Medicare Part C and Part D Data Validation (42 C.F.R. 422.516g and 423.514g) - (CMS-10305)
Medicare Part C and Part D Data Validation (42 CFR 422.516g and 423.514g) (CMS-10305)
Appendix3V5 110614.v2
Medicare Part C and Part D Data Validation (42 C.F.R. 422.516g and 423.514g) - (CMS-10305)
OMB: 0938-1115
Medicare Part C and Part D Reporting Requirements
Data Validation Procedure Manual
Appendix 3: Data Extraction and Sampling Instructions
Version 5.0
Prepared by:
Centers for Medicare & Medicaid Services
Center for Medicare
Medicare Drug Benefit and C & D Data Group
1 Overview 1
2 Conceptual Framework for Data Extraction 2
3 Data Extraction Process Detail 3
3.1 Extraction of the Census 3
3.2 Extraction of the Sample Data 5
3.3 Requirement for Extraction and Review of Source Data 8
4 Additional Guidance 10
4.1 Sampling Guidance When selecting a random sample 10
List of Exhibits
Exhibit 1 Data Type Definitions 2
Exhibit 2 Conceptual Framework for Data Extraction 3
Exhibit 3 Application of the Census Process 4
Exhibit 4 Application of Sampling Process 6
Exhibit 5 Requirement for Extraction and Review of Source Data 8
Exhibit 6 Validation Standards Applicable to Extracted Data 9
Exhibit 7 Sampling Units and Minimum Sample Size for “Final Stage List” 10
The purpose of this document is to provide guidance to reviewers regarding drawing and evaluating census and/or sample files to support validation of Part C and Part D reporting sections.
Please note that all revisions since the 2013 data validation cycle are identified by underlined and/or strikethrough text.
This document describes guidelines and methodologies for extracting sponsoring organizations’ data for data validation review. Two methods of data extraction are available to data validation contractors (reviewers). The first method is referred to as the census. For example, extracting all records used in the calculation of data elements for a specific reporting section would constitute extracting a census of data. When possible, reviewers should attempt to extract the full census. Extracting the census will enable the reviewer to determine with the greatest precision whether reporting sections were submitted accurately. The second method used for data extraction is a random sample. The random sample is a subset of the census data. If extraction of the census proves to be too burdensome due to the size or complexity of the data for a specific reporting section, a sample of records should be extracted instead.
The use of one or both of the extraction methods described above are key for reviewers as they validate the quality of the data used to calculate Part C and Part D reporting sections. Examples of characteristics evaluated using the census data include appropriate date ranges, appropriate data inclusions and exclusions, correctness of data values, and handling of missing values. When extracting a census is not practical, the use of a large enough random sample can accomplish the same goals, although the reviewer will need to rely on statistically valid estimates rather than evaluating the entire population. For both methods, reviewers must examine source data as a means of verifying that the organization’s underlying data are correct: for example, reviewing customer service call logs or member letters to verify that grievances were properly categorized as grievances and to verify the grievance categories applied were correct.
The reviewer will determine whether or not supervision is required while the sponsoring organization extracts census and/or sample files. It is also left to the reviewer’s discretion as to the feasibility of the sponsoring organization extracting census and/or sample files before, during, or after the site visit. However, it is mandatory that reviewers follow the instructions in this document. If the sponsoring organization’s staff is extracting the data, it is highly recommended that the reviewer supervise the data extraction process to ensure these instructions are followed correctly. If the reviewer is unable to supervise the data extraction process, the reviewer should obtain documentation from the sponsoring organization describing how the extraction process was performed. For example, if a random sample is extracted, the reviewer should request and validate the programming code used to extract the sample data. If a full census is extracted, the reviewer should validate that the record counts match between the census extraction and the source and final stage data files.
Throughout the document, there are several closely related terms used to describe different data types. Exhibit 1 provides data type definitions. All terms are applicable to both census and random sample methods.
Exhibit 1 Data Type Definitions
Data Type |
Definition |
Examples |
Source Data |
The initial source for all data used to create all subsequent databases that will be used to report on each reporting section |
Claims adjudication data, provider files, customer service call logs, enrollment files |
Preliminary (Census or Sample) Data |
Data stored in a data warehouse that has been cleaned and prepared for analysis |
Databases created from cleaned source data |
Interim Data |
Data sets stored in the data warehouse and joined to other data sets residing in the data warehouse |
Grievance data set joined with enrollment data for each beneficiary filing a grievance |
Final Stage Data |
The last detailed data set before calculation of counts or sums for each reporting section |
Data set containing a row for each grievance filed, with fields such as member ID, type of grievance, date filed, date resolved, etc. |
Exhibit 2 shows conceptually how sponsoring organizations create aggregated data for submission into the Health Plan Management System (HPMS) and where data extraction is incorporated into the data validation review process.1
Exhibit 2 Conceptual Framework for Data Extraction
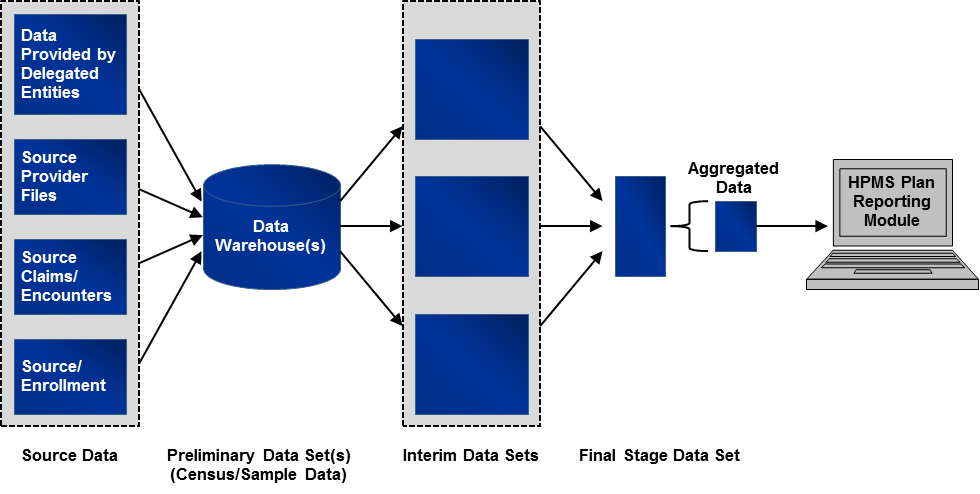
While actual reporting approaches vary significantly from organization to organization, and even between reporting sections, the general reporting approach can be described as follows:
Source data reside on operational systems, such as claims adjudication systems, provider files, enrollment files, and data systems maintained by delegated entities.
Source data are often uploaded to an analytic data warehouse where data are cleaned and put into database structures to support analysis.
The data in the warehouse are extracted to create an interim data set, which often contains manipulated and merged data.
Data from interim data sets are combined into a final stage data set.
This final stage data set is aggregated to create sums and counts, which are then entered into the HPMS Plan Reporting Module.
The data extraction process produces at least two (and for some reporting sections it could be three) sets of validation data for each reporting section. The first comes from the endpoint of the calculation, and the second is a corresponding set of extracts drawn from a data or analytic warehouse or operational system which produce underlying data. The third is a sample extract of source data (e.g., customer service call logs) that underlie the data residing in a data warehouse. If interim data sets are produced, the reviewer may consider extracting these data to ensure that data sets have been joined properly. Details on extracting and evaluating the data are outlined in the next section.
Data extraction of the full census will be conducted at the organization’s contract level, and in some instances, at the plan level. Extraction of a full census will provide the reviewer with the most precise evaluation of how accurately an organization reports its Part C or Part D data. Extracting the full census is the most straightforward of the two data extraction methods. The process illustrated in Exhibit 3 applies to all reporting sections where it is deemed practical to extract a census.
Exhibit 3 Application of the Census Process
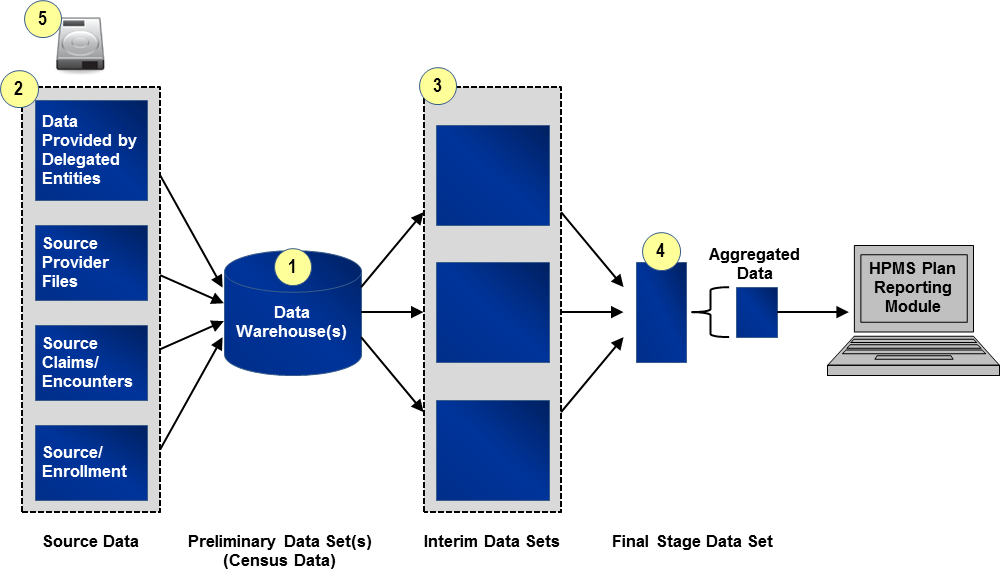
Identify and Extract “Preliminary Census Data Set(s)”: “Preliminary Census Data Set(s)” will include all files containing records extracted from one of the originating data source(s) (e.g., organization’s internal data warehouse, enrollment system). The “Preliminary Census Data Set(s)” will include all fields referenced in the programming code used to calculate the reporting section. To identify appropriate source data and fields and date ranges for the “Preliminary Census Data Set(s),” the reviewer will refer to the source/programming code, saved data queries, data dictionaries, analysis plans, etc. provided by the organization.
Identify and Extract Source Data: For some reporting sections within some organizations, the source data may already have been extracted from the source systems (e.g., enrollment system) as part of step one described above. For other reporting sections such as grievances or coverage determinations and exceptions, the reviewer will need to extract and examine data sources such as customer service call logs or pharmacy claim files. This step is important because it allows the reviewer the opportunity to validate that the underlying data are correct and were accurately uploaded or entered into the data warehouse. See Section 3.3 for instructions on example source data and sample sizes.
Identify and Extract “Interim Census Data Set(s)” (If applicable): Where applicable, the reviewer will identify “Interim Census Data Sets,” that is, data sets that have undergone a cleaning process after initial entry into a data warehouse and before being joined to create the “Final Stage Data Set(s).” All “Interim Census Data Sets” should be identified and clearly labeled so that the relationship between data extracts is identified and distinguishable.
Identify and Extract “Final Stage Data Set(s)”: The reviewer will identify the last clean and detailed (line item level) data set used prior to aggregating counts and sums for the data reporting section. This is the cleanest and last line item level file before data aggregation for entry into HPMS and is referred to as the “Final Stage Data Set.” Note that in some cases, multiple “Final Stage Data Sets” will be identified.
Write and Encrypt Data to Secure Storage Device: The organization will transfer all data files collected to a secure storage device. Organizations undergoing review should coordinate with reviewers to ensure that the organization’s security software does not interfere with data transfer. Files requested before or after the site visit can be transferred via a secure web portal or by other methods that comply with regulations governing secure storage and transfer of Personal Health Information (PHI). See Section 4.2 for instructions on the file format.
In general, sampling will be conducted at the organization’s contract level, but for some reporting sections sampling will take place at the plan benefit package (PBP) level. In cases where organizations have multiple contracts that use the same data sources and processes for each contract, only one sample is required. This one sample must be randomly drawn from pooled data from all contracts so that it is representative of the systems and processes across the contracts. For organizations with multiple contracts, where data sources and processes differ among contracts, separate samples are required for each unique contract. Based on information obtained during the review, the reviewer will determine whether the data sources are the same and processes are standardized across an organization’s multiple contracts; this will aid in determining whether one or more samples need to be drawn. It is the responsibility of the reviewer to determine the appropriate sample size for each reporting section which is why including a statistician on the reviewer team is required. For guidance on the minimum sample size, see Section 4.1. The above description for extracting a sample from a contract or several contracts applies in exactly the same way for reporting sections that are reported at the PBP level, where sampling is required for a PBP or several PBPs.
Drawing the sample data follows the same six-step process for each reporting section. Details on each step of the process are outlined and illustrated at a high-level in Exhibit 4.
Exhibit 4 Application of Sampling Process
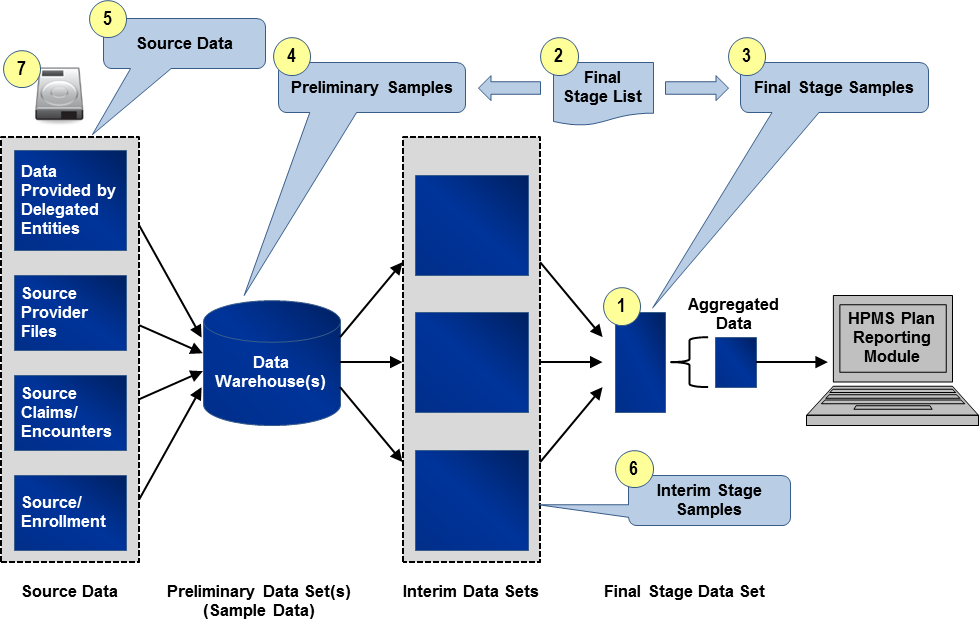
Identify “Final Stage Data Set(s)”: The reviewer will identify the last clean and detailed (line item level) data set used prior to aggregating counts and sums for the reporting section. This is the cleanest and last line item level file before data aggregation for entry into HPMS and is referred to as the “Final Stage Data Set.” As with the process of extracting the census, in some cases, multiple “Final Stage Data Sets” will be identified.
Draw Random Sample to Create “Final Stage List”: The reviewer will work with a knowledgeable organization resource to draw a random list of distinct sampling units (e.g., member IDs, Provider IDs) from the appropriate “Final Stage Data Set(s).” This list is called the “Final Stage List” and is required for extracting the source and final stage sample data. Reviewers should use standard statistical practices when determining sample sizes. Sampling units and sample size for the “Final Stage List” will vary by reporting section. In cases where there are multiple “Final Stage Data Sets,” the reviewer will ensure that the “Final Stage List” is representative of all the “Final Stage Data Sets.”
Generally the selection of the “Final Stage List” should be pulled using simple random sampling. For guidance on these methods, see Section 4.1. The reviewer may apply more complex approaches if needed (stratified samples, for example). Determination of the appropriate size and type of random sample must follow sound statistical principles and be well-documented.
Create “Final Stage Sample(s)”: Using the “Final Stage List,” the organization will provide the reviewer a “Final Stage Sample.” The “Final Stage Sample” will be extracted from the “Final Stage Data Set” and will include all records associated with the identified sampling units in the “Final Stage List.” The “Final Stage Sample” will contain all fields from the “Final Stage Data Set.” In cases where there are multiple “Final Stage Data Sets,” there will be multiple “Final Stage Samples.”
As an example, the Grievances “Final Stage Sample” will include all records and fields in the “Final Stage Data Set” associated with the distinct Case IDs identified in the Grievances “Final Stage List.”
Create “Preliminary Sample(s)”: Using the “Final Stage List,” the organization will provide for the reviewer one or more “Preliminary Samples.” Each “Preliminary Sample” will be a file containing records extracted from one of the originating data source(s) (e.g., organization’s internal data warehouse, enrollment system), and it will include all records within the reporting period(s) associated with the identified sampled units in the “Final Stage List.” The “Preliminary Sample(s)” will include all fields referenced in the programming code used to calculate the reporting section. To identify appropriate originating data sources and fields for the “Preliminary Sample(s),” the reviewer will refer to the source/programming code, saved data queries, data dictionaries, analysis plans, or other documentation provided by the organization.
As an example, the Serious Reportable Adverse Events (SRAEs) reporting section may have at least two “Preliminary Samples.” One will consist of all claims from the reporting period associated with the distinct Member IDs identified in the SRAEs “Final Stage List.” The second will consist of all enrollment records in the reporting period associated with these Member IDs.
Note:
The actual number of records in the “Final Stage Sample(s)”
and “Preliminary Sample(s)” will vary, and in many cases,
it will be substantially larger than the “Final Stage List”
sample size. For example, the “Final Stage List” of
Member IDs for the SRAEs reporting
section will likely result in “Preliminary Samples” of
more than the total number of Member IDs because of multiple claims
and enrollment records for each member.
Note: If the data are the same as the “Final Stage Data Set,” the “Final Stage Sample” will be sufficient.
Extract Source Data: Similar to the census process, for certain reporting sections and within some organizations, the source data may have already been extracted as part of step four described above. If not, sample source data will need to be extracted in order to validate that the underlying data are correct and were accurately uploaded or entered into the data warehouse. See Section 3.3 for instructions on example source data and sample sizes.
Create “Interim Stage Sample(s)”: Where applicable, the reviewer will identify “Interim Stage Data Sets”, that is, data sets that have undergone a cleaning process after initial entry into a source system and before being joined to create the “Final Stage Data Set(s).” The reviewer will apply the same methodology for extraction of the “Preliminary Sample” as described in Step 4. All “Interim Stage Samples” should be identified and clearly labeled so that the relationship between data extracts is identified and distinguishable.
Write and Encrypt Data to Secure Storage Device: The organization will transfer all data files to a secure storage device. Organizations undergoing review should coordinate with reviewers to ensure that the organization’s security software does not interfere with data transfer. Files requested before or after the site visit can be transferred via a secure web portal or by other methods that comply with regulations governing secure storage and transfer of Personal Health Information (PHI). See Section 4.2 for instructions on the file format.
Review and validation of source data ensure that organizations are accurate in the numbers being reported by ensuring that CMS regulations and guidance are being followed and that the underlying data are correct and have been uploaded correctly. Reviewers must review this data and sponsoring organizations and delegated entities must make available necessary source data files and documents. Exhibit 5 provides guidance on examples of source data needed for each reporting section. A sample size of 30 is required unless that sample size is not available. There may be other source data as this list is not all inclusive. It is expected that the reviewer will pull the sample from across the different data sources versus pulling all source data from one location. The source data should represent a random sub-sample of the data underlying the census/sample records pulled from the data warehouse. While the sample size may not allow for the greatest precision in detecting an error, it will serve as an additional verification step. For some of these reporting sections, the source data are the same data that are included in the data warehouse and therefore this new requirement should not impact those reporting sections and the reviewer should continue using the census or sample sizes previously recommended.
Exhibit 5 Requirement for Extraction and Review of Source Data
Reporting Section |
Example Source Data |
Part C Reporting Sections |
|
Grievances |
Customer Service Call Logs Member Letters Case Notes |
Organization Determinations / Reconsiderations |
Adjudicated Claims Customer Service Call Logs Member/Provider/Authorized Representative Requests |
Plan Oversight of Agents |
Marketing Personnel Files Compliance Records (e.g., Investigation and/or Disciplinary Action Logs) Enrollment Files Complaint data Agent/broker testing and training data |
Special Needs Plans (SNPs) Care Management |
Enrollment Files (Data Elements 13.1 and 13.2) Electronic or paper copies of the completed health risk assessment tool, evidence of communication (facsimile, e-mail, letter, etc.) with providers for verification of care (reports from specialists, copies of medical records, copies of medical histories, etc.), the OASIS assessment tool for beneficiaries receiving home care, or the MDS assessment tool for beneficiaries in long-term care facilities (Data Elements 13.3-13.4) |
Part D Reporting Sections |
|
Medication Therapy Management (MTM) Programs |
Claims Files (to confirm changes to drug therapy) Evidence of communication (e.g., prescriber letters to confirm medication reviews, prescriber interventions, and changes to drug therapy) Member Files (to confirm LTC residency) MTM Program files (if separate from Member Files) (to confirm targeting criteria, enrollment, opt out dates, reasons) |
Grievances |
Customer Service Call Logs Member Letters Case Notes |
Coverage Determinations/Exceptions |
Adjudicated Claims Customer Service Call Logs Member/Provider/Authorized Representative Requests Case Notes |
Redeterminations |
Customer Service Call Logs Member/Provider/Authorized Representative Requests |
Long-Term Care (LTC) Utilization |
LTC and Retail Pharmacy Contracts (Data Elements A-B, D (a-d)) Pharmacy Claim Files (Data Elements C-E) |
Plan Oversight of Agents |
Marketing Personnel Files Compliance Records (e.g., Investigation and/or Disciplinary Action Logs) Enrollment Files Complaint data Agent/broker testing and training data |
The reviewer will use each reporting section’s full census or samples from source, interim, and final stage data sets to validate against the applicable Part C and/or Part D reporting requirements. Specific validation checks requiring census or sample data are included in Validation Standard 2 in the “Data Validation Standards” and the “Findings Data Collection Form (FDCF).” Validation Standard 2 is reproduced below in Exhibit 6. The validation of all criteria except for meeting deadlines will be conducted using the extracted data.
Exhibit 6 Validation Standards Applicable to Extracted Data
VALIDATION STANDARDS |
|
2 |
A review of source documents (e.g., programming code, spreadsheet formulas, analysis plans, saved data queries, file layouts, process flows) and census or sample data, whichever is applicable, indicates that data elements for each reporting section are accurately identified, processed, and calculated.
Criteria for Validating Reporting Section Criteria (Refer to reporting section criteria section below):
|
As specified in the “Data Validation Standards” and “FDCF”, reviewers should evaluate the data in conjunction with the programming code, spreadsheet formulas, analysis plans, saved data queries, file layouts, process flows, provided by the organization. The reviewer should evaluate the data submissions for overall data accuracy for missing information, invalid fields, implausible fields (range checks), demographic errors, or other errors causing linkage or data aggregation failures. All results of the data validation findings should be recorded in the HPMS Plan Reporting Data Validation Module (PRDVM) (and “FDCF”, if used), including the number and percentage of errors or variance from HPMS-filed data found when examining the source data. For purposes of recording results in the PRDVM and FDCF (if used), an error is any discrepancy that either impacted the number of events reported or has the potential to impact the number of events reported in future reporting periods. These errors must be reported in the “Review Results” area of the PRDVM and FDCF and include the sample size selected for the source data.
Important Note: Sample data can be used to validate individual records (e.g., to validate the values of specific data elements), however, the total counts or sums for data elements cannot be determined without using the census data. The following are examples of how sample data can be used to validate specific data elements:
To verify that calculations are performed correctly
To ensure date ranges are correct
To evaluate whether specific records have been filtered or categorized properly
To verify that any manual manipulation of the source and final stage data are accurate
To evaluate missing data and the impact on the calculation of derived data fields
To verify that the organization is properly defining terms per CMS regulations, guidance, and Reporting Requirements Technical Specifications
The calculation of each data element requires the organization to pull data from key data sources. The validation samples will reflect the same process, but will be limited to relatively small samples of data.
Conceptually, selecting a simple random sample follows this process:
Use a pseudo-random number generator (e.g., SAS ranuni function or MS Excel’s
Random Number Generator in the Data Analysis dialog box) to assign a uniform random number to each record in the key data source.2
Sort the records by the new random number, from lowest value to highest value.
After identifying sample size (n), write the key fields from the first n records of the sorted key data source to a new file.
Alternate Approach: Organizations using SAS for standard calculation
may opt to use Proc SURVEYSELECT.

In cases where reviewers need to extract a random sample, Exhibit 7 provides guidance on the proper sample units and the minimum sample sizes for each reporting section. As mentioned above, reviewers should use sound statistical principles when determining the appropriate sample size.
Exhibit 7 Sampling Units and Minimum Sample Size for “Final Stage List”
Reporting Section |
Sampling Unit |
Sample Size1F3 |
Part C |
||
Grievances |
Case ID |
150 |
Organization Determinations/Reconsiderations |
Case ID |
150 |
Plan Oversight of Agents
|
Plan Assigned Agent/Broker Identification Number (Under “Agent/Broker” Data Elements A-R) |
150
|
Beneficiary HICN or RRB Number (Under “New Enrollments” Data Elements A-P) |
150 |
|
Special Needs Plans (SNPs) Care Management |
Member ID |
205 |
Part D |
||
Grievances |
Case ID |
150 |
Coverage Determinations and Redeterminations |
Case ID |
150 |
Long-Term Care (LTC) Utilization |
Claim ID |
150 |
Plan Oversight of Agents |
Plan Assigned Agent/Broker Identification Number |
150 |
Beneficiary HICN or RRB Number (Under “New Enrollments” Data Elements A-P) |
||
Note: The Medication Therapy Management Program reporting section is removed from this table because there should be no requirement to sample the data in lieu of a census file since the beneficiary upload file can serve as the census file. Sampling may not be necessary for other reporting sections also such as Plan Oversight of Agents if census files are available.
The organization must write all data files to tab-delimited or comma-delimited text files with variable names in the first row, and transfer these files to the reviewer’s secure storage device. The organization must also provide the reviewer a file layout or data dictionary for the data files in either Word documents or Excel spreadsheets on the same secure storage device. Naming conventions should be consistent between files and their corresponding layout (e.g., if a sample for Part C Grievances is extracted and labeled “PartCGrievanceSample.txt”, the corresponding layout should be named PartCGrievanceLayout.doc). An example file layout is illustrated in Exhibit 8.
Name |
Description |
Data Type/Length |
Data Values |
Calculation |
M_ID |
Member ID |
Character (16) |
|
Unique counts |
DOR |
Grievance Date of Receipt |
DateMMDDYYYY |
|
Date |
M_Status |
Member Status |
Numeric (2) |
1=Enrolled; 2=Disenrolled |
|
The organization is responsible for ensuring that it has established mutually agreeable methods for sharing proprietary and/or secure (PHI/PII) information with the reviewer and that the reviewer complies with all HIPAA privacy and security requirements.
1 Please note that the Medication Therapy Management Programs reporting section is no longer submitted through the HPMS Plan Reporting Module. The beneficiary file is uploaded into Gentran. This change does not impact the processes described below.
2 Note: Random number generators require seed numbers as input, but often have options to use the system clock as a seed. It is recommended that the organization key in a literal number as a seed, to assure the sample can be replicated if necessary; these seeds could change from year to year, but should be documented.
3 Depending on the size of the organization, some reporting sections will have populations that are smaller than the recommended sample size. In these cases, the entire population will be used for selecting the “Final Stage List.”
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | AppHDataExtractSamplInstV4 |
| Subject | Data Validation Procedure Manual |
| Author | Centers for Medicare & Medicaid Services |
| File Modified | 0000-00-00 |
| File Created | 2021-01-26 |
© 2026 OMB.report | Privacy Policy