NAHMS 339 Fecal Data Collection Record
Equine 2015 Study
NAHMS 339 Equine Fecal Collection CER_omb
Equine 2015 Study
OMB: 0579-0269

NAHMS
Equine 2015
Fecal
Data
Collection Record
National
Animal Health Monitoring System
2150
Centre Ave, Bldg B
Fort
Collins, CO 80526
Form
Approved
OMB
Number 0579-0269
EXP.
DATE: XX/20XX


Animal and Plant
Health Inspection
Service
Veterinary
Services
Farm ID: (6 digits) |
Kit #: |
Collector’s name and phone # |
Date: (mm/dd/yy) |
|
|
|
|
1. How many resident equids are on this premises? _____ head
2. How many samples are being submitted to the lab? _____
# resident horses # horses to sample
Fewer than 10 All
10–19 10
20–49 15
50 or more 20
3. [Interviewer’s assessment. Do not ask this question of the owner.]
What is the overall cleanliness of the equine housing/pasture area compared to other equine operations in your area?
1 Poor 2 Average 3 Excellent
4. Sample collection
Place labels on whilrpak bags and write farm ID, animal name/unique ID, and sample number on each bag
Turn a whirlpak bag inside out over your hand (wear gloves provided)
Pick up a small handfull (golf ball sized) of fecal material from the ground
Turn the bag right side out, squeeze any extra air out of the bag and close firmly
Place all the labeled sample bags inside the large ziploc bag and seal the bag
Keep samples cool (do not freeze the samples) and ship on ice within 24 hours
Paperwork and shipping
Complete the information on the data collection form for each horse sampled
Send the original white copy to your NAHMS coordinator within 3 business days
Include the yellow copy on top of the styrofoam lid and ship with samples
Ship overnight on Mon-Wed only. No shipping Thr-Sat.
The lab cannot receive samples Fri-Sun.
According
to the Paperwork Reduction Act of 1995, an agency may not conduct or
sponsor, and a person is not required to respond to, a collection of
information unless it displays a valid OMB control number. The valid
OMB control number for this information collection is 0579-0269. The
time required to complete this information collection is estimated
to average 1 hour per response, including the time for reviewing
instructions, searching existing data sources, gathering and
maintaining the data needed, and completing and reviewing the
collection of information.
NAHMS-339

July
2014
Reference codes for Fecal Collection
Gender codes |
|
1 – Intact male (stallion or colt) |
4 – Pregnant female |
2 – Castrated male (gelding) |
5 – Spayed female |
3 – Non-pregnant female (mare or filly) |
|
|
|
Primary use codes |
|
1 – Pleasure |
4 – Racing |
2 – Show or competition (not betting) |
5 – Farm or ranch work |
3 – Breeding |
6 – Other (specify in column) |
|
|
Equine type |
|
1 = horse |
4= pony |
2 = mule |
5 = miniature horse |
3 = donkey |
6 = other |
|
|
Breed codes |
|
1 – Appaloosa |
9 – Quarter horse |
2 – Arabian |
10 – Standardbred |
3 – Draft breed |
11 – Tennessee Walker |
4 – Miniature horse |
12 – Thoroughbred |
5 – Morgan |
13 – Warmblood breeds |
6 – Mustang |
14 – Other registered breed (specify on collection form) |
7 – Paint |
15 – Other nonregistered breed (specify on collection form) |
8 – Saddlebred |
|
|
|
Antibiotics (see separate sheet) |
|
|
|
Route of administration of antibiotic |
|
1 = oral |
2 = injected (into muscle or joint, or IV) |
Sample # |
Animal name or unique ID |
A. Age
(months
|
B. Gender (See code sheet.) |
C. Primary use (See code sheet.) |
D. Equine Type (See code sheet.) |
E. Breed (See code sheet.) |
F. Fecal score 1=normal
2=soft/ 3=watery 4=bloody 5-other, describe |
G. Body condition score 1=thin 2=normal 3=fat |
H.
Did this animal receive an antibiotic in the last (Yes/No)
[If
No, SKIP |
I.
Did this animal receive an antibiotic in the last (Yes/No)
[If
No, SKIP |
J.
Which antibiotic(s) were given in the last 30 days |
K.
Route of administration of antibiotic(s) if given in the last 30
days |
1 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
2 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
3 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
4 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
5 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
6 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
7 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
8 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
9 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
10 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
Sample # |
Animal name or unique ID |
A. Age
(months
|
B. Gender (See code sheet.) |
C. Primary use (See code sheet.) |
D.
Equine Type |
E. Breed (See code sheet.) |
F. Fecal score 1=normal
2=soft/ 3=watery 4=bloody 5-other, describe |
G. Body condition score 1=thin 2=normal 3=fat |
H.
Did this animal receive an antibiotic in the last (Yes/No)
[If
No, SKIP |
I.
Did this animal receive an antibiotic in the last (Yes/No)
[If
No, SKIP |
J.
Which antibiotic(s) were given in the last 30 days |
K.
Route of administration of antibiotic(s) if given in the last 30
days |
11 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
12 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
13 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
14 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
15 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
16 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
17 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
18 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
19 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
20 |
|
___ mo OR ___ yr |
|
|
|
|
|
|
|
|
|
|
Code |
Proprietary name |
Established |
Manufacturer |
Picture |
1 |
Amiglyde-V |
Amikacin |
Zoetis |
|
2 |
Amoxicillin |
Amoxicillin |
Ranbaxy |
|
3 |
Ampicillin |
Ampicillin |
Various manufacturers |
|
4 |
Zithromax |
Azithromycin |
Pfizer |
|
5 |
Mefoxin |
Cefoxitin |
Merck |
|
6 |
Ceftazidime |
Ceftazidime |
Various manufacturers |
|
7 |
Simplicef
|
Cefpodoxime
|
Zoetis |
|
8 |
Excede (Ceftiofur Crystalline Free Acid) |
Ceftiofur
|
Zoetis |
|
9 |
Naxcel (Ceftiofur Sodium) |
Ceftiofur |
Zoetis |
|
10 |
Cefazolin (Injectable and Powder)
|
Cefazolin
|
Various manufacturers |
|
11 |
Ceftriaxone
|
Ceftriaxone
|
Various manufacturers |
|
12 |
ToDay |
Cephapirin
|
Boehringer Ingelheim |
|
13 |
Chloromy-cetin (Human) |
Chloramphenicol
|
Pfizer |
|
14 |
Biaxin
|
Clarithromycin
|
Abbott Laboratories |
|
15 |
Doxycycline Hyclate (Powder, Tablet and Suspension)
Vibramycin |
Doxycycline
|
Various manufacturers |
|
16 |
Baytril 100 |
Enrofloxacin
|
Bayer |
|
17 |
Erygel |
Erythromycin
|
Merz Pharma-ceutical |
|
18 |
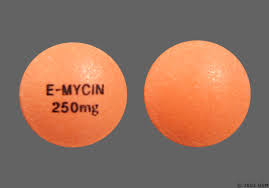
E-Mycin |
Erythromycin |
Pacific Pharma-ceutical |
|
19 |
Nuflor |
Florfenicol
|
Merck |
|
20 |
Legacy |
Gentamicin |
AgriLabs |
|
21 |
GentaMax |
Gentamicin |
Phoenix |
|
22 |
Gentamicin Sulfate |
Gentamicin |
Vet One |
|
23 |
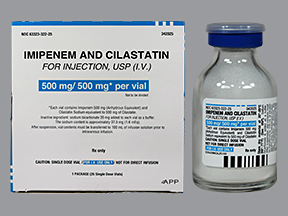
Primaxin IV |
Imipenem |
Merck |
|
24 |
Flagyl (Human form) |
Metronidazole |
Pfizer |
|
25 |
Bio-Mycin |
Oxytetracycline |
Boehringer Ingelheim |
|
26 |
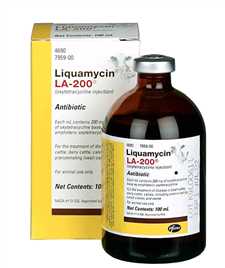
Liquamycin LA-100 Liquamycin LA-200 |
Oxytetracycline |
Zoetis |
|
27 |
Terra-Vet |
Oxytetracycline |
Aspen |
|
28 |
Penicillin G Potassium USP |
Penicillin G Potassium |
Agri Laboratories, Agripharm |
|
29 |
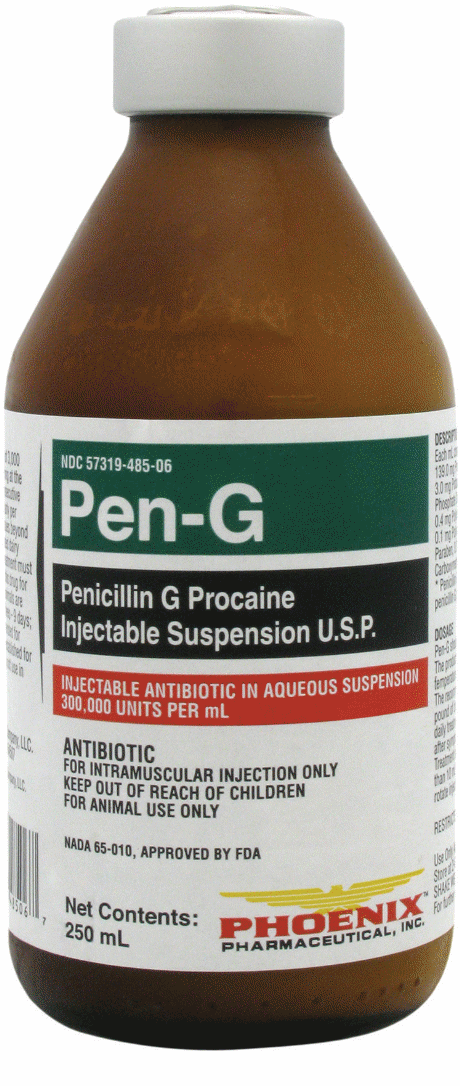
Pen-G |
Penicillin G Procaine |
Phoenix |
|
30 |
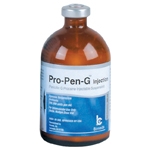
Pro-Pen-G |
Penicillin G Procaine |
Bimeda |
|
31 |
PenOne Pro |
Penicillin G Procaine |
Vet One |
|
32 |
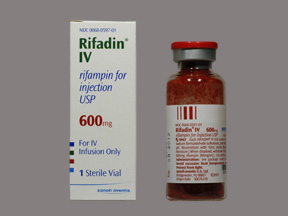
Rifadin |
Rifampin |
Aventis Pharma-ceuticals |
|
33 |
Rimactane |
Rifampin |
Novartis |
|
34 |
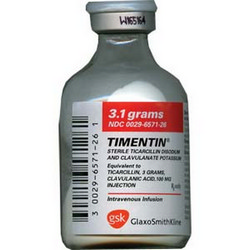
Timentin |
Ticarcillin |
GlaxoSmith-Kline |
|
35 |
Uniprim |
Trimethoprim Sulfadiazine
|
Neogen |
|
36 |
Tucoprim |
Trimethoprim Sulfadiazine
|
Zoetis |
|
37 |
SMZ Tablets |
Trimethoprim Sulfadiazine
|
Various manufacturers |
|
38 |
SMZ/TMP |
Sulfamethoxazole Trimethoprim |
Various manufacturers |
|
39
|
Vanococin |
Vancomycin
|
Baxter Healthcare, ViroPharma, Sandoz |
|
40 |
Other |
- |
- |
- |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | pasture.PDF |
| Author | Unknown |
| File Modified | 0000-00-00 |
| File Created | 2021-01-26 |
© 2026 OMB.report | Privacy Policy