Outpatient Dialysis Practices Survey
Emergency Epidemic Investigation Data Collections - Expedited Reviews
Outpatient Dialysis Center Practices Survey
BSI_CA
OMB: 0920-1011
Outpatient Dialysis Center Practices Survey
Form Approved
OMB No. 0920-1011
Exp. Date: 3-31-2017
Facility ID#: _ |
*Survey Year: |
A. Dialysis Center Information |
|
|
|
A.1. General |
|
*1. Ownership of your dialysis center (choose one):
*2. Location/hospital affiliation of your dialysis center (choose one):
*3. Types of dialysis services offered (select all that apply):
*4. Number of in-center hemodialysis stations: *5. Is your center part of a group or chain of dialysis centers? Yes No
*6. Do you (the person primarily responsible for collecting data for this survey) perform Yes No patient care in the dialysis center? *7. Is there someone at your dialysis center in charge of infection control? Yes No
*8. Is there a dedicated vascular access nurse/coordinator (either full or part-time) at your Yes No center? |
|
A.2. Isolation and Screening |
|
*9. Does your center have capacity to isolate patients with hepatitis B?
|
|
Public reporting burden of this collection of information is estimated to average 1.75 hours per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information including suggestions for reducing this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road NE, MS D-74 Atlanta, Georgia 30333; ATTN: PRA (0920-1011) |
|
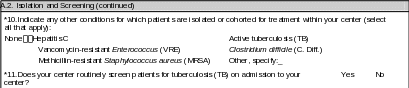
A.3. Patient Records |
*12. Does your center routinely maintain records of patients’ hemodialysis station Yes No assignment? *13. Does your center routinely maintain records of patients’ hemodialysis machine Yes No assignment? *14. If a patient from your center was hospitalized, how often is your center able to determine if a bloodstream infection contributed to their hospital admission?
*15. How often is your center able to obtain a patient’s microbiology lab records from a hospitalization?
|
Please respond to the following questions based on information from your center for the first week of February (applies to current or most recent February relative to current date). |
B. Patient and staff census |
*16. Was your center operational during the first week of February? Yes No *17. How many MAINTENANCE, NON-TRANSIENT dialysis PATIENTS were assigned to your center during the first week of February? _ Of these, indicate the number who received:
*18. How many PATIENT CARE staff (full time, part time, or affiliated with) worked in your center during the first week of February? Include only staff who had direct contact with dialysis patients or equipment: Specify the number of persons by category:
|
C. Vaccines |
*19. Of the patients counted in question 17, how many received:
*20. Of your MAINTENANCE, NON-TRANSIENT hemodialysis patients from question 17 (17a + 17b), how many received at least 3 doses of hepatitis B vaccine (ever)? |
Page 3 of 7 |
|
*21. |
Of the patient care staff members counted in question 18, how many received: |
a. At least 3 doses of hepatitis B vaccine (ever)? |
|
b. The influenza (flu) vaccine for the current/most recent flu season? _ |
|
*22. |
Does your center use standing orders to allow nurses to administer some or all vaccines to patients without a specific physician order? |
|
|
|
|
*23. |
Which type of pneumococcal vaccine does your center offer to patients? (choose one) |
|
|
|
|
|
|
|
|
|
|
D. Hepatitis B and C |
|
|
|
D.1. Hepatitis B |
|
*24. Of the MAINTENANCE, NON-TRANSIENT in-center hemodialysis PATIENTS from question 17a: a. How many were hepatitis B surface ANTIGEN (HBsAg) positive in the first week of February? |
|
i. Of these patients who were hepatitis B surface ANTIGEN (HBsAg) positive in the first week of February, how many were positive when first admitted to your center? _ b. How many patients converted from hepatitis B surface ANTIGEN (HBsAg) negative to positive during the prior 12 months (i.e., in the past year, how many patients had newly acquired hepatitis B virus infection, not as a result of vaccination)? Do not include patients who were antigen positive before they were first dialyzed in your center: _ |
|
D.2. Hepatitis C |
|
*25. Does your center routinely screen hemodialysis patients for hepatitis C antibody Yes No (anti-HCV) on admission to your center? (Note: This is NOT hepatitis B core antibody) *26. Does your center routinely screen hemodialysis patients for hepatitis C antibody (anti- Yes No HCV) at any other time?
*27. Of the MAINTENANCE, NON-TRANSIENT in-center hemodialysis patients counted in question 17a,
|
|
E. Dialysis Policies and Practices |
|
|
|
E.1. Dialyzer Reuse |
|
*28. Does your center reuse dialyzers for some or all patients? Yes No If yes,
|
|
|
|
E.1. Dialyzer Reuse (continued) |
|
E.2. Dialysate |
*29. What type of dialysate is used for in-center hemodialysis patients at your center? (choose one)
*30. Does your center routinely test dialysate from the patient’s machine for culture and Yes No endotoxin whenever a patient has a pyrogenic reaction? |
E.3. Priming Practices |
*31. Does your center use hemodialysis machine Waste Handling Option (WHO) ports? Yes No *32. Are any patients in your center “bled onto the machine” (i.e., where blood is allowed to Yes No reach or almost reach the prime waste receptacle or WHO port)? |
E.4. Injection Practices |
*33. What form of erythropoiesis stimulating agent (ESA) is most often used in your center?
*34. Where are medications most commonly drawn into syringes to prepare for patient administration? (choose one)
*35. Do technicians administer any IV medications or infusates (e.g., heparin, saline) in your Yes No center? |
E.5. Antibiotic Use |
*36. Indicate whether your center uses any of the following means to restrict or ensure appropriate antibiotic use: Yes No
|
E.6. Prevention Activities |
*37. Has your center participated in any national or regional infection prevention-related Yes No initiatives?
*38. Does your center follow CDC-recommended Core Interventions to prevent bloodstream infections in hemodialysis patients?
*39. Does your center perform hand hygiene audits of staff monthly (or more frequently)? Yes No *40. Does your center perform observations of staff vascular access care and catheter Yes No accessing practices quarterly (or more frequently)? *41. Does your center perform staff competency assessments for vascular access care and Yes No catheter accessing annually (or more frequently)? |
E.7. Peritoneal Dialysis |
*42. For peritoneal dialysis catheters, is antimicrobial ointment routinely applied to the exit site during dressing change?
|
F. Vascular Access |
|
F.1. General Vascular Access Information |
*43. Of your MAINTENANCE, NON-TRANSIENT hemodialysis patients from question 17 (17a + 17b), how many received hemodialysis through each of the following access types during the first week of February?
|
F.2. Arteriovenous (AV) Fistulas or Grafts |
*44. Before prepping the fistula or graft site for cannulation, the site is most often cleansed with:
_ |
F.2. Arteriovenous (AV) Fistulas or Grafts (continued) |
*45. Before cannulation of a fistula or graft, the site is most often prepped with (select the one most commonly used):
*46. How many of your fistula patients undergo buttonhole cannulation?
If any,
|
F.3. Hemodialysis Catheters |
*47. Before accessing the hemodialysis catheter, the catheter hubs are most commonly prepped with (select the one most commonly used):
*48. Are catheter hubs routinely scrubbed after the cap is removed and before accessing Yes No the catheter (or before accessing the catheter via a needleless connector device, if one is used)? |
F.3. Hemodialysis Catheters (continued) |
*49. When the catheter dressing is changed, the exit site (i.e., place where the catheter enters the skin) is most commonly prepped with (select the one most commonly used):
*50. For hemodialysis catheters, is antimicrobial ointment routinely applied to the Yes No exit site during dressing change?
*51. Job classification of staff members who most often perform hemodialysis catheter care (i.e., access catheters or perform exit site care) in your center (choose one):
*52. Are antimicrobial lock solutions routinely used to prevent hemodialysis catheter infections in your center?
If yes,
*53. Are needleless closed connector devices used on hemodialysis catheters in your Yes No center? If yes,
patients: patients only patients only Both *54. Are any of the following used for hemodialysis catheters in your center? (select all that apply)
|
Comments: |
Disclaimer: Use of trade names and commercial sources is for identification only and does not imply endorsement. |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | CDC |
| File Modified | 0000-00-00 |
| File Created | 2021-01-26 |
© 2026 OMB.report | Privacy Policy