other |
12.85 |
13.29 |
23.71 |
Source: CMS hospice claims files, 2012.
Nearly three-quarters (72.6%) of respondents were female, 44.8% were age 65 or older, and 5.8% were black. Nearly half (46.6%) were children of the hospice patient, while one-third were the spouse or partner. Table 3.5 compares the field test respondents to respondents in the 2013 national FEHC survey repository. Maintained by the National Hospice and Palliative Care Organization (NHPCO), the repository characterizes FEHC respondents from hospices that voluntarily submit data for the purpose of benchmarking. The distribution of respondent characteristics is generally similar; a slightly greater proportion of HECS field test respondents were non-white.
Table 3.5. Characteristics of Field Test Respondents and Respondents in the 2013 Family Evaluation of Hospice Care survey repository
Characteristic |
Field Test Sample
|
2013 National FEHC Sample |
N |
1,136 |
228,134 |
Respondent's Age |
|
|
65 years or older |
44.8% |
48.22% |
64 years or younger |
55.2% |
51.76% |
Respondent’s Race |
|
|
White |
88.79% |
93.26% |
Black or African-American |
5.75% |
4.06% |
Asian or Pacific Islander |
1.34% |
1.05% |
Multiracial or other |
4.12% |
1.60% |
Respondent's Ethnicity |
|
|
Hispanic |
4.67% |
3.06% |
Non-Hispanic |
95.3% |
96.93% |
Respondent's Gender |
|
|
Male |
27.43% |
28.08% |
Female |
72.57% |
71.91% |
Respondent's Highest Education Reached |
|
|
8th grade or less |
1.13% |
1.27% |
Some high school |
4.15% |
3.74% |
High school graduate or GED |
28.58% |
31.10% |
1-3 years of college |
33.68% |
28.57% |
4-year college graduate |
15.28% |
15.31% |
More than a 4-year college degree |
17.17% |
19.97% |
Respondent's Relationship to the Patient |
|
|
Spouse / Partner |
33.10% |
37.83% |
Child |
46.57% |
45.68% |
Parent |
2.02% |
3.26% |
Sibling |
4.67% |
4.34% |
Other relative |
9.16% |
4.50% |
Friend |
2.55% |
1.83% |
Other |
1.94% |
2.52% |
Source: Family Evaluation of Hospice Care 2013 National Summary Report. National Hospice and Palliative Care Organization, 2014.
Response Rates
Unit nonresponse occurs when an eligible sampled individual does not respond to any of the items in a survey. Item nonresponse occurs when a respondent to the survey inappropriately skips an item. Both types of nonresponse result in lower sample size and statistical power for analyses. If the propensity to not respond is associated with a particular response or series of responses to items in the survey, biased estimates may result from analyses. In this section, we focus on unit response rates and specifically examine rates of nonresponse and characteristics associated with nonresponse in the field test. Item response rates will be examined in the next section.
For the survey, decedents were sampled from hospice programs. Primary caregivers (i.e., family members or friends familiar with the decedent’s care) of the decedents were surveyed to gather information about patient and caregiver experiences with hospice care. Though we seek to identify hospice-level, caregiver-level and decedent-level predictors of unit response, note that CAHPS in general does not use unit nonresponse weighting to compare between entities. Prior research (Elliott, Zaslavsky, Goldstein, Lehrman, Hambarsoomians, et al., 2009; Elliott, Edwards, Angeles, Hambarsoomians & Hays, 2005) examining between-entity comparisons after case-mix adjustment has shown that there is no evidence that nonresponse weighting improves the accuracy of comparisons. In addition, with small samples, the design effect imposed by weighting for nonresponse can be very costly and thus may increase the variability of estimates with little reduction in bias. Although there may be some systematic bias in response (e.g., respondents in general tend to be those that respond more positively), this type of bias would not bias the comparisons between entities. We employ simple hospice-by-setting-level design and nonresponse weights in the field test but do not apply person-level nonresponse weights. Person-level nonresponse weights are generally not used in CAHPS public reporting or accountability analyses but are sometimes employed in research projects, such as those involving inference at the national level.
Predictors of Unit Response
We consider response propensities by several hospice, caregiver, and decedent characteristics.
Hospice-level predictors include ownership (nonprofit, for-profit, or government); region (Northeast, South, Midwest, West, or Puerto Rico); urban or rural location; chain status (yes or no); size defined by the mean number of deaths per month in the nonflu season among Medicare beneficiaries (small [ten to fewer than 30 deaths per month] or medium or large [30 or more deaths per month]); rate of live discharge from 2012 hospice Medicare claims (less than 10 percent, 10 to less than 20 percent, 20 percent to less than 30 percent, 30 percent to less than 40 percent, or 40 percent or more); and mean length of stay from 2012 hospice Medicare claims in days (20 to 39, 40 to 59, 60 to 79, or 80 or more).
Caregiver-level predictors include relationship to the decedent (spouse or partner, child or stepchild, parent, other family member, friend, or other nonfamily, with the latter two categories collapsed for regression due to small size) and time elapsed between death and first mailing of the field hospice CAHPS survey in days (63 to 74, 75 to 85, 86 to 97, or 98 or more). The FEHC survey is widely used to survey next-of-kin caregivers; some hospices were able to suspend administration of the FEHC survey for some or all of our sampling period. We additionally considered response rates according to whether the sampled caregiver had previously received the FEHC survey.
Decedent-level predictors include age at death in years (18 to 54, 55 to 64, 65 to 69, 70 to 74, 75 to 79, 80 to 84, 85 to 89, or 90 or more); sex (male or female); race and ethnicity (non-Hispanic white, black, Hispanic, Asian or Pacific Islander, multiracial, or other); payer for hospice care (Medicare only, Medicaid only or Medicaid and private, Medicare and Medicaid, private only, Medicare and private, uninsured or no payer, or other); final setting of care (home, nursing home, acute care hospital, or hospice IPU); length of final episode of hospice care (less than one week, one week to less than two weeks, two weeks to less than four weeks, one month to less than two months, two months to less than four months, four months to less than six months, or six or more months); and primary diagnosis (cancer, dementia or neurological disease, cardiovascular disease, renal failure, liver failure, chronic obstructive pulmonary disease [COPD], or other).
Hospice-level predictors are drawn from CMS administrative records and contain no missingness. Caregiver- and decedent-level predictors are drawn from administrative data provided by each hospice and may be missing some observations. No values were missing for time elapsed between death and first mailing, previous receipt of the FEHC, patient’s age, patient’s gender, or final setting of care. Relationship between caregiver and decedent and payer for hospice care were missing in less than 1 percent of cases. Higher rates of missingness were observed for length of final episode of hospice care (4.4 percent), patient’s race or ethnicity (4.7 percent), and primary diagnosis (16.5 percent). Missing data were imputed as the mean value within hospice where possible. Where all data from a hospice were missing, the grand mean was used.
Analysis of Unit Response
We report response rates overall and by each level of the hospice, caregiver, and decedent characteristics above. In order to test for adjusted differences in response rates, we estimated linear mixed models of unit response regressed on fixed effects for caregiver and decedent characteristics and random effects for hospice.
Analysis of Response Mode
We report response rates by mail and by phone and phone response rates contingent on not responding by mail (contingent phone response rates), overall and by care setting. Among respondents, we estimated adjusted associations of early response by mail versus late response by phone with caregiver and decedent characteristics using logistic regression of response by mail on all caregiver and decedent characteristics and fixed effects for hospice. To check the effect of phone follow-up to the survey mailing on balance of respondent characteristics with respect to eligible cases, we present the distribution of caregiver and decedent characteristics within all eligible sampled cases, respondents by mail only, and all respondents. We test for differences in distributions between all eligible cases and each of the other two groups.
RESULTS
Unit Response
Table 3.6 presents counts of sampled, ineligible, eligible, and complete cases and response rates overall and by final setting of care. The overall response rate among the eligibles was 53.6 percent. The response rate among respondents from the home setting was slightly higher (56.5 percent) than in the other three care settings (51.3 to 52.9 percent). Response rates from the two settings of care that received the inpatient survey were similar, with response rates of 52.4 percent for acute care hospitals and 51.3 percent for hospice IPUs.
Table 3.6. Response Rates by Setting and Survey Versiona
|
Overall |
Home setting and survey |
Nursing Home setting and survey |
Inpatient survey |
Acute Care Hospital setting |
Freestanding Hospice IPU setting |
Surveyed |
2,267 |
729 |
639 |
899 |
198 |
701 |
Administrative Ineligible N (% of Surveyed) |
80 (3.5%) |
23 (3.2%) |
15 (2.3%) |
42 (4.7%) |
22 (11.1%) |
20 (2.9%) |
Nonparticipating Ineligible N (% of Surveyed) |
66 (2.9%) |
9 (1.2%) |
25 (3.9%) |
32 (3.6%) |
8 (4.0%) |
24 (3.4%) |
Eligible N (% of Surveyed) |
2,121 (93.6%) |
697 (95.6%) |
599 (93.7%) |
825 (91.8%) |
168 (83.7%) |
657 (93.7%) |
Completes |
1,136 |
394 |
317 |
425 |
88 |
337 |
Response Rate among Eligible |
53.6% |
56.5% |
52.9% |
51.5% |
52.4% |
51.3% |
a. The home and nursing home settings were each surveyed with their own instrument. Both the acute care hospital and freestanding hospice IPU settings were surveyed with the inpatient survey. Both the acute care hospital and freestanding hospice IPU settings were surveyed with the inpatient survey. Overall column reflects the combined total of the home, nursing home and inpatient setting surveys.
Eligible respondents who answered at least one item were considered unit respondents. We distinguish two types of ineligibility among sampled cases. Administrative ineligibility resulted from incomplete or inaccurate administrative data at the time of sampling. Any case in which a sampled caregiver did not have a valid address in the administrative data was dropped. In some sampled cases, decedent and caregiver names were identical; in order to avoid sending a letter addressed to a decedent, these cases were dropped. An added challenge for the field test was that some hospices were inexperienced with providing data sets for sample generation. These hospices inadvertently made errors in their files or neglected to provide key variables until after sampling was complete. Data on length-of-stay eligibility arrived from these hospices after sampling; sampled cases determined to be ineligible based on this additional information were dropped from the data collection. Decedent and caregiver information was mismatched for a subset of cases from one hospice, and the sampled caregivers linked to the wrong decedents were also dropped. These last categories of administrative ineligibility (i.e., sample-file errors or missing data) are less likely to occur once national implementation is under way because data requirements will be streamlined to reflect lessons learned from the field test and hospices will have the opportunity to become accustomed to the requirements over time.
Because the goal of the survey was to collect information on experiences with hospice care, sampled caregivers who were not familiar with their family members’ hospice care were deemed nonparticipating ineligible informants. Responses to three survey items were used to define nonparticipating ineligibility: (1) a negative response to the question “Did your family member receive care from the hospice listed on the survey cover letter?” (2) a response of ‘never’ to the question “While your family member was in hospice care, how often did you take part in or oversee care for him or her?” or (3) a write-in response to the item “How are you related to the person listed on the survey cover letter?” indicating no personal relationship. Nonresponders to the survey or respondents who did not answer these items were considered eligible for the survey. Any phone case in which a sampled caregiver indicated before the first survey question that his or her family member had not received care at the listed hospice or indicated that he or she was not involved in the patient’s hospice care and that there was no one else within that household who had been involved was also classified as nonparticipating ineligible. The percentage of the total sample that was deemed nonparticipating ineligible is lower for caregivers sampled for the home setting (1.2 percent) than for those in the other three settings (3.4 to 4.0 percent).
There were a total of 985 eligible nonresponding cases. Approximately 209 (21.2 percent) of these cases were unlocatable during the field period because of bad address or bad telephone numbers (approximately 9 percent of all sampled cases); just over 16 percent refused to complete the survey, and the remainder were unresolved after five telephone attempts.
Table 3.7 through Table 3.9 present composition of the eligible sample, respondents, and response rates by hospice, caregiver, and decedent characteristics. Hospice-level characteristics that were statistically significantly associated with response rate include geographic location, with the response rate in Puerto Rico (36.7 percent) lower than that in the rest of the United States (53.8 percent) and the response rate for hospices in rural areas (65.1 percent) higher than that for urban areas (52.8 percent). A higher response rate is observed among nonchain hospices (55.2 percent) than among chain hospices (49.1 percent).
Table 3.7. Composition of Eligible Sample and Response Rates, by Hospice Characteristic
Characteristic |
N Hospices |
Eligible N Cases Sampled |
Percentage of Eligible in This Category |
Response Rate in This Category (%) |
Respondent N |
Percentage of Completes in This Category |
P-Value of Bivariate Tests of Equal Response Rates in Each Category |
Ownership |
|
|
|
|
|
|
0.0756 |
Nonprofit |
17 |
1,405 |
66.2 |
55.2 |
776 |
68.3 |
|
For profit |
14 |
678 |
32.0 |
50.6 |
343 |
30.2 |
|
Government |
2 |
38 |
1.8 |
44.7 |
17 |
1.5 |
|
Region |
|
|
|
|
|
|
0.0005 |
Northeast |
6 |
184 |
8.7 |
58.2 |
107 |
9.4 |
|
South |
10 |
680 |
32.1 |
49.4 |
336 |
29.6 |
|
Midwest |
11 |
688 |
32.4 |
59.2 |
407 |
35.8 |
|
West |
5 |
539 |
25.4 |
51.0 |
275 |
24.2 |
|
Puerto Rico |
1 |
30 |
1.4 |
36.7 |
11 |
1.0 |
|
Rural or urban |
|
|
|
|
|
|
0.0071 |
Urban |
29 |
1,992 |
93.9 |
52.8 |
1,052 |
92.6 |
|
Rural |
4 |
129 |
6.1 |
65.1 |
84 |
7.4 |
|
Chain |
|
|
|
|
|
|
0.0372 |
No |
22 |
1,543 |
72.7 |
55.2 |
852 |
75.0 |
|
Yes |
11 |
578 |
27.3 |
49.1 |
284 |
25.0 |
|
Small |
8 |
203 |
9.6 |
58.1 |
118 |
10.4 |
|
Medium or large |
25 |
1,918 |
90.4 |
53.1 |
1,018 |
89.6 |
|
Rate of live discharge, from 2012 hospice Medicare claims |
0.2560 |
||||||
Less than 10% |
4 |
270 |
12.7 |
54.1 |
146 |
12.9 |
|
10% to less than 20% |
15 |
1,081 |
51.0 |
55.0 |
595 |
52.4 |
|
20% to less than 30% |
9 |
556 |
26.2 |
51.6 |
287 |
25.3 |
|
30% to less than 40% |
4 |
184 |
8.7 |
52.7 |
97 |
8.5 |
|
40% or higher |
1 |
30 |
1.4 |
36.7 |
11 |
1.0 |
|
Mean length of stay, days, from 2012 hospice Medicare claims |
0.6147 |
||||||
20–39 |
9 |
741 |
34.9 |
51.8 |
384 |
33.8 |
|
40–59 |
19 |
1,210 |
57.1 |
54.8 |
663 |
58.4 |
|
60–79 |
2 |
53 |
2.5 |
50.9 |
27 |
2.4 |
|
80+ |
3 |
117 |
5.5 |
53.0 |
62 |
5.5 |
|
Table 3.8. Composition of Eligible Sample and Response Rates, by Caregiver Characteristic
Characteristic |
Eligible N Cases Sampled |
Percentage of Eligible in This Category |
Response Rate in This Category |
Respondent N |
Percentage of Completes in This Category |
P-Value of Bivariate Tests of Equal Response Rates in Each Category |
Relationship to decedent |
|
|
|
|
|
<.0001 |
Spouse or partner |
631 |
29.8 |
62.4 |
394 |
34.7 |
|
Child or stepchild |
1,080 |
50.9 |
49.6 |
535 |
47.1 |
|
Parent |
32 |
1.5 |
56.2 |
18 |
1.6 |
|
Other family member |
301 |
14.2 |
49.7 |
149 |
13.1 |
|
Friend |
71 |
3.4 |
52.1 |
37 |
3.3 |
|
Other non–family member |
6 |
0.3 |
33.3 |
2 |
0.2 |
|
Days elapsed from death to first mailing |
0.5483 |
|||||
63 to 74 |
509 |
24.0 |
54.4 |
277 |
24.4 |
|
75 to 85 |
491 |
23.1 |
54.6 |
268 |
23.6 |
|
86 to 97 |
550 |
25.9 |
54.5 |
300 |
26.4 |
|
98 or more |
571 |
26.9 |
51.0 |
291 |
25.6 |
|
Previous receipt of the FEHC survey |
0.0147 |
|||||
No |
213 |
10.0 |
61.5 |
131 |
11.5 |
|
Yes |
1,908 |
90.0 |
52.7 |
1,005 |
88.5 |
|
Table 3.9. Composition of Eligible Sample and Response Rates, by Decedent Characteristic
Characteristic |
Eligible N Cases Sampled |
Percentage of Eligible in This Category |
Response Rate in This Category |
Respondent N |
Percentage of Completes in This Category |
P-Value of Bivariate Tests of Equal Response Rates in Each Category |
Age at death |
|
|
|
|
|
<.0001 |
18–54 |
130 |
6.1 |
46.2 |
60 |
5.3 |
|
55–64 |
212 |
10.0 |
50.5 |
107 |
9.4 |
|
65–69 |
169 |
8.0 |
39.1 |
66 |
5.8 |
|
70–74 |
220 |
10.4 |
52.3 |
115 |
10.1 |
|
75–79 |
261 |
12.3 |
49.8 |
130 |
11.4 |
|
80–84 |
331 |
15.6 |
51.1 |
169 |
14.9 |
|
85–89 |
387 |
18.2 |
57.4 |
222 |
19.5 |
|
90 or older |
411 |
19.4 |
65.0 |
267 |
23.5 |
|
Sex |
|
|
|
|
|
0.5041 |
Male |
979 |
46.2 |
54.3 |
532 |
46.8 |
|
Female |
1,142 |
53.8 |
52.9 |
604 |
53.2 |
|
Race and ethnicity |
|
|
|
|
|
0.0001 |
White |
1,772 |
83.5 |
55.5 |
983 |
86.6 |
|
Black |
157 |
7.4 |
39.9 |
63 |
5.5 |
|
Hispanic |
104 |
4.9 |
38.8 |
40 |
3.6 |
|
Asian or Pacific Islander |
34 |
1.6 |
52.8 |
18 |
1.6 |
|
Multiracial |
27 |
1.3 |
59.3 |
16 |
1.4 |
|
Other |
26 |
1.2 |
58.7 |
15 |
1.4 |
|
Payer for hospice care |
|
|
|
|
|
0.3689 |
Medicare only |
1,666 |
78.5 |
54.3 |
904 |
79.6 |
|
Medicaid only or Medicaid and private |
60 |
2.8 |
46.7 |
28 |
2.5 |
|
Medicare and Medicaid |
101 |
4.8 |
51.6 |
52 |
4.6 |
|
Private only |
107 |
5.0 |
48.6 |
52 |
4.6 |
|
Medicare and private |
46 |
2.2 |
65.2 |
30 |
2.6 |
|
Uninsured or no payer |
50 |
2.4 |
46.0 |
23 |
2.0 |
|
Other |
91 |
4.3 |
51.6 |
47 |
4.1 |
|
Final setting of care |
|
|
|
|
|
0.2590 |
Home |
697 |
32.9 |
56.5 |
394 |
34.7 |
|
Nursing home |
599 |
28.2 |
52.9 |
317 |
27.9 |
|
Acute care hospital |
168 |
7.9 |
52.4 |
88 |
7.7 |
|
Hospice IPU |
657 |
31.0 |
51.3 |
337 |
29.7 |
|
Length of final episode of hospice care |
0.0001 |
|||||
Less than 1 week |
685 |
32.3 |
47.2 |
324 |
28.5 |
|
1 week to less than 2 weeks |
373 |
17.6 |
56.2 |
210 |
18.4 |
|
2 weeks to less than 4 weeks |
290 |
13.7 |
55.8 |
162 |
14.2 |
|
1 month to less than 2 months |
269 |
12.7 |
49.9 |
134 |
11.8 |
|
2 months to less than 4 months |
205 |
9.7 |
59.8 |
123 |
10.8 |
|
4 months to less than 6 months |
99 |
4.7 |
57.6 |
57 |
5.0 |
|
6 or more months |
201 |
9.5 |
63.5 |
127 |
11.2 |
|
Primary diagnosis |
|
|
|
|
|
0.0378 |
Cancer |
852 |
40.2 |
50.1 |
427 |
37.5 |
|
Dementia or neurological disease |
368 |
17.3 |
58.5 |
215 |
18.9 |
|
Cardiovascular disease |
420 |
19.8 |
57.0 |
239 |
21.1 |
|
Renal failure |
61 |
2.9 |
50.8 |
31 |
2.7 |
|
Liver failure |
68 |
3.2 |
55.7 |
38 |
3.3 |
|
COPD |
108 |
5.1 |
54.5 |
59 |
5.2 |
|
Other |
275 |
13.0 |
53.9 |
148 |
13.0 |
|
The response rate tended to be higher when the caregiver was the decedent’s spouse or partner (62.4 percent) or parent (56.2 percent) than when he or she was a child or stepchild (49.6 percent) or other family member (49.7 percent). Non–family members (i.e., friends) had a response rate of 50.6 percent. Previous mailing of the FEHC survey was associated with a lower response rate (61.5 percent if no previous mailing, 52.7 percent if previous mailing). Note that, for national implementation, we anticipate that the FEHC will be suspended and therefore expect higher response rates overall than what was observed in the field test.
Age and race of the respondent are frequently associated with survey response rates. In this case, we do not have these variables for nonrespondents, but age and race of decedents may be proxies for the ages and races of their caregivers. Response rates were higher for older decedents (57.4 percent for those 85 to 90 years old, 65.0 percent for those 90 or older) than for younger decedents (39.1 to 52.3 percent for those 18 to 54 years old). Response rates for black (39.9 percent) and Hispanic (38.8 percent) decedents were lower than for other racial and ethnic groups. The response rate for Hispanic decedents in Puerto Rico (36.7 percent) was lower than for Hispanic decedents outside Puerto Rico (39.7 percent).
There is a tendency toward higher response rates for long final episode of hospice care (63.5 percent in cases of six or more months, compared with 47.2 to 59.8 percent for shorter periods). Response rates where the primary diagnosis was dementia or other neurological condition (58.5 percent) or cardiovascular disease (57.0 percent) were higher than for other diagnoses.
Table 3.10 presents linear regression results examining probability of response by caregiver and decedent characteristics, with hospice included as a random effect. Specifically, this model examined the probability of response; note that we have used linear regression as an appropriate approximation to logistic regression to facilitate interpretation (i.e., coefficients can be interpreted as an increase in probability or percentage). The standard deviation of the hospice random effect was 3.7%, indicating that about 95% of hospices would have a response rate in the range of 46.2-61.0% (mean +/- 2SD), adjusting for case-level fixed effects in the model.
Table 3.10. Mixed Linear Regression Model of Unit Response
N |
2,121 |
Response Rate |
53.6% |
Random Effectsa |
|
Hospice-level STD DEV |
3.7% |
Fixed Effects (95% CI) |
|
Caregiver Characteristics |
|
Relationship to decedent |
|
Spouse/partner |
0.19 (0.13, 0.24) *** |
Child/step-child [ref] |
0.00 |
Parent |
0.22 (0.04, 0.41) * |
Other family member |
0.05 (-0.01, 0.12) |
Friend/ Other non-family member |
0.10 (-0.01, 0.22) |
Days elapsed from death to first mailing |
|
63 to 74 [ref] |
0.00 |
75 to 85 |
0.02 (-0.04, 0.08) |
86 to 97 |
0.01 (-0.05, 0.07) |
98 or more |
-0.01 (-0.07, 0.05) |
Previous receipt of the FEHC survey |
-0.08 (-0.16, -0.001) * |
Decedent Characteristics |
|
Age at death |
|
18-54 |
-0.25 (-0.38, -0.12) *** |
55-64 |
-0.19 (-0.29, -0.09) *** |
65-69 |
-0.29 (-0.38, -0.20) *** |
70-74 |
-0.17 (-0.25, -0.08) *** |
75-79 |
-0.18 (-0.26, -0.10) *** |
80-84 |
-0.17 (-0.24, -0.09) *** |
85-89 |
-0.08 (-0.15, -0.01) * |
90 or older [ref] |
0.00 |
Sex Male |
-0.01 (-0.05, 0.04) |
Race/Ethnicity |
|
White [ref] |
0.00 |
Black |
-0.08 (-0.17, 0.00) |
Hispanic |
-0.14 (-0.24, -0.03) ** |
Asian/Pacific Islander |
0.02 (-0.15, 0.19) |
Multiracial |
0.06 (-0.13, 0.25) |
Other |
0.10 (-0.10, 0.30) |
Payer for Hospice Care |
|
Medicare only [ref] |
0.00 |
Medicaid only/Medicaid and private |
-0.01 (-0.16, 0.13) |
Medicare and Medicaid |
-0.01 (-0.11, 0.09) |
Private only |
-0.02 (-0.14, 0.09) |
Medicare and private |
0.13 (-0.02, 0.27) |
Uninsured/no payer |
-0.02 (-0.18, 0.13) |
Other |
0.03 (-0.09, 0.14) |
Final Setting of Care |
|
Home [ref] |
0.00 |
Nursing Home |
-0.05 (-0.11, 0.01) |
Acute Care Hospital |
0.00 (-0.09, 0.08) |
Hospice Inpatient Unit |
-0.01 (-0.07, 0.05) |
Length of final episode of hospice care |
|
Less than 1 week [ref] |
0.00 |
1 to less than 2 weeks |
0.10 (0.04, 0.17) ** |
2 to less than 4 weeks |
0.10 (0.03, 0.17) ** |
1 to less than 2 months |
0.04 (-0.03, 0.12) |
2 to less than 4 months |
0.13 (0.05, 0.21) ** |
4 to less than 6 months |
0.10 (-0.01, 0.21) |
6 or more months |
0.15 (0.07, 0.23) *** |
Primary Diagnosis |
|
Cancer [ref] |
0.00 |
Dementia/Neurological |
0.07 (0.00, 0.14) |
Cardiovascular diseases |
0.06 (0.00, 0.13) |
Renal failure |
0.06 (-0.08, 0.20) |
Liver failure |
0.09 (-0.05, 0.23) |
COPD |
0.06 (-0.05, 0.17) |
Other |
0.04 (-0.04, 0.11) |
*p<0.05
**p<0.01 ***p<0.001
a. Square root of variance component
Fixed effects for caregiver and decedent characteristics are interpreted as within-hospice associations after adjustment for all other variables in the model. Spouses, partners, and parents had response rates 19 to 22 percentage points higher than children and stepchildren. Previous receipt of the FEHC survey was associated with an 8-percentage-point lower response rate. Lower response rates were observed for younger decedents, with response rates in cases in which the decedent was 18 to 54 years old 25 percentage points lower than when the decedent was 90 or older. Compared with non-Hispanic whites, the response rate was 14 percentage points lower in cases in which the decedent was Hispanic and 8 percentage points lower in cases in which the decedent was black (p = 0.053). There was a tendency to higher response rates for longer final episode of hospice care: Response rates for cases with a final episode of hospice care of six months or longer were 15 percentage points higher than cases of less than one week.
Response Mode
Table 3.11 presents rates of response overall and by final setting of care. Among all respondents, 69 percent responded by mail. Thirty-seven percent of eligible cases responded by mail (ranges from 35.0 percent for hospice IPUs to 38.6 percent for home). Of cases not responding by mail, 26.3 percent responded by phone (ranges from 23.1 percent for acute care hospital setting to 29.2 percent for home setting).
Table 3.11. Unit Response Rates, by Final Setting of Care (%)
Respondent |
Home |
Nursing Home |
Acute Care Hospital |
Freestanding Hospice IPU |
Overall |
Among all eligibles |
|
|
|
|
|
38.6 |
36.9 |
38.1 |
35.0 |
37.0 |
|
Phone |
17.9 |
16.0 |
14.3 |
16.3 |
16.6 |
Total |
56.5 |
52.9 |
52.4 |
51.3 |
53.6 |
Telephone response among eligibles not responding by mail |
29.2 |
25.4 |
23.1 |
25.1 |
26.3 |
NOTE: There was no evidence of difference in mode of response by final setting of care among respondents (Χ2 = 0.83; degrees of freedom (df) = 3; p = 0.84). |
|||||
We also ran logistic regression models of response by mail rather than phone on caregiver and decedent characteristics, among respondents. Hospice was included as a fixed effect (results not shown). Increased time between death and the beginning of mailing of the HECS (odds ratio [OR] = 0.67 for those in the highest quartile compared to the lowest); youngest age category (OR = 0.21 for decedent’s age 18 to 54 years compared with decedent’s age 90 or older); and black, Asian, or Pacific Islander race or ethnicity (0.42 and 0.36, respectively, compared with non-Hispanic whites) were associated with a lower tendency to respond by mail. That is, a longer time between the decedent’s date of death and the date of first mailing tended to result in a lower probability of response by mail and thus a higher probability of response by phone among those caregivers.
Table 3.12 and Table 3.13 show the distribution of caregiver and decedent characteristics among all eligible cases, respondents by mail, and all respondents. Statistically significant differences between distributions among all eligible, among respondents by mail, and overall are shown. Several characteristics are associated with differential response rates and additionally with differential mode preference within respondents. For instance, responses rates are lower where the decedent is 18 to 54 years old, and the odds of response by mail within respondents in this group are lower than in other age groups. Thus, adding a telephone mode yields a closer distribution among all respondents (5.3 percent with decedent 18 to 54 years old) compared with the eligible sampled pool (6.1 percent) than a mail mode alone (3.6 percent) for this characteristic. A similar pattern is seen for black decedents (7.4 percent of eligible sampled group, 4.0 percent of respondents by mail, and 5.5 percent of all respondents) and Hispanic decedents (4.9 percent of eligible sampled group, 2.8 percent of respondents by mail, and 3.6 percent of all respondents), although both race and ethnicity groups are statistically significantly underrepresented in the final sample compared with the eligible group.
Table 3.12. Comparison of Eligibles, Respondents by Mail, and All Respondents, by Caregiver Characteristic
Characteristic |
Percentage of Eligible in This Category |
Percentage of Mail Completes in This Category |
Percentage of All Completes in This Category |
Relationship to decedent |
|
|
|
Spouse or partner |
29.8 |
36.1*** |
34.7*** |
Child or stepchild |
50.9 |
47.1** |
47.1*** |
Parent |
1.5 |
1.4 |
1.6 |
Other family member |
14.2 |
12.5 |
13.1 |
Friend |
3.4 |
2.8 |
3.3 |
Other non–family member |
0.3 |
0.1 |
0.2 |
Days elapsed from death to first mailing |
|||
63 to 74 |
24.0 |
25.3 |
24.4 |
75 to 85 |
23.1 |
24.2 |
23.6 |
86 to 97 |
25.9 |
26.4 |
26.4 |
98 or more |
26.9 |
24.1* |
25.6 |
Previous receipt of the FEHC survey |
|||
No |
10.0 |
12.2* |
11.5* |
Yes |
90.0 |
87.8* |
88.5* |
NOTE: * = p < 0.05. ** = p < 0.01. *** = p < 0.001. |
|||
Table 3.13. Comparison of Eligibles, Respondents by Mail, and All Respondents, by Decedent Characteristic
Characteristic |
Percentage of Eligible in This Category |
Percentage of Mail Completes in This Category |
Percentage of All Completes in This Category |
Age at death (years) |
|
|
|
18–54 |
6.1 |
3.6*** |
5.3 |
55–64 |
10.0 |
8.5 |
9.4 |
65–69 |
8.0 |
6.0* |
5.8*** |
70–74 |
10.4 |
9.3 |
10.1 |
75–79 |
12.3 |
12.2 |
11.4 |
80–84 |
15.6 |
15.3 |
14.9 |
85–89 |
18.2 |
19.8 |
19.5 |
90 or older |
19.4 |
25.3*** |
23.5*** |
Sex |
|
|
|
Male |
46.2 |
47.6 |
46.8 |
Female |
53.8 |
52.4 |
53.2 |
Race or ethnicity |
|
|
|
White |
83.5 |
89.6*** |
86.6*** |
Black |
7.4 |
4.0*** |
5.5*** |
Hispanic |
4.9 |
2.8*** |
3.6** |
Asian or Pacific Islander |
1.6 |
1.0 |
1.6 |
Multiracial |
1.3 |
1.1 |
1.4 |
Other |
1.2 |
1.4 |
1.4 |
Payer for hospice care |
|
|
|
Medicare only |
78.5 |
82.4** |
79.6 |
Medicaid only or Medicaid and private |
2.8 |
1.9 |
2.5 |
Medicare and Medicaid |
4.8 |
3.7 |
4.6 |
Private only |
5.0 |
4.0 |
4.6 |
Medicare and private |
2.2 |
2.6 |
2.6 |
Uninsured or no payer |
2.4 |
1.8 |
2.0 |
Other |
4.3 |
3.7 |
4.1 |
Final setting of care |
|
|
|
Home |
32.9 |
34.3 |
34.7 |
Nursing home |
28.2 |
28.2 |
27.9 |
Acute care hospital |
7.9 |
8.2 |
7.7 |
Hospice IPU |
31.0 |
29.3 |
29.7 |
Length of final episode of hospice care |
|||
Less than 1 week |
32.3 |
28.9** |
28.5*** |
1 week to less than 2 weeks |
17.6 |
16.8 |
18.4 |
2 week to less than 4 weeks |
13.7 |
13.9 |
14.2 |
1 month to less than 2 months |
12.7 |
12.3 |
11.8 |
2 months to less than 4 months |
9.7 |
10.7 |
10.8 |
4 months to less than 6 months |
4.7 |
5.3 |
5.0 |
6 or more months |
9.5 |
12.1** |
11.2** |
Primary diagnosis |
|
|
|
Cancer |
40.2 |
36.7** |
37.6** |
Dementia or neurological disease |
17.4 |
20.2** |
18.9* |
Cardiovascular disease |
19.8 |
20.8 |
21.0 |
Renal failure |
2.9 |
2.7 |
2.7 |
Liver failure |
2.3 |
3.1 |
2.1 |
COPD |
4.7 |
4.8 |
4.8 |
Other |
12.8 |
13.5 |
12.8 |
NOTE: * = p < 0.05. ** = p < 0.01. *** = p < 0.001. |
|||
DISCUSSION
Analysis of unit nonresponse demonstrated higher eligibility rates and response rates among those in the home setting (even though the survey instrument is longer). Multivariate regression analyses showed that the relationship between the survey caregiver and the decedent, previous mailing of the FEHC survey, decedent age at death, decedent race and ethnicity, and length of final episode of hospice care are all significantly associated with the probability of response. In particular, spouses and parents were more likely to respond than children, those who were mailed the FEHC survey were less likely to respond, caregivers of older decedents were more likely to respond than those of younger decedents, and caregivers of Hispanic decedents were less likely to respond than those caring for decedents of other race or ethnicity categories. In addition, caregivers of decedents who had longer final episodes of hospice care were more likely to respond than those with shorter episodes. Given the anticipated suspension of the FEHC during national implementation of the HECS, we may expect improved response rates in national implementation. Specifically, FEHC mailing was associated with an 8.8-percent lower response rate than from those who were not mailed the FEHC in this field test, and about 90 percent of eligible caregivers were mailed the FEHC; given our observed overall response rate of 53.6 percent and the same administration procedures and field period, in the absence of the FEHC, we would expect a response rate of about 61.4 percent.
Nonresponding cases include refusals, the majority of which were identified during telephone data collection and directly from the sampled caregiver rather than an informant on the caregiver’s behalf. Approximately 19 percent of caregivers who refused did not provide specific reasons for refusal, either simply hanging up or indicating they were not interested. Telephone interviewers could code more than one reason for refusal. Where reasons were provided, the most frequently cited were that the caregiver was too busy (cited by 34.4 percent of refusals) or not emotionally ready to discuss the patient’s care (cited by 31.3 percent of refusals). Some caregivers indicated that they had previously provided information, perhaps thinking about the FEHC, and would not do so again (cited by 14.4 percent of refusals). It seems likely that at least a portion of these refusals would have completed if they had not previously received the FEHC. A smaller proportion of refusing caregivers (11.3 percent) declined to participate citing that they did not know enough about the patients’ care; just over half of this group noted that the time the decedent spent in care was too short to properly comment. This follows along with the finding that caregivers of decedents with shorter stays were less likely to respond.
Caregivers with a longer time between decedent death and the beginning of mailing of the HECS; caregivers of younger decedents; and caregivers of black, Asian, and Pacific Islander decedents were less likely to respond by mail than by phone. Given that a longer time between the decedent’s date of death and the date of first mailing tended to result in a lower probability of response by mail and thus a higher probability of response by phone and that mail mode is generally less costly than phone mode, this might suggest a recommendation that mailings go out more quickly than what we implemented in this field test. For example, these results suggest that delays between death and mailing that were in the highest quartile, a delay of 98 days or more, should be avoided in national implementation.
In addition, one-fifth of eligible nonresponding cases were unlocatable during the field test. Because caregivers may move or change contact information after patient death, this further underscores the need for fielding the survey in a timely manner after patient death. The number of unlocatable cases also highlights the need for hospices to give attention to verification of caregiver contact information and to consider collecting and maintaining multiple sources of contact information for caregivers.
These response analyses also show that, although caregivers of black and Hispanic decedents are less likely to respond to the survey in general than caregivers of white decedents are, caregivers of black and Asian decedents who do respond are more likely to respond by phone than by mail. With such small minority representation in the field test and likely across hospices in general, this highlights the importance of telephone follow-up to ensure that such groups are represented. Use of the telephone mode in addition to the mail mode yielded a group of respondents that was more similar to the eligible sample in terms of race and ethnicity of the decedent and in terms of other characteristics, including relationship to decedent, age of decedent, and payer for hospice care, although differences still persist between all respondents and the eligible sampled group (Klein, Elliott, Haviland, Saliba, Burkhart, et al., 2011).
As previously noted, CAHPS in general does not use unit nonresponse weighting to compare entities because of the lack of evidence that such weighting improves accuracy, the potential to increase variability of estimates, and the fact that response biases do not generally bias comparisons between entities. In addition to our recommendation not to incorporate nonresponse weighting in this field test, we also recommend exclusion of nonresponse weights in national implementation when providing comparable scores across hospices.
Item Nonresponse and Ceiling Effects
As discussed in the prior section, unit nonresponse occurs when an eligible sampled individual does not respond to any item on a survey. Item nonresponse occurs when a unit respondent inappropriately skips an item. Both types of nonresponse result in lower sample size and statistical power for analyses. If the propensity to not respond is associated with what response would have been given, biased estimates may result from analyses. In this section, we focus on item nonresponse. Specifically, we look at rates of item nonresponse and characteristics associated with item nonresponse in the HECS field test.
In addition, we investigate floor and ceiling effects by examining both the number of respondents validating extreme response categories expressed as a proportion of valid responses obtained and the intraclass correlation coefficients (ICCs). ICCs measure the amount of variability in response among hospices. Low ICCs indicate highly similar mean scores across hospices relative to variability within hospices and may indicate that an item was poorly understood and requires modifications. However, a low ICC in combination with a very high or very low mean score may indicate a ceiling or floor effect (i.e., a situation in which most hospices score near the maximum or minimum, limiting that question’s ability to distinguish performance between hospices).
METHODS
Predictors of Item Nonresponse
We consider nonresponse by several hospice, caregiver, and decedent characteristics.
Hospice-level predictors include ownership (nonprofit, for profit, or government); region (Northeast, South, Midwest, West, or Puerto Rico); urban or rural location; chain status (yes or no); size (small [ten to fewer than 30 deaths per month] or medium or large [30 or more deaths per month); rate of live discharge from 2012 hospice Medicare claims (less than 10 percent, 10 to less than 20 percent, 20 percent to less than 30 percent, 30 percent to less than 40 percent, or more than 40 percent); and mean length of stay from 2012 hospice Medicare claims in days (20 to 39, 40 to 59, 60 to 79, or 80 or more).
Caregiver-level predictors include relationship to the decedent (spouse or partner, child or stepchild, parent, other family member, friend, or other nonfamily, with the latter two categories collapsed for regression due to small size) and time elapsed between death and first mailing of the field-test HECS in days (63 to 74, 75 to 85, 86 to 97, or 98 or more). The FEHC survey is widely used to survey next-of-kin caregivers; as noted above, some hospices were able to suspend administration of the FEHC survey for some or all of our sampling period. We additionally considered response rates according to whether the sampled caregiver had previously received the FEHC survey.
Decedent-level predictors include age at death in years (18 to 54, 55 to 64, 65 to 69, 70 to 74, 75 to 79, 80 to 84, 85 to 89, or 90 or more); sex (male or female); race and ethnicity (non-Hispanic white, black, Hispanic, Asian or Pacific Islander, multiracial, or other); payer for hospice care (Medicare only, Medicaid only or Medicaid and private, Medicare and Medicaid, private only, Medicare and private, uninsured or no payer, or other); final setting of care (home, nursing home, acute care hospital, or hospice IPU); length of final episode of hospice care (less than one week, one week to less than two weeks, two weeks to less than four weeks, one month to less than two months, two months to less than four months, four months to less than six months, or six or more months); and primary diagnosis (cancer, dementia or neurological disease, cardiovascular disease, renal failure, liver failure, COPD, or other).
Hospice-level predictors are drawn from CMS administrative records and contain no missingness. Caregiver- and decedent-level predictors are drawn from administrative data provided by each hospice and may be missing some observations. No values were missing for time elapsed between death and first mailing, previous receipt of the FEHC, patient’s age, patient’s sex, or final setting of care. Relationship between caregiver and decedent and payer for hospice care were missing in less than 1 percent of cases. Higher rates of missingness were observed for length of final episode of hospice care (4.4 percent), decedent’s race and ethnicity (4.7 percent), and primary diagnosis (16.5 percent). Missing data were imputed as the mean value within hospice where possible. Where all data from a hospice were missing, the grand mean was used.
Analysis of Item Nonresponse
We consider item nonresponse among unit respondents. We report number and proportion of missingness to eligible items overall, by response mode, and by final setting of care. If a gatekeeper question is skipped, we assume that the respondent would have been eligible to respond to its dependent items using standard CAHPS forward-cleaning rules.
In order further to investigate nonresponse, we created a data set that is unique by respondent and eligible item and performed logistic regression of inappropriate item missingness on survey length (number of eligible items), the position of the item on the survey (scaled from 0 to 1), caregiver and decedent characteristics, and fixed effects for hospice. In addition to all caregiver characteristics listed above, we included two variables that measure the caretaker’s involvement in care. The first is response to the item “While your family member was in hospice care, how often did you take part or oversee care for him or her?” with response levels ‘sometimes,’ ‘usually,’ and ‘always.’ The response option ‘never’ is offered; however, a response of ‘never’ makes a case nonparticipating ineligible for the survey (i.e., the case is excluded from analysis). This item was missing in 2.8 percent of cases and was imputed as the mean value within hospice. The second variable was an indicator that the caretaker’s address was in the same state as the hospice. We account for clustering of item nonresponse within respondent.
For each item, we calculated the proportion of nonlegitimate skips (i.e., skips not dictated by the survey’s skip-logic instructions) among respondents eligible to respond and the mean position of the item among eligible items across all surveys. We regressed proportion of nonlegitimate skips on mean position and investigated items with at least 3 percentage points more nonlegitimate skips than would be predicted by position within the survey.
For each item, we report the number of applicable completed surveys, the number and proportion of legitimate skips (i.e., skips dictated by the survey’s skip-logic instructions), the number of legitimate responses, the number of nonlegitimate skips, and the proportion of nonlegitimate skips overall and by final setting of care.
Analysis of Floor and Ceiling Effects
We investigate floor and ceiling effects by first examining the number of respondents validating extreme response categories expressed as a proportion of valid responses in the lowest and highest possible categories for each evaluative item on the survey. We additionally examine these proportions within each care setting (results not shown). Second, we calculate the estimated ICC, along with its standard error (SE) and confidence interval (CI), for each evaluative item, which measures the amount of variability in response among hospices. Because several hospices had very few respondents to certain items, we conducted a sensitivity analysis that restricted the ICC estimation to hospices with at least 20 respondents to the item being examined; the threshold of 20 respondents reduced difficulties in estimating ICCs. We evaluate the potential bias and loss in precision with this restricted ICC estimate. Low ICCs indicate highly similar mean scores across hospices relative to variability within hospices and may indicate that an item was poorly understood and requires modifications. However, a low ICC in combination with a very high or very low mean score may indicate a ceiling or floor effect (i.e., a situation in which most hospices score near the maximum or minimum, limiting that question’s ability to distinguish performance between hospices). Therefore, items with both a low ICC and a very high or very low mean score are flagged as having a potential floor or ceiling effect.
RESULTS
Item Nonresponse
Overall, respondents were eligible to answer an average of 59.3 items (range = 46 to 71), did not answer 3.4 of them, and had an item nonresponse rate of 5.5 percent (Table 3.14). There was a slightly higher mean and SD of item nonresponse rate among respondents by telephone (mean = 5.8 percent, SD = 15.7 percent) than mail (mean = 5.3 percent, SD = 13.1 percent). Note that it is common in CAHPS settings to see much higher item nonresponse by phone due to break-off (i.e., respondent hanging up before call is completed). On average, respondents in the home setting were eligible to answer more items (62.9) than the other settings of care (56.0 to 58.4). However, respondents in the home setting had a lower rate of item nonresponse (3.7 percent) than the other settings of care (6.1 percent for nursing home and freestanding hospice IPU, 8.3 percent for acute care hospital).
Table 3.14. Item Nonresponse Rates by Mode and by Final Setting of Care
Item |
All Respondents |
Mode |
Final Setting of Care |
||||
All Mail Respondents |
All Telephone Respondents |
Home |
Nursing Home |
Acute Care Hospital |
Freestanding Hospice IPU |
||
N |
1,136 |
784 |
352 |
394 |
317 |
88 |
337 |
Number of eligible items, of 80 total: mean (SD) |
59.3 (4.8) |
59.2 (4.8) |
59.3 (4.8) |
62.9 (4.5) |
56.0 (3.7) |
58.1 (3.7) |
58.4 (3.2) |
Number of nonlegitimate missing: mean (SD) |
3.4 (8.9) |
3.2 (8.3) |
3.6 (10.3) |
2.4 (6.8) |
3.6 (9.7) |
5.1 (11.6) |
3.7 (9.5) |
Percentage of eligible items missing: mean (SD) |
5.5 (13.9) |
5.3 (13.1) |
5.8 (15.7) |
3.7 (10.2) |
6.1 (15.4) |
8.3 (17.9) |
6.1 (14.9) |
Table 3.15 shows a multivariate logistic regression model of nonlegitimate skips among unit respondents on item, caregiver, and decedent characteristics. An increase in both the overall length of the survey (OR of 1.35 for an increase of one item) and the position of an item within a survey (OR = 2.42 for the last item compared with the first item) were associated with higher inappropriate missingness. Caregivers who were spouses or partners (OR = 1.64) and non–family members (OR = 4.25) of decedents had higher rates of inappropriate skips than caregivers who were children of decedents. Lower odds of inappropriate missingness were observed among the youngest decedent age categories (OR = 0.22 for 18- to 54-year-olds, OR = 0.50 for 55- to 64-year-olds) than in the oldest category. This observed pattern in item nonresponse by caregiver relationship and decedent age may be driven largely by the fact that these caregivers may be older themselves and older age is often associated with higher item nonresponse in CAHPS. Caregivers of Asian and Pacific Islander decedents had lower odds of inappropriate missingness (OR = 0.31) than caregivers of white decedents; this observation is unusual but may be difficult to interpret with our small presentation by Asians and Pacific Islanders. Further examination of this result would be of interest in national implementation. Caregivers of decedents covered by Medicaid or Medicaid and private insurance had a higher inappropriate missingness rate (OR = 2.71) than those covered by Medicare only. Coverage by Medicaid is likely an indication of low SES, and it is common in CAHPS settings for one to observe higher item nonresponse among respondents with low SES. Compared with the home care setting, the other three settings of care had much higher odds of inappropriate missingness (OR = 10.8 for nursing home, 7.87 for acute care hospital, and 5.97 for hospice IPU). Caregivers of decedents with longer final episodes of hospice care tended to have lower item missingness (OR = 0.35 for four to six months and 0.37 for more than months, compared with less than one week). Caregivers of decedents with a primary diagnosis of dementia or neurological disease (OR = 2.04) or cardiovascular disease (OR = 1.56) tended to have higher rates of inappropriate missingness than caregivers of decedents with cancer as their primary diagnoses. Lower rates of inappropriate missingness were observed among caregivers who reported ‘usually’ (OR = 0.41) or ‘always’ (OR = 0.46) taking part in care for family member than for those who ‘sometimes’ took part in care. It is interesting to note that, once we account for the level of caregiver involvement in hospice care, the variable indicating whether the caregiver lived in the same state as the hospice has no association with item response. This supports other analyses (described in the CMA section below) that demonstrate that caregiver location in the same state or city as the hospice seems to be a proxy for region rather than a proxy for the degree of involvement in care.
Table 3.15. Probability of item nonresponse: Logistic regression of non-legitimate missingness among all unit respondersa
-
Odds ratios (95% CI)
N
1,136
Survey length (# of applicable items)
1.35 (1.28, 1.43) ***
Survey position
(rescaled to 0-1)
2.42 (1.83, 3.20) ***
Caregiver Characteristics
Relationship to decedent
Spouse/partner
1.64 (1.13, 2.37) **
Child/step-child [ref]
1.00
Parent
1.98 (0.71, 5.53)
Other family member
1.29 (0.81, 2.06)
Friend/ Other non-family member
4.25 (2.18, 8.28) ***
Days elapsed from death to first mailing
63 to 74 [ref]
1.00
75 to 85
0.86 (0.57, 1.28)
86 to 97
1.01 (0.69, 1.48)
98 or more
1.18 (0.80, 1.74)
Previous receipt of the FEHC survey
1.09 (0.39, 3.04)
Decedent Characteristics
Age at death
18-54
0.22 (0.09, 0.55) **
55-64
0.50 (0.28, 0.89) *
65-69
0.48 (0.22, 1.03)
70-74
0.76 (0.46, 1.26)
75-79
0.67 (0.41, 1.08)
80-84
0.58 (0.36, 0.93) *
85-89
1.00 (0.65, 1.53)
90 or older [ref]
1.00
Sex
Male
1.02 (0.74, 1.42)
Race/Ethnicity
White [ref]
1.00
Black
0.66 (0.34, 1.27)
Hispanic
0.42 (0.15, 1.15)
Asian/Pacific Islander
0.31 (0.11, 0.89) *
Multiracial
1.59 (0.64, 3.99)
Other
1.42 (0.62, 3.26)
Payer for Hospice Care
Medicare only [ref]
1.00
Medicaid only/Medicaid and private
2.71 (1.06, 6.92) *
Medicare and Medicaid
1.23 (0.68, 2.22)
Private only
1.95 (0.87, 4.40)
Medicare and private
0.88 (0.36, 2.13)
Uninsured/no payer
1.22 (0.40, 3.74)
Other
2.00 (0.80, 5.00)
Final Setting of Care
Home [ref]
1.00
Nursing Home
10.8 (5.71, 20.44) ***
Acute Care Hospital
7.87 (4.10, 15.11) ***
Hospice Inpatient Unit
5.97 (3.37, 10.57) ***
Length of final episode of hospice care
Less than 1 week [ref]
1.00
1 to less than 2 weeks
0.70 (0.46, 1.05)
2 to less than 4 weeks
0.49 (0.30, 0.79) **
1 to less than 2 months
0.49 (0.28, 0.87) *
2 to less than 4 months
0.58 (0.35, 0.97) *
4 to less than 6 months
0.35 (0.13, 0.94) *
6 or more months
0.37 (0.21, 0.65) ***
Primary Diagnosis
Cancer [ref]
1.00
Dementia/Neurological
2.04 (1.35, 3.10) ***
Cardiovascular diseases
1.56 (1.01, 2.43) *
Renal failure
1.40 (0.50, 3.96)
Liver failure
1.81 (0.73, 4.49)
COPD
1.38 (0.70, 2.73)
Other
1.77 (1.17, 2.67) **
How often did you take part or oversee care for your family member?
Sometimes [ref]
1.00
Usually
0.41 (0.26, 0.62) ***
Always
0.46 (0.33, 0.63) ***
Caretaker lives in same state as hospice
1.16 (0.70, 1.91)
a. This model also controls for hospice as a fixed effect (results not shown). Adjusts for clustering of items within respondents.
*p<0.05 **p<0.01 ***p<0.001
Figure 3.1 shows a scatterplot of the percentage of inappropriate missingness against the mean position of an item. The dashed line shows the simple regression line of percentage of inappropriate missingness or skips on mean position. Items with a 3-percentage-point or greater inappropriate skip rate after adjusting for survey position are labeled. A one-unit increase in the position of an item is associated with a 0.19-percent greater inappropriate skip rate. Item position explained about 10 percent of the variability in response rates.
F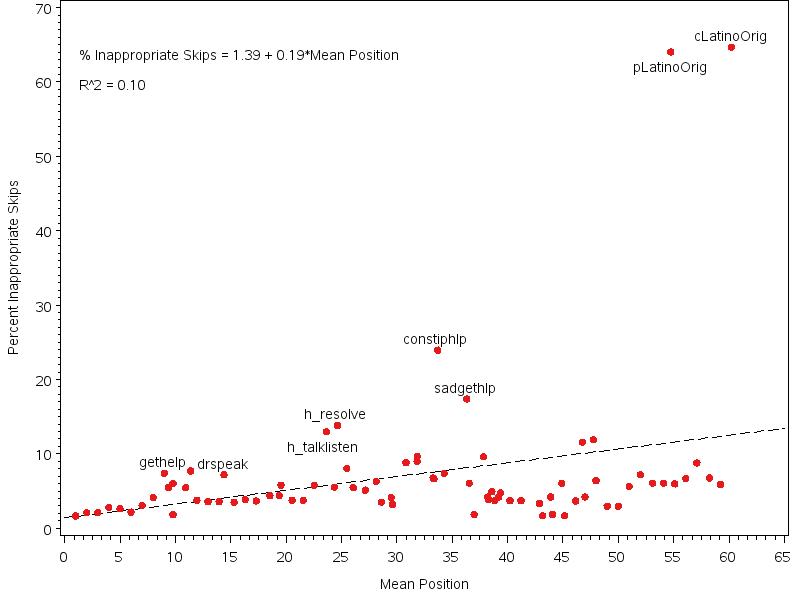 igure
3.1. Plot of Percentage Inappropriate Skips by Item Mean Position,
all items
igure
3.1. Plot of Percentage Inappropriate Skips by Item Mean Position,
all items
Two items labeled in Figure 3.1 have very high inappropriate skip rates: pLatinoOrig (64.1 percent) and cLatinoOrig (64.7 percent). Both are dependent items in a skip pattern, with the gatekeeper item asking whether the family member and caretaker, respectively, were Latino. These dependent items ask for the Latino label that best describes the family member or caretaker. Very few respondents endorsed Latino ethnicity either for their family members or for themselves, and most of them answered the Latino group items appropriately: Forty-six respondents indicated that their family members were Latino, and all but one of those selected a Latino label. Fifty-three respondents indicated that they themselves were Latino, and all of them selected a Latino label. The high inappropriate skip rate is due to people who skip both the gatekeeper and dependent item: These people are assumed to be eligible to answer the dependent item. Eighty-one people skipped both the gatekeeper and dependent items for family member’s Latino origin, and 98 skipped both items for their own Latino origins.
Because of concerns about how these two items may be skewing results, this analysis was re-run dropping these two items; Figure 3.2 shows the resulting figure. Only 4 percent of the variability in inappropriate skip rate was then due to item position. Several items had high rates of inappropriate skip after accounting for position in the surveys. Most of these items were dependent items in a skip pattern; high rates of missingness could indicate confusion about the skip pattern or applicability. Additionally, these items all had high rates of missingness in nonhome care settings, perhaps indicating that caregivers whose family members were in nonhome care settings did not feel that they had sufficient information to answer the item. In Table 3.16, we describe each item labeled in Figure 3.1 (minus the two items with high inappropriate skip rates) and the item nonresponse rate across settings. Note that a dependent item means that the item required the respondent to “pass” a gatekeeper or screener question indicating that he or she was eligible to answer the dependent item. Only those who answered the gatekeeper item with a certain response pattern were eligible for the dependent item. A gatekeeper item is the item that determines whether the respondent is eligible for subsequent dependent questions.
Table 3.16. Inappropriate Skips and Item Nonresponse Rates
Label |
|
Item Wording |
Type |
Nonresponse Rate (%) |
h_talklisten |
|
How often did the hospice team members listen carefully to you when you talked with them about problems with your family member’s hospice care? |
Dependent |
8.1 (home) to 25.0 (acute care hospital |
h_resolve |
|
How often were problems with your family member’s hospice care resolved as soon as you needed? |
Dependent |
8.1 (home) to 25.0 (acute care hospital) |
breathhlp |
|
How often did your family member get the help he or she needed for trouble breathing? |
Dependent |
6.4 (home) to 11.8 (nursing home) |
breathinfo |
|
How often did you get the information you needed from the hospice team about your family member’s trouble breathing? |
Dependent |
6.8 (home) to 11.2 (nursing home) |
constip |
|
While your family member was in hospice care, did your famiy member ever have trouble with constipation? |
Gatekeeper |
4.3 (home) to 13.1 (hospice IPU) |
constiphlp |
|
How often did your family member get the help he or she needed for trouble with constipation? |
Dependent |
8.6 (home) to 39.7 (hospital IPU) |
sadgethlp |
|
How often did your family member receive the help he or she needed from the hospice team for feelings of anxiety or sadness? |
Dependent |
10.2 (home) to 26.3 (hospital IPU) |
beliefrespect |
|
How often did the hospice team treat your family member’s religious or spiritual beliefs with respect? |
Dependent |
7.0 (home) to 16.9 (acute care hospital) |
csptreligion |
|
While your family member was in hospice care, how much support for your religious and spiritual beliefs did you get from the hospice team? |
Dependent |
6.9 (home) to 32.1 (acute care hospital) |
cbeliefrespect |
|
How often did the hospice team treat your religious or spiritual beliefs with respect? |
Dependent |
6.9 (home) to 30.2 (acute care hospital) |
Figure 3.2. Plot of Percentage Inappropriate Skips by Item Mean Position, omitting Latino origin items
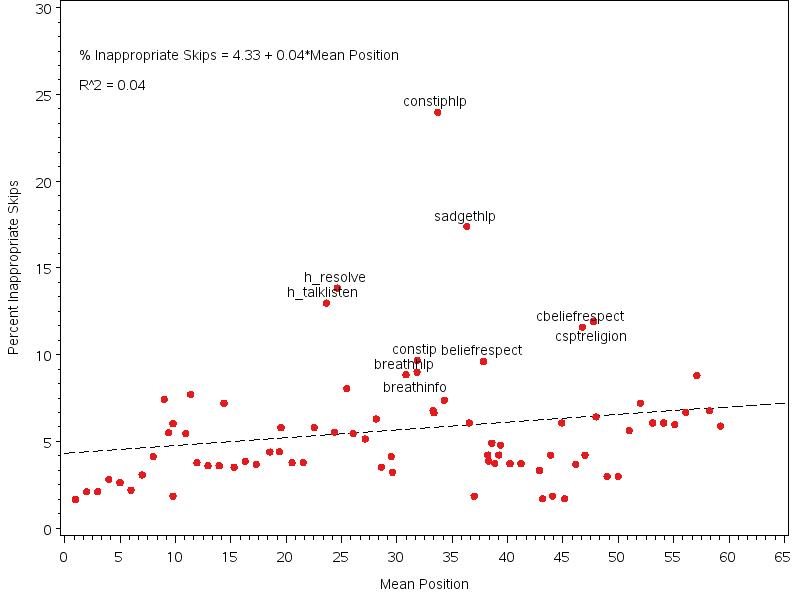
Table E.1 in Appendix E reports the number of applicable completed surveys, the number and proportion of legitimate skips, the number of legitimate responses, the number of nonlegitimate skips, and the proportion of nonlegitimate skips overall and by final setting of care. For many items, the inappropriate item skip rate is much lower for respondents in the home care setting than in the other three settings. Some health conditions were rare, and many respondents appropriately skipped the dependent items evaluating the hospice (for instance, 56.7 percent of respondents appropriately skipped an evaluative item on treatment of constipation, and 54.0 percent appropriately skipped an evaluative item on getting help for sadness). This decreases the power to test hospice’s help for those conditions.
Floor and Ceiling Effects
Table 3.17 shows the percentage of responses in the lowest and highest categories for each evaluative item. There were no items with 90 percent or more responses in the lowest category. Items with more than 90 percent of responses in the highest category were as follows:
• While your family member was in hospice care, did the hospice team give you and your family member enough privacy?
• While your family member was in hospice care, how often did you have a hard time speaking with or understanding members of the hospice team because you spoke different languages?
• While your family member was in hospice care, how often did the hospice team treat your family member with dignity and respect?
• Did the hospice team get in the way of you spending time with your family member while he or she was dying?
• While your family member was in hospice care, were his or her room and bathroom kept clean?
• While your family member was in hospice care, was his or her room a comfortable place for you to be together?
• While your family member was in hospice care, was your family member’s room a calm and soothing place for him or her?
• Did your family member get the equipment as soon as he or she needed it?
• Was the equipment picked up in a timely manner when your family member no longer needed it?
• How often did the hospice team treat your religious or spiritual beliefs with respect?
• While your family member was in hospice care, how much support for your religious and spiritual beliefs did you get from the hospice team?
• While your family member was in hospice care, how much emotional support did you get from the hospice team?
Table 3.17. Ceiling Effects: Percentage of respondents in the lowest and highest possible category and ICC
Item |
% Lowest Category |
% Highest Category |
N respondents |
N Hospices |
ICC
among all hospices (95% CI) |
N respondents after restricting |
N Hospices after restricting |
ICC
among hospices with 20+respondents total (95% CI) |
Starting Hospice Care |
|
|
|
|
|
|
|
|
Did the hospice team explain the kinds of care and services they could give you and your family member? |
1.3 |
88.5 |
1111 |
33 |
0.0057 (-0.013, 0.0244) |
941 |
20 |
0.0099 (-0.0148, 0.0346) |
Did your family member begin getting hospice care too early, at the right time, or too late?
|
11.7 |
88.3 |
1101 |
33 |
0.000 (-0.0002, 0.0002) |
933 |
20 |
0.0000 (0.0000, 0.0000) |
Your family member's hospice care |
|
|
|
|
|
|
|
|
How often did you get the help you needed from the hospice team during evenings, weekends, or holidays? |
2.0 |
75.9 |
560 |
33 |
0.0000 (0.0000, 0.0000) |
459 |
20 |
0.0000 (0.0000, 0.0000) |
While your family member was in hospice care, how often did the hospice team keep you informed about when they would arrive to care for your family member?
|
0.7 |
76.6 |
369 |
33 |
0.0228 (-0.0301, 0.0757) |
261 |
20 |
0.0119 (-0.0315, 0.0552) |
While your family member was in hospice care, how often did the nursing home staff and hospice team work well together to care for your family member? |
1.4 |
69.6 |
257 |
27 |
0.0000 (0.0000, 0.0000) |
232 |
20 |
0.0000 (0.0000, 0.0000) |
Personal care needs include bathing, dressing, eating meals and changing bedding. While your family member was in hospice care, how often did your family member get as much help with personal care as he or she needed?
|
2.6 |
75.8 |
654 |
27 |
0.0318 (-0.0133, 0.0769) |
612 |
20 |
0.0231 (-0.015, 0.0612) |
While your family member was in hospice care, were your family member’s personal care needs ever not taken care of because the nursing home staff expected the hospice team to take care of those needs?
|
15.0 |
85.0 |
251 |
27 |
0.0000 (0.0000, 0.0000) |
228 |
20 |
0.0000 (0.0000, 0.0000) |
While your family member was in hospice care, when you or your family member asked for help from the hospice team, how often did you get help as soon as you needed it?
|
1.5 |
77.8 |
1074 |
33 |
0.0123 (-0.0137, 0.0383) |
912 |
20 |
0.0157 (-0.0156, 0.0471) |
While your family member was in hospice care, did the hospice team give you and your family member enough privacy? |
0.4 |
94.8 |
1093 |
33 |
0.0059 (-0.011, 0.0227) |
929 |
20 |
0.0089 (-0.0123, 0.03) |
While your family member was in hospice care, how often did you have a hard time speaking with or understanding members of the hospice team because you spoke different languages? |
0.7 |
94.9 |
1095 |
33 |
0.0000 (0.0000, 0.0000) |
930 |
20 |
0.0000 (0.0000, 0.0000) |
While your family member was in hospice care, did the hospice team seem informed and up-to-date about your family member’s condition and care? |
1.3 |
88.6 |
1095 |
33 |
0.0297 (-0.0078, 0.0671) |
930 |
20 |
0.0253 (-0.0143, 0.0649) |
While your family member was in hospice care, did you speak to a doctor as often as you needed? |
15.3 |
65.3 |
373 |
22 |
0.0779 (0.0002, 0.1556)* |
357 |
15 |
0.0885 (-0.0031, 0.1801) |
While your family member was in hospice care, how often did the hospice team explain things in a way that was easy to understand? |
1.1 |
80.9 |
1096 |
33 |
0.0162 (-0.0121, 0.0445) |
931 |
20 |
0.018 (-0.0142, 0.0502) |
While your family member was in hospice care, how often did the hospice team keep you informed about your family member’s condition? |
1.6 |
77.0 |
1092 |
33 |
0.0316 (-0.0023, 0.0656) |
929 |
20 |
0.0501 (-0.0015, 0.1017) |
While your family member was in hospice care, how often did anyone from the hospice team give you confusing or contradictory information about your family member’s condition or care? |
1.3 |
88.3 |
1094 |
33 |
0.0026 (-0.0113, 0.0166) |
930 |
20 |
0.0033 (-0.0121, 0.0187) |
While your family member was in hospice care, how often was the information you were given about your family member by the nursing home staff different from the information you were given by the hospice team? |
1.6 |
71.7 |
260 |
27 |
0.0000 (0.0000, 0.0000) |
236 |
20 |
0.0000 (0.0000, 0.0000) |
While your family member was in hospice care, how often did the hospice team respect your needs and preferences? |
0.8 |
86.5 |
1086 |
33 |
0.0144 (-0.0114, 0.0402) |
923 |
20 |
0.0225 (-0.0128, 0.0578) |
While your family member was in hospice care, how often did the hospice team spend enough time with your family member? |
1.2 |
74.0 |
1070 |
33 |
0.0126 (-0.0102, 0.0354) |
909 |
20 |
0.0193 (-0.0114, 0.0499) |
While your family member was in hospice care, how often did the hospice team treat your family member with dignity and respect? |
0.1 |
93.7 |
1093 |
33 |
0.0000 (0.0000, 0.0000) |
930 |
20 |
0.0000 (0.0000, 0.0000) |
While your family member was in hospice care, how often did you feel that the hospice team really cared about your family member? |
1.2 |
87.1 |
1093 |
33 |
0.0000 (0.0000, 0.0000) |
929 |
20 |
0.0000 (0.0000, 0.0000) |
How often did the hospice team listen carefully to you when you talked with them about problems with your family member’s hospice care? |
0.9 |
81.0 |
402 |
33 |
0.0187 (-0.0476, 0.0851) |
339 |
20 |
0.0139 (-0.0454, 0.0733) |
How often were problems with your family member’s hospice care resolved as soon as you needed? |
2.3 |
67.9 |
398 |
33 |
0.0000 (0.0000, 0.0000) |
335 |
20 |
0.0000 (0.0000, 0.0000) |
Did your family member get as much help with pain as he or she needed? |
2.6 |
84.3 |
730 |
33 |
0.0000 (0.0000, 0.0000) |
614 |
20 |
0.0000 (0.0000, 0.0000) |
Did you get the information you needed from the hospice team about your family member’s pain medicine? |
3.1 |
86.2 |
993 |
33 |
0.0000 (-0.0058, 0.0058) |
845 |
20 |
0.002 (-0.0159, 0.0199) |
Side effects of pain medicine include things like sleepiness. Did any member of the hospice team discuss side effects of pain medicine with you or your family member? |
10.3 |
72.3 |
981 |
33 |
0.0071 (-0.019, 0.0332) |
835 |
20 |
0.0022 (-0.0177, 0.0222) |
Did the hospice team give you enough training about what side effects to watch for from pain medicine? |
7.0 |
75.4 |
328 |
33 |
0.0169 (-0.0285, 0.0623) |
234 |
20 |
0.0286 (-0.0334, 0.0907) |
Did the hospice team give you enough training about if and when to give more pain medicine to your family member? |
5.6 |
83.8 |
329 |
33 |
0.0316 (-0.0291, 0.0923) |
235 |
20 |
0.0501 (-0.0391, 0.1392) |
How often did your family member get the help he or she needed for trouble breathing? |
0.9 |
83.6 |
597 |
33 |
0.0248 (-0.0122, 0.0617) |
499 |
20 |
0.0452 (-0.0124, 0.1027) |
How often did you get the information you needed from the hospice team about your family member’s trouble breathing? |
2.4 |
75.1 |
596 |
33 |
0.0056 (-0.0243, 0.0355) |
499 |
20 |
0.0076 (-0.0253, 0.0405) |
Did the hospice team give you enough training about how to help your family member if he or she had trouble breathing? |
5.3 |
81.1 |
210 |
33 |
0.0000 (0.0000, 0.0000) |
147 |
20 |
0.0000 (0.0000, 0.0000) |
How often did your family member get the help he or she needed for trouble with constipation? |
1.6 |
72.1 |
374 |
33 |
0.0171 (-0.0359, 0.0702) |
302 |
20 |
0.0217 (-0.0399, 0.0833) |
While your family member was in hospice care, did he or she show any feelings of anxiety or sadness? |
57.3 |
42.7 |
1059 |
33 |
0.0403 (-0.0006, 0.0811) |
898 |
20 |
0.0381 (-0.0058, 0.0819) |
How often did your family member receive the help he or she needed from the hospice team for feelings of anxiety or sadness? |
3.6 |
65.1 |
432 |
33 |
0.0028 (-0.0269, 0.0326) |
356 |
20 |
0.005 (-0.0308, 0.0408) |
Did the hospice team give you enough training about what to do if your family member became restless or agitated? |
8.3 |
71.9 |
232 |
32 |
0.0257 (-0.0274, 0.0788) |
161 |
20 |
0.0156 (-0.0415, 0.0728) |
Moving your family member includes things like helping him or her turn over in bed, or get in and out of bed or a wheelchair. Did the hospice team give you enough training about how to safely move your family member? |
7.6 |
76.0 |
283 |
33 |
0.0186 (-0.0261, 0.0634) |
199 |
20 |
0.025 (-0.0302, 0.0802) |
How often did the hospice team treat your family member’s religious or spiritual beliefs with respect? |
1.8 |
89.5 |
770 |
33 |
0.0191 (-0.0139, 0.0521) |
652 |
20 |
0.0224 (-0.0187, 0.0636) |
Did the hospice team give you as much information as you wanted about what to expect while your family member was dying? |
6.5 |
78.3 |
1092 |
33 |
0.0287 (-0.0051, 0.0624) |
927 |
20 |
0.0362 (-0.0077, 0.0801) |
Was the information provided in a way that was easy to understand? |
0.1 |
89.1 |
1011 |
33 |
0.0000 (0.0000, 0.0000) |
854 |
20 |
0.0000 (0.0000, 0.0000) |
When your family member died, was the hospice team with you, or available as soon as you needed? |
7.5 |
84.9 |
346 |
33 |
0.0419 (-0.0302, 0.1139) |
246 |
20 |
0.0397 (-0.0518, 0.1312) |
Did the hospice team get in the way of you spending time with your family member while he or she was dying? |
1.9 |
97.0 |
385 |
22 |
0.0000 (0.0000, 0.0000) |
369 |
20 |
0.0000 (0.0000, 0.0000) |
The hospice environment |
|
|
|
|
|
|
|
|
While your family member was in hospice care, were his or her room and bathroom kept clean? |
0.2 |
97.5 |
385 |
21 |
0.2785 (0.0731, 0.484)* |
368 |
14 |
0.0000 (0.0000, 0.0000) |
While your family member was in hospice care, was his or her room a comfortable place for you to be together? |
0.3 |
94.6 |
387 |
22 |
0.0286 (-0.0414, 0.0985) |
370 |
14 |
0.0238 (-0.0386, 0.0863) |
While your family member was in hospice care, was your family member’s room a calm and soothing place for him or her? |
0.0 |
92.6 |
387 |
22 |
0.0759 (-0.0551, 0.2069) |
370 |
14 |
0.0643 (-0.0479, 0.1765) |
Special medical equipment |
|
|
|
|
|
|
|
|
Did your family member get the equipment as soon as he or she needed it? |
3.6 |
96.4 |
346 |
33 |
0.2001 (-0.1315, 0.5316) |
244 |
20 |
0.216 (-0.1605, 0.5925) |
Was the equipment picked up in a timely manner when your family member no longer needed it? |
4.2 |
95.8 |
339 |
33 |
0.0603 (-0.2288, 0.3493) |
240 |
20 |
0.0517 (-0.2228, 0.3263) |
Your own experience with hospice |
|
|
|
|
|
|
|
|
While your family member was in hospice care, how often did the hospice team listen carefully to you? |
0.9 |
83.2 |
1098 |
33 |
0.0295 (-0.005, 0.0641) |
931 |
20 |
0.0341 (-0.0068, 0.075) |
While your family member was in hospice care, how often did the hospice team spend enough time with you? |
2.3 |
69.1 |
1088 |
33 |
0.0001 (-0.0009, 0.001) |
921 |
20 |
0.0044 (-0.0185, 0.0273) |
How often did the hospice team treat your religious or spiritual beliefs with respect? |
0.9 |
91.3 |
549 |
33 |
0.0000 (0.0000, 0.0000) |
460 |
20 |
0.0000 (0.0000, 0.0000) |
Support for religious or spiritual beliefs includes talking, praying, quiet time, or other ways of meeting your religious or spiritual needs. While your family member was in hospice care, how much support for your religious and spiritual beliefs did you get from the hospice team? |
3.8 |
96.2 |
547 |
33 |
0.0000 (0.0000, 0.0000) |
460 |
20 |
0.0000 (0.0000, 0.0000) |
While your family member was in hospice care, how much emotional support did you get from the hospice team? |
7.0 |
93.0 |
1088 |
33 |
0.0484 (-0.0575, 0.1542) |
922 |
20 |
0.0059 (-0.0828, 0.0947) |
In the weeks after your family member died, how much emotional support did you get from the hospice team? |
11.0 |
89.0 |
1063 |
33 |
0.0236 (-0.038, 0.0851) |
898 |
20 |
0.0065 (-0.0322, 0.0451) |
Overall rating of care |
|
|
|
|
|
|
|
|
Using any number from 0 to 10, where 0 is the worst hospice care possible and 10 is the best hospice care possible, what number would you use to rate your family member’s hospice care? |
0.6 |
67.8 |
1102 |
33 |
0.0321 (-0.003, 0.0671) |
935 |
20 |
0.036 (-0.0063, 0.0783) |
Would you recommend this hospice to your friends and family? |
1.8 |
84.9 |
1102 |
33 |
0.0167 (-0.0096, 0.0431) |
936 |
20 |
0.0147 (-0.0122, 0.0416) |
* 95% CI does not cross 0.
Estimated ICCs were generally very small for most items, indicating that there is very little variability between hospices. However, with the small number of respondents and small number of hospices with enough respondents to each item, our ability to precisely estimate ICCs in the field test may be limited. All items listed above with more than 90 percent of respondents in the highest category also had estimated ICC with a 95-percent CI that overlapped zero, indicating very little or no variability between hospices, with the exception of “While your family member was in hospice care, were his or her room and bathroom kept clean?” which had an estimated ICC and 95-percent CI of 0.2785 (0.0731, 0.484). In addition to this item, overall, there was only one other item with a moderate ICC estimate that was significantly different from zero: the item asking whether the caregiver spoke with a doctor as often as he or she needed to, with an ICC (95-percent CI) of 0.0779 (0.0002, 0.1556).
On average, restricting the calculation of the ICC to hospices with at least 20 responses to an item results in the use of only 84 percent of respondents and 63 percent of hospices. Among the items for which the ICC SE is estimable in both the full sample and the restricted sample, the estimated ICC SE increases in 72 percent of the items when using the restricted sample compared with the full sample. The average absolute increase in ICC SE after the restriction was 0.0026, and the average multiplicative increase was 1.68. There are two items for which the ICC SE is estimable in the full sample and then not estimable or poorly estimated in the restricted sample and 14 items for which the ICC SE is either not estimable or poorly estimated in both the full sample and the restricted sample. Although examining the ICC calculated both with all hospices and the restricted set is useful, bias and precision issues probably arise by restricting to hospices with at least 20 respondents.
DISCUSSION
Item nonresponse analyses showed that overall item missingness among eligible items was 5.5 percent, with a lower item missingness rate observed in the home care setting even though the survey instrument for this setting is longer (62.9 eligible items compared with 56.0 to 58.4 for the other care settings). Higher nonresponse in the nonhome care settings was not restricted to setting-specific items asked only in the nursing home and inpatient survey instruments. This pattern may be due to caregivers of decedents in the home care setting being more familiar with their family members’ care than caregivers of patients in other settings. Item missingness tended to be higher with an increased number of applicable items and for those items that appeared later in the survey instrument. Although there was a slightly higher item nonresponse rate among respondents by phone than by mail, it is common in CAHPS settings to see much higher item nonresponse by phone due to break-off (i.e., respondent hanging up before call is completed) than what was observed in this field test. This may indicate that break-off is less likely in the hospice survey because of the emotional content of the survey. Among unit respondents, several characteristics were associated with higher item missingness, including caregivers who were spouses or partners and non–family members (i.e., friends) of the decedent, caregivers of decedents covered by Medicaid or Medicaid and private insurance, caregivers of decedents in nursing home and inpatient care settings, and caregivers of decedents with primary diagnoses of dementia, neurological disease, or cardiovascular disease. Among unit respondents, several characteristics were associated with lower item missingness, including caregivers of younger decedents, caregivers of Asian or Pacific Islander decedents, caregivers of decedents with longer final episodes of hospice care, and caregivers who reported they usually or always took part in care of the decedent. This observed pattern in item nonresponse by caregiver relationship and decedent age may be driven largely by the fact that these caregivers may be older themselves and older age is often associated with higher item nonresponse in CAHPS. In addition, the fact that observed rates of inappropriate missingness were lower among caregivers who reported ‘usually’ or ‘always’ taking part in care for family member than among those who ‘sometimes’ took part in care is not surprising because these respondents likely know more about the care that was received.
The analysis of floor and ceiling effects showed that 12 items had a high proportion of responses in the highest category, and 11 of these 12 also had very small ICC estimates, indicating a ceiling effect for these 11 items. For these 11 items, the ability to distinguish performance between hospices based on responses to these items is very limited. Given the anticipated larger number of respondents per hospice and larger number of hospices in national implementation, ICC estimates may be better calculated in national implementation.
Psychometric Analyses and Development of Composites
In this section, we describe the analytic process used to develop the multi- and single-item measures of core concepts (i.e., composites). Composites are collections of items on the survey that assess similar content domains. When a set of items measure a given content domain, combining those items into a composite allows for a more precise estimate of a respondent’s care experience than would be possible from any single item and allows fewer measures to be presented to consumers, reducing cognitive burden. The analyses below are intended to establish composites from the available items, where appropriate. This section of the report uses the n=1,136 field test survey data collected across the home, inpatient, and nursing home settings. The sequence of analyses reported here includes factor analytic models to establish domains of interest (i.e., composites) and item- and scale-level correlations to ensure that the domains measure distinct content. The remainder of this section also highlights points during the analytic process at which items were removed from the proposed composites. For a discussion of all items removed or retained on the final survey, see Chapter Four of this report.
METHODS
Prior to data delivery, the project team established a set of preliminary content domains and corresponding survey items. This step was taken both to ensure that our decisionmaking process was not overly influenced by potentially sample-dependent empirical findings, and also to ensure that our analytic findings were led by substantive theory. To accomplish this, team members first independently reviewed the set of survey items and grouped together items that assessed similar content. This resulted in a number of potential domains that were then consolidated by forming a set of hypothetical domains around content clusters for which there was a high-degree of agreement amongst team members. The process revealed a number of hypothetical domains, some of which are consistent with core CAHPS measures (e.g., Communication and Getting timely care), and others of which represented content specific to the hospice environment (e.g., Emotional support and Getting hospice care training).
Prior to evaluating the factor structure of the HECS items, we considered the results of the ceiling effects analysis described above. This analysis identified multiple items for which approximately 95 percent or more of the sample selected the highest response category (e.g., home setting items assessing delivery and removal of special medical equipment). Because these items provide very little variability and would preclude factor analytic review, they were not considered for development of composites.
We used the hypothetical domains to conduct a series of patient-level factor analyses in order to identify unidimensional domains (i.e., multi-item composites). This step assesses whether it is appropriate to compute a single score from a given collection of items. The hypothetical domains identified by the research team served as the starting point for these factor analytic models. First, we conducted single-factor confirmatory factor analytic (CFA) models using weighted least squares mean- and variance-adjusted estimation while taking into account the categorical nature of the items. These models generally closely fit the data across composites; however, they also revealed items with low loadings that were typically administered only on a single setting-specific version of the survey. Low factor loadings are an indication that an item is unrelated or weakly related to the content measured by the other items in the composite, and often suggest that an item should be removed from consideration. Therefore, after several iterations of fitting single-factor models we elected to remove 16 items that either did not contribute to the reliability of the measures, or had relatively little variance because of ceiling effects using a lower threshold that the 95 percent cutoff used for initial exclusions. The preliminary multi-item domains that emerged from this step were as follows:
Hospice team communication
Care coordination1
Getting timely care
Treating your family member with respect
Providing emotional support
Understanding the side effects of pain medication
Getting help for symptoms
Next, multifactor CFAs were fit to the items remaining in the composites in order to evaluate the correlations among the domains after accounting for measurement error (disattenuated correlations). Disattenuated correlations estimate the true relationships among the domains as if they were measured without error. Results from this model indicated that the factors were highly correlated (r = .50 to .94). The correlations tended to be highest for factors corresponding to the Hospice team communication domain, indicating that this domain is central to the survey content. In a CFA framework highly related factors are often further evaluated to determine whether a single-factor representation is adequate. Thus, to further explore the degree to which the composites reflect unique content, we evaluated a series of exploratory factor analytic (EFA) solutions. These solutions mostly confirmed our prior findings: The items were generally highly intercorrelated, which tends to support a single-factor representation of the collection of items; nonetheless, however, unique factors or composites emerged that are generally consistent with the core domains listed above.
Item and scale-level correlations
Because the factor analytic models suggested highly related composites and it was necessary to further reduce the length of the survey, we next conducted item and scale-level analysis using a classical test theory approach. The aims of this analytic step were to remove items from scales that were overly related to the Hospice team communication composite and to remove items from scales while still maintaining adequate score reliability. We first removed several items that contributed little to the reliability of the domain scores at the patient-level (e.g., “information provided was easy to understand”, “hospice team explained care”, “hospice team gave you privacy”, “hospice team treated your religious or spiritual beliefs with respect”, etc.). Next, as expected given our CFA findings, evaluations of correlations between items and scales revealed that many items across composites were highly related to scores on the Hospice team communication composite (the general dominant scale identified in the EFA step). This led to 5 additional items being removed and ensured that the resulting composites would measure content that is distinct from the Hospice team communication composite, despite being related to Hospice team communication (“hospice team spent enough time with you”, “hospice team seemed informed”, “problems with care were resolved as soon as needed”, “hospice team respected your needs”, and “received information regarding pain medicine”).
Setting-specific composites
As a final step in composite development, we reviewed the utility of developing composites that were unique to the nursing home and home settings. (All items specific to the inpatient setting were dropped from analytic consideration due to ceiling effects.) First, we evaluated the possibility of combining three items unique to the Nursing Home survey with the single-item Care Coordination measure. Results from this analysis did not support combining these items into a single scale. Furthermore, after reviewing item content with CMS, the team relabeled the single-item care coordination measure, “did anyone form the hospice team give you confusing or contradictory information about your family member’s condition or care,” Information Continuity, which better describes the content assessed by the item.
As
a final step in composite development, we considered combining four
items that assess whether the hospice team provided family members
training on how to handle various challenges of caring for the family
member in the home setting (i.e., “Getting hospice care
training”). This domain was finalized after a one-factor
model provided strong evidence of unidimensionality.
RESULTS AND DISCUSSION
The analytic process resulted in the development of multi-item composites and single-item measures of key HECS domains. Below we present the domains, the number of items per domain, and Cronbach’s alpha, a 0 to 1 index that increases with the number of items in a domain and their average correlation with one another. Higher values indicate better measurement of the underlying construct that the composite is intended to measure. In particular we have developed a 5-item measure of Hospice team communication (alpha = .89), a 4-item measure of Getting help for symptoms (alpha = .80), along with separate two-item measures of Getting timely care, Treating your family member with respect, and Providing emotional support (alpha range = .68 to .72), single-item measures of Information continuity and Understanding the side effects of pain medication, and finally, the 4-item measure of Getting hospice care training (alpha = .87) that is unique to the home setting. Table 3.18 lists the survey items in each of the multi- and single-item composites that were developed from the HECS field test survey along with the item-total correlations. The correlations in Table 3.18 show the relationship between each item within a composite and the overall composite (with the given item removed). The table is arranged by magnitude of the correlations, so items at the top of the each composite are more reflective of the content being measured. As expected given the composite reliabilities, the item-total correlations are highest in magnitude for the Hospice team communication and Getting hospice training composites.
Table 3.18. Survey items comprising multi- and single-item composites and item-total correlations |
|
Composite and item |
Item-total correlation |
Hospice team communication |
|
How often did the hospice team listen carefully to you when you talked with them about problems with your family member’s hospice care? |
0.90 |
While your family member was in hospice care, how often did the hospice team listen carefully to you? |
0.80 |
While your family member was in hospice care, how often did the hospice team explain things in a way that was easy to understand? |
0.71 |
While your family member was in hospice care, how often did the hospice team keep you informed about your family member’s condition? |
0.67 |
While your family member was in hospice care, how often did the hospice team keep you informed about when they would arrive to care for your family member? |
0.65 |
Getting timely care |
|
While your family member was in hospice care, when you or your family member asked for help from the hospice team, how often did you get help as soon as you needed it? |
0.55 |
How often did you get the help you needed from the hospice team during evenings, weekends, or holidays? |
0.55 |
Treating your family member with respect |
|
While your family member was in hospice care, how often did the hospice team treat your family member with dignity and respect? |
0.58 |
While your family member was in hospice care, how often did you feel that the hospice team really cared about your family member? |
0.58 |
Providing emotional support |
|
In the weeks after your family member died, how much emotional support did you get from the hospice team? |
0.53 |
While your family member was in hospice care, how much emotional support did you get from the hospice team? |
0.53 |
Providing Support for Religious and Spiritual Beliefs |
|
Support for religious or spiritual beliefs includes talking, praying, quiet time, or other ways of meeting your religious or spiritual needs. While your family member was in hospice care, how much support for your religious and spiritual beliefs did you get from the hospice team? |
---- |
Getting help for symptoms |
|
How often did your family member receive the help he or she needed from the hospice team for feelings of anxiety or sadness? |
0.70 |
Did your family member get as much help with pain as he or she needed? |
0.60 |
How often did your family member get the help he or she needed for trouble with constipation? |
0.58 |
How often did your family member get the help he or she needed for trouble breathing? |
0.57 |
Information continuity |
|
While your family member was in hospice care, how often did anyone from the hospice team give you confusing or contradictory information about your family member’s condition or care?
|
---- |
Understanding the side effects of pain medication
|
|
Side effects of pain medicine include things like sleepiness. Did any member of the hospice team discuss side effects of pain medicine with you or your family member? |
---- |
Getting hospice care training (home setting only) |
|
Did the hospice team give you enough training about what to do if your family member became restless or agitated? |
0.79 |
Did the hospice team give you enough training about if and when to give more pain medicine to your family member? |
0.71 |
Did the hospice team give you enough training about how to help your family member if he or she had trouble breathing? |
0.70 |
Did the hospice team give you enough training about what side effects to watch for from pain medicine? |
0.68 |
Table 3.19 displays the correlation matrix between the multi- and single-item composites. The scales are generally moderately intercorrelated. No single scale or cluster of scales stands out as being particularly highly or weakly intercorrelated. There is a slight tendency for the inter-correlations to be highest for the Hospice team communication (r = 0.38 to 0.66). This is due in part to the survey generally assessing the communication between the hospice team and the family, but is also reflective of the high internal consistency of this composite. The inter-correlations are somewhat lower for the Information continuity (r = 0.23 to 0.38) and Providing emotional support (r = 0.16 to 0.53) composites, indicating that these domains measure content that is distinct on the survey.
Table 3.19. Correlations among composites and single-item indicators. |
|||||||||
|
Hospice team communication |
Information Continuity |
Getting Timely Care |
Treating your family member with respect |
Emotional Support |
Understanding pain medication side effects |
Getting help for symptoms |
Support for religious and spiritual beliefs |
Training (Home specific) |
Hospice team communication |
1 |
|
|
|
|
|
|
|
|
Information continuity |
0.38 |
1 |
|
|
|
|
|
|
|
Getting timely care |
0.58 |
0.30 |
1 |
|
|
|
|
|
|
Treating your family member with respect |
0.61 |
0.27 |
0.48 |
1 |
|
|
|
|
|
Emotional support |
0.42 |
0.23 |
0.36 |
0.34 |
1 |
|
|
|
|
Understanding pain medication side effects |
0.45 |
0.23 |
0.27 |
0.30 |
0.16 |
1 |
|
|
|
Getting help for symptoms |
0.58 |
0.32 |
0.51 |
0.50 |
0.40 |
0.34 |
1 |
|
|
Support for religious and spiritual beliefs |
0.32 |
0.32 |
0.22 |
0.27 |
0.53 |
0.12 |
0.31 |
1 |
|
Training (Home specific) |
0.66 |
0.32 |
0.50 |
0.50 |
0.32 |
0.68 |
0.60 |
0.20 |
1 |
Note: All correlations are significant at p < .01.
Case Mix Adjustment
Previous research, both within and outside of CAHPS, has identified respondent characteristics that are not under the control of the entities being assessed but tend to be related to survey responses. For example, individuals who are older, those with less education and those in better overall and mental health generally tend to give more positive ratings and reports of care in Medicare CAHPS (MCAHPS). Hence, entities with disproportionate numbers of patients with such characteristics (favorable case mix) are advantaged relative to those with a less favorable case mix. To ensure that comparisons between hospices reflect differences in performance rather than differences in case mix, responses must be adjusted for such characteristics.
In this section, we make recommendations for case-mix adjustment (CMA) of hospices participating in the field test, examine adjusted scores, and describe the impact of adjustment. Note that these are preliminary recommendations based solely on the field test and may be further informed by information obtained from national implementation. In general, only respondent characteristics that are determined not to be endogenous (i.e., not to be related to satisfaction or quality of care) should be considered as potential case-mix adjustors. Given this particular setting and available information, we considered both respondent and decedent characteristics as potential case-mix adjustors.
METHODS
We compiled a list of all potential variables/characteristics that could be used for adjustment that were available either through administrative records or from the survey. Variables were removed from consideration if they were potentially endogenous or are not generally considered for case-mix adjustment in CAHPS for other reasons. All remaining candidate adjustors were then evaluated for use in adjustment. Missing values were imputed using within-hospice means for all analyses. To be a necessary case-mix adjustor, a respondent or decedent characteristic must both be a significant predictor of response and vary in distribution across hospices. For example, in previous analyses of MCAHPS, gender was sometimes predictive of response but gender proportions varied little across Medicare Advantage contracts and therefore had little impact on comparisons. For each potential case-mix adjustor, we examined 1) the variation among hospices using ICCs, 2) the predictive power and statistical significance of the adjustor, and 3) the marginal impact of the adjustor on overall adjustment. The marginal impact of the adjustor synthesizes information from 1) and 2) to capture the overall impact of the adjustor. In addition, we examine the overall impact of adjustment. Finally, we use these results to make recommendations for CMA of hospice-level scores for hospices participating in the field test and make recommendations, where possible, regarding CMA for national implementation.
RESULTS
Candidate list of case-mix adjustors
We started with a list of all variables obtained either through administrative records or by survey relating to the following:
decedent and respondent demographics: age, sex, race and ethnicity, education, relationship between decedent and respondent, whether the respondent lived in the same state or city as the hospice, respondent’s primary language
characteristics of decedent’s condition and care: first and last settings of care; length of final hospice episode; payer for hospice care; primary diagnosis; whether the decedent started getting care too early, at the right time, or too late (survey item)
characteristics of survey administration: Spanish-language survey, survey version, prior mailing of FEHC survey, time between death and first mailing
information collected on the survey about respondent’s knowledge and prior experience with hospice care
whether this was the respondent’s first experience with hospice for a friend or family member
how often the respondent took part in or oversaw care.
Table 3.20 lists all variables considered, the model in which they were included (if any), reasons for exclusion, and comments and concerns expressed by the RAND team during model development. Variables that could be influenced by the hospice or by the quality of care or were for other reasons not viewed as potential case-mix adjustors were not included in analyses, and the reason for exclusion was noted. For example, final setting of care (or survey version) is not included as a candidate adjustor because of endogeneity concerns. Although the distribution of care settings varies substantially among hospices (e.g., some hospice programs have a large proportion of patients in nursing homes while others have a large proportion in freestanding IPUs) and overall ratings of care vary substantially by care setting (with freestanding IPUs receiving higher unadjusted ratings than other settings, and nursing homes faring the worst), adjustment for setting may obscure true differences in quality of care assuming that there is no inherent disadvantage for one care setting versus another. Of the remaining variables, there were three—payer type, first experience with hospice care, and lag time between death and first mailing—for which we do not have strong endogeneity concerns, but there was some ambivalence about including them in case-mix models; these were excluded in the midsized model but were included in the full model to weigh their influence.
Table 3.20. Potential Case-Mix Adjustors
Potential CMA |
Data Source |
Model Tested |
Concerns or Comments |
Language of completed survey |
Administrative |
Full |
MCAHPS uses Chinese language of survey completion; hospice to test English and Spanish |
Survey version |
Administrative |
None |
Version is related to final setting of care, concerns about endogeneity |
Decedent characteristic |
|
|
|
Age |
Administrative |
Full |
No concern |
Sex |
Administrative |
Full |
Often makes no difference but will consider |
Race and ethnicity |
Survey and administrative |
None |
Some stakeholders object to use of race and ethnicity; indirect measures, such as language and education, are often preferred; may be revisited in light of recent NQF report on adjustment for SES |
Education |
Survey |
Full |
No concern |
Payer for hospice care |
Administrative |
Full |
Hospices may be linked to payers; retain in full model as reflecting patient’s SES |
Primary diagnosis |
Administrative |
Full |
No concern |
Final setting of care |
Survey and administrative |
None |
Concerns about endogeneity |
Length of final episode of hospice care |
Administrative |
None |
Potential concerns about endogeneity |
First location where patient received care |
Administrative |
None |
May be difficult to identify consistently in hospice administrative records |
Respondent characteristic |
|
|
|
Age |
Survey |
Full |
No concern |
Sex |
Survey |
Full |
Often makes no difference but test |
Race and ethnicity |
Survey |
None |
May prefer indirect measures, such as language or education |
Education |
Survey |
Full |
No concern |
Language spoken at home |
Survey |
Full |
HCAHPS uses home language; hospice to test |
Prior experience with hospice care |
Survey |
Midsized |
|
Relationship to patient |
Survey and administrative |
Full |
No concern |
Lag time between death and first mailing |
Administrative |
Midsized |
|
Address in the same state as the hospice |
Administrative |
Full |
|
Address in the same city as the hospice |
Administrative |
None |
Associated with respondents’ rating of hospices for willingness to recommend and communication scale, but this association disappears when controlling for census division. May act as a proxy for region of country. |
Prior receipt of FEHC |
Administrative |
Full |
Will cease to be relevant in national implementation |
Other survey item |
|
|
|
While your family member was in hospice care, how often did you take part in or oversee care for him or her? |
Survey |
Midsized |
|
Did your family member begin getting hospice care too early, at the right time, or too late? |
Survey |
None |
Concerns about endogeneity or retrospective judgment influenced by care; item included on field-test survey for construct validity only, so variable will not be available on final survey |
|
|||
NOTE: NQF = National Quality Forum.
Variation of candidate adjustors among hospices
ICCs were used to assess variation in respondent and decedent characteristics among hospices (see Table 3.21). ICCs were calculated both for the full set of hospices and among the subset of hospices with at least 20 respondents. Note that a minimum of 20 respondents per hospice over the field-test time period would correspond to a hospice with about 175 respondents over a one-year time period. Very little to no variation was observed for decedent sex, respondent age, first experience with hospice care, lag time between death and first mailing, and whether the respondent was in the same state as the hospice. However, it is possible that lag time may exhibit variation among hospices in the future when there is more than one vendor administering the survey; therefore, this variable should be considered for CMA in future implementations. There was moderate variation in decedent age, decedent education, whether the decedent’s primary diagnosis was dementia versus other diagnoses, respondent education, and whether the respondent was a spouse or partner to the patient or had some other relationship type. There was substantial variation among hospices in language of completed survey (though this was not the case when the ICCs were restricted to hospices with at least 20 respondents); payer for hospice, particularly for Medicare only, Medicare and Medicaid, Medicare and private, and other; language spoken at home (though this was not the case when the ICCs were restricted to hospices with at least 20 respondents); and prior receipt of the FEHC.
Table 3.21. Hospice-Level Intraclass Correlation Coefficients of Potential Case-Mix Adjustors to Be Included in the Models
Potential CMA |
From the HECS Field Test |
From the 2009 FEHC Repositorya |
|
ICC, All Hospices |
ICC, Hospices with 20+ Respondents |
||
Survey |
|
|
|
Language of completed survey |
0.740 |
0.000 |
|
Decedent characteristic |
|
|
|
Age |
0.060 |
0.064 |
|
Sex |
0.000 |
0.000 |
|
Education |
0.078 |
0.031 |
|
Payer for hospice care |
|
|
|
Medicare only |
0.356 |
0.258 |
|
Medicaid only or Medicaid and private |
0.087 |
0.089 |
|
Medicare and Medicaid |
0.431 |
0.500 |
|
Private only |
0.138 |
0.146 |
|
Medicare and private |
0.488 |
0.498 |
|
Uninsured or no payer |
0.189 |
0.194 |
|
Other |
0.609 |
0.396 |
|
Primary diagnosis |
|
|
|
Cancer |
0.023 |
0.012 |
0.043 |
Dementia or neurological disease |
0.071 |
0.077 |
0.030 |
Cardiovascular disease |
0.000 |
0.000 |
0.004 |
Renal failure |
0.032 |
0.025 |
0.004 |
Liver failure |
0.060 |
0.057 |
0.003 |
COPD |
0.019 |
0.030 |
|
Other |
0.004 |
0.002 |
|
Respondent characteristic |
|
|
|
Age |
0.000 |
0.000 |
|
Sex |
0.028 |
0.023 |
0.002 |
Education |
0.063 |
0.056 |
0.066 |
First experience with hospice care |
0.006 |
0.001 |
|
Language spoken at home |
|
|
|
English only |
0.777 |
0.081 |
|
Spanish or Spanish and English |
0.713 |
0.096 |
|
Other or other and English |
0.052 |
0.020 |
|
Patient’s relationship to caregiver |
|
|
|
Spouse or partner |
0.044 |
0.027 |
0.028 |
Parent |
0.022 |
0.020 |
0.019 |
Other older relative |
0.000 |
0.000 |
|
Sibling or child |
0.000 |
0.000 |
0.025 (child) |
Friend or other |
0.004 |
0.004 |
|
Lag time between death and first mailing |
0.078 |
0.037 |
|
In the same state as the hospice |
0.013 |
0.006 |
|
Prior mailing of FEHC |
0.831 |
0.855 |
|
a Note that the data from the FEHC survey represent a different set of hospices from that examined in the field test; in addition, FEHC analyses employ a slightly different ICC estimation method such that direct comparison of ICCs should be made with caution. |
|||
Our ability to accurately estimate ICCs and draw conclusions that are applicable nationwide may be limited because of the lack of national representativeness of hospices participating in the field test, the small number of hospices, and the small number of respondents overall. It is possible that characteristics that do not exhibit variation in the field test will exhibit variation in national implementation. In an effort to investigate this prior to national implementation, we examined ICC estimates obtained from the 2009 FEHC survey in the 1,045 hospices with at least 30 respondents. For the respondent characteristic indicating whether the respondent was a spouse or partner of the decedent, we observed an estimated ICC of 0.027 among all hospices, while the FEHC observed a similar ICC estimate of 0.028 (95-percent CI: 0.025 to 0.032). For the decedent sex, we observed essentially no variation at all among hospices, while the FEHC observed an estimated ICC of 0.007 (95-percent CI: 0.006 to 0.008). Table 3.21 shows additional available ICC estimates from the FEHC survey. Note that the data from the FEHC survey represent a different set of hospices from that examined in the field test, and FEHC analyses employ a slightly different ICC estimation method such that direct comparison of ICCs should be conducted with caution. This additional information may support a recommendation to retain certain characteristics in the model that we suspect may have more variation in national implementation, given FEHC estimates.
Predictive Power at the Patient Level Within Hospices
We selected six quality-of-care measures to examine predictive power of the candidate adjustors: two single-item measures (Overall rating [Table 3.22] and Willingness to recommend [Table 3.23]) that reflect overall experience with care and four multiple-item measures that focus on specific aspects of care (Hospice team communication, Treating your family member with respect, Providing emotional support, and Getting help for symptoms [Tables 3.24 through 3.27]). The outcomes of Overall rating and Willingness to recommend were selected because of their overall importance and similarity across surveys; the remaining four composite measures were selected because they had slightly lower overall means than other measures, and high means limit the ability to detect CMA effects at the respondent level. For all regression models, dummy variables and fixed effects for hospices were included; SEs were not adjusted for clustering. In addition, regression models included only those hospices with 20 or more respondents because of limited power to detect within-hospice associations between adjustors and outcomes for hospices with fewer than 20 respondents. We first regressed each quality-of-care measure on each adjustor in separate linear models that included dummy variables and fixed effects for hospices so that the resulting coefficients were estimates of the within-hospice univariate effect of the adjustor. All quality-of-care measures were standardized such that they had a mean of zero and a variance of one (Z-score). Therefore, a regression coefficient of 0.85, for example, can be interpreted as an increase of 0.85 SD of the outcome for a one-unit increase in the predictor.
Table 3.22. Summary of Models and Impact Analysis for Potential CMA and Overall Rating
|
Overall rating (935 observations in 20 hospices) Mean=93.3, SD=13.5 Comparing full model to adjusting only for FEHC: 1-R^2=1.605% (1.017-3.878%), Kendall Tau=0.884 |
||||
|
Standardized beta coefficients (se) |
Impact analysis |
|||
|
One-at-a-time |
Mid-sized model |
Full Model |
Leave one out (1-R^2) with mid-sized model |
Leave one out (1-R^2) with full model |
Survey |
|
|
|
|
|
Language of completed survey |
0.1874 ( 0.4653 ) |
-0.4978 ( 0.7123 ) |
-0.6084 ( 0.7187 ) |
0.009% |
0.016% |
Decedent |
|
|
|
|
|
Age |
0.0124 ( 0.0143 ) |
0.0141 ( 0.0214 ) |
0.0303 ( 0.0234 ) |
0.021% |
0.078% |
Sex |
-0.0293 ( 0.0613 ) |
0.0166 ( 0.0691 ) |
0.0177 ( 0.0695 ) |
0.001% |
0.001% |
Education |
-0.0387 ( 0.0233 ) |
-0.0313 ( 0.0258 ) |
-0.0353 ( 0.0259 ) |
0.059% |
0.084% |
Payer for Hospice Care |
|
|
|
|
0.408% |
Medicare only [ref] |
|
|
|
|
|
Medicaid only/Medicaid and private |
0.0428 ( 0.1956 ) |
|
0.1312 ( 0.2150 ) |
|
|
Medicare and Medicaid |
-0.1841 ( 0.1522 ) |
|
-0.192 ( 0.1556 ) |
|
|
Private only |
0.0154 ( 0.1471 ) |
|
0.1365 ( 0.1643 ) |
|
|
Medicare and private |
0.1354 ( 0.2032 ) |
|
0.1412 ( 0.2085 ) |
|
|
Uninsured/no payer |
0.1029 ( 0.2092 ) |
|
0.2335 ( 0.2200 ) |
|
|
Other |
0.2701 ( 0.2031 ) |
|
0.3537 ( 0.2147 ) |
|
|
Primary Dx |
|
|
|
0.458% |
0.421% |
Cancer [ref] |
|
|
|
|
|
Dementia/Neurological |
-0.1125 ( 0.0964 ) |
-0.1754 ( 0.1033 ) |
-0.1580 ( 0.1041 ) |
|
|
Cardiovascular diseases |
-0.0554 ( 0.0919 ) |
-0.1136 ( 0.0989 ) |
-0.1144 ( 0.0994 ) |
|
|
Renal failure |
0.1392 ( 0.1938 ) |
0.1083 ( 0.1974 ) |
0.1216 ( 0.1982 ) |
|
|
Liver failure |
-0.3608 ( 0.1989 ) |
-0.3410 ( 0.2028 ) |
-0.3564 ( 0.2064 ) |
|
|
COPD |
0.1022 ( 0.1602 ) |
0.0673 ( 0.1632 ) |
0.0868 ( 0.1639 ) |
|
|
Other |
0.0058 ( 0.1058 ) |
-0.0336 ( 0.1096 ) |
-0.0169 ( 0.1103 ) |
|
|
Respondent |
|
|
|
|
|
Age |
0.0226 ( 0.0244 ) |
0.0323 ( 0.0339 ) |
0.0295 ( 0.0341 ) |
0.013% |
0.012% |
Sex |
0.1148 ( 0.0689 ) |
0.1417 ( 0.0727 ) |
0.1442 ( 0.0732 ) * |
0.121% |
0.181% |
Education |
-0.033 ( 0.0273 ) |
-0.0261 ( 0.0308 ) |
-0.0224 ( 0.0311 ) |
0.042% |
0.033% |
First experience with hospice care |
0.0661 ( 0.0659 ) |
|
0.0747 ( 0.0683 ) |
|
0.041% |
Language spoken at home |
|
|
|
0.047% |
0.057% |
English only [ref] |
|
|
|
|
|
Spanish or Spanish/English |
0.3550 ( 0.3545 ) |
0.6013 ( 0.5450 ) |
0.6228 ( 0.5491 ) |
|
|
Other, or Other/English |
-0.0154 ( 0.2164 ) |
-0.0103 ( 0.2213 ) |
-0.088 ( 0.2260 ) |
|
|
Patient's relationship to caregiver |
|
|
|
0.093% |
0.135% |
Spouse/partner |
-0.0816 ( 0.0703 ) |
-0.1288 ( 0.1059 ) |
-0.1305 ( 0.1071 ) |
|
|
Parent [ref] |
|
|
|
|
|
Other older relative |
-0.0898 ( 0.1264 ) |
-0.0743 ( 0.1278 ) |
-0.0785 ( 0.1281 ) |
|
|
Sibling or child |
0.0894 ( 0.1213 ) |
0.1323 ( 0.1489 ) |
0.1307 ( 0.1504 ) |
|
|
Friend/ Other |
-0.0938 ( 0.1323 ) |
-0.1118 ( 0.1441 ) |
-0.0808 ( 0.1454 ) |
|
|
Lag time between death and first mailing |
-0.0114 ( 0.0274 ) |
|
-0.0167 ( 0.0285 ) |
|
0.147% |
In the same state as the hospice |
0.0507 ( 0.1347 ) |
0.1093 ( 0.1401 ) |
0.1033 ( 0.1407 ) |
0.017% |
0.018% |
Prior receipt of FEHC |
-0.2607 ( 0.3138 ) |
-0.2538 ( 0.3152 ) |
-0.1839 ( 0.3214 ) |
7.188% |
4.037% |
Table 3.23. Summary of Models and Impact Analysis for Potential CMA and Willingness to Recommend
|
Willing to recommend (936 observations in 20 hospices) Mean=93.3, SD=18.0 Comparing full model to adjusting only for FEHC: 1-R^2=1.208% (0.766-2.923%), Kendall Tau=0.863 |
||||
|
Standardized beta coefficients (se) |
Impact analysis |
|||
|
One-at-a-time |
Mid-sized model |
Full Model |
Leave one out (1-R^2) with mid-sized model |
Leave one out (1-R^2) with full model |
Survey |
|
|
|
|
|
Language of completed survey |
0.2501 ( 0.4855 ) |
-0.1986 ( 0.7454 ) |
-0.2299 ( 0.7538 ) |
0.002% |
0.004% |
Decedent |
|
|
|
|
|
Age |
0.0010 ( 0.0149 ) |
0.0059 ( 0.0224 ) |
0.0123 ( 0.0245 ) |
0.006% |
0.019% |
Sex |
-0.0725 ( 0.0639 ) |
-0.0502 ( 0.0724 ) |
-0.0514 ( 0.0729 ) |
0.017% |
0.017% |
Education |
-0.0217 ( 0.0243 ) |
-0.0294 ( 0.0270 ) |
-0.0306 ( 0.0272 ) |
0.078% |
0.092% |
Payer for Hospice Care |
|
|
|
|
0.373% |
Medicare only [ref] |
|
|
|
|
|
Medicaid only/Medicaid and private |
0.0666 ( 0.2042 ) |
|
0.1111 ( 0.2254 ) |
|
|
Medicare and Medicaid |
-0.0900 ( 0.1576 ) |
|
-0.0798 ( 0.1617 ) |
|
|
Private only |
-0.1125 ( 0.1569 ) |
|
-0.0948 ( 0.1752 ) |
|
|
Medicare and private |
0.0614 ( 0.2122 ) |
|
0.0335 ( 0.2187 ) |
|
|
Uninsured/no payer |
0.0598 ( 0.2184 ) |
|
0.1104 ( 0.2306 ) |
|
|
Other |
0.2901 ( 0.2120 ) |
|
0.3196 ( 0.2250 ) |
|
|
Primary Dx |
|
|
|
1.321% |
1.244% |
Cancer [ref] |
|
|
|
|
|
Dementia/Neurological |
-0.1982 ( 0.1008 ) * |
-0.2435 ( 0.1083 ) * |
-0.2391 ( 0.1094 ) * |
|
|
Cardiovascular diseases |
-0.0948 ( 0.0958 ) |
-0.1224 ( 0.1034 ) |
-0.1266 ( 0.1041 ) |
|
|
Renal failure |
0.0810 ( 0.2022 ) |
0.0655 ( 0.2065 ) |
0.0764 ( 0.2078 ) |
|
|
Liver failure |
-0.1901 ( 0.2066 ) |
-0.1641 ( 0.2114 ) |
-0.1827 ( 0.2156 ) |
|
|
COPD |
0.053 ( 0.1671 ) |
0.0321 ( 0.1707 ) |
0.0402 ( 0.1719 ) |
|
|
Other |
-0.021 ( 0.1104 ) |
-0.0325 ( 0.1146 ) |
-0.0293 ( 0.1158 ) |
|
|
Respondent |
|
|
|
|
|
Age |
0.0073 ( 0.0255 ) |
0.0210 ( 0.0354 ) |
0.0179 ( 0.0357 ) |
0.008% |
0.007% |
Sex |
0.1209 ( 0.072 ) |
0.1139 ( 0.0762 ) |
0.1206 ( 0.0769 ) |
0.130% |
0.206% |
Education |
0.0099 ( 0.0285 ) |
0.0196 ( 0.0322 ) |
0.0228 ( 0.0326 ) |
0.037% |
0.051% |
First experience with hospice care |
0.0179 ( 0.0688 ) |
|
0.0258 ( 0.0717 ) |
|
0.007% |
Language spoken at home |
|
|
|
0.037% |
0.038% |
English only [ref] |
|
|
|
|
|
Spanish or Spanish/English |
0.2882 ( 0.3700 ) |
0.4198 ( 0.5704 ) |
0.4066 ( 0.5760 ) |
|
|
Other, or Other/English |
-0.0471 ( 0.2259 ) |
-0.0391 ( 0.2316 ) |
-0.0844 ( 0.2372 ) |
|
|
Patient's relationship to caregiver |
|
|
|
0.151% |
0.165% |
Spouse/partner |
-0.0851 ( 0.0732 ) |
-0.0849 ( 0.1109 ) |
-0.0728 ( 0.1125 ) |
|
|
Parent [ref] |
|
|
|
|
|
Other older relative |
-0.2321 ( 0.1308 ) |
-0.2292 ( 0.1329 ) |
-0.2348 ( 0.1335 ) |
|
|
Sibling or child |
0.0135 ( 0.1282 ) |
0.0416 ( 0.1568 ) |
0.0332 ( 0.1588 ) |
|
|
Friend/ Other |
-0.0335 ( 0.1370 ) |
-0.0513 ( 0.1501 ) |
-0.0381 ( 0.1516 ) |
|
|
Lag time between death and first mailing |
-0.0060 ( 0.0286 ) |
|
-0.0106 ( 0.0299 ) |
|
0.097% |
In the same state as the hospice |
-0.0119 ( 0.1406 ) |
0.0515 ( 0.1467 ) |
0.0495 ( 0.1476 ) |
0.007% |
0.007% |
Prior receipt of FEHC |
-0.1115 ( 0.3276 ) |
-0.1085 ( 0.3298 ) |
-0.0705 ( 0.3372 ) |
2.102% |
0.910% |
Table 3.24. Summary of Models and Impact Analysis for Potential CMA and the Hospice Team Communication Scale
|
Hospice team communication scale (948 observations in 20 hospices) Mean=91.0, SD=16.7 Comparing full model to adjusting only for FEHC: 1-R^2=1.108% (0.702-2.681%), Kendall Tau=0.926 |
||||
|
Standardized beta coefficients (se) |
Impact analysis |
|||
|
One-at-a-time |
Mid-sized model |
Full Model |
Leave one out (1-R^2) with mid-sized model |
Leave one out (1-R^2) with full model |
Survey |
|
|
|
|
|
Language of completed survey |
-0.1799 ( 0.4866 ) |
-1.0666 ( 0.7461 ) |
-1.1361 ( 0.7513 ) |
0.022% |
0.028% |
Decedent |
|
|
|
|
|
Age |
-0.0182 ( 0.0148 ) |
-0.0064 ( 0.0223 ) |
0.0105 ( 0.0243 ) |
0.003% |
0.006% |
Sex |
0.0007 ( 0.0637 ) |
0.0220 ( 0.0720 ) |
0.0261 ( 0.0722 ) |
0.002% |
0.002% |
Education |
-0.0321 ( 0.0244 ) |
-0.0247 ( 0.027 ) |
-0.0316 ( 0.0271 ) |
0.023% |
0.043% |
Payer for Hospice Care |
|
|
|
|
0.392% |
Medicare only [ref] |
|
|
|
|
|
Medicaid only/Medicaid and private |
0.1844 ( 0.1995 ) |
|
0.1710 ( 0.2201 ) |
|
|
Medicare and Medicaid |
-0.2929 ( 0.1538 ) |
|
-0.3101 ( 0.1580 ) * |
|
|
Private only |
0.2091 ( 0.1531 ) |
|
0.2330 ( 0.1713 ) |
|
|
Medicare and private |
0.2715 ( 0.2113 ) |
|
0.2606 ( 0.2176 ) |
|
|
Uninsured/no payer |
0.0348 ( 0.2179 ) |
|
0.0270 ( 0.2298 ) |
|
|
Other |
0.3486 ( 0.2115 ) |
|
0.3276 ( 0.2238 ) |
|
|
Primary Dx |
|
|
|
0.470% |
0.393% |
Cancer [ref] |
|
|
|
|
|
Dementia/Neurological |
-0.2132 ( 0.1006 ) * |
-0.2233 ( 0.1079 ) * |
-0.1923 ( 0.1086 ) |
|
|
Cardiovascular diseases |
-0.0736 ( 0.0950 ) |
-0.0775 ( 0.1023 ) |
-0.0709 ( 0.1025 ) |
|
|
Renal failure |
0.0175 ( 0.2027 ) |
0.0041 ( 0.2066 ) |
0.0127 ( 0.2071 ) |
|
|
Liver failure |
-0.0290 ( 0.2080 ) |
-0.0440 ( 0.2123 ) |
-0.0432 ( 0.2155 ) |
|
|
COPD |
-0.1173 ( 0.1656 ) |
-0.1415 ( 0.1689 ) |
-0.1268 ( 0.1694 ) |
|
|
Other |
-0.0005 ( 0.1094 ) |
-0.0025 ( 0.1134 ) |
0.0211 ( 0.1141 ) |
|
|
Respondent |
|
|
|
|
|
Age |
-0.0347 ( 0.0255 ) |
-0.0211 ( 0.0354 ) |
-0.0240 ( 0.0355 ) |
0.004% |
0.006% |
Sex |
0.0376 ( 0.0721 ) |
0.0718 ( 0.0761 ) |
0.0720 ( 0.0765 ) |
0.020% |
0.030% |
Education |
-0.0341 ( 0.0285 ) |
-0.0292 ( 0.0322 ) |
-0.0266 ( 0.0324 ) |
0.032% |
0.028% |
First experience with hospice care |
0.0304 ( 0.0685 ) |
|
0.0461 ( 0.0709 ) |
|
0.010% |
Language spoken at home |
|
|
|
0.040% |
0.044% |
English only [ref] |
|
|
|
|
|
Spanish or Spanish/English |
0.2383 ( 0.3709 ) |
0.7196 ( 0.5709 ) |
0.7261 ( 0.5739 ) |
|
|
Other, or Other/English |
-0.0093 ( 0.2264 ) |
-0.0557 ( 0.2318 ) |
-0.1204 ( 0.2362 ) |
|
|
Patient's relationship to caregiver |
|
|
|
0.085% |
0.105% |
Spouse/partner |
-0.0776 ( 0.0730 ) |
-0.1200 ( 0.1105 ) |
-0.1307 ( 0.1116 ) |
|
|
Parent [ref] |
|
|
|
|
|
Other older relative |
-0.1818 ( 0.1292 ) |
-0.1808 ( 0.1310 ) |
-0.1799 ( 0.1311 ) |
|
|
Sibling or child |
0.0703 ( 0.1267 ) |
0.0395 ( 0.1558 ) |
0.0376 ( 0.1569 ) |
|
|
Friend/ Other |
-0.1271 ( 0.1372 ) |
-0.1531 ( 0.1498 ) |
-0.1143 ( 0.1507 ) |
|
|
Lag time between death and first mailing |
-0.0264 ( 0.0284 ) |
|
-0.0251 ( 0.0296 ) |
|
0.179% |
In the same state as the hospice |
0.1064 ( 0.1396 ) |
0.1537 ( 0.1455 ) |
0.1578 ( 0.1458 ) |
0.018% |
0.022% |
Prior receipt of FEHC |
-0.3787 ( 0.3076 ) |
-0.3684 ( 0.3096 ) |
-0.3251 ( 0.3161 ) |
9.839% |
8.328% |
Table 3.25. Summary of Models and Impact Analysis for Potential CMA and the Treating your Family Member with Respect Scale
|
Treating your family member with respect scale (933 observations in 20 hospices) Mean=95.8, SD=12.0 Comparing full model to adjusting only for FEHC: 1-R^2=6.540% (4.125-15.460%), Kendall Tau=0.850 |
||||
|
Standardized beta coefficients (se) |
Impact analysis |
|||
|
One-at-a-time |
Mid-sized model |
Full Model |
Leave one out (1-R^2) with mid-sized model |
Leave one out (1-R^2) with full model |
Survey |
|
|
|
|
|
Language of completed survey |
-0.6548 ( 0.488 ) |
-1.2352 ( 0.7507 ) |
-1.3162 ( 0.7563 ) |
0.132% |
0.150% |
Decedent |
|
|
|
|
|
Age |
-0.0089 ( 0.015 ) |
-0.0061 ( 0.0227 ) |
0.0101 ( 0.0247 ) |
0.008% |
0.016% |
Sex |
-0.0452 ( 0.0644 ) |
-0.0108 ( 0.0729 ) |
-0.0184 ( 0.0732 ) |
0.001% |
0.003% |
Education |
-0.0355 ( 0.0247 ) |
-0.0444 ( 0.0275 ) |
-0.0504 ( 0.0276 ) |
0.204% |
0.251% |
Payer for Hospice Care |
|
|
|
|
3.864% |
Medicare only [ref] |
|
|
|
|
|
Medicaid only/Medicaid and private |
0.0682 ( 0.2007 ) |
|
0.0793 ( 0.2218 ) |
|
|
Medicare and Medicaid |
-0.3218 ( 0.1564 ) * |
|
-0.3502 ( 0.1607 ) * |
|
|
Private only |
0.1287 ( 0.1558 ) |
|
0.1422 ( 0.1745 ) |
|
|
Medicare and private |
-0.0837 ( 0.2127 ) |
|
-0.0760 ( 0.2193 ) |
|
|
Uninsured/no payer |
0.2789 ( 0.2191 ) |
|
0.3195 ( 0.2315 ) |
|
|
Other |
0.2401 ( 0.2127 ) |
|
0.2946 ( 0.2258 ) |
|
|
Primary Dx |
|
|
|
0.331% |
0.184% |
Cancer [ref] |
|
|
|
|
|
Dementia/Neurological |
-0.1163 ( 0.1019 ) |
-0.1217 ( 0.1093 ) |
-0.0881 ( 0.1100 ) |
|
|
Cardiovascular diseases |
-0.1078 ( 0.0961 ) |
-0.1238 ( 0.1037 ) |
-0.1097 ( 0.104 ) |
|
|
Renal failure |
0.0965 ( 0.2078 ) |
0.0695 ( 0.2123 ) |
0.0794 ( 0.2128 ) |
|
|
Liver failure |
-0.1158 ( 0.2091 ) |
-0.0983 ( 0.2137 ) |
-0.0925 ( 0.2171 ) |
|
|
COPD |
-0.1568 ( 0.1665 ) |
-0.1756 ( 0.1701 ) |
-0.1464 ( 0.1706 ) |
|
|
Other |
-0.0784 ( 0.1108 ) |
-0.0754 ( 0.1151 ) |
-0.0367 ( 0.1159 ) |
|
|
Respondent |
|
|
|
|
|
Age |
-0.0111 ( 0.0257 ) |
-0.0012 ( 0.0358 ) |
-0.0051 ( 0.036 ) |
0.000% |
0.001% |
Sex |
0.1453 ( 0.0728 ) * |
0.1529 ( 0.0771 ) * |
0.1501 ( 0.0776 ) |
0.277% |
0.279% |
Education |
-0.0105 ( 0.0288 ) |
-0.0052 ( 0.0326 ) |
-0.0056 ( 0.0328 ) |
0.003% |
0.003% |
First experience with hospice care |
-0.0159 ( 0.0693 ) |
|
0.0021 ( 0.0719 ) |
|
0.000% |
Language spoken at home |
|
|
|
0.061% |
0.063% |
English only [ref] |
|
|
|
|
|
Spanish or Spanish/English |
-0.2008 ( 0.3723 ) |
0.4324 ( 0.5744 ) |
0.4729 ( 0.5778 ) |
|
|
Other, or Other/English |
0.0909 ( 0.2273 ) |
0.0396 ( 0.2334 ) |
-0.0201 ( 0.2380 ) |
|
|
Patient's relationship to caregiver |
|
|
|
0.045% |
0.066% |
Spouse/partner |
-0.0272 ( 0.0739 ) |
-0.0492 ( 0.1119 ) |
-0.0253 ( 0.1131 ) |
|
|
Parent [ref] |
|
|
|
|
|
Other older relative |
-0.0125 ( 0.1311 ) |
0.0033 ( 0.1330 ) |
-0.0036 ( 0.1331 ) |
|
|
Sibling or child |
-0.0476 ( 0.1275 ) |
-0.0790 ( 0.1571 ) |
-0.0737 ( 0.1583 ) |
|
|
Friend/ Other |
0.0905 ( 0.1404 ) |
0.0809 ( 0.1529 ) |
0.1255 ( 0.1539 ) |
|
|
Lag time between death and first mailing |
0.0345 ( 0.0288 ) |
|
0.0333 ( 0.0301 ) |
|
0.790% |
In the same state as the hospice |
-0.0043 ( 0.1414 ) |
0.0237 ( 0.1480 ) |
0.0015 ( 0.1484 ) |
0.002% |
0.000% |
Prior receipt of FEHC |
-0.2133 ( 0.3194 ) |
-0.2273 ( 0.3224 ) |
-0.2918 ( 0.3284 ) |
13.374% |
18.218% |
Table 3.26. Summary of Models and Impact Analysis for Potential CMA and the Providing Emotional Support Scale
|
Providing emotional support scale (929 observations in 20 hospices) Mean=91.2, SD=24.7 Comparing full model to adjusting only for FEHC: 1-R^2=3.510% (2.219-8.411%), Kendall Tau=0.947 |
||||
|
Standardized beta coefficients (se) |
Impact analysis |
|||
|
One-at-a-time |
Mid-sized model |
Full Model |
Leave one out (1-R^2) with mid-sized model |
Leave one out (1-R^2) with full model |
Survey |
|
|
|
|
|
Language of completed survey |
-2.7098 ( 0.4838 ) *** |
-3.1048 ( 0.7383 ) *** |
-3.1140 ( 0.7465 ) *** |
0.718% |
0.880% |
Decedent |
|
|
|
|
|
Age |
0.0114 ( 0.0151 ) |
0.0275 ( 0.0222 ) |
0.0302 ( 0.0243 ) |
0.155% |
0.125% |
Sex |
0.0070 ( 0.0650 ) |
0.0358 ( 0.0720 ) |
0.0294 ( 0.0725 ) |
0.014% |
0.008% |
Education |
0.0119 ( 0.0247 ) |
-0.0091 ( 0.0268 ) |
-0.0106 ( 0.0270 ) |
0.011% |
0.016% |
Payer for Hospice Care |
|
|
|
|
0.540% |
Medicare only [ref] |
|
|
|
|
|
Medicaid only/Medicaid and private |
0.1587 ( 0.2114 ) |
|
0.2660 ( 0.2277 ) |
|
|
Medicare and Medicaid |
-0.1636 ( 0.1596 ) |
|
-0.1729 ( 0.1602 ) |
|
|
Private only |
-0.0247 ( 0.1571 ) |
|
-0.0442 ( 0.1720 ) |
|
|
Medicare and private |
0.0166 ( 0.2149 ) |
|
0.1005 ( 0.2165 ) |
|
|
Uninsured/no payer |
-0.3552 ( 0.2214 ) |
|
-0.0999 ( 0.2286 ) |
|
|
Other |
-0.0258 ( 0.2148 ) |
|
0.0163 ( 0.2230 ) |
|
|
Primary Dx |
|
|
|
0.407% |
0.571% |
Cancer [ref] |
|
|
|
|
|
Dementia/Neurological |
-0.0241 ( 0.1025 ) |
-0.0889 ( 0.1076 ) |
-0.0794 ( 0.1086 ) |
|
|
Cardiovascular diseases |
-0.1333 ( 0.0973 ) |
-0.2084 ( 0.1028 ) * |
-0.2035 ( 0.1034 ) * |
|
|
Renal failure |
-0.0858 ( 0.2050 ) |
-0.0896 ( 0.2046 ) |
-0.0973 ( 0.2059 ) |
|
|
Liver failure |
-0.3995 ( 0.2110 ) |
-0.3898 ( 0.2112 ) |
-0.3991 ( 0.2155 ) |
|
|
COPD |
0.0712 ( 0.1676 ) |
0.0253 ( 0.1673 ) |
0.0212 ( 0.1685 ) |
|
|
Other |
-0.0500 ( 0.1123 ) |
-0.1075 ( 0.1142 ) |
-0.0969 ( 0.1153 ) |
|
|
Respondent |
|
|
|
|
|
Age |
0.0443 ( 0.0258 ) |
0.0114 ( 0.0351 ) |
0.0099 ( 0.0354 ) |
0.004% |
0.003% |
Sex |
0.1682 ( 0.0733 ) * |
0.1903 ( 0.0762 ) * |
0.1843 ( 0.0769 ) * |
0.545% |
0.715% |
Education |
0.0238 ( 0.0291 ) |
0.0013 ( 0.0322 ) |
0.0053 ( 0.0325 ) |
0.000% |
0.004% |
First experience with hospice care |
-0.0364 ( 0.0701 ) |
|
-0.0110 ( 0.0714 ) |
|
0.002% |
Language spoken at home |
|
|
|
0.368% |
0.478% |
English only [ref] |
|
|
|
|
|
Spanish or Spanish/English |
-1.3992 ( 0.3711 ) *** |
0.3851 ( 0.5649 ) |
0.4051 ( 0.5703 ) |
|
|
Other, or Other/English |
-0.5411 ( 0.2266 ) * |
-0.5172 ( 0.2293 ) * |
-0.539 ( 0.2347 ) * |
|
|
Patient's relationship to caregiver |
|
|
|
0.304% |
0.339% |
Spouse/partner |
0.0299 ( 0.0743 ) |
0.0023 ( 0.1099 ) |
0.0132 ( 0.1115 ) |
|
|
Parent [ref] |
|
|
|
|
|
Other older relative |
-0.0378 ( 0.1326 ) |
-0.0532 ( 0.1318 ) |
-0.0525 ( 0.1323 ) |
|
|
Sibling or child |
0.1904 ( 0.1299 ) |
0.2732 ( 0.1555 ) |
0.2625 ( 0.1571 ) |
|
|
Friend/ Other |
-0.2108 ( 0.1420 ) |
-0.1701 ( 0.1515 ) |
-0.1626 ( 0.1531 ) |
|
|
Lag time between death and first mailing |
0.0371 ( 0.0291 ) |
|
0.0184 ( 0.0297 ) |
|
0.382% |
In the same state as the hospice |
0.0914 ( 0.1440 ) |
0.1833 ( 0.1468 ) |
0.1873 ( 0.1477 ) |
0.109% |
0.122% |
Prior receipt of FEHC |
0.1443 ( 0.3328 ) |
0.1793 ( 0.3274 ) |
0.1416 ( 0.3346 ) |
6.968% |
4.317% |
Table 3.27. Summary of Models and Impact Analysis for Potential CMA and the Getting Help for Symptoms Scale
|
Getting help for symptoms scale (801 observations in 20 hospices) Mean=90.0, SD=18.8 Comparing full model to adjusting only for FEHC: 1-R^2=5.254% (3.318-12.495%), Kendall Tau=0.874 |
||||
|
Standardized beta coefficients (se) |
Impact analysis |
|||
|
One-at-a-time |
Mid-sized model |
Full Model |
Leave one out (1-R^2) with mid-sized model |
Leave one out (1-R^2) with full model |
Survey |
|
|
|
|
|
Language of completed survey |
0.2322 ( 0.4978 ) |
-0.4909 ( 0.7643 ) |
-0.6154 ( 0.7717 ) |
0.017% |
0.026% |
Decedent |
|
|
|
|
|
Age |
-0.0155 ( 0.0164 ) |
-0.0239 ( 0.0247 ) |
-0.0218 ( 0.0271 ) |
0.102% |
0.057% |
Sex |
0.0133 ( 0.0710 ) |
0.0395 ( 0.0802 ) |
0.0248 ( 0.0807 ) |
0.018% |
0.006% |
Education |
-0.0360 ( 0.0271 ) |
-0.0350 ( 0.0302 ) |
-0.0377 ( 0.0304 ) |
0.153% |
0.176% |
Payer for Hospice Care |
|
|
|
|
1.473% |
Medicare only [ref] |
|
|
|
|
|
Medicaid only/Medicaid and private |
0.2309 ( 0.2231 ) |
|
0.1337 ( 0.2481 ) |
|
|
Medicare and Medicaid |
-0.2547 ( 0.1646 ) |
|
-0.3227 ( 0.1696 ) |
|
|
Private only |
-0.0679 ( 0.1670 ) |
|
-0.0946 ( 0.1896 ) |
|
|
Medicare and private |
0.3652 ( 0.2265 ) |
|
0.3124 ( 0.2338 ) |
|
|
Uninsured/no payer |
0.0479 ( 0.2415 ) |
|
0.0293 ( 0.2565 ) |
|
|
Other |
0.1356 ( 0.2379 ) |
|
0.1178 ( 0.2514 ) |
|
|
Primary Dx |
|
|
|
0.294% |
0.367% |
Cancer [ref] |
|
|
|
|
|
Dementia/Neurological |
0.0057 ( 0.1157 ) |
0.0577 ( 0.1233 ) |
0.0850 ( 0.1242 ) |
|
|
Cardiovascular diseases |
0.0847 ( 0.1049 ) |
0.1306 ( 0.1134 ) |
0.1359 ( 0.1138 ) |
|
|
Renal failure |
-0.0076 ( 0.2370 ) |
0.0502 ( 0.2411 ) |
0.0175 ( 0.2423 ) |
|
|
Liver failure |
-0.2674 ( 0.2370 ) |
-0.2820 ( 0.2414 ) |
-0.2826 ( 0.2450 ) |
|
|
COPD |
0.1132 ( 0.1841 ) |
0.1536 ( 0.1869 ) |
0.1419 ( 0.1877 ) |
|
|
Other |
0.0435 ( 0.1240 ) |
0.0896 ( 0.1295 ) |
0.0995 ( 0.1306 ) |
|
|
Respondent |
|
|
|
|
|
Age |
0.0248 ( 0.0281 ) |
0.0307 ( 0.0388 ) |
0.0325 ( 0.0390 ) |
0.026% |
0.026% |
Sex |
0.1915 ( 0.0805 ) * |
0.2080 ( 0.0856 ) * |
0.1982 ( 0.0862 ) * |
0.466% |
0.393% |
Education |
-0.0451 ( 0.0318 ) |
-0.0277 ( 0.0361 ) |
-0.0236 ( 0.0364 ) |
0.085% |
0.051% |
First experience with hospice care |
0.0334 ( 0.0760 ) |
|
0.0126 ( 0.0794 ) |
|
0.002% |
Language spoken at home |
|
|
|
0.106% |
0.113% |
English only [ref] |
|
|
|
|
|
Spanish or Spanish/English |
0.3896 ( 0.3796 ) |
0.6473 ( 0.5857 ) |
0.6871 ( 0.5904 ) |
|
|
Other, or Other/English |
-0.1071 ( 0.2449 ) |
-0.0683 ( 0.2513 ) |
-0.0893 ( 0.2576 ) |
|
|
Patient's relationship to caregiver |
|
|
|
0.078% |
0.119% |
Spouse/partner |
0.0340 ( 0.0817 ) |
-0.0390 ( 0.1217 ) |
-0.0286 ( 0.1234 ) |
|
|
Parent [ref] |
|
|
|
|
|
Other older relative |
-0.0319 ( 0.153 ) |
-0.0083 ( 0.156 ) |
0.0085 ( 0.1564 ) |
|
|
Sibling or child |
0.1506 ( 0.1404 ) |
0.0838 ( 0.1728 ) |
0.0982 ( 0.1747 ) |
|
|
Friend/ Other |
0.1236 ( 0.1465 ) |
0.0726 ( 0.1609 ) |
0.1060 ( 0.1624 ) |
|
|
Lag time between death and first mailing |
0.0265 ( 0.0317 ) |
|
0.0262 ( 0.0332 ) |
|
0.487% |
In the same state as the hospice |
-0.0300 ( 0.1630 ) |
-0.0294 ( 0.1689 ) |
-0.0163 ( 0.1698 ) |
0.002% |
0.001% |
Prior receipt of FEHC |
-0.2246 ( 0.3541 ) |
-0.2174 ( 0.3577 ) |
-0.2882 ( 0.3651 ) |
8.994% |
14.570% |
First we consider one-at-a-time models. Results are presented in the first column of Tables 3.22 through 3.27. For Overall rating, none of the candidate case-mix adjustors were significantly predictive of the respondent’s rating. For Willingness to recommend and the Hospice Team Communication scale, a primary diagnosis of dementia was associated with lower rating (compared to cancer). For the Treating Your Family Member with Respect scale, male respondents were more likely to respond more positively while respondents for decedents who had Medicare and Medicaid as their payer for hospice care were more likely to respond more negatively (compared to Medicare only). For the Providing Emotional Support scale, language of completed survey, respondent gender, and language spoken at home were significantly associated with response with those using a Spanish language survey and those respondents who do not speak only English at home responding more negatively and male respondents responding more positively. For Getting help for symptoms scale, only respondent sex was associated with response with male respondents rending to respond more positively.
Second, we fit two multivariate regression models for each quality of care measure, one that included only those case-mix adjustors that were not controversial or still under discussion, which were payer for hospice care, lag time, and first experience with hospice care (midsized model), and one which included all case-mix adjustors (full model). Results for the mid-sized model and the full model are presented in columns 2 and 3, respectively, of Tables 3.22 through 3.27. For Overall rating, none of the case-mix adjustors were significantly predictive of the respondent’s rating in the mid-sized model while only respondent sex was significant in the full model (though potential spurious results due to multiple testing should be considered). For Willingness to recommend, only a primary diagnosis of dementia was predictive of response in the mid-sized model and in the full model. For example, the standardized regression coefficient for dementia in the full model was 0.24 reflecting a small effect (on Cohen’s D scale) of dementia on response. For the Hospice team communication scale, a primary diagnosis of dementia was still predictive of response in the mid-sized model while only Medicare and Medicaid as a payer type was significant in the full model such that those respondents where the decedent had dual payer status responded more negatively. For the Treating your family member with respect scale, male respondents were still more likely to respond more positively in the mid-sized model while respondents for decedents who had Medicare and Medicaid as their payer for hospice care were more likely to respond more negatively (compared to Medicare only) in the full model. In both the mid-sized and full models for the Providing emotional support scale, language of completed survey, primary diagnosis of cardiovascular disease, respondent sex, and language spoken at home were significantly associated with response. For the Getting help for symptoms scale, only respondent sex was associated with response in both the mid-sized and full models. Note that these results may suffer from limited power due to our small sample size (fewer than 1000 respondents), small ICCs and inclusion of hospice fixed effects in these models. We will likely have increased power to detect associations when examining CMA in national implementation. It is interesting to note that analyses of survey data from the FEHC also demonstrate significant associations between patient experience responses and dementia diagnosis (not shown).
Marginal effects of adjustors on hospice-level scores
We evaluated the impact of each case-mix adjustor variable on adjustments in the midsized model and in the full model by calculating the correlation, R, between the adjusted hospice-level scores from the midsized model and the adjusted hospice-level scores from the midsized model minus the CMA variable of interest, and similarly for the full model. The quantity 1 – R2 then represents the proportion of adjustment attributable to that variable. This quantity integrates information from the ICC estimates and the standardized regression coefficients to provide an overall assessment of each adjustor’s marginal impact on hospice-level scores. Only variables that vary among hospices and have predictive power at the patient level will have an effect on hospice-level scores. Results are shown in the two rightmost columns of Tables 3.22 through 3.27. Results showed that most variables contributed very little, with the exception of prior receipt of the FEHC survey and, for some outcomes, payer type. Note that, because regression models included only those hospices that had 20 or more respondents, such characteristics as language of completed survey and language spoken at home, which had much smaller ICCs when estimated with the restricted set of hospices than with the full set of hospices, had very little marginal effects but may have more marginal impact in national implementation with more hospices and more respondents per hospice available for analysis.
Overall adjustment impact on hospice level scores
In order to investigate CMA’s overall effect on each quality-of-care measure, we compared hospice-level estimates after adjusting for a null model and the full model. Generally, the FEHC survey will not be in use during national implementation of the HECS; therefore, we adjust only for use of the FEHC survey in our null model. We calculated two statistics between hospice-level scores from the null and the full models: (1) 1 – R2 (95-percent CI), which reflects the proportion of adjustment attributable only to the full model and (2) Kendall Tau, which is a rank-based correlation coefficient that expresses the fraction of hospice pairs whose relative rankings were reversed by adjustment, scaled from 1 for no changes to −1 for a complete reversal of rankings.
Results of the comparison between adjustments for the null and the full models are shown in the header for each quality-of-care measure in Tables 3.22 through 3.27. The 1 – R2 estimated for several of the measures are low, indicating that full adjustment has little effect on scores: Overall rating (1 – R2 = 1.605 percent, 95-percent CI = 1.017 to 3.878 percent), Willingness to recommend (1 – R2 = 1.208 percent, 95-percent CI = 0.766 to 2.923 percent), and the Hospice team communication scale (1 – R2 = 1.108 percent, 95-percent CI = 0.702 to 2.681 percent). For the other three measures, 1 – R2 is higher: Treating your family member with respect scale (1 – R2 = 6.540 percent, 95-percent CI = 4.125 to 15.460 percent), Providing emotional support scale (1 – R2 = 3.510 percent, 95-percent CI = 2.219 to 8.411 percent), and the Getting help for symptoms scale (1 – R2 = 5.254 percent, 95-percent CI = 3.318 to 12.495 percent). Kendall Tau comparing scores between null and full adjustments for the six quality-of-care measures range from 0.850 for the respect scale to 0.947 for the emotional-support scale, meaning that only between 2.7 and 7.5 percent of hospice pairs would switch in terms of relative rankings due to adjustment.
Tests for nonlinearity of ordinal adjustors
In addition, we assessed the appropriate parameterization for ordinal variables, which could be included either as a series of dummy variables or as a linear variable. Specifically, for respondent age and education, decedent age and education and lag time we tested the null hypothesis that adding categorical variables does not improve prediction beyond inclusion of the linear form of the variable. Results shown in Table 3.28 demonstrated weak evidence of nonlinearity for decedent education for three out of six quality of care measures. Therefore, if this variable is included in a CMA model for national implementation, we recommend testing this hypothesis again to determine whether this variable should be included as dummy variables.
Table 3.28. Parameterization of Ordinal CMA: p-values of test of hull hypothesis that categorical variables does not improve prediction beyond inclusion of the linear form.
|
df of joint test |
Overall rating (N=1,102) |
Willingness to recommend (N=1,102) |
Hospice Team Communication Scale (N=1,117) |
Treating Your Family Member with Respect Scale (N=1,097) |
Providing Emotional Support Scale (N=1,096) |
Getting Help for Symptoms Scale (N=948) |
|
|
|
|
|
|
|
|
Decedent |
|
|
|
|
|
|
|
Agea |
6 |
0.0745 |
0.4494 |
0.2409 |
0.1901 |
0.2876 |
0.1237 |
Educationb |
4 |
0.1632 |
0.0476 |
0.0490 |
0.0247 |
0.0796 |
0.3393 |
Respondent |
|
|
|
|
|
|
|
Agec |
6 |
0.4158 |
0.7819 |
0.2041 |
0.4887 |
0.4168 |
0.1301 |
Educationd |
4 |
0.5637 |
0.5531 |
0.7084 |
0.8980 |
0.1189 |
0.6975 |
Lag time between death and first mailinge |
2 |
0.2218 |
0.7182 |
0.6013 |
0.5173 |
0.4730 |
0.6378 |
Bold values indicate p-value < 0.05 and evidence of non-linearity for parameterization.
a. Ordinal parameterization: 1=18to54, 2=55to64, 3=65to69, 4=70to74, 5=75to79, 6=80to84, 7=85to89, 8=90+
b. Ordinal parameterization: 1=LT 8th grade, 2=Some HS, 3=HS, 4=Some College, 5=BA, 6=GT BA
c. Ordinal parameterization: 1=18to24, 2=25to34, 3=35to44, 4=45to54, 5=55to64, 6=65to74, 7=75to84, 8= more than 85
d. Ordinal parameterization: 1=LT 8th grade, 2=Some HS, 3=HS, 4=Some College, 5=BA, 6=GT BA
e. Ordinal parameterization: 1=63 to 74 days, 2=75 to 85 days, 3=86 to 97 days, 4= more than 98 days
DISCUSSION
Overall, little to moderate variation in the following respondent and decedent characteristics was observed among hospices in the field test: language of completed survey, payer type, language spoken at home, prior receipt of the FEHC, decedent age, decedent education, primary diagnosis of dementia or neurological condition versus other, and respondent education. A small number of characteristics were significantly associated with at least one of the six outcomes examined in either a univariate or multivariate model: respondent sex, primary diagnosis of dementia or neurological condition versus other, payer type, language spoken at home, primary diagnosis of cardiovascular disease versus other, and language of completed survey. Only prior receipt of the FEHC demonstrated substantial marginal impact on adjustment of hospice-level scores.
Though decedent age, decedent sex, decedent education, respondent age, and respondent education neither were significantly associated with any examined outcomes nor had moderate or large (standardized regression coefficient greater than 0.20 SD) nonsignificant effects, one might consider retaining them in the survey for CMA or other purposes. First, other CAHPS surveys, including MCAHPS and CAHPS for Accountable Care Organization (ACOs) observe substantial variation in respondent age and respondent education among entities being evaluated and significant associations with ratings and reports of care and thus adjust for such respondent characteristics. Our potentially limited power in the field test to observe such effects leads us to recommend retaining these items in the survey for further evaluation in national implementation. Second, although improved power in national implementation will also allow further evaluation of decedent age, sex, and education as case-mix adjustors, one would also be interested in retaining these items in the survey regardless of adjustment potential to allow for description and reporting of observed true differences in quality of care by these characteristics at a national level. Similarly, this reasoning also supports the retention of survey items related to decedent race and ethnicity. Although this decedent characteristic was ruled out for CMA consideration, it should be retained in the survey so that potential disparities in quality of care can be examined moving forward. Respondent race and ethnicity, on the other hand, were not considered for adjustment and would likely not be needed for future analyses. Furthermore, among respondents who answered survey items relating to the respondent’s race and ethnicity and the decedent’s race and ethnicity, race and ethnicity matched in 94.8 percent of cases.
Of the three candidate case-mix adjustors that were excluded from the midsized model—payer type, first experience with hospice care, and lag time between death and first mailing—first experience with hospice care and lag time did not vary among hospices, were not significantly associated with any examined outcomes, and had no moderate or large (standardized regression coefficient greater than 0.20 SD) nonsignificant effects. (This item, which asks whether this was the respondent’s first experience with hospice care, will not be included on the survey in national implementation.) Therefore, we recommend that these characteristics no longer be considered as potential case-mix adjustors. Payer type, however, demonstrated substantial variation among hospices and was significantly associated with multiple outcomes. Therefore, we recommend including this variable in the final CMA model. Note that this is similar to the inclusion of Medicaid dual eligibility in the CMA models for MCAHPS and CAHPS for ACOs.
Although the characteristic indicating whether a respondent was located in the same state as the hospice was included in our initial list of candidate adjustors and examined in these analyses, further discussion of this variable, along with potential inclusion of a variable indicating whether the respondent was located in the same city as the hospice, has led us to recommend that both variables be excluded from CMA consideration because they seem to be proxies for census region. In general, stakeholders do not tend to support adjustment for region in CAHPS, and, to maintain consistency with other CAHPS survey initiatives, we recommend not including variables that directly or indirectly measure region. Finally, although respondent’s relationship to decedent was not significantly associated with any examined outcomes and varied very little among hospices, we recommend including this characteristic provisionally in the CMA model for the field test and recommend further examination in national implementation.
For the purposes of providing hospice-level scores for hospices participating in the field test, we recommend a CMA model that includes the following:
language of completed survey
decedent age
decedent education
decedent sex
payer type (all categories)
primary diagnosis (all categories)
respondent age
respondent education
respondent sex
language spoken at home (all categories)
relationship to decedent (all categories)
prior receipt of FEHC survey.
This recommended CMA model should be further examined and evaluated in national implementation. Prior receipt of the FEHC is unlikely to be relevant in the context of national implementation. Future considerations could include discussion about whether one should categorize primary diagnosis as dementia or neurological condition versus cardiovascular disease versus other, categorize payer type as Medicare only versus Medicare and Medicaid versus Medicaid only or Medicaid and private, categorize language spoken at home as English only versus other, and categorize relationship to decedent as spouse or partner versus other.
Association between Hospice, Decedent and Caregiver Characteristics and Hospice Experience of Care Survey Outcomes
We explore a range of hospice, patient, and caregiver characteristics that may be associated with differences in care experiences. At the hospice level, we examine the following characteristics with particular interest:
region because regional differences in care patterns, such as variation in length of stay and the use of general inpatient level of care hospice services among persons dying on hospice, may be associated with differences in patient and caregiver experience
hospice size because there is some evidence from the FEHC survey that caregiver experiences with smaller hospice programs are better than experiences with larger hospices (RAND team analysis)
chain status because care patterns, including nursing intensity and programs for minority care and outreach (Lorenz, Ettner, Rosenfeld, Carlisle, Liu, et al., 2004]), may differ for hospice programs that are members of national chains
profit status because intensity of care may be higher among for-profit hospices (Lorenz, Ettner, Rosenfeld, Carlisle, Leake, et al., 2002; Wachterman et al., 2011; Carlson, Gallo, and Bradley, 2004) and care experiences may be better for patients in higher-volume for-profits than in lower-volume for-profits (Miller et al., 2008).
At the decedent level, we focus in particular on setting of care and patient race/ethnicity. Quality of hospice care in the nursing home setting is challenged by higher rates of enrollment of dementia patients with longer lengths of stay and the inherent difficulty of coordinating hospice and nursing home care for dying patients. In addition, analysis of visit data finds that hospice patients residing in nursing homes are less likely to receive skilled nursing services than hospice patients in other settings of care. Previous research has focused on differences between nursing home deaths with and without hospice services, finding that hospice care is associated with improvement in the management of pain, decreased utilization, and improved reports of perceptions that bereaved family members have of the quality of care for persons dying of dementia. Analysis of survey data from the FEHC repository has consistently found lower overall ratings of experiences of care in nursing homes than of care in other settings.
METHODS
We investigated the associations between hospice and patient characteristics and (1) overall rating, (2) willingness to recommend hospice care, and (3) each multi-item composite or single-item measure, by first examining unadjusted mean responses by hospice or patient characteristic. We then used multivariate regressions to examine the adjusted mean response by characteristic, adjusted for appropriate case mix, and tested whether adjusted response differed by hospice or patient characteristic. The case-mix adjustors included were language of completed survey, decedent age, decedent education, decedent sex, payer type, primary diagnosis of decedent, respondent age, respondent education, respondent sex, language spoken at home, caregivers’ relationship to decedent, and prior receipt of the FEHC survey (see CMA section above for more information). Hospice characteristics examined were ownership (not for profit, for profit, or government), geographic region (Northeast, South, Midwest, West, or Puerto Rico), rural or urban hospice location, chain status, and hospice size (small versus medium or large). Respondent and decedent characteristics examined were final setting of care (home, nursing home, acute care hospital, or hospice IPU), survey version, days elapsed from death to first survey mailing, decedent race and ethnicity, length of final episode of hospice care, respondent race and ethnicity, whether this was the respondent’s first experience with hospice, respondent involvement in care (respondent report sometimes, usually, or always overseeing care), whether the respondent was in the same state as the hospice, and whether the respondent was in the same city as the hospice. All dependent variables were scaled from 0 to 100. Because respondents were sampled from hospice programs, a robust variance adjustment of the SEs was used, as well as simple weights, to account for sampling and nonresponse.
RESULTS AND DISCUSSION
Table 3.29 presents the unadjusted mean scores for each of the outcomes of interest on a 0 to 100 scale. Overall, across hospice, decedent and caregiver characteristics, the mean overall rating of hospice care was 93.0 out of 100. Mean scores for each composite were generally high, ranging from 81.0 for Understanding the side of effects of pain medication and 85.2 for Getting hospice care training to 94.9 for Information continuity and 95.7 for Treating your family member with respect.
Table 3.29. Overall Unadjusted Mean Scores for Overall Rating, Willingness to Recommend, and Composites
Outcome |
N |
Unadjusted Person-Level Mean (SD) |
Overall rating |
1,102 |
93.0 (19.9) |
Recommend hospice |
1,102 |
93.1 (25.4) |
Hospice Team Communication |
1,117 |
91.2 (23.0) |
Getting Timely Care |
1,077 |
90.2 (26.5) |
Treating Your Family Member with Respect |
1,097 |
95.7 (17.1) |
Providing Emotional Support |
1,096 |
91.0 (34.1) |
Providing Support for Religious and Spiritual Beliefs |
547 |
96.2 (26.0) |
Getting Help for Symptoms |
948 |
90.2 (25.5) |
Information Continuity |
1,094 |
94.9 (21.7) |
Understanding the Side Effects of Pain Medication |
981 |
81.0 (45.2) |
Hospice Care Training (home setting only) |
362 |
85.2 (35.1) |
Hospice Characteristics
Table 3.30 presents adjusted mean overall rating of hospice by hospice characteristics. Adjusted means varied greatly by hospice region with lower adjusted means for overall rating and willingness to recommend for hospices in the Northeast and Puerto Rico. Regional results should be interpreted with caution given that field test hospices may not be representative of hospices within their regions, and that Puerto Rico results reflect only one hospice. Chain hospices also tended to have lower adjusted mean scores compared to non-chain hospices. Differences in adjusted mean scores by hospice size were not observed for any outcomes examined.
Table 3.30. Adjusted mean response for overall rating of hospice by hospice characteristics
|
|
|
Overall
Hospice Rating (N=1,102) |
|
|
|
|
CMA Adjusted Mean |
p-value, adjusted |
|
N (%) hospices |
N (%) Respondents |
|
|
Hospice Characteristics |
|
|
|
|
Ownership |
|
|
|
0.0383 |
Non-profit |
17 (51.5%) |
776 (68.3%) |
94.3 |
|
For-profit |
14 (42.4%) |
343 (30.2%) |
90.4 |
|
Government |
2 (6.1%) |
17 (1.5%) |
93.6 |
|
Region |
|
|
|
0.0498 |
Northeast |
6 (18.2%) |
107 (9.4%) |
88.4 |
|
South |
10 (30.3%) |
336 (29.6%) |
93.5 |
|
Midwest |
11 (33.3%) |
407 (35.8%) |
93.5 |
|
West |
5 (15.2%) |
275 (24.2%) |
93.7 |
|
Puerto Rico |
1 (3.0%) |
11 (1.0%) |
85.9 |
|
Rural/Urban |
|
|
|
0.6919 |
Urban |
29 (87.9%) |
1,502 (92.6%) |
93.1 |
|
Rural |
4 (12.1%) |
84 (7.4%) |
92.3 |
|
Chain |
|
|
|
0.019 |
No |
22 (66.7%) |
852 (75.0%) |
94.1 |
|
Yes |
11 (33.3%) |
284 (25.0%) |
90.3 |
|
Size |
|
|
|
0.5297 |
Small [ref] |
8 (24.2%) |
118 (10.4%) |
91.9 |
|
Medium/Large |
25 (75.8%) |
1,1018 (89.6%) |
93.2 |
|
Rate of live discharge, from 2012 hospice Medicare claims |
|
|
|
0.082 |
Less than 10% |
4 (12.1%) |
146 (12.9%) |
95.2 |
|
10% to less than 20% |
15 (45.5%) |
595 (52.4%) |
92.7 |
|
20% to less than 30% |
9 (27.3%) |
287 (25.3%) |
93.3 |
|
30% or higher |
5 (15.2%) |
108 (9.5%) |
91.4 |
|
Mean length of stay, days, from 2012 hospice Medicare claims |
|
|
|
0.0607 |
20-39 |
9 (27.3%) |
384 (33.8%) |
94.8 |
|
40-59 |
19 (57.6%) |
663 (58.4%) |
92.1 |
|
60-79 |
2 (6.1%) |
27 (2.4%) |
88.9 |
|
80+ |
3 (9.1%) |
62 (5.5%) |
93.0 |
|
Table 3.31 shows the case-mix adjusted mean overall rating by patient and respondent characteristics. The adjusted mean is lower for patients for whom the last setting of care was the nursing home, and higher for patients for whom the last setting of care was the freestanding IPU. There are no other significant differences in overall rating by other patient or respondent characteristics.
Table 3.31. Adjusted mean response for overall rating of hospice by patient and respondent characteristics
|
|
Overall
Hospice Rating (N=1,102) |
|
|
N (%) Respondents |
CMA Adjusted Mean |
p-value, adjusted |
Patient/Respondent characteristics |
|
|
|
Final Setting of Care |
|
|
<.0001 |
Home |
394 (34.7%) |
92.2 |
|
Nursing Home |
317 (27.9%) |
90.2 |
|
Acute Care Hospital |
88 (7.7%) |
93.0 |
|
Hospice Inpatient Unit |
337 (29.7%) |
96.6 |
|
Days elapsed from death to first mailing |
|
|
0.1484 |
63 to 74 |
277 (24.4%) |
92.8 |
|
75 to 85 |
268 (23.6%) |
94.2 |
|
86 to 97 |
300 (26.4%) |
91.5 |
|
98 or more |
291 (25.6%) |
93.6 |
|
Decedent Race/Ethnicity |
|
|
0.4268 |
White |
915 (80.6%) |
92.8 |
|
Black |
60 (5.3%) |
94.4 |
|
Hispanic |
46 (4.1%) |
92.4 |
|
Native American |
3 (0.3%) |
92.8 |
|
Asian/PI |
17 (1.5%) |
92.9 |
|
Multiracial |
26 (2.3%) |
93.8 |
|
Unknown |
69 (6.1%) |
95.7 |
|
Length of final episode of hospice care |
|
|
0.3685 |
Less than 1 week |
307 (28.2%) |
94.0 |
|
1 to less than 2 weeks |
201 (18.5%) |
93.5 |
|
2 to less than 4 weeks |
155 (14.3%) |
92.8 |
|
1 to less than 2 months |
128 (11.8%) |
92.7 |
|
2 to less than 4 months |
118 (10.9%) |
91.1 |
|
4 to less than 6 months |
55 (5.1%) |
93.8 |
|
6 or more months |
123 (11.3%) |
91.0 |
|
Respondent Race/Ethnicity |
|
|
0.1391 |
White |
898 (79.1%) |
92.9 |
|
Black |
55 (4.8%) |
94.5 |
|
Hispanic |
53 (4.7%) |
91.5 |
|
Native American |
5 (0.4%) |
95.4 |
|
Asian/PI |
14 (1.2%) |
91.2 |
|
Multiracial |
34 (3.0%) |
96.9 |
|
Unknown |
77 (6.8%) |
93.5 |
|
Respondent's first experience with hospice |
|
|
0.2364 |
No |
356 (32.2%) |
92.3 |
|
Yes |
750 (67.8%) |
93.5 |
|
Respondent rating of how often he/she oversaw care |
|
|
0.7417 |
Sometimes |
183 (16.6%) |
93.3 |
|
Usually |
165 (15.0%) |
92.4 |
|
Always |
756 (68.5%) |
93.1 |
|
Respondent address in same state as hospice |
|
|
0.8968 |
No |
61 (5.4%) |
92.9 |
|
Yes |
1075 (94.6%) |
93.0 |
|
Respondent address in same city as hospice |
|
|
0.0971 |
No |
882 (77.6%) |
92.7 |
|
Yes |
254 (22.4%) |
94.3 |
|
Table 3.32 displays the adjusted means for overall rating, willingness to recommend and each composite measure by final setting of care. In general, adjusted mean ratings and reports of experience are best in the freestanding hospice IPU, and worst in the nursing home setting.
Table 3.32. Adjusted Mean Response for Each Developed Composite, Overall Rating, and Willingness to Recommend, by Final Setting of Care
Outcome |
Home |
Nursing Home |
Acute Care Hospital |
Hospice IPU |
N respondents |
394 |
317 |
88 |
337 |
Overall rating** |
92.2 (90.2, 94.2) |
90.2 (87.7, 92.6) |
93.0 (89.8, 96.1) |
96.6 (95.4, 97.8) |
Recommend hospice** |
92.0 (89.1, 94.8) |
90.7 (88.2, 93.3) |
91.2 (88.1, 94.3) |
96.9 (95.8, 98.0) |
Hospice Team Communication* |
91.0 (89.1, 92.8) |
88.5 (86.1, 90.9) |
89.4 (86.4, 92.4) |
94.4 (92.7, 96.2) |
Getting Timely Care** |
89.2 (87.2, 91.3) |
87.3 (85.0, 89.6) |
86.7 (82.5, 91.0) |
94.7 (93.0, 96.5) |
Treating Your Family Member with Respect |
95.2 (93.7, 96.7) |
95.3 (93.4, 97.2) |
94.8 (92.8, 96.8) |
98.9 (95.3, 98.4) |
Providing Emotional Support* |
90.2 (87.5, 92.8) |
88.6 (84.7, 92.6) |
92.5 (88.7, 96.3) |
94.5 (92.1, 96.9) |
Providing Support for Religious and Spiritual Beliefs* |
95.0 (92.4, 97.7) |
95.2 (91.6, 98.8) |
101.5 (98.5, 104.6) |
98.1 (95.9, 100.3) |
Getting Help for Symptoms** |
89.8 (86.8, 92.9) |
86.2 (84.0, 88.5) |
86.3 (81.3, 91.3) |
95.3 (92.0, 98.6) |
Information Continuity |
94.4 (92.6, 96.3) |
94.9 (92.9, 96.9) |
94.0 (91.4, 96.7) |
95.5 (93.8, 97.2) |
Understanding the Side Effects of Pain Medication** |
89.5 (87.1, 92.0) |
71.1 (66.6, 76.7) |
73.7 (62.2, 85.2) |
81.0 (77.2, 84.8) |
NOTE: ** = p ≤ 0.001. * = p ≤ 0.05. |
||||
In keeping with prior analyses reported by the Medicare Payment Advisory Commission (MedPAC) regarding important concerns with provision of hospice care in nursing homes, we find that reported experiences of care are typically worse in the nursing home setting, particularly with regard to Understanding the side effects of pain medication, Getting help for symptoms, Getting timely care, and Hospice team communication. Such differences may be associated with different visit patterns in the nursing home setting (i.e., fewer visits from skilled nursing staff). The field test findings support that experiences of care in freestanding hospice IPUs are rated best by caregivers. Previously, Casarett and colleagues noted that palliative care units were rated higher by bereaved family members than palliative care consultations in the VA (Casarett et al., 2011). There were few significant associations between patient and respondent characteristics and outcomes; observed differences in composite scores by race / ethnicity will be explored further in later analyses.
Open-Ended Responses
All versions of the field test instrument included an open-ended survey item meant to elicit detailed comments from respondents on both exemplars and problems related to the care the patient received from the hospice. One purpose of including the open-ended question was to determine if any domains not represented by the field test questions should be considered for inclusion in the final survey. Specifically, the question asked; "In thinking about your experiences with hospice, was there anything that went especially well or that you wish had gone differently for you and your family member? Please tell us about those experiences." A total of 833 respondents provided answers to this question; approximately 62 percent of mail survey respondents (n=486) and approximately 90 percent of telephone survey respondents (n=320) provided a codeable open-ended text response. 2
The open-ended text responses were analyzed to identify general themes. Text responses were first coded as positive or negative. Positive and negative comments were furthered coded into 14 themes; themes were identified based on the survey content and some emerged from the text itself. Many caregivers’ comments were coded into more than one theme and text responses could include both negative and positive aspects. The most prevalent themes identified in the text included concern and respect, communication, emotional support, access, staff and team care, medication, knowledge imparted to caregiver, and religious support. Table 3.33 lists the coded themes and provides counts as well as examples of comments for each.
Table 3.33. Themes Identified in Open Ended Responses
Category |
Example Text |
Total Number of comments |
Concern and respect |
By the time my mother needed hospice after moving from the hospital to a nursing home she was not aware of anything being done for her. But the concern shown for her and me couldn't have been more heartfelt - the co-ordination between hospice people and the other health care providers was very efficient - everyone worked very hard to see our needs were met. (POSITIVE) |
233 |
Communication |
We had to initiate all conversations about dad's care. At the hospital, no one ever suggested hospice to us. We finally asked a distant family chaplain what do we do? She suggested palliative care. Once hospice finally started the nurses were wonderful! Communication between us, nurses and doctors was poor. The doctor gave contradictory information. Our concerns are not with hospice but with the doctors. No one wanted to take responsibility for his dying (NEGATIVE) |
136 |
Emotional support |
All staff were genuinely caring. While in facility some phone calls were not returned, some requests not followed through on (brain tissue donation - "we ran out of time"). My greatest disappointment has been that after my husband's death i have not gotten some calls returned and things that were said they would do have not been, or were not clear on what was to be done. Ending my more than year-long relationship with hospice with a weird, unfinished business feeling. (NEGATIVE) |
131 |
Access |
We always got speedy responses when we called to ask our questions. (POSITIVE) |
129 |
Staff/ team care |
Whenever we were worried about anything, we thought we would tell them the next day. When we went in the next day, it was already done without us even telling them about it. It was as if they read our minds. (POSITIVE) |
64 |
Medication |
I would have preferred that the nursing home nurse staff had been more attentive to any changes in care and the way new meds were prescribed. They seemed to want to do things their way and not how hospice had ordered; several times i had to call the hospice rn for her to clarify things with the nursing staff at [name]. The [nursing home name] staff didn't read the patient's charts to see if/when hospice made changes. This caused some serious discomfort to the patient. (NEGATIVE) |
61 |
Knowledge imparted to caregiver |
I was by myself; I needed someone to be there to explain everything to me very simply. The young lady just bathed my mom and the people just came to drop off the equipment. It was like they just did their job, nothing more or less. I was very appreciative, but I needed more knowledge and support from the hospice team. (NEGATIVE) |
53 |
Religious support |
They weren't there when he died and they didn't call after either. I asked for a chaplain they didn't send a chaplain. They didn't tell me about morphine patches. I didn't know we had options for consistent pain relief. We barely saw the hospice team. (NEGATIVE) |
52 |
Continuity of care |
The nurse was great, explained everything in detail. Night nurse on call was also very helpful. The nurse also informed staff at nursing home. Very happy with all care. (POSITIVE) |
39 |
Equipment |
When my mother needed special equipment, it was generally delivered within a few hours. (POSITIVE) |
23 |
Bathing |
I thought the hospice aide who provided personal care and hygiene did an excellent job. (POSITIVE) |
19 |
Respite |
The respite care that they provided for me was most beneficial. Also the personal care they provided for my husband. They were all professional and caring. (POSITIVE) |
8 |
Distance to hospice |
Granddaughter did most of the caretaking and did most everything for hospice team. The hospice was located too far away from the area to get to the home in a timely fashion. Went to this hospice because of family ties. They were good, just so far away. Nurse's aid came in for once and the woman who administered medicine was twice a week. Not enough. (NEGATIVE) |
6 |
Reimbursement/ payment |
The only thing that I wish would have went better was when we had a situation where hospice was supposed to pay for my husband's medication. They did, but the paperwork wasn't in place right away so we had to pay out of pocket. [HOSPICE] said they would reimburse us but it took quite a lot of time for that to happen. It was difficult to get a hold of anyone, but when we did, we did get reimbursed. (NEGATIVE) |
5 |
The four most common themes were concern and respect, communication, emotional support, and access. These same four themes were the most common across all settings of care (home, inpatient, nursing home). The concern and respect theme included comments about the warmth, kindness, and attentiveness shown by hospice team members. Respondents' comments about communication covered communication both before and after the death, such as explanations of the hospice process, updates on the patient's condition, and follow up calls after the patient's death. Emotional support was a frequently mentioned topic, and included giving the caregivers breaks, spending time talking and reminiscing with caregivers, and providing grief counseling. Access was also an important theme, and included responsiveness to requests and the availability of hospice team members when they were needed.
The two themes with the most positive comments overall were concern and respect (211 positive comments overall) and emotional support (98 overall). Themes with the most negative comments overall were communication (69 negative comments overall) and access (70 overall). Examining the negative and positive responses by mode, the mail survey also had the most positive comments for the themes of concern and respect (144 positive comments) and emotional support (76). For the telephone survey, the two most common positive comments were concern and respect (67) and communication (35). For both modes, the themes with the most negative comments were communication and access.
The open-ended questions elicited rich and detailed responses regarding these themes, but for the most part addressed issues for which survey questions already existed. The field test instrument included several questions that addressed each of the four most prevalent themes.
Review of the comments to identify any prevalent concerns not included in the survey questions brought to light two interesting findings. Although the field test instrument included multiple questions regarding spiritual support, most of them were omitted from the final survey after analyses showed ceiling effects for these items. Respondents frequently spontaneously mentioned chaplain care in the open-ended questions; because of the significance of that this type of care presumably has for caregivers, an item regarding religious or spiritual support was recommended for inclusion on the final survey instrument.
Finally, we identified one prevalent concern across settings that was not included as a survey question; caregivers reported disappointment and distress related to not being given notification by the hospice team that death was imminent, and therefore failing to be able to be present for the passing of their loves ones (n=20). This concern was identified in early qualitative development work; however, it was determined that such a question would not be a good candidate for inclusion because hospices cannot be held accountable for predicting the time of death.
Chapter Four: Final Survey Instrument
We identified items to maintain for the final survey instrument using several general guidelines. First, we removed items that were included on the field-test instrument solely to facilitate tests of construct validity (e.g., “Did your family member begin getting hospice care too early, at the right time, or too late?”) and those that exhibited little variation or ceiling effects. Some items with limited variation were maintained because of the importance that the measured constructs have for hospice stakeholders or consumers (e.g., an item regarding spiritual and religious support). For parallel items regarding caregivers’ and decedents’ experiences (e.g., “How often did the hospice team listen carefully to you?” and “to your family member?”), we generally included the item directed to the caregiver respondent rather than the decedent on the grounds that respondents’ answers regarding their own experiences have greater face validity than proxy answers on behalf of family members. Finally, we retained items, such as respondent and decedent race and education, that may be used for CMA or other analytic purposes. Table F.1 in Appendix F indicates which survey items were included in each composite and notes other reasons for inclusion or removal of items from the final survey.
Because few setting-specific items were maintained for the final version of the survey instrument and because it is simpler and less expensive to administer one survey instrument in national implementation than to administer multiple setting-specific versions, the three setting-specific survey instruments administered during the field test were consolidated into one instrument designed to measure experiences with care in all settings in which the patient received care. Items specific to the nursing home setting are presented under the heading “Hospice Care Received in a Nursing Home,” and tailored nonapplicable responses are offered for items specific to the home setting. No inpatient-specific items were maintained for the final survey. The final recommended survey instrument is 47 items long.
Chapter Five: Recommendations for National Implementation
Based on the experiences in the field test, and the input of a subsequent TEP convened for the National Implementation of the HECS contract, we recommend the following procedures for national implementation.
Survey eligibility criteria
The following groups of patients discharged from hospice are eligible for inclusion in the sampling universe:
decedents over the age of 18
decedents with death at least 48 hours following last admission to hospice care
decedents for whom there is a caregiver of record
decedents whose caregiver is someone other than a non-familial legal guardian; and
decedents for whom the caregiver has a U.S. or U.S. Territory home address.
Decedents or caregivers of decedents who request that they not be contacted (those who sign “no publicity” requests while under the care of hospice or otherwise directly request not to be contacted) will be excluded. Patients whose last admission to hospice resulted in a live discharge will be excluded.
These eligibility criteria closely match those of the field test with the notable exception that the required length of stay of 48 hours is not restricted to the final setting of hospice care as it was in during the field test. This recommendation follows from the decision to implement one consolidated survey, rather than setting-specific versions, in national implementation. During the field test we needed to ensure that patients had a minimum of 48 hours in the last setting of care, to ensure that caregivers had enough experience to respond to the setting-specific questions. With the one consolidated survey, all caregiver respondents, even those whose family member experienced a transition in care setting, should be able to respond to all questions. Approximately 99% of transitions in care setting occur within the same hospice organization (analysis of 2012 CMS hospice claims data); therefore, respondents reporting on care experiences across settings are highly likely to be reporting about the hospice named on the survey cover.
Timing of Survey Administration
We recommend that the 42-day data collection period begin 2 to 3 months following patient death. This will result in caregivers being surveyed between 2 and 4.5 months after their family member’s death. This recommendation is in keeping with the field test, but modified to reflect monthly data submission by hospices to vendors during national implementation. Survey administration should begin two calendar months following the completion of the data submission month (e.g., on April 1 for deaths occurring anytime between January 1 and January 31). The time lag is designed to be respectful of caregiver grief while allowing for adequate recall of hospice care experiences, and keeping to a minimum the proportion of the sample frame that will have changed contact information in the period following the death.
Sampling Procedures and Methods of Sampling
The field test did not examine alternative methods of sampling; however, given that many hospices participating in national implementation will have a small patient volume, we make the following recommendation:
Hospices with fewer than 50 decedents during the prior calendar year should be exempt from the survey data collection and reporting requirements. Hospices with 50 to 699 decedents in the prior year (n = 2,326 in 2012) should be required to survey all cases. Large hospices with 700 or more decedents in the prior year (n = 274 in 2012) should be required to survey a minimum sample of 700 using an equal-probability design. Prior to the introduction of the HECS, most hospices sponsoring the FEHC survey administered it to all cases (a census). While we do not recommend requiring census administration, this option should be available to hospices that wish to continue it.
Our sampling recommendations are derived from the assumptions, based on the HECS field test, that approximately 85% of cases will be eligible, and that approximately 50% of those in the sample frame will respond. These rates will result in an estimated 300 completed questionnaires for each large hospice and between 21 and 300 completed questionnaires for hospices with at least 50 decedents during the calendar year. Assuming a total of 300 completes within each hospice and an intraclass correlation coefficient (ICC) of 0.01, which measures the amount of variability between hospices, we would achieve an interunit reliability of 0.75. Note that in Medicare CAHPS (MCAHPS) a reliability of 0.75 is regarded as a minimal acceptable standard.
Mode of Survey Administration
The HECS field test did not examine the effects of survey mode on patterns or rates of response. As such, we recommend that hospices be allowed to administer the survey using one of the three mode protocols currently in use for other CMS CAHPS data collection efforts, such as HCAHPS. Specifically, the three recommended modes are: mail only (one mailed survey followed by an additional mailed survey to non-responders 21 days later); telephone only (up to five telephone attempts); and mixed mode (one mailed survey followed by telephone follow-up to non-responders 21 days later with up to five telephone attempts). During the first year of national implementation, a mode experiment will be conducted to assess the degree to which results from the three modes of survey administration are comparable, and to develop analytic adjustments to compensate for any differences across modes if needed.
Data Requirements
We recommend that hospices be required to supply monthly data files to their vendors containing the following types of data elements for hospice patients who died within a calendar month while under the care of the hospice program (first day of month through last day of month).
Information about the hospice patient
patient name (first, middle (if available), last) and prefix/suffix
date of birth
date of death
sex
race/ethnicity
primary diagnosis
admission date for final episode of hospice care
payers (primary, secondary, other)
last location / setting of care (i.e., home, assisted living facility, nursing home, acute care hospital, freestanding hospice inpatient unit)
Information about the primary caregiver
caregiver name (first, middle (if available), last) and prefix/suffix
contact information, including mailing address, telephone numbers, email address (if available)
relationship to hospice patient (i.e., spouse/partner, child, sibling, etc.)
Survey vendors should conduct all sampling activities. Hospices should be required to document the complete list of all patients/caregivers for whom information has been withheld from the survey vendor for any reason, and to provide counts of patients by each of the ineligible categories to allow for tracking. Ineligible categories are:
patient was discharged alive
decedent was over the age of 18
decedent’s death was less than 48 hours following last admission to hospice care
decedent has no caregiver of record
decedent’s caregiver is a non-familial legal guardian
decedent’s caregiver has an address outside the U.S. or U.S. Territories; and
decedent or caregiver requested not to be contacted (i.e., signed “no publicity” requests or otherwise directly requested not to be contacted).
Appendix A: Members of the Technical Expert Panel
Name |
Position as of December 2012 |
David Casarett, MD, MA TEP Co-Chair |
Director of Hospice and Palliative Care and Associate Professor of Medicine, University of Pennsylvania Health System |
Paul Cleary, PhD TEP Co-Chair |
Dean, Yale School of Public Health |
Bradley Beukema, MS |
Consultant and Chaplain, Montgomery Hospice |
Karen Mikula, RN, BSN, CPHQ |
Senior Director of Quality Initiatives, VITAS Innovative Hospice Care |
Naomi Naierman, MPA |
President and Chief Executive Officer, American Hospice Foundation |
Scott Shreve, DO |
National Director of Hospice and Palliative Care, Department of Veterans Affairs |
Eugenia Smither, RN, CHC, CHP, CHE |
Corporate Compliance Officer/Vice President of Compliance and Quality Improvement, Hospice of the Bluegrass |
Shoshanna Sofaer, DrPH |
Robert P. Luciano Professor of Health Care Policy, School of Public Affairs, Baruch College |
Carol Spence, PhD |
Vice President, Research and Quality, National Hospice and Palliative Care Organization |
John Thoma |
CEO, Hospice of Wake County, Hospice of Harnett County, Horizons Palliative Care, and Horizons Home Care |
Appendix B: Hospice Experience Survey – Home Version
Hospice Experience Survey – Home Version (72 items)
Please answer the questions in this survey about the care this patient received from this hospice:
[name of hospice label goes here]
All of the questions in the survey will ask about experience with this hospice.
According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0938-1208. The time required to complete this information collection is estimated to average 16 minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Mail Stop C4-26-05, Baltimore, Maryland 21244-1850.
Survey Instructions
Please give this survey to the person in your household who knows the most about the hospice care received by the person listed on the survey cover letter.
Answer all the questions by checking the box to the left of your answer.
You are sometimes told to skip over some questions in this survey. When this happens you will see an arrow with a note that tells you what question to answer next, like this:
Yes
![]() If Yes, go to Question 1.
If Yes, go to Question 1.
No
The Hospice Patient
How are you related to the person listed on the survey cover letter?
My spouse or partner
My parent
My mother-in-law or father-in-law
My grandparent
My aunt or uncle
My sister or brother
My child
My friend
Other (please print): ________________________
For this survey, the phrase “family member” refers to the person listed on the survey cover letter. Did your family member receive care from the hospice listed on the survey cover letter?
Yes
No
![]() If No, please stop and return the survey in the envelope provided.
If No, please stop and return the survey in the envelope provided.
What was the last location in which your family member received care from this hospice?
Home
Assisted living facility
Nursing home
Hospital
Hospice facility / hospice house
Other
Your Role
While your family member was in hospice care, how often did you take part in or oversee care for him or her?
Never
![]() If Never, please stop and return the survey in the envelope
provided.
If Never, please stop and return the survey in the envelope
provided.
Sometimes
Usually
Always
Was your family member’s hospice care your first experience with hospice services for a close friend or family member?
Yes
No
Starting Hospice Care
For this survey, the hospice team includes all the nurses, doctors, social workers, chaplains and other people who provided hospice care to your family member. Please do not include hospice volunteers.
Did the hospice team explain the kinds of care and services they could give you and your family member?
Yes, definitely
Yes, somewhat
No
Did your family member begin getting hospice care too early, at the right time, or too late?
Too early
At the right time
Too late
Your Family Member’s Hospice Care
As you answer the rest of the questions in this survey, please think only about your family member’s experience with this hospice in the last location in which he or she received hospice care.
While your family member was in hospice care, did you need to contact the hospice team during evenings, weekends, or holidays for questions or help with your family member’s care?
Yes
No![]() If No, please go to Question 10.
If No, please go to Question 10.
How often did you get the help you needed from the hospice team during evenings, weekends, or holidays?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team keep you informed about when they would arrive to care for your family member?
Never
Sometimes
Usually
Always
While your family member was in hospice care, when you or your family member asked for help from the hospice team, how often did you get help as soon as you needed it?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did the hospice team give you and your family member enough privacy?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, how often did you have a hard time speaking with or understanding members of the hospice team because you spoke different languages?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did the hospice team seem informed and up-to-date about your family member’s condition and care?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, how often did the hospice team explain things in a way that was easy to understand?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team keep you informed about your family member’s condition?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did anyone from the hospice team give you confusing or contradictory information about your family member’s condition or care?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team respect your needs and preferences?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team spend enough time with your family member?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team treat your family member with dignity and respect?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did you feel that the hospice team really cared about your family member?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did you talk with the hospice team about any problems with your family member’s hospice care?
Yes
No![]() If No, please go to Question 25.
If No, please go to Question 25.
How often did the hospice team listen carefully to you when you talked with them about problems with your family member’s hospice care?
Never
Sometimes
Usually
Always
How often were problems with your family member’s hospice care resolved as soon as you needed?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did he or she have any pain?
Yes
No![]() If No, please go to Question 27.
If No, please go to Question 27.
Did your family member get as much help with pain as he or she needed?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, did he or she receive any pain medicine?
Yes
No![]() If No, please go to Question 32.
If No, please go to Question 32.
Did you get the information you needed from the hospice team about your family member’s pain medicine?
Yes, definitely
Yes, somewhat
No
Side effects of pain medicine include things like sleepiness. Did any member of the hospice team discuss side effects of pain medicine with you or your family member?
Yes, definitely
Yes, somewhat
No
Did the hospice team give you enough training about what side effects to watch for from pain medicine?
Yes, definitely
Yes, somewhat
No
Did the hospice team give you enough training about if and when to give more pain medicine to your family member?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, did your family member ever have trouble breathing or receive treatment for trouble breathing?
Yes
No![]() If No, please go to Question 36.
If No, please go to Question 36.
How often did your family member get the help he or she needed for trouble breathing?
Never
Sometimes
Usually
Always
How often did you get the information you needed from the hospice team about your family member’s trouble breathing?
Never
Sometimes
Usually
Always
Did the hospice team give you enough training about how to help your family member if he or she had trouble breathing?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, did your family member ever have trouble with constipation?
Yes
No![]() If No, please go to Question 38.
If No, please go to Question 38.
How often did your family member get the help he or she needed for trouble with constipation?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did he or she show any feelings of anxiety or sadness?
Yes
No
Did your family member need help with feelings of anxiety or sadness?
Yes
No![]() If No, please go to Question 41.
If No, please go to Question 41.
How often did your family member receive the help he or she needed from the hospice team for feelings of anxiety or sadness?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did he or she ever become restless or agitated?
Yes
No
![]() If No, please go to Question 43.
If No, please go to Question 43.
Did the hospice team give you enough training about what to do if your family member became restless or agitated?
Yes, definitely
Yes, somewhat
No
Moving your family member includes things like helping him or her turn over in bed, or get in and out of bed or a wheelchair. Did the hospice team give you enough training about how to safely move your family member?
Yes, definitely
Yes, somewhat
No
I did not need to move my family member
While your family member was in hospice care, did any member of the hospice team discuss your family member’s religious or spiritual beliefs?
Yes
No![]() If No, please go to Question 46.
If No, please go to Question 46.
How often did the hospice team treat your family member’s religious or spiritual beliefs with respect?
Never
Sometimes
Usually
Always
Did the hospice team give you as much information as you wanted about what to expect while your family member was dying?
Yes, definitely
Yes, somewhat
No
![]() If No, please go to Question 48.
If No, please go to Question 48.
Was the information provided in a way that was easy to understand?
Yes, definitely
Yes, somewhat
No
When your family member died, was the hospice team with you, or available as soon as you needed?
Yes, definitely
Yes, somewhat
No
Did not need the hospice team
Special Medical Equipment
Special medical equipment includes things like hospital beds, wheelchairs, or oxygen. While your family member was in hospice care, did your family member need special medical equipment?
Yes
No
![]() If No, please go to Question 52.
If No, please go to Question 52.
Did your family member get the equipment as soon as he or she needed it?
Yes
No
Was the equipment picked up in a timely manner when your family member no longer needed it?
Yes
No
Your Own Experience with Hospice
While your family member was in hospice care, how often did the hospice team listen carefully to you?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team spend enough time with you?
Never
Sometimes
Usually
Always
While your family member was in hospice care, were your religious or spiritual beliefs discussed with any member of the hospice team?
Yes
No![]() If No, please go to Question 57.
If No, please go to Question 57.
How often did the hospice team treat your religious or spiritual beliefs with respect?
Never
Sometimes
Usually
Always
Support for religious or spiritual beliefs includes talking, praying, quiet time, or other ways of meeting your religious or spiritual needs. While your family member was in hospice care, how much support for your religious and spiritual beliefs did you get from the hospice team?
Too little
Right amount
Too much
While your family member was in hospice care, how much emotional support did you get from the hospice team?
Too little
Right amount
Too much
In the weeks after your family member died, how much emotional support did you get from the hospice team?
Too little
Right amount
Too much
Overall Rating of Hospice Care
Please answer the following questions about your family member’s care from the hospice named on the cover letter. Do not include care from other hospices in your answers.
Using any number from 0 to 10, where 0 is the worst hospice care possible and 10 is the best hospice care possible, what number would you use to rate your family member’s hospice care?
0 Worst hospice care possible
1
2
3
4
5
6
7
8
9
10 Best hospice care possible
Would you recommend this hospice to your friends and family?
Definitely no
Probably no
Probably yes
Definitely yes
In thinking about your experiences with hospice, was there anything that went especially well or that you wish had gone differently for you and your family member? Please tell us about those experiences.
____________________________________
____________________________________
____________________________________
____________________________________
____________________________________
____________________________________
____________________________________
____________________________________
About Your Family Member
What is the highest grade or level of school that your family member completed?
8th grade or less
Some high school but did not graduate
High school graduate or GED
Some college or 2-year degree
4-year college graduate
More than 4-year college degree
Don’t know
Was your family member of Hispanic, Latino/a or Spanish origin or descent?
Yes
No![]() If No, please go to Question 65.
If No, please go to Question 65.
Which group best describes your family member?
Mexican, Mexican American, Chicano/a
Puerto Rican
Cuban
Another Hispanic, Latino/and, or Spanish Origin
What was your family member’s race? Please mark one or more.
White
Black or African American
American Indian or Alaska Native
Asian Indian
Chinese
Filipino
Japanese
Korean
Vietnamese
Other Asian
Native Hawaiian
Guamanian or Chamorro
Samoan
Other Pacific Islander
About You
What is your age?
18 to 24
25 to 34
35 to 44
45 to 54
55 to 64
65 to 74
75 to 84
85 or older
Are you male or female?
Male
Female
What is the highest grade or level of school that you have completed?
8th grade or less
Some high school but did not graduate
High school graduate or GED
Some college or 2-year degree
4-year college graduate
More than 4-year college degree
Don’t know
Are you of Hispanic, Latino/a, or Spanish origin or descent?
Yes
No![]() If No, please go to Question 71.
If No, please go to Question 71.
Which group best describes you?
Mexican, Mexican American, Chicano
Puerto Rican
Cuban
Another Hispanic, Latino/a, or Spanish Origin
What is your race? Please mark one or more.
White
Black or African American
American Indian or Alaska Native
Asian Indian
Chinese
Filipino
Japanese
Korean
Vietnamese
Other Asian
Native Hawaiian
Guamanian or Chamorro
Samoan
Other Pacific Islander
What language do you mainly speak at home?
English
Spanish
Chinese
Some other language:
Please print: ________________________
Thank you.
Please return the completed survey in the postage-paid envelope.
Appendix C: Hospice Experience Survey – Nursing Home Version
Hospice Experience Survey – Nursing Home Version (65 items)
Please answer the questions in this survey about the care this patient received from this hospice:
[name of hospice label goes here]
All of the questions in the survey will ask about experience with this hospice.
According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0938-1208. The time required to complete this information collection is estimated to average 15 minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Mail Stop C4-26-05, Baltimore, Maryland 21244-1850.
Survey Instructions
Please give this survey to the person in your household who knows the most about the hospice care received by the person listed on the survey cover letter.
Answer all the questions by checking the box to the left of your answer.
You are sometimes told to skip over some questions in this survey. When this happens you will see an arrow with a note that tells you what question to answer next, like this:
Yes
![]() If Yes, go to Question 1.
If Yes, go to Question 1.
No
The Hospice Patient
How are you related to the person listed on the survey cover letter?
My spouse or partner
My parent
My mother-in-law or father-in-law
My grandparent
My aunt or uncle
My sister or brother
My child
My friend
Other:
Please print: __________________
For this survey, the phrase “family member” refers to the person listed on the survey cover letter. Did your family member receive care from the hospice listed on the survey cover letter?
Yes
No
![]() If No, please stop and return the survey in the envelope provided.
If No, please stop and return the survey in the envelope provided.
What was the last location in which your family member received care from this hospice?
Home
Assisted living facility
Nursing home
Hospital
Hospice facility / hospice house
Other
Your Role
While your family member was in hospice care, how often did you take part in or oversee care for him or her?
Never
![]() If Never, please stop and return the survey in the envelope
provided.
If Never, please stop and return the survey in the envelope
provided.
Sometimes
Usually
Always
Was your family member’s hospice care your first experience with hospice services for a close friend or family member?
Yes
No
Starting Hospice Care
For this survey, the hospice team includes all the nurses, doctors, social workers, chaplains and other people who provided hospice care to your family member. Please do not include hospice volunteers.
Did the hospice team explain the kinds of care and services they could give you and your family member?
Yes, definitely
Yes, somewhat
No
Did your family member begin getting hospice care too early, at the right time, or too late?
Too early
At the right time
Too late
Your Family Member’s Hospice Care
As you answer the rest of the questions in this survey, please think only about your family member’s experience with this hospice in the last location in which he or she received hospice care.
While your family member was in hospice care, did you need to contact the hospice team during evenings, weekends, or holidays for questions or help with your family member’s care?
Yes
No![]() If No, please go to Question 10.
If No, please go to Question 10.
How often did you get the help you needed from the hospice team during evenings, weekends, or holidays?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the nursing home staff and hospice team work well together to care for your family member?
Never
Sometimes
Usually
Always
Personal care needs include bathing, dressing, eating meals and changing bedding. While your family member was in hospice care, how often did your family member get as much help with personal care as he or she needed?
Never
Sometimes
Usually
Always
While your family member was in hospice care, were your family member’s personal care needs ever not taken care of because the nursing home staff expected the hospice team to take care of those needs?
Yes
No
While your family member was in hospice care, when you or your family member asked for help from the hospice team, how often did you get help as soon as you needed it?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did the hospice team give you and your family member enough privacy?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, how often did you have a hard time speaking with or understanding members of the hospice team because you spoke different languages?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did the hospice team seem informed and up-to-date about your family member’s condition and care?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, how often did the hospice team explain things in a way that was easy to understand?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team keep you informed about your family member’s condition?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did anyone from the hospice team give you confusing or contradictory information about your family member’s condition or care?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often was the information you were given about your family member by the nursing home staff different from the information you were given by the hospice team?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team respect your needs and preferences?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team spend enough time with your family member?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team treat your family member with dignity and respect?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did you feel that the hospice team really cared about your family member?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did you talk with the hospice team about any problems with your family member’s hospice care?
Yes
No![]() If No, please go to Question 28.
If No, please go to Question 28.
How often did the hospice team listen carefully to you when you talked about problems with your family member’s hospice care?
Never
Sometimes
Usually
Always
How often were problems with your family member’s hospice care resolved as soon as you needed?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did he or she have any pain?
Yes
No![]() If No, please go to Question 30.
If No, please go to Question 30.
Did your family member get as much help with pain as he or she needed?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, did he or she receive any pain medicine?
Yes
No![]() If No, please go to Question 33.
If No, please go to Question 33.
Did you get the information you needed from the hospice team about your family member’s pain medicine?
Yes, definitely
Yes, somewhat
No
Side effects of pain medicine include things like sleepiness. Did any member of the hospice team discuss side effects of pain medicine with you or your family member?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, did your family member ever have trouble breathing or receive treatment for trouble breathing?
Yes
No![]() If No, please go to Question 36.
If No, please go to Question 36.
How often did your family member get the help he or she needed for trouble breathing?
Never
Sometimes
Usually
Always
How often did you get the information you needed from the hospice team about your family member’s trouble breathing?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did your family member ever have trouble with constipation?
Yes
No![]() If No, please go to Question 38.
If No, please go to Question 38.
How often did your family member get the help he or she needed for trouble with constipation?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did he or she show any feelings of anxiety or sadness?
Yes
No
Did your family member need help with feelings of anxiety or sadness?
Yes
No![]() If No, please go to Question 41.
If No, please go to Question 41.
How often did your family member receive the help he or she needed from the hospice team for feelings of anxiety or sadness?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did any member of the hospice team discuss your family member’s religious or spiritual beliefs?
Yes
No![]() If No, please go to Question 43.
If No, please go to Question 43.
How often did the hospice team treat your family member’s religious or spiritual beliefs with respect?
Never
Sometimes
Usually
Always
Did the hospice team give you as much information as you wanted about what to expect while your family member was dying?
Yes, definitely
Yes, somewhat
No
![]() If No, please go to Question 45.
If No, please go to Question 45.
Was the information provided in a way that was easy to understand?
Yes, definitely
Yes, somewhat
No
Your Own Experience with Hospice
While your family member was in hospice care, how often did the hospice team listen carefully to you?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team spend enough time with you?
Never
Sometimes
Usually
Always
While your family member was in hospice care, were your religious or spiritual beliefs discussed with any member of the hospice team?
Yes
No![]() If No, please go to Question 50.
If No, please go to Question 50.
How often did the hospice team treat your religious or spiritual beliefs with respect?
Never
Sometimes
Usually
Always
Support for religious or spiritual beliefs includes talking, praying, quiet time, or other ways of meeting your religious or spiritual needs. While your family member was in hospice care, how much support for your religious and spiritual beliefs did you get from the hospice team?
Too little
Right amount
Too much
While your family member was in hospice care, how much emotional support did you get from the hospice team?
Too little
Right amount
Too much
In the weeks after your family member died, how much emotional support did you get from the hospice team?
Too little
Right amount
Too much
Overall Rating of Hospice Care
Please answer the following questions about your family member’s care from the hospice named on the cover letter. Do not include care from other hospices in your answers.
Using any number from 0 to 10, where 0 is the worst hospice care possible and 10 is the best hospice care possible, what number would you use to rate your family member’s hospice care?
0 Worst hospice care possible
1
2
3
4
5
6
7
8
9
10 Best hospice care possible
Would you recommend this hospice to your friends and family?
Definitely no
Probably no
Probably yes
Definitely yes
In thinking about your experiences with hospice, was there anything that went especially well or that you wish had gone differently for you and your family member? Please tell us about those experiences.
____________________________________
____________________________________
____________________________________
____________________________________
____________________________________
____________________________________
____________________________________
About Your Family Member
What is the highest grade or level of school that your family member completed?
8th grade or less
Some high school but did not graduate
High school graduate or GED
Some college or 2-year degree
4-year college graduate
More than 4-year college degree
Don’t know
Was your family member of Hispanic, Latino/a, or Spanish origin or descent?
Yes
No![]() If No, please go to Question 58.
If No, please go to Question 58.
Which group best describes your family member?
Mexican, Mexican American, Chicano
Puerto Rican
Cuban
Another Hispanic, Latino/a, or Spanish Origin
What was your family member’s race? Please mark one or more.
White
Black or African American
American Indian or Alaska Native
Asian Indian
Chinese
Filipino
Japanese
Korean
Vietnamese
Other Asian
Native Hawaiian
Guamanian or Chamorro
Samoan
Other Pacific Islander
About You
What is your age?
18 to 24
25 to 34
35 to 44
45 to 54
55 to 64
65 to 74
75 to 84
85 or older
Are you male or female?
Male
Female
What is the highest grade or level of school that you have completed?
8th grade or less
Some high school but did not graduate
High school graduate or GED
Some college or 2-year degree
4-year college graduate
More than 4-year college degree
Don’t know
Are you of Hispanic, Latino/a or Spanish origin or descent?
Yes
No![]() If No, please go to Question 64.
If No, please go to Question 64.
Which group best describes you?
Mexican, Mexican American, Chicano/a
Puerto Rican
Cuban
Another Hispanic, Latino/a, or Spanish Origin
What is your race? Please mark one or more.
White
Black or African American
American Indian or Alaska Native
Asian Indian
Chinese
Filipino
Japanese
Korean
Vietnamese
Other Asian
Native Hawaiian
Guamanian or Chamorro
Samoan
Other Pacific Islander
What language do you mainly speak at home?
English
Spanish
Chinese
Some other language:
Please print: ________________________
Thank you.
Please return the completed survey in the postage-paid envelope.
Appendix D: Hospice Experience Survey – Inpatient Version
Hospice Experience Survey – Inpatient Version (67 items)
Please answer the questions in this survey about the care this patient received from this hospice:
[name of hospice label goes here]
All of the questions in the survey will ask about experience with this hospice.
According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0938-1208. The time required to complete this information collection is estimated to average 15 minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Mail Stop C4-26-05, Baltimore, Maryland 21244-1850.
Survey Instructions
Please give this survey to the person in your household who knows the most about the hospice care received by the person listed on the survey cover letter.
Answer all the questions by checking the box to the left of your answer.
You are sometimes told to skip over some questions in this survey. When this happens you will see an arrow with a note that tells you what question to answer next, like this:
Yes
![]() If Yes, go to Question 1.
If Yes, go to Question 1.
No
The Hospice Patient
How are you related to the person listed on the survey cover letter?
My spouse or partner
My parent
My mother-in-law or father-in-law
My grandparent
My aunt or uncle
My sister or brother
My child
My friend
Other:
Please print: __________________
For this survey, the phrase “family member” refers to the person listed on the survey cover letter. Did your family member receive care from the hospice listed on the survey cover letter?
Yes
No
![]() If No, please stop and return the survey in the envelope provided.
If No, please stop and return the survey in the envelope provided.
What was the last location in which your family member received care from this hospice?
Home
Assisted living facility
Nursing home
Hospital
Hospice facility / hospice house
Other
Your Role
While your family member was in hospice care, how often did you take part in or oversee care for him or her?
Never
![]() If Never, please stop and return the survey in the envelope
provided.
If Never, please stop and return the survey in the envelope
provided.
Sometimes
Usually
Always
Was your family member’s hospice care your first experience with hospice services for a close friend or family member?
Yes
No
Starting Hospice Care
For this survey, the hospice team includes all the nurses, doctors, social workers, chaplains and other people who provided hospice care to your family member. Please do not include hospice volunteers.
Did the hospice team explain the kinds of care and services they could give you and your family member?
Yes, definitely
Yes, somewhat
No
Did your family member begin getting hospice care too early, at the right time, or too late?
Too early
At the right time
Too late
Your Family Member’s Hospice Care
As you answer the rest of the questions in this survey, please think only about your family member’s experience with this hospice in the last location in which he or she received hospice care.
While your family member was in hospice care, did you need to contact the hospice team during evenings, weekends, or holidays for questions or help with your family member’s care?
Yes
No![]() If No, please go to Question 10.
If No, please go to Question 10.
How often did you get the help you needed from the hospice team during evenings, weekends, or holidays?
Never
Sometimes
Usually
Always
Personal care needs include bathing, dressing, eating meals and changing bedding. While your family member was in hospice care, how often did your family member get as much help with personal care as he or she needed?
Never
Sometimes
Usually
Always
While your family member was in hospice care, when you or your family member asked for help from the hospice team, how often did you get help as soon as you needed it?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did the hospice team give you and your family member enough privacy?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, how often did you have a hard time speaking with or understanding members of the hospice team because you spoke different languages?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did the hospice team seem informed and up-to-date about your family member’s condition and care?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, did you speak to a doctor as often as you needed?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, how often did the hospice team explain things in a way that was easy to understand?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team keep you informed about your family member’s condition?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did anyone from the hospice team give you confusing or contradictory information about your family member’s condition or care?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team respect your needs and preferences?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team spend enough time with your family member?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team treat your family member with dignity and respect?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did you feel that the hospice team really cared about your family member?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did you talk with the hospice team about any problems with your family member’s hospice care?
Yes
No![]() If No, please go to Question 26.
If No, please go to Question 26.
How often did the hospice team listen carefully to you when you talked about problems with your family member’s hospice care?
Never
Sometimes
Usually
Always
How often were problems with your family member’s hospice care resolved as soon as you needed?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did he or she have any pain?
Yes
No![]() If No, please go to Question 28.
If No, please go to Question 28.
Did your family member get as much help with pain as he or she needed?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, did he or she receive any pain medicine?
Yes
No![]() If No, please go to Question 31.
If No, please go to Question 31.
Did you get the information you needed from the hospice team about your family member’s pain medicine?
Yes, definitely
Yes, somewhat
No
Side effects of pain medicine include things like sleepiness. Did any member of the hospice team discuss side effects of pain medicine with you or your family member?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, did your family member ever have trouble breathing or receive treatment for trouble breathing?
Yes
No![]() If No, please go to Question 34.
If No, please go to Question 34.
How often did your family member get the help he or she needed for trouble breathing?
Never
Sometimes
Usually
Always
How often did you get the information you needed from the hospice team about your family member’s trouble breathing?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did your family member ever have trouble with constipation?
Yes
No![]() If No, please go to Question 36.
If No, please go to Question 36.
How often did your family member get the help he or she needed for trouble with constipation?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did he or she show any feelings of anxiety or sadness?
Yes
No
Did your family member need help with feelings of anxiety or sadness?
Yes
No![]() If No, please go to Question 39.
If No, please go to Question 39.
How often did your family member receive the help he or she needed from the hospice team for feelings of anxiety or sadness?
Never
Sometimes
Usually
Always
While your family member was in hospice care, did any member of the hospice team discuss your family member’s religious or spiritual beliefs?
Yes
No![]() If No, please go to Question 41.
If No, please go to Question 41.
How often did the hospice team treat your family member’s religious or spiritual beliefs with respect?
Never
Sometimes
Usually
Always
Did the hospice team give you as much information as you wanted about what to expect while your family member was dying?
Yes, definitely
Yes, somewhat
No
![]() If No, please go to Question 43.
If No, please go to Question 43.
Was the information provided in a way that was easy to understand?
Yes, definitely
Yes, somewhat
No
Did the hospice team get in the way of you spending time with your family member while he or she was dying?
Yes, definitely
Yes, somewhat
No
The Hospice Environment
While your family member was in hospice care, were his or her room and bathroom kept clean?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, was his or her room a comfortable place for you to be together?
Yes, definitely
Yes, somewhat
No
While your family member was in hospice care, was your family member’s room a calm and soothing place for him or her?
Yes, definitely
Yes, somewhat
No
Your Own Experience with Hospice
While your family member was in hospice care, how often did the hospice team listen carefully to you?
Never
Sometimes
Usually
Always
While your family member was in hospice care, how often did the hospice team spend enough time with you?
Never
Sometimes
Usually
Always
While your family member was in hospice care, were your religious or spiritual beliefs discussed with any member of the hospice team?
Yes
No![]() If No, please go to Question 52.
If No, please go to Question 52.
How often did the hospice team treat your religious or spiritual beliefs with respect?
Never
Sometimes
Usually
Always
Support for religious or spiritual beliefs includes talking, praying, quiet time, or other ways of meeting your religious or spiritual needs. While your family member was in hospice care, how much support for your religious and spiritual beliefs did you get from the hospice team?
Too little
Right amount
Too much
While your family member was in hospice care, how much emotional support did you get from the hospice team?
Too little
Right amount
Too much
In the weeks after your family member died, how much emotional support did you get from the hospice team?
Too little
Right amount
Too much
Overall Rating of Hospice Care
Please answer the following questions about your family member’s care from the hospice named on the cover letter. Do not include care from other hospices in your answers.
Using any number from 0 to 10, where 0 is the worst hospice care possible and 10 is the best hospice care possible, what number would you use to rate your family member’s hospice care?
0 Worst hospice care possible
1
2
3
4
5
6
7
8
9
10 Best hospice care possible
Would you recommend this hospice to your friends and family?
Definitely no
Probably no
Probably yes
Definitely yes
In thinking about your experiences with hospice, was there anything that went especially well or that you wish had gone differently for you and your family member? Please tell us about those experiences.
____________________________________
____________________________________
____________________________________
____________________________________
____________________________________
____________________________________
____________________________________
About Your Family Member
What is the highest grade or level of school that your family member completed?
8th grade or less
Some high school but did not graduate
High school graduate or GED
Some college or 2-year degree
4-year college graduate
More than 4-year college degree
Don’t know
Was your family member of Hispanic, Latino and/or Spanish origin or descent?
Yes
No![]() If No, please go to Question 60.
If No, please go to Question 60.
Which group best describes your family member?
Mexican, Mexican American, Chicano
Puerto Rican
Cuban
Another Hispanic, Latino and/or Spanish Origin
What was your family member’s race? Please mark one or more.
White
Black or African American
American Indian or Alaska Native
Asian Indian
Chinese
Filipino
Japanese
Korean
Vietnamese
Other Asian
Native Hawaiian
Guamanian or Chamorro
Samoan
Other Pacific Islander
About You
What is your age?
18 to 24
25 to 34
35 to 44
45 to 54
55 to 64
65 to 74
75 to 84
85 or older
Are you male or female?
Male
Female
What is the highest grade or level of school that you have completed?
8th grade or less
Some high school but did not graduate
High school graduate or GED
Some college or 2-year degree
4-year college graduate
More than 4-year college degree
Don’t know
Are you of Hispanic, Latino/a, or Spanish origin or descent?
Yes
No![]() If No, please go to Question 66.
If No, please go to Question 66.
Which group best describes you?
Mexican, Mexican American, Chicano
Puerto Rican
Cuban
Another Hispanic, Latino/a, or Spanish Origin
What is your race? Please mark one or more.
White
Black or African American
American Indian or Alaska Native
Asian Indian
Chinese
Filipino
Japanese
Korean
Vietnamese
Other Asian
Native Hawaiian
Guamanian or Chamorro
Samoan
Other Pacific Islander
What language do you mainly speak at home?
English
Spanish
Chinese
Some other language:
Please print: ________________________
Thank you.
Please return the completed survey in the postage-paid envelope.
Appendix E: Item Response Rates Among Unit Respondents
Table E.1. Item Response Rates Among Unit Respondents
Item |
Applicable Completed Surveys |
Appropriate Skip (N) |
Appropriate Skip (%) |
N Legitimate Responses |
Nonlegitimate Skips (N) |
Nonlegitimate Skips (%) |
||||
Overall |
Home |
Nursing Home |
Acute Care Hospital |
Hospice IPU |
||||||
The hospice patient |
|
|
|
|
|
|
|
|
|
|
How related to decedent |
1,136 |
0 |
0.0 |
1,117 |
19 |
1.7 |
0.8 |
1.3 |
2.3 |
3.0 |
Receive care from the hospice listed |
1,136 |
0 |
0.0 |
1,112 |
24 |
2.1 |
1.3 |
1.6 |
4.5 |
3.0 |
Last location of care |
1,136 |
0 |
0.0 |
1,112 |
24 |
2.1 |
0.5 |
1.9 |
3.4 |
3.9 |
Your role |
|
|
|
|
|
|
|
|
|
|
How often you oversaw care |
1,136 |
0 |
0.0 |
1,104 |
32 |
2.8 |
0.8 |
3.5 |
5.7 |
3.9 |
Your first experience with hospice |
1,136 |
0 |
0.0 |
1,106 |
30 |
2.6 |
1.0 |
1.9 |
4.5 |
4.7 |
Starting hospice care |
|
|
|
|
|
|
|
|
|
|
Hospice explained the kinds of care |
1,136 |
0 |
0.0 |
1,111 |
25 |
2.2 |
0.8 |
1.9 |
2.3 |
4.2 |
Began getting hospice care too early, at the right time, or too late |
1,136 |
0 |
0.0 |
1,101 |
35 |
3.1 |
2.0 |
3.5 |
3.4 |
3.9 |
Your family member’s hospice care |
|
|
|
|
|
|
|
|
|
|
Needed to contact the hospice during evenings, weekends, or holidays |
1,136 |
0 |
0.0 |
1,089 |
47 |
4.1 |
3.3 |
4.4 |
4.5 |
4.7 |
Got help from the hospice during evenings, weekends, or holidays |
1,136 |
531 |
46.7 |
560 |
45 |
7.4 |
4.3 |
9.3 |
14.3 |
11.2 |
Informed about when hospice team would arrive |
376 |
0 |
0.0 |
369 |
7 |
1.9 |
1.9 |
|
|
|
Nursing home staff and hospice team worked well together |
272 |
0 |
0.0 |
257 |
15 |
5.5 |
|
5.5 |
|
|
Got as much help with personal care as needed |
696 |
0 |
0.0 |
654 |
42 |
6.0 |
|
5.3 |
7.3 |
6.4 |
Personal care not done because nursing home staff expected the hospice team to take care of those needs |
272 |
0 |
0.0 |
251 |
21 |
7.7 |
|
7.7 |
|
|
Got help as soon as you needed it |
1,136 |
0 |
0.0 |
1,074 |
62 |
5.5 |
2.5 |
7.3 |
8.0 |
6.5 |
Got enough privacy |
1,136 |
0 |
0.0 |
1,093 |
43 |
3.8 |
3.6 |
3.5 |
4.5 |
4.2 |
Different languages |
1,136 |
0 |
0.0 |
1,095 |
41 |
3.6 |
2.8 |
3.2 |
3.4 |
5.0 |
Hospice seemed informed about condition and care |
1,136 |
0 |
0.0 |
1,095 |
41 |
3.6 |
2.8 |
3.5 |
3.4 |
4.7 |
Spoke to a doctor as often as you needed |
402 |
0 |
0.0 |
373 |
29 |
7.2 |
|
|
5.3 |
7.7 |
Hospice explained things in a way that was easy to understand |
1,136 |
0 |
0.0 |
1,096 |
40 |
3.5 |
2.3 |
3.5 |
3.4 |
5.0 |
Hospice kept you informed about condition |
1,136 |
0 |
0.0 |
1,092 |
44 |
3.9 |
3.0 |
2.8 |
4.5 |
5.6 |
Confusing or contradictory information about condition |
1,136 |
0 |
0.0 |
1,094 |
42 |
3.7 |
3.0 |
3.2 |
4.5 |
4.7 |
Information from nursing home staff and hospice team differed |
272 |
0 |
0.0 |
260 |
12 |
4.4 |
|
4.4 |
|
|
Respected your needs and preferences |
1,136 |
0 |
0.0 |
1,086 |
50 |
4.4 |
2.8 |
5.0 |
3.4 |
5.9 |
Hospice spent enough time with your family member |
1,136 |
0 |
0.0 |
1,070 |
66 |
5.8 |
3.0 |
6.6 |
10.2 |
7.1 |
Hospice treated your family member with dignity and respect |
1,136 |
0 |
0.0 |
1,093 |
43 |
3.8 |
2.8 |
3.5 |
4.5 |
5.0 |
Hospice cared about your family member |
1,136 |
0 |
0.0 |
1,093 |
43 |
3.8 |
2.5 |
3.8 |
4.5 |
5.0 |
Talked with the hospice about any problems with hospice care |
1,136 |
0 |
0.0 |
1,070 |
66 |
5.8 |
4.1 |
6.6 |
8.0 |
6.5 |
Hospice listened carefully to you about problems with care |
1,136 |
674 |
59.3 |
402 |
60 |
13.0 |
8.1 |
12.8 |
25.0 |
17.6 |
Problems resolved as soon as you needed |
1,136 |
674 |
59.3 |
398 |
64 |
13.9 |
8.1 |
15.2 |
25.0 |
18.5 |
Family member had any pain |
1,136 |
0 |
0.0 |
1,073 |
63 |
5.5 |
4.3 |
7.9 |
5.7 |
4.7 |
Got help for pain |
1,136 |
342 |
30.1 |
730 |
64 |
8.1 |
7.0 |
10.2 |
6.8 |
7.8 |
Family member received any pain medicine |
1,136 |
0 |
0.0 |
1,074 |
62 |
5.5 |
3.3 |
7.6 |
6.8 |
5.6 |
Got needed info about pain medicine |
1,136 |
89 |
7.8 |
993 |
54 |
5.2 |
2.0 |
8.0 |
7.1 |
5.6 |
Hospice discussed side effects of pain medicine |
1,136 |
89 |
7.8 |
981 |
66 |
6.3 |
2.8 |
9.4 |
7.1 |
7.2 |
Hospice trained about side effects of pain medicine |
376 |
36 |
9.6 |
328 |
12 |
3.5 |
3.5 |
|
|
|
Hospice trained when to give more pain medicine |
376 |
36 |
9.6 |
329 |
11 |
3.2 |
3.2 |
|
|
|
Family member had trouble breathing |
1,136 |
0 |
0.0 |
1,089 |
47 |
4.1 |
2.8 |
5.4 |
4.5 |
4.5 |
Got help for trouble breathing |
1,136 |
481 |
42.3 |
597 |
58 |
8.9 |
6.4 |
11.8 |
8.9 |
9.2 |
Got needed info from the hospice team about trouble breathing |
1,136 |
481 |
42.3 |
596 |
59 |
9.0 |
6.8 |
11.2 |
8.9 |
9.7 |
Hospice trained about trouble breathing |
376 |
151 |
40.2 |
210 |
15 |
6.7 |
6.7 |
|
|
|
Family member had trouble with constipation |
1,136 |
0 |
0.0 |
1,026 |
110 |
9.7 |
4.3 |
12.0 |
12.5 |
13.1 |
Got needed help for constipation |
1,136 |
644 |
56.7 |
374 |
118 |
24.0 |
8.6 |
33.6 |
35.5 |
39.7 |
Family member was sad |
1,136 |
0 |
0.0 |
1,059 |
77 |
6.8 |
4.3 |
6.9 |
9.1 |
8.9 |
Family member needed help with sadness |
1,136 |
0 |
0.0 |
1,052 |
84 |
7.4 |
4.6 |
8.8 |
11.4 |
8.3 |
Got needed help for sadness |
1,136 |
613 |
54.0 |
432 |
91 |
17.4 |
10.2 |
18.1 |
24.3 |
26.3 |
Family member was restless or agitated |
376 |
0 |
0.0 |
369 |
7 |
1.9 |
1.9 |
|
|
|
Hospice trained about what to do if restless or agitated |
376 |
132 |
35.1 |
232 |
12 |
4.9 |
4.9 |
|
|
|
Hospice trained how to move |
376 |
82 |
21.8 |
283 |
11 |
3.7 |
3.7 |
|
|
|
Hospice discussed your family member’s religious beliefs |
1,136 |
0 |
0.0 |
1,067 |
69 |
6.1 |
4.3 |
7.3 |
10.2 |
5.9 |
Treated your family member’s religious beliefs with respect |
1,136 |
284 |
25.0 |
770 |
82 |
9.6 |
7.0 |
11.0 |
16.9 |
9.6 |
Information about expectations while your family member was dying |
1,136 |
0 |
0.0 |
1,092 |
44 |
3.9 |
2.8 |
4.7 |
4.5 |
4.2 |
Information provided in a way that was easy to understand |
1,136 |
74 |
6.5 |
1,011 |
51 |
4.8 |
4.0 |
5.9 |
4.8 |
4.7 |
Hospice with you as soon as you needed after death |
376 |
24 |
6.4 |
346 |
6 |
1.7 |
1.7 |
|
|
|
Hospice got in way while he or she was dying |
402 |
0 |
0.0 |
385 |
17 |
4.2 |
|
|
6.6 |
3.7 |
The hospice environment |
|
|
|
|
|
|
|
|
|
|
Room and bathroom kept clean |
402 |
0 |
0.0 |
385 |
17 |
4.2 |
|
|
9.2 |
3.1 |
Room comfortable |
402 |
0 |
0.0 |
387 |
15 |
3.7 |
|
|
7.9 |
2.8 |
Room calm |
402 |
0 |
0.0 |
387 |
15 |
3.7 |
|
|
6.6 |
3.1 |
Special medical equipment |
|
|
|
|
|
|
|
|
|
|
Needed special medical equipment |
376 |
0 |
0.0 |
369 |
7 |
1.9 |
1.9 |
|
|
|
Got the equipment as soon as needed |
376 |
24 |
6.4 |
346 |
6 |
1.7 |
1.7 |
|
|
|
Equipment picked up in a timely manner |
376 |
24 |
6.4 |
339 |
13 |
3.7 |
3.7 |
|
|
|
Your own experience with hospice |
|
|
|
|
|
|
|
|
|
|
Hospice listened carefully to you |
1,136 |
0 |
0.0 |
1,098 |
38 |
3.3 |
1.3 |
3.8 |
9.1 |
3.9 |
Hospice spent enough time with you |
1,136 |
0 |
0.0 |
1,088 |
48 |
4.2 |
2.0 |
5.4 |
8.0 |
4.7 |
Hospice discussed your religious or spiritual beliefs |
1,136 |
0 |
0.0 |
1,067 |
69 |
6.1 |
3.6 |
7.6 |
17.0 |
4.7 |
Hospice treated your religious beliefs with respect |
1,136 |
515 |
45.3 |
549 |
72 |
11.6 |
6.9 |
14.6 |
32.1 |
8.9 |
Support for your religious beliefs from hospice |
1,136 |
515 |
45.3 |
547 |
74 |
11.9 |
6.9 |
15.3 |
30.2 |
10.1 |
Emotional support from hospice for caretaker before death |
1,136 |
0 |
0.0 |
1,088 |
48 |
4.2 |
2.0 |
6.0 |
9.1 |
3.9 |
Emotional support from hospice for caretaker after death |
1,136 |
0 |
0.0 |
1,063 |
73 |
6.4 |
3.6 |
6.9 |
11.4 |
8.0 |
Overall rating of care |
|
|
|
|
|
|
|
|
|
|
Rate hospice, 0 = worst and 10 = best |
1,136 |
0 |
0.0 |
1,102 |
34 |
3.0 |
1.0 |
3.8 |
9.1 |
3.0 |
Recommend this hospice |
1,136 |
0 |
0.0 |
1,102 |
34 |
3.0 |
1.0 |
4.7 |
9.1 |
2.1 |
About your family member |
|
|
|
|
|
|
|
|
|
|
Family member’s education |
1,136 |
0 |
0.0 |
1,072 |
64 |
5.6 |
3.6 |
6.3 |
11.4 |
5.9 |
Family member Hispanic |
1,136 |
0 |
0.0 |
1,054 |
82 |
7.2 |
6.6 |
7.9 |
10.2 |
6.5 |
Family member’s Hispanic group |
1,136 |
1008 |
88.7 |
46 |
82 |
64.1 |
51.9 |
82.8 |
69.2 |
64.7 |
Family member’s race |
1,136 |
0 |
0.0 |
1,067 |
69 |
6.1 |
6.1 |
4.7 |
9.1 |
6.5 |
About you |
|
|
|
|
|
|
|
|
|
|
Caregiver’s age |
1,136 |
0 |
0.0 |
1,067 |
69 |
6.1 |
6.1 |
5.4 |
9.1 |
5.9 |
Caregiver’s gender |
1,136 |
0 |
0.0 |
1,068 |
68 |
6.0 |
6.3 |
5.0 |
9.1 |
5.6 |
Caregiver’s education |
1,136 |
0 |
0.0 |
1,060 |
76 |
6.7 |
7.1 |
5.4 |
9.1 |
6.8 |
Caregiver Hispanic |
1,136 |
0 |
0.0 |
1,036 |
100 |
8.8 |
7.9 |
9.1 |
12.5 |
8.6 |
Caregiver’s Hispanic group |
1,136 |
983 |
86.5 |
54 |
99 |
64.7 |
56.4 |
85.3 |
62.5 |
60.4 |
Caregiver’s race |
1,136 |
0 |
0.0 |
1,059 |
77 |
6.8 |
5.6 |
5.7 |
12.5 |
7.7 |
Caregiver’s home language |
1,136 |
0 |
0.0 |
1,069 |
67 |
5.9 |
5.3 |
5.4 |
9.1 |
6.2 |
Appendix F: Summary of Changes to Field Test Survey
Table F.1. Summary of Changes to Field Test Survey
HECS Field Test Survey Item |
Home |
Inpatient |
Nursing Home |
Keep/Drop in Final Survey? |
Notes |
The hospice patient |
|
|
|
|
|
How are you related to the person listed on the survey cover letter? |
X |
X |
X |
Keep |
|
Did your family member receive care from the hospice listed on the survey cover letter? |
X |
X |
X |
Drop |
In keeping with other CMS efforts, survey responses would have been kept regardless of whether the respondent answered yes or no to this item. |
What was the last location in which your family member received care from this hospice? |
X |
X |
X |
Keep |
|
Your role |
|
|
|
|
|
While your family member was in hospice care, how often did you take part in or oversee care for him or her? |
X |
X |
X |
Keep |
Needed to identify knowledgeable respondent; on field test, those responding "never" were instructed to stop survey. For national implementation, these respondents will complete demographic questions only. |
Was your family member’s hospice care your first experience with hospice services for a close friend or family member? |
X |
X |
X |
Drop |
Included in field test survey for construct validity only; not evaluative |
Starting hospice care |
|
|
|
|
|
Did the hospice team members explain the kinds of care and services they could give you and your family member? |
X |
X |
X |
Drop |
Little variation or ceiling effect |
Did your family member begin getting hospice care too early, at the right time, or too late? |
X |
X |
X |
Drop |
Included in field test survey for construct validity only; not evaluative |
Your family member’s hospice care |
|
|
|
|
|
While your family member was in hospice care, did you need to contact the hospice team during evenings, weekends, or holidays for questions or help with your family member’s care? |
X |
X |
X |
Keep |
Gatekeeper to next question |
How often did you get the help you needed from the hospice team during evenings, weekends, or holidays? |
X |
X |
X |
Keep |
Although this item has a ceiling effect, responsiveness on evenings and weekends has been previously shown to help identify low-performing hospices. |
While your family member was in hospice care, how often did the hospice team members keep you informed about when they would arrive to care for your family member? |
X |
|
|
Keep |
Home-only item; will be tested in cognitive interviews to determine whether tailored inapplicable response is needed |
While your family member was in hospice care, how often did the nursing home staff and hospice team work well together to care for your family member? |
|
|
X |
Keep |
Nursing home only; will be tested in cognitive interviews to determine best tailored inapplicable response or skip pattern |
Personal care needs include bathing, dressing, eating meals and changing bedding. While your family member was in hospice care, how often did your family member get as much help with personal care as he or she needed? |
|
X |
X |
Supplemental set |
Hospices may not feel they are reasonably accountable for personal care. |
While your family member was in hospice care, were your family member’s personal care needs ever not taken care of because the nursing home staff expected the hospice team to take care of those needs? |
|
|
X |
Drop |
Confusing question; may be difficult for respondents to accurately attribute failed care to nursing home versus hospice staff |
While your family member was in hospice care, when you or your family member asked for help from the hospice team, how often did you get help as soon as you needed it? |
X |
X |
X |
Keep |
|
While your family member was in hospice care, did the hospice team give you and your family member enough privacy? |
X |
X |
X |
Drop |
Little variation or ceiling effect |
While your family member was in hospice care, how often did you have a hard time speaking with or understanding members of the hospice team because you spoke different languages? |
X |
X |
X |
Supplemental set |
Little variation or ceiling effect; consider for supplemental item set; could be relevant for hospices with need to assess cultural competence |
While your family member was in hospice care, did the hospice team seem informed and up to date about your family member’s condition and care? |
X |
X |
X |
Drop |
Very highly correlated with items in Hospice Team Communication composite |
While your family member was in hospice care, did you speak to a doctor as often as you needed? |
|
X |
|
Supplemental set |
Only remaining inpatient-specific item, so dropped to streamline survey |
While your family member was in hospice care, how often did the hospice team explain things in a way that was easy to understand? |
X |
X |
X |
Keep |
|
While your family member was in hospice care, how often did the hospice team keep you informed about your family member’s condition? |
X |
X |
X |
Keep |
|
While your family member was in hospice care, how often did anyone from the hospice team give you confusing or contradictory information about your family member’s condition or care? |
X |
X |
X |
Keep |
|
While your family member was in hospice care, how often was the information you were given about your family member by the nursing home staff different from the information you were given by the hospice team? |
|
|
X |
Keep |
Nursing home only; will be tested in cognitive interviews to determine best tailored inapplicable response or skip pattern |
While your family member was in hospice care, how often did the hospice team respect your needs and preferences? |
X |
X |
X |
Drop |
Very highly correlated with items in Hospice Team Communication composite |
While your family member was in hospice care, how often did the hospice team spend enough time with your family member? |
X |
X |
X |
Drop |
Very highly correlated with items in Hospice Team Communication composite |
While your family member was in hospice care, how often did the hospice team treat your family member with dignity and respect? |
X |
X |
X |
Keep |
Important construct in qualitative work |
While your family member was in hospice care, how often did you feel that the hospice team really cared about your family member? |
X |
X |
X |
Keep |
Important construct in qualitative work |
While your family member was in hospice care, did you talk with the hospice team about any problems with your family member’s hospice care? |
X |
X |
X |
Keep |
Gatekeeper to next question |
How often did the hospice team members listen carefully to you when you talked with them about problems with your family member’s hospice care? |
X |
X |
X |
Keep |
|
How often were problems with your family member’s hospice care resolved as soon as you needed? |
X |
X |
X |
Drop |
Very highly correlated with other items in Hospice Team Communication composite |
While your family member was in hospice care, did he or she have any pain? |
X |
X |
X |
Keep |
Gatekeeper to next question |
Did your family member get as much help with pain as he or she needed? |
X |
X |
X |
Keep |
|
While your family member was in hospice care, did he or she receive any pain medicine? |
X |
X |
X |
Keep |
Gatekeeper to question about side effects of pain medicine |
Did you get the information you needed from the hospice team about your family member’s pain medicine? |
X |
X |
X |
Drop |
|
Side effects of pain medicine include things like sleepiness. Did any member of the hospice team discuss side effects of pain medicine with you or your family member? |
X |
X |
X |
Keep |
One-item assessment of pain medication and shared decisionmaking |
Did the hospice team give you enough training about what side effects to watch for from pain medicine? |
X |
|
|
Keep |
Home-only item |
Did the hospice team give you enough training about whether and when to give more pain medicine to your family member? |
X |
|
|
Keep |
Home-only item |
While your family member was in hospice care, did your family member ever have trouble breathing or receive treatment for trouble breathing? |
X |
X |
X |
Keep |
Gatekeeper to next question |
How often did your family member get the help he or she needed for trouble breathing? |
X |
X |
X |
Keep |
|
How often did you get the information you needed from the hospice team about your family member’s trouble breathing? |
X |
X |
X |
Drop |
|
Did the hospice team give you enough training about how to help your family member if he or she had trouble breathing? |
X |
|
|
Keep |
Home-only item |
While your family member was in hospice care, did your family member ever have trouble with constipation? |
X |
X |
X |
Keep |
Gatekeeper to next question |
How often did your family member get the help he or she needed for trouble with constipation? |
X |
X |
X |
Keep |
|
While your family member was in hospice care, did he or she show any feelings of anxiety or sadness? |
X |
X |
X |
Drop |
Two gatekeepers for question about anxiety and sadness symptom assessment; more yes responses to need-help gatekeeper than to this one |
Did your family member need help with feelings of anxiety or sadness? |
X |
X |
X |
Keep |
Gatekeeper to next question |
How often did your family member receive the help he or she needed from the hospice team for feelings of anxiety or sadness? |
X |
X |
X |
Keep |
|
While your family member was in hospice care, did he or she ever become restless or agitated? |
X |
|
|
Keep |
|
Did the hospice team give you enough training about what to do if your family member became restless or agitated? |
X |
|
|
Keep |
Home-only item |
Moving your family member includes things like helping him or her turn over in bed or get in and out of bed or a wheelchair. Did the hospice team give you enough training about how to safely move your family member? |
X |
|
|
Keep |
|
While your family member was in hospice care, did any member of the hospice team discuss your family member’s religious or spiritual beliefs? |
X |
X |
X |
Drop |
Gatekeeper to next question |
How often did the hospice team treat your family member’s religious or spiritual beliefs with respect? |
X |
X |
X |
Drop |
Keep question about respondent’s religious and spiritual beliefs: greater face validity |
Did the hospice team give you as much information as you wanted about what to expect while your family member was dying? |
X |
X |
X |
Keep |
|
Was the information provided in a way that was easy to understand? |
X |
X |
X |
Drop |
Highly correlated with prior question |
When your family member died, was the hospice team with you or available as soon as you needed? |
X |
|
|
Drop |
Little variation or ceiling effect |
Did the hospice team get in the way of you spending time with your family member while he or she was dying? |
|
X |
|
Drop |
Little variation or ceiling effect |
The hospice environment |
|
|
|
|
|
While your family member was in hospice care, were his or her room and bathroom kept clean? |
|
X |
|
Supplemental set |
Little variation; although ICC is significant, 97.5% of field test respondents selected highest response category |
While your family member was in hospice care, was his or her room a comfortable place for you to be together? |
|
X |
|
Drop |
Little variation or ceiling effect |
While your family member was in hospice care, was your family member’s room a calm and soothing place for him or her? |
|
X |
|
Drop |
Little variation or ceiling effect |
Special medical equipment |
|
|
|
|
|
Special medical equipment includes things like hospital beds, wheelchairs, and oxygen. While your family member was in hospice care, did your family member need special medical equipment? |
X |
|
|
Supplemental set |
|
Did your family member get the equipment as soon as he or she needed it? |
X |
|
|
Supplemental set |
Little variation or ceiling effect; however, this rarely occurring problem is of great concern to families |
Was the equipment picked up in a timely manner when your family member no longer needed it? |
X |
|
|
Supplemental set |
Little variation or ceiling effect; however, this rarely occurring problem is of great concern to families |
Your own experience with hospice |
|
|
|
|
|
While your family member was in hospice care, how often did the hospice team listen carefully to you? |
X |
X |
X |
Keep |
|
While your family member was in hospice care, how often did the hospice team spend enough time with you? |
X |
X |
X |
Drop |
Very highly correlated with other items in Hospice Team Communication composite |
While your family member was in hospice care, were your religious or spiritual beliefs discussed with any member of the hospice team? |
X |
X |
X |
Drop |
Alternative religious or spiritual item preferred because requires fewer items on the survey to evaluate religious or spiritual care |
How often did the hospice team treat your religious or spiritual beliefs with respect? |
X |
X |
X |
Drop |
Alternative religious or spiritual item preferred because requires fewer items on the survey to evaluate religious or spiritual care |
Support for religious or spiritual beliefs includes talking, praying, quiet time, and other ways of meeting your religious or spiritual needs. While your family member was in hospice care, how much support for your religious and spiritual beliefs did you get from the hospice team? |
X |
X |
X |
Keep |
Although this item has limited variation, religious and spiritual support is a vital part of the hospice benefit, and assessment of it is valued by hospice staff, particularly chaplains. |
While your family member was in hospice care, how much emotional support did you get from the hospice team? |
X |
X |
X |
Keep |
Important construct in qualitative work |
In the weeks after your family member died, how much emotional support did you get from the hospice team? |
X |
X |
X |
Keep |
Important construct in qualitative work |
Overall rating of care |
|
|
|
|
|
Using any number from 0 to 10, where 0 is the worst hospice care possible and 10 is the best hospice care possible, what number would you use to rate your family member’s hospice care? |
X |
X |
X |
Keep |
|
Would you recommend this hospice to your friends and family? |
X |
X |
X |
Keep |
Parallel to other CAHPS surveys; appreciated by providers |
In thinking about your experiences with hospice, was there anything that went especially well or that you wish had gone differently for you and your family member? Please tell us about those experiences. |
X |
X |
X |
Supplemental set |
CMS will not require an open-ended item. |
About your family member |
|
|
|
|
|
What is the highest grade or level of school that your family member completed? |
X |
X |
X |
Keep |
May be needed for CMA |
Was your family member of Hispanic, Latino/a, or Spanish origin or descent? |
X |
X |
X |
Keep |
May be needed for CMA; combine with next question |
Which group best describes your family member? |
X |
X |
X |
Keep |
|
What was your family member’s race? Please mark one or more. |
X |
X |
X |
Keep |
May be needed for CMA |
About you |
|
|
|
|
|
What is your age? |
X |
X |
X |
Keep |
May be needed for CMA |
Are you male or female? |
X |
X |
X |
Keep |
May be needed for CMA |
What is the highest grade or level of school that you have completed? |
X |
X |
X |
Keep |
May be needed for CMA |
Are you of Hispanic, Latino/a, or Spanish origin or descent? |
X |
X |
X |
Drop |
Highly correlated with family-member ethnicity |
Which group best describes you? |
X |
X |
X |
Drop |
Highly correlated with family-member ethnicity |
What is your race? Please mark one or more. |
X |
X |
X |
Drop |
Highly correlated with family-member race |
What language do you mainly speak at home? |
X |
X |
X |
Keep |
May be needed for CMA |
References
Berenson,
Robert. A., Peter J. Pronovost, and Harlan. M.
Krumholz, Achieving the Potential of Health Care Performance
Measures: Timely Analysis of Immediate Health Policy Issues,
Princeton, N.J.: Urban Institute and the Robert Wood Johnson
Foundation, May 2013. As of June 14,
2014:
http://www.rwjf.org/en/research-publications/find-rwjf-research/2013/05/achieving-the-potential-of-health-care-performance-measures.html
Carlson, Melissa D. A., William T. Gallo, and Elizabeth H. Bradley, “Ownership Status and Patterns of Care in Hospice: Results from the National Home and Hospice Care Survey,” Medical Care, Vol. 42, No. 5, May 2004, pp. 432–438.
Casarett, David, Megan Johnson, Dawn Smith, and Diane Richardson, “The Optimal Delivery of Palliative Care: A National Comparison of the Outcomes of Consultation Teams Vs Inpatient Units,” Archives of Internal Medicine, Vol. 171, No. 7, April 11, 2011, pp. 649–655.
Crofton, Christine, James S. Lubalin, and Charles Darby, “Consumer Assessment of Health Plans Study (CAHPS): Foreword,” Medical Care, Vol. 37, No. 3 Suppl., March 1999, pp. MS1–MS9.
Darby, Charles, Ron D. Hays, and Phillip Kletke, “Development and Evaluation of the CAHPS Hospital Survey,” Health Services Research, Vol. 40, No. 6, Pt. 2, December 2005, pp. 1973–1976.
Elliott, Marc N., Carol Edwards, January Angeles, Katrin Hambarsoomians, and Ron D. Hays, “Patterns of Unit and Item Nonresponse in the CAHPS® Hospital Survey,” Health Services Research, Vol. 40, No. 6, Pt. 2, December 2005, pp. 2096–2119.
Elliott, Marc N., Alan M. Zaslavsky, Elizabeth Goldstein, W. Lehrman, Katrin Hambarsoomians, Megan K. Beckett, and Laura Giordano, “Effects of Survey Mode, Patient Mix, and Nonresponse on CAHPS Hospital Survey Scores,” Health Services Research, Vol. 44, No. 2, Pt. 1, April 2009, pp. 501–518.
Friedberg, Marc W., Gillian K. SteelFisher, Melinda Karp, and Eric C. Schneider, “Physician Groups’ Use of Data from Patient Experience Surveys,” Journal of General Internal Medicine, Vol. 26, No. 5, May 2011, pp. 498–504.
Goldstein, Elizabeth, Paul D. Cleary, Kathryn M. Langwell, Alan M. Zaslavsky, and Amy Heller, “Medicare Managed Care CAHPS®: A Tool for Performance Improvement,” Health Care Financing Review, Vol. 22, No. 3, Spring 2001, pp. 101–107.
Institute
of Medicine, Crossing the Quality Chasm: A New Health System for
the 21st Century, Washington, D.C.: National Academy Press, 2001.
As of June 14, 2014:
http://www.nap.edu/books/0309072808/html/
Jones, Jennifer M., Christine J. McPherson, Camilla Zimmermann, Gary Rodin, Lisa W. Le, and S. Robin Cohen, “Assessing Agreement Between Terminally Ill Cancer Patients’ Reports of Their Quality of Life and Family Caregiver and Palliative Care Physician Proxy Ratings,” Journal of Pain and Symptom Management, Vol. 42, No. 3, September 2011, pp. 354–365.
Klein, David J., Marc N. Elliott, Amelia M. Haviland, Debra Saliba, Q. Burkhart, Carol Edwards, and Alan M. Zaslavsky, “Understanding Nonresponse to the 2007 Medicare CAHPS Survey,” Gerontologist, Vol. 51, No. 6, 2011, pp. 843–855.
Kolstad, Jonathan T., and Michael E. Chernew, “Quality and Consumer Decision Making in the Market for Health Insurance and Health Care Services,” Medical Care Research and Review, Vol. 66, No. 1 Suppl., February 2009, pp. 28S–52S.
Kutner, Jean S., Lucinda L. Bryant, Brenda L. Beaty, and Diane L. Fairclough, “Symptom Distress and Quality-of-Life Assessment at the End of Life: The Role of Proxy Response,” Journal of Pain and Symptom Management, Vol. 32, No. 4, October 2006, pp. 300–310.
Lorenz, Karl A., Steven M. Asch, Kenneth E. Rosenfeld, Hui Liu, and Susan L. Ettner, “Hospice Admission Practices: Where Does Hospice Fit in the Continuum of Care?” Journal of the American Geriatrics Society, Vol. 52, No. 5, May 2004, pp. 725–730.
Lorenz, Karl A., Susan L. Ettner, Kenneth E. Rosenfeld, David M. Carlisle, B. Leake, and Steven M. Asch, “Cash and Compassion: Profit Status and the Delivery of Hospice Services,” Journal of Palliative Medicine, Vol. 5, No. 4, August 2002, pp. 507–514.
Lorenz, Karl A., Susan L. Ettner, Kenneth E. Rosenfeld, David Carlisle, Hui Liu, and Steven M. Asch, “Accommodating Ethnic Diversity: A Study of California Hospice Programs,” Medical Care, Vol. 42, No. 9, September 2004, pp. 871–874.
Miller, Susan C., Dan K. Kiely, Joan M. Teno, Stephen R. Connor, and Susan L. Mitchell, “Hospice Care for Patients with Dementia: Does Volume Make a Difference?” Journal of Pain and Symptom Management, Vol. 35, No. 3, March 2008, pp. 283–291.
Nicosia, Nancy, Elaine Reardon, Karl Lorenz, Joanne Lynn, and Melinda B. Buntin, “The Medicare Hospice Payment System: A Consideration of Potential Refinements,” Health Care Financing Review, Vol. 30, No. 4, Summer 2009, pp. 47–59.
U.S.
Department of Health and Human Services, Centers for Medicare and
Medicaid Services, “Medicare Program; Request for Information
to Aid in the Design and Development of a Survey Regarding Patient
and Family Member/Friend Experiences with Hospice Care,”
Federal Register, Vol. 78, No. 17, January 25,
2013, pp. 5458–5459. As of June 16,
2014:
http://www.gpo.gov/fdsys/pkg/FR-2013-01-25/html/2013-01299.htm
Wachterman, Melissa W., Edward R. Marcantonio, Roger B. Davis, and Ellen P. McCarthy, “Association of Hospice Agency Profit Status with Patient Diagnosis, Location of Care, and Length of Stay,” Journal of the American Medical Association, Vol. 305, No. 5, February 2, 2011, pp. 472–479.
1 This core domain was modified based on later analyses and is currently represented by a single item measuring Information Continuity.
2 A small number of comments were not coded as they did not provide any substantive detail.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Rebecca Anhang Price |
| File Modified | 0000-00-00 |
| File Created | 2021-01-27 |
© 2026 OMB.report | Privacy Policy