NAHMS 336 Blood Instructions and Data Collection Record
Equine 2015 Study
NAHMS 336 Equine Blood CER_omb
Equine 2015 Study
OMB: 0579-0269

NAHMS
Equine 2015
BLOOD
Instructions
and
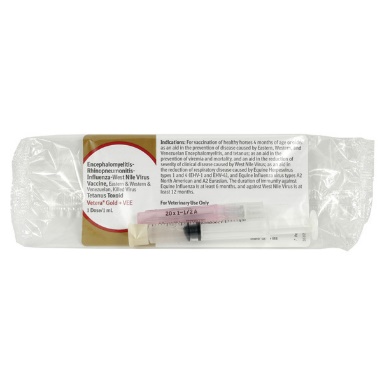
Data
Collection Record
National
Animal Health Monitoring System
2150
Centre Ave, Bldg B
Fort
Collins, CO 80526
Form
Approved
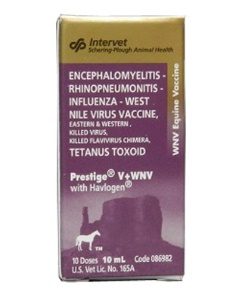
OMB
Number 0579-0269
EXP.
DATE: XX/20XX


Animal and Plant
Health Inspection
Service
Veterinary
Services
Instructions
1. Collect samples from resident equids only and based on the following criteria:
# resident equids # equids to sample
Fewer than 10 All
10–19 10
20–49 15
50 or more 20
2. Randomly select equids that represent the resident equine inventory on the premises in terms of age, sex, breed, and use. Include foals and stallions.
3. Wipe the venopuncture site with alcohol before taking the sample. Wear clean gloves for each
horse.
4. Write the farm ID, horse name/unique ID, and sample number on the label and place it lengthwise on the tube. Complete the attached data collection record.
5. Shipping samples: Keep samples cool and ship overnight on ice within 24 hours of collection using the enclosed FedEx Priority Overnight shipping label.
6. Make sure kit number on form matches kit number on tubes. Be sure to write State/operation# on all pages. Place the yellow and pink copies of the collection record on top of the styrofoam lid before closing the box. Send the white copy to your NAHMS Coordinator within 3 business days.
According
to the Paperwork Reduction Act of 1995, an agency may not conduct or
sponsor, and a person is not required to respond to, a collection of
information unless it displays a valid OMB control number. The valid
OMB control number for this information collection is 0579-0269. The
time required to complete this information collection is estimated
to average 1.5 hours per response, including the time for reviewing
instructions, searching existing data sources, gathering and
maintaining the data needed, and completing and reviewing the
collection of information.
NAHMS-336

JULY
2014
Reference codes for blood collection
G. Direct contact with other equids within last 30 days |
Direct physical contact with equids not resident to this premises. This includes the addition of new equids to the herd and through commingling with other equids on or off the premises, such as a horse show on or off the premises. |
|
H. |
1 – Colic or other digestive 2 – Respiratory problems 3 – Behavioral problems (unusual demeanor affected use, health, or safety) 4 – Neurologic problems (incoordination, spinal problem, wobbler, seizures, West Nile virus, EHM, EPM) 5 – Lameness 6 – Infectious disease unrelated to specific body condition 7 – Fever (T>101.5°F in adult, T>102.5°F in foal) 8 – Abortion or fetal reabsorption 9 – Other (specify) |
|
J & K. EHV-1 vaccination history |
Enter the dates and EHV product used for vaccination against EHV-1 (herpesvirus, also called rhino) in the previous 12 months. Enter NA if never vaccinated and DK if Don’t know. |
NAHMS ID #: ____________ Blood Kit Number: ______________ Collection date: ____________________
Collector name: _____________________________ Phone number: ____________________
Number of resident horses on the operation today: (Check one.)
_____ Fewer than 10 resident horses; collect samples from all _____ 20–49 horses; collect 15 samples Total samples submitted: _____
_____ 10–19 horses; collect 10 samples _____ 50+ horses; collect 20 samples
Sample # |
A. Animal name or unique ID |
B.
Age
|
C. Gender code 1–5 |
D. Primary use code 1–6 |
E. Equid type code 1-6 |
F. Breed code 1–15 |
G.
Direct
contact w/nonresident equids w/in last |
H.
Has
this equid had any health issues in last (List all that apply. If none, enter 0.) |
I.
#
EHV vacc in last |
J. What was the last date of EHV vacc |
K. EHV vacc product (enter code) |
|||||
1 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
2 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
3 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
4 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
5 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
6 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
7 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
8 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
9 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
10 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
Gender:
1
– Intact male 2 – Castrated male (gelding)
3
– Nonpregnant female 4 – Pregnant female 5 – Spayed female |
Primary
use of horse: 1 – Pleasure 2 – Show or competition 3 – Breeding 4 – Racing 5 – Farm/ranch work 6 – Other |
Equid type: 1 – Horse 2 – Mule 3 – Donkey 4 – Pony 5 – Miniature horse 6 – Other |
Breed:
(if
“other,” specify 1 – Appaloosa 2 – Arabian 3 – Draft breeds 4 – Miniature horse 5 – Morgan 6 – Mustang
|
7 – Paint 8 – Quarter horse 9 – Saddlebred 10 – Standardbred 11 – Tennessee Walker 12 – Thoroughbred
|
13 – Warmblood breeds 14 – Other registered breed (specify) 15 – Other nonregistered breed (specify) |
|||||||||||
NAHMS ID #: Blood Kit Number: ______________ Collection date: ____________________
Collector name: _____________________________ Phone number: ____________________
Collect 15 samples if there are 20 to 49 resident horses. Collect 20 samples if there are 50+ resident horses. |
Sample # |
A. Animal name or unique ID |
B.
Age
|
C. Gender code 1–5 |
D. Primary use code 1–6 |
E. Equid type code 1-6 |
F. Breed code 1–15 |
G.
Direct
contact w/nonresident equids w/in last |
H.
Has
this equid had any health issues in last (List all that apply. If none, enter 0.) |
I.
#
EHV vacc in last |
J. What was the last date of EHV vacc |
K. EHV vacc product (enter code) |
|||||
11 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
12 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
13 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
14 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
15 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
16 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
17 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
18 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
19 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
20 |
|
___ mo OR ___ yr |
|
|
|
|
Yes1 No3 |
|
|
___
/ ___ |
|
|||||
Gender:
1
– Intact male 2 – Castrated male (gelding)
3
– Nonpregnant female 4 – Pregnant female 5 – Spayed female |
Primary
use of horse: 1 – Pleasure 2 – Show or competition 3 – Breeding 4 – Racing 5 – Farm/ranch work 6 – Other |
Equid type: 1 – Horse 2 – Mule 3 – Donkey 4 – Pony 5 – Miniature horse 6 – Other |
Breed:
(if
“other,” specify 1 – Appaloosa 2 – Arabian 3 – Draft breeds 4 – Miniature horse 5 – Morgan 6 – Mustang
|
7 – Paint 8 – Quarter horse 9 – Saddlebred 10 – Standardbred 11 – Tennessee Walker 12 – Thoroughbred
|
13 – Warmblood breeds 14 – Other registered breed (specify) 15 – Other nonregistered breed (specify) |
|||||||||||
Code |
Est name |
True name |
Picture |
Trade name |
Route of admin |
1 |
Boehringer Ingelheim |
Equine Rhinopneumonitis-Influenza Vaccine, Killed Virus |
|
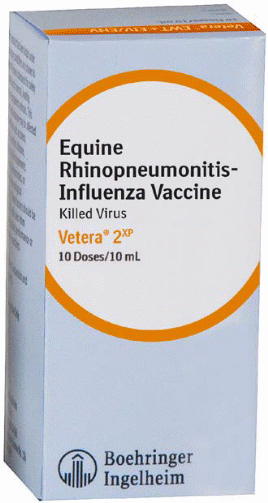
Vetera 2xp |
Intramuscular |
2 |
Boehringer Ingelheim |
Equine Rhinopneumonitis-Influenza Vaccine, Killed Virus |
|
Calvenza-03 EIV/EHV |
Intramuscular |
3 |
Boehringer Ingelheim |
Equine Rhinopneumonitis Vaccine, Modified Live Virus |
|
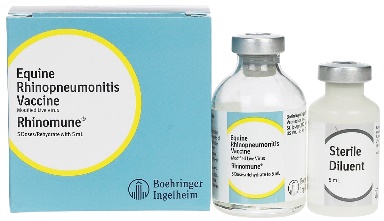
Rhinomune |
Intramuscular |
4 |
Boehringer Ingelheim |
Equine Rhinopneumonitis Vaccine, Killed Virus |
|
Calvenza EHV |
Intramuscular/Intranasal |
5 |
Boehringer Ingelheim |
Equine Rhinopneumonitis Vaccine, Killed Virus |
|
Vetera EHVxp-1, EHVxp-4 |
Intramuscular |
6 |
Boehringer Ingelheim |
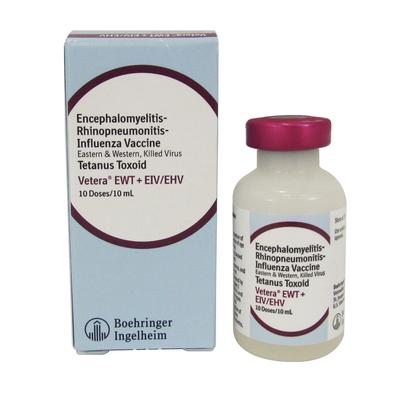
Encephalomyelitis-Rhinopneumonitis-Influenza Vaccine, Eastern & Western, Killed Virus, Tetanus Toxoid |
Vetera EWT + EIV/EHV |
Intramuscular |
Code |
Est name |
True name |
Picture |
Trade name |
Route of admin |
7 |
Boehringer Ingelheim |
Encephalomyelitis-Rhinopneumonitis-Influenza Vaccine, Eastern & Western, Killed Virus, Tetanus Toxoid |
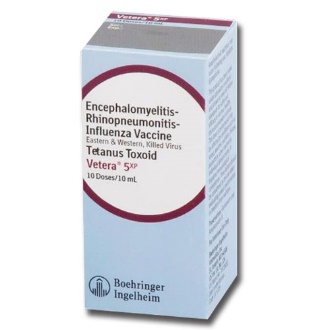
|
Vetera 5xp |
Intramuscular |
8 |
Boehringer Ingelheim |
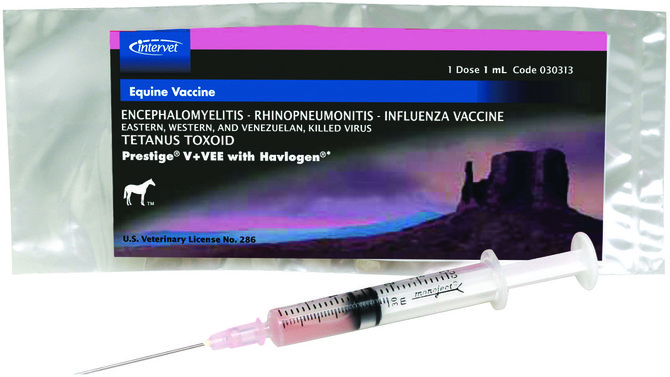
Encephalomyelitis-Rhinopneumonitis-Influenza Vaccine, Eastern & Western & Venezuelan, Killed Virus, Tetanus Toxoid |
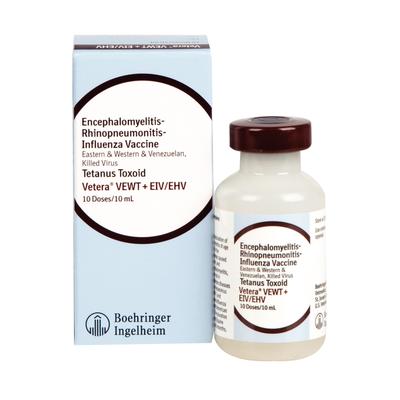
Vetera VEWT + EIV/EHV |
Intramuscular |
|
9 |
Boehringer Ingelheim |
Encephalomyelitis-Rhinopneumonitis-Influenza Vaccine, Eastern & Western & Venezuelan, Killed Virus, Tetanus Toxoid |
|
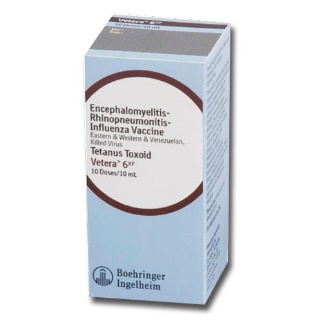
Vetera 6xp |
Intramuscular |
10 |
Boehringer Ingelheim |
Encephalomyelitis-Rhinopneumonitis-Influenza-West Nile Virus Vaccine, Eastern & Western, Killed Virus, Tetanus Toxoid |
|
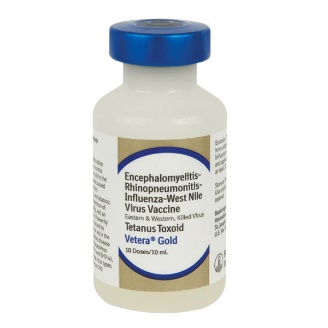
Vetera Gold |
Intramuscular |
11 |
Boehringer Ingelheim |
Encephalomyelitis-Rhinopneumonitis-Influenza-West Nile Virus Vaccine, Eastern & Western, Killed Virus, Tetanus Toxoid |
|
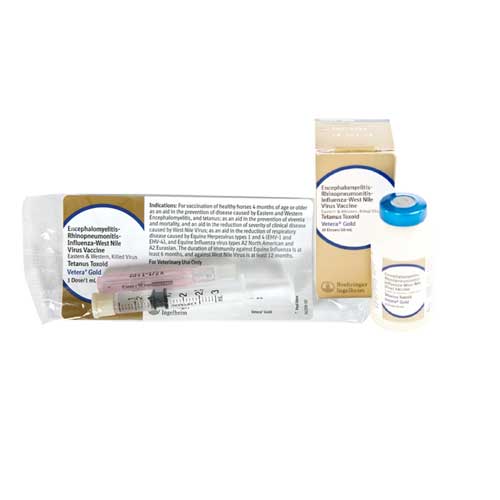
Vetera Goldxp |
Intramuscular |
Code |
Est name |
True name |
Picture |
Trade name |
Route of admin |
12 |
Boehringer Ingelheim |
Encephalomyelitis-Rhinopneumonitis-Influenza-West Nile Virus Vaccine, Eastern & Western & Venezuelan, Killed Virus, Tetanus Toxoid |
|
Vetera Gold + VEE |
Intramuscular |
13 |
Boehringer Ingelheim |
Encephalomyelitis-Rhinopneumonitis-Influenza-West Nile Virus Vaccine, Eastern & Western & Venezuelan, Killed Virus, Tetanus Toxoid |
|
Vetera Goldxp + VEE |
Intramuscular |
14 |
Merck Animal Health |
Equine Rhinopneumonitis-Influenza Vaccine, Killed Virus |
|
Prestige II |
Intramuscular |
15 |
Merck Animal Health |
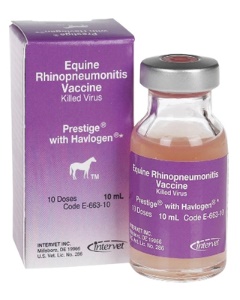
Equine Rhinopneumonitis Vaccine, Killed Virus Prevention of Abortion |
|
Prodigy with Havlogen |
Intramuscular |
16 |
Merck Animal Health |
Equine Rhinopneumonitis Vaccine, Killed Virus |
|
Prestige with Havlogen |
Intramuscular |
Code |
Est name |
True name |
Picture |
Trade name |
Route of admin |
17 |
Merck Animal Health |
Encephalomyelitis-Rhinopneumonitis Vaccine, Eastern & Western, Killed Virus, Tetanus Toxoid |
|
Prestige IV |
Intramuscular |
18 |
Merck Animal Health |
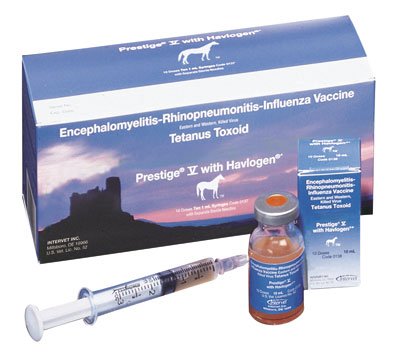
Encephalomyelitis-Rhinopneumonitis-Influenza Vaccine, Eastern & Western, Killed Virus, Tetanus Toxoid |
|
Prestige V with Havlogen |
Intramuscular |
19 |
Merck Animal Health |
Encephalomyelitis-Rhinopneumonitis-Influenza Vaccine, Eastern & Western & Venezuelan, Killed Virus, Tetanus Toxoid |
|
Prestige V + VEE |
Intramuscular |
20 |
Merck Animal Health |
Encephalomyelitis-Rhinopneumonitis-Influenza-West Nile Virus Vaccine, Eastern & Western, Killed Virus, WNV |
|
Prestige V+WNV with Havlogen |
Intramuscular |
21 |
Zoetis Inc. |
Equine Rhinopneumonitis-Influenza Vaccine, Killed Virus |
|
Fluvac Innovator EHV-4, EHV-1 |
Intramuscular |
Code |
Est name |
True name |
Picture |
Trade name |
Route of admin |
22 |
Zoetis Inc |
Equine Rhinopneumonitis Vaccine, Killed Virus Prevention of Abortion |
|
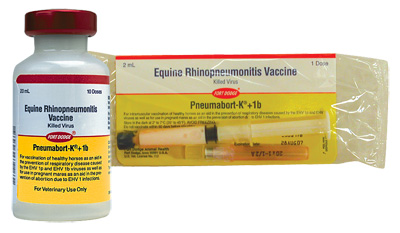
Pneumabort-K+1b |
Intramuscular |
23 |
Zoetis Inc |
Equine Rhinopneumonitis Vaccine, Killed Virus |
|
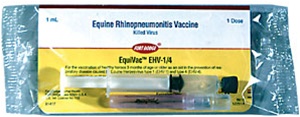
EquiVac Innovator EHV-1, EHV-4 |
Intramuscular |
24 |
Zoetis Inc |
Encephalomyelitis-Rhinopneumonitis-Influenza Vaccine, Eastern & Western, Killed Virus, Tetanus Toxoid |
|
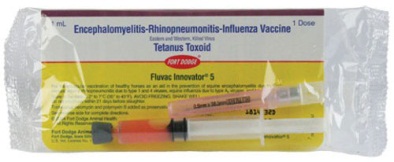
Fluvac Innovator 5 |
Intramuscular |
25 |
Zoetis Inc |
Encephalomyelitis-Rhinopneumonitis-Influenza Vaccine, Eastern & Western & Venezuelan, Killed Virus, Tetanus Toxoid |
|
Fluvac Innovator 6 |
Intramuscular |
26 |
Zoetis Inc |
Encephalomyelitis –West Nile Virus Vaccine, Eastern & Western & Venezuelan, Killed Virus, Tetanus Toxoid |
|
West Nile Innovator + VEWT |
Intramuscular |
27 |
Other |
- |
- |
- |
- |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | pasture.PDF |
| Author | Unknown |
| File Modified | 0000-00-00 |
| File Created | 2021-01-26 |
© 2026 OMB.report | Privacy Policy