• Attachment A - Informed Consent - Leaders Training Module
Attachment A - Leader module 10-6-14 .docx
Improving Hospital Informed Consent with Training on Effective Tools and Strategies
• Attachment A - Informed Consent - Leaders Training Module
OMB: 0935-0228
I mproving
Hospital Informed Consent with an Informed Consent Toolkit
mproving
Hospital Informed Consent with an Informed Consent Toolkit
Contract # HHSA290201000031I / TO 3
Deliverable 3.1.2
FINAL
Informed Consent Toolkit
Leaders Storyboard
For Pilot Test
October 6, 2014
Prepared for:
Cindy Brach, MPP
Agency for Healthcare Research and Quality
Submitted by:
Abt Associates Inc.
55 Wheeler
Street
Cambridge, MA 02138
Introduction
In medical care, informed consent is a process of communication between clinician and patient that results in the patient's authorization or agreement to undergo a specific medical intervention. All too frequently, however, patients do not understand the benefits, harms, and risks, alternatives of their treatments even after signing a consent form.
In response to this challenge, Abt Associates, the Joint Commission, the Fox Chase Cancer Center and Temple University have been contracted by AHRQ to develop, test and make available to hospitals and the medical community an improved Toolkit on informed consent to medical treatment. This toolkit will draw from several sources, including:
A Practical Guide for Informed Consent, developed by Suzanne Miller and Linda Fleisher (Temple University) with funding by the Robert Wood Johnson Foundation.
Rozovsky F. Consent to Treatment: A Practical Guide, 4th Ed.(2013). Aspen Publishers.
Preliminary research including an updated environmental scan of the peer-reviewed and grey literature on informed consent
Input from an expert and stakeholder panel
Published sources will be referenced in a “resources” section of the toolkit.
The toolkit will be delivered in the form of two training modules, each providing approximately 1 hour of continuing medical education, to be pilot-tested through the Joint Commission’s Learning Management System. One training module will be designed for health care professionals, the other for hospital leaders.
The present document is the draft toolkit for hospital leaders in quality, safety, risk management, medicine, nursing, interpreter services, and other areas. It is presented as a storyboard. Once the storyboard is finalized, it will go into production and, upon satisfactory completion of the production process, it will be uploaded into the learning management system for pilot-testing.
Project name |
AHRQ Informed Consent Toolkit |
Course Title |
Making Informed Consent an Informed Choice: Training for Health Care Leaders |
Slide 1: Welcome and Overview |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
Present the education accreditation notes in a smaller font or on a scrolling screen to limit learner interference |
Making Informed Consent an Informed Choice: Training for Health Care Leaders
Informed Consent requires clear communication about choices. It is not a signature on a form.
Goal
Informed Consent Informed Choice
Overview
This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of The Joint Commission/Joint Commission Resources and the Centers for Disease Control and Prevention (CDC). Joint Commission Resources is accredited by the ACCME to provide continuing medical education for physicians”. – ACCME Accreditation Statement Policy |
Making Informed Consent an Informed Choice: Training for Health Care Leaders.
Welcome and THANK YOU for your interest in improving the informed consent process for your patients. Informed consent for medical treatment requires clear communication about choices. It’s not a signature on a form. It’s a communication process in which a patient is given information about his or her options for medical treatments or procedures, and then selects the option that is the best fit for his or her goals and values.
The goal of this training is to help you make informed consent an informed choice in your hospital. In this training, you will find:
This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of The Joint Commission/Joint Commission Resources and the Centers for Disease Control and Prevention (CDC). Joint Commission Resources is accredited by the ACCME to provide continuing medical education for physicians”. – ACCME Accreditation Statement Policy |
Slide 2: Course Scope |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
In the resources section: please link to these resources on informed consent to research: http://www.hhs.gov/ohrp/education/index.html http://www.ahrq.gov/funding/policies/informedconsent/index.html
Please also include this legal reference on end-of-life care: Rozovsky, FA (2013). Refusing treatment, dying and death, and the elderly. Section 10.6, pp.10-23 –10-30. In: Consent to Treatment: A Practical Guide. 4th ed. New York, NY: Aspen Publishers: Wolters-Kluwer Law & Business. |
Course Scope
This course focuses on informed consent for medical treatment It does not focus on:
See “Resources” for references on these topics.
|
Course Scope
Please note that this course focuses on informed consent to medical treatment.
It does not focus on blanket consent forms that patients sign upon admission to a hospital, since such forms provide very little information to patients.
It also does not focus on informed consent for research, nor on advance directives for end-of-life care.
If you wish to learn more about informed consent for research or advance directives, please see the “resources” section of this course.
|
Slide 3: Learning Objectives |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
|
Learning Objectives:
|
At the end of this course, you will be able to:
|
Slide 4: Contents of CE activity |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
|
Course Contents
Section 1: Principles of Informed Consent Purpose: Examine existing problems with the process of informed consent for health care, principles of informed consent and implications for a good informed consent process
Section 2: Crafting and disseminating your informed consent policy Purpose: Assess current policies, develop and disseminate improved informed consent policies
Section 3: Building systems to improve the informed consent process Purpose: Describe systems and resources that need to be put in place to support the effort to improve the informed consent process
Section 4: Championing change - Developing and implementing an action plan Purpose: Learn how to generate the organizational will and momentum to improve the informed consent process in your hospital
All sections of this activity are required for continuing education credit.
|
Course Contents The information in this course is organized into the following sections: Section 1: Principles of Informed Consent The purpose of Section 1 is to examine existing problems with the process of informed consent for health care, principles of informed consent and implications for a good informed consent process
Section 2: Crafting and disseminating your informed consent policy Section 2’s purpose is to assess current policies, develop and disseminate improved informed consent policies
Section 3: Building systems to improve the informed consent process The third section’s purpose is to describe systems and resources that need to be put in place to support the effort to improve the informed consent process
Section 4: Championing change - Developing and implementing an action plan The purpose of Section 4 is to learn how to generate the organizational will and momentum to improve the informed consent process in your hospital All sections of this activity are required for continuing education credit.
|
Slide 5: Course Navigation |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
Will need to update navigation instructions as necessary.
Please provide an option for closed captioning so that the module will be 508 compliant
Note to programmers – use BACK not PREV for the button name. |
Course Navigation
|
Before you get started, take a moment to learn how to navigate in this course:
If you exit the module before it is over, you’ll be asked if you want to resume (where you left off) the next time you watch the module.
|
Slide 6: Authors and Disclosures |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
Scroll to view authors/planners |
Authors and Disclosures As an organization accredited by the ACCME and the ANCC, Joint Commission Resources requires everyone who is a planner or faculty/presenter/author to disclose all relevant conflicts of interest with any commercial interest. Nurse Planners Name Jill Chmielewski, RN, BSN, MJ Title: Associate Project Director, The Joint Commission Disclosure: Jill Chmielewski has no conflict of interest to disclose.
Physician Planner Name: Daniel Castillo, MD Title: Medical Director, Division of Healthcare Quality Evaluation, The Joint Commission. Disclosure: Dr. Castillo has no conflict of interest to disclose.
Planning Committee Members Name: Cindy Brach, MPP Title: Senior Health Policy Researcher, Agency for Healthcare Research and Quality Disclosure: Ms. Brach has no conflict of interest to disclose.
Name: Melanie Wasserman, PhD, MPA Title: Senior Associate, Abt Associates Disclosure: Dr. Wasserman has no conflict of interest to disclose.
Name: Salome, Chitavi, PhD Title: Project Director, The Joint Commission Disclosure: Dr. Chitavi has no conflict of interest to disclose.
Name: Linda Fleisher, PhD, MPH Title: Senior Scientist, The Children’s Hospital of Philadelphia Disclosure: Dr. Fleisher has no conflict of interest to disclose.
Name: Suzanne Miller, PhD Title: Professor, Fox Chase Cancer Center Disclosure: Dr. Miller has no conflict of interest to disclose.
Name: Sarah Shoemaker PhD, PharmD Title: Senior Associate, Abt Associates Disclosure: Dr. Shoemaker has no conflict of interest to disclose.
Name: Dina Moss, MPP Title: Senior Science Analyst, Agency for Healthcare Research and Quality Disclosure: Ms. Moss has no conflict of interest to disclose.
Technical Advisory Panel Name: Mary Ann Abrams, MD, MPH Title: Clinical Assistant Professor, Department of Pediatrics, Ohio State University College of Medicine and Nationwide Children’s Hospital Disclosure: Dr. Abrams has no conflict of interest to disclose.
Name: David Andrews Title: Patient Advisor, Georgia Regents Medical Center Disclosure: Mr. Andrews has no conflict of interest to disclose.
Name: Ellen Fox, MD Title: Executive Director, National Center for Ethics in Health Care, U.S. Department of Veterans Affairs Disclosure: Dr. Fox has no conflict of interest to disclose.
Name: Barbara Giardino, RN, BSN, MJ, CPHRM, CPPS Title: Risk Manager, Rockford Health System, Illinois Disclosure: Ms. Giardino has no conflict of interest to disclose.
Name: Jamie Oberman, MD Title: Navy Medical Corps Career Planner, Office of the Medical Corps Chief, Bureau of Medicine and Surgery Disclosure: Dr. Oberman has no conflict of interest to disclose.
Name: Yael Schenker, MD, MAS Title: Assistant Professor of Medicine, Division of General Internal Medicine, Section of Palliative Care and Medical Ethics, University of Pittsburgh Disclosure: Dr. Schenker has no conflict of interest to disclose.
Name: Faye Sheppard, RN, MSN, JD, CPHRM, CPPS, FASHRM Title: Principal, Patient Safety Resources, Inc. Disclosure: Ms. Sheppard, Esq. has no conflict of interest to disclose.
Name: Jana Towne, BSN, MHCA Title: Nurse Executive, Whiteriver Indian Hospital Disclosure: Ms. Towne has no conflict of interest to disclose.
Name: Dale Collins Vidal, MD, MS Title: Professor of Surgery, Giesel School of Medicine at Dartmouth and Chief of Plastic Surgery, Dartmouth Hitchcock Medical Center Disclosure: Dr. Collins Vidal has no conflict of interest to disclose.
Name: Matthew Wynia, MD, MPH, FACP Title: Director, Institute for Ethics & Center for Patient Safety, American Medical Association Disclosure: Dr. Wynia has no conflict of interest to disclose.
Credits Available ACCME ANCC ACHE IACET
There are no fees for participating in or receiving credit for this online educational course.
A certificate of CE/CME is available for print at the end of each module.
Original release date: xx-xx-xxxx Last reviewed: xx-xx-xxxx Termination date: xx-xx-xxxx Joint Commission Resources is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. Joint Commission Resources takes responsibility for the content, quality, and scientific integrity of the CME activity. Joint Commission Resources designates this educational activity for the listed contact hours of AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity. Joint Commission Resources is also accredited as a provider of continuing nursing education by the American Nurses Credentialing Center’s Commission on Accreditation. Joint Commission Resources designates this continuing nursing education activity for the above listed contact hours. Joint Commission Resources is provider approved by the California Board of Registered Nursing, provider number CEP 6381, for the listed contact hours. Joint Commission Resources is authorized to award the listed hours of pre-approved ACHE Qualified Education credit for this program toward advancement or recertification in the American College of Healthcare Executives. Participants in this program wishing to have the continuing education hours applied toward ACHE Qualified Education credit should indicate their attendance when submitting application to the American College of Healthcare Executives for advancement or recertification. The Joint Commission Enterprise has been accredited as an Authorized Provider by the International Association for Continuing Education and Training (IACET). |
Authors and Disclosures As an organization accredited by the ACCME and the ANCC, Joint Commission Resources requires everyone who is a planner or faculty/presenter/author to disclose all relevant conflicts of interest with any commercial interest. Nurse Planners Name Jill Chmielewski, RN, BSN, MJ Title: Associate Project Director, The Joint Commission Disclosure: Jill Chmielewski has no conflict of interest to disclose.
Physician Planner Name: Daniel Castillo, MD Title: Medical Director, Division of Healthcare Quality Evaluation, The Joint Commission. Disclosure: Dr. Castillo has no conflict of interest to disclose.
Planning Committee Members Name: Cindy Brach, MPP Title: Senior Health Policy Researcher, Agency for Healthcare Research and Quality Disclosure: Ms. Brach has no conflict of interest to disclose.
Name: Melanie Wasserman, PhD, MPA Title: Senior Associate, Abt Associates Disclosure: Dr. Wasserman has no conflict of interest to disclose.
Name: Salome, Chitavi, PhD Title: Project Director, The Joint Commission Disclosure: Dr. Chitavi has no conflict of interest to disclose.
Name: Linda Fleisher, PhD, MPH Title: Senior Scientist, The Children’s Hospital of Philadelphia Disclosure: Dr. Fleisher has no conflict of interest to disclose.
Name: Suzanne Miller, PhD Title: Professor, Fox Chase Cancer Center Disclosure: Dr. Miller has no conflict of interest to disclose.
Name: Sarah Shoemaker PhD, PharmD Title: Senior Associate, Abt Associates Disclosure: Dr. Shoemaker has no conflict of interest to disclose.
Name: Dina Moss, MPP Title: Senior Science Analyst, Agency for Healthcare Research and Quality Disclosure: Ms. Moss has no conflict of interest to disclose.
Technical Advisory Panel Name: Mary Ann Abrams, MD, MPH Title: Clinical Assistant Professor, Department of Pediatrics, Ohio State University College of Medicine and Nationwide Children’s Hospital Disclosure: Dr. Abrams has no conflict of interest to disclose.
Name: David Andrews Title: Patient Advisor, Georgia Regents Medical Center Disclosure: Mr. Andrews has no conflict of interest to disclose.
Name: Ellen Fox, MD Title: Executive Director, National Center for Ethics in Health Care, U.S. Department of Veterans Affairs Disclosure: Dr. Fox has no conflict of interest to disclose.
Name: Barbara Giardino, RN, BSN, MJ, CPHRM, CPPS Title: Risk Manager, Rockford Health System, Illinois Disclosure: Ms. Giardino has no conflict of interest to disclose.
Name: Jamie Oberman, MD Title: Navy Medical Corps Career Planner, Office of the Medical Corps Chief, Bureau of Medicine and Surgery Disclosure: Dr. Oberman has no conflict of interest to disclose.
Name: Yael Schenker, MD, MAS Title: Assistant Professor of Medicine, Division of General Internal Medicine, Section of Palliative Care and Medical Ethics, University of Pittsburgh Disclosure: Dr. Schenker has no conflict of interest to disclose.
Name: Faye Sheppard, RN, MSN, JD, CPHRM, CPPS, FASHRM Title: Principal, Patient Safety Resources, Inc. Disclosure: Ms. Sheppard, Esq. has no conflict of interest to disclose.
Name: Jana Towne, BSN, MHCA Title: Nurse Executive, Whiteriver Indian Hospital Disclosure: Ms. Towne has no conflict of interest to disclose.
Name: Dale Collins Vidal, MD, MS Title: Professor of Surgery, Giesel School of Medicine at Dartmouth and Chief of Plastic Surgery, Dartmouth Hitchcock Medical Center Disclosure: Dr. Collins Vidal has no conflict of interest to disclose.
Name: Matthew Wynia, MD, MPH, FACP Title: Director, Institute for Ethics & Center for Patient Safety, American Medical Association Disclosure: Dr. Wynia has no conflict of interest to disclose.
Credits Available ACCME ANCC ACHE IACET
There are no fees for participating in or receiving credit for this online educational course.
A certificate of CE/CME is available for print at the end of each module.
Original release date: xx-xx-xxxx Last reviewed: xx-xx-xxxx Termination date: xx-xx-xxxx Joint Commission Resources is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. Joint Commission Resources takes responsibility for the content, quality, and scientific integrity of the CME activity. Joint Commission Resources designates this educational activity for the listed contact hours of AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity. Joint Commission Resources is also accredited as a provider of continuing nursing education by the American Nurses Credentialing Center’s Commission on Accreditation. Joint Commission Resources designates this continuing nursing education activity for the above listed contact hours. Joint Commission Resources is provider approved by the California Board of Registered Nursing, provider number CEP 6381, for the listed contact hours. Joint Commission Resources is authorized to award the listed hours of pre-approved ACHE Qualified Education credit for this program toward advancement or recertification in the American College of Healthcare Executives. Participants in this program wishing to have the continuing education hours applied toward ACHE Qualified Education credit should indicate their attendance when submitting application to the American College of Healthcare Executives for advancement or recertification. The Joint Commission Enterprise has been accredited as an Authorized Provider by the International Association for Continuing Education and Training (IACET). |
Slide 7: Principles of Informed Consent |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
|
Section 1: Principles of Informed Consent
A good informed consent process can:
Problems with informed consent:
|
Section 1: Principles of Informed Consent
Patients and health care teams alike benefit when a patient’s consent to treatment is fully informed as the result of a clear, comprehensive and engaging communication process.
A good informed consent process has many benefits. It helps patients to make informed decisions, strengthens the therapeutic relationship, and can improve follow-up and after-care. When patients and their families understand the benefits, harms, and risks in advance, they can be partners in patient safety, and they can better cope with any poor outcomes that may happen as a result of treatment. This makes it less likely that the patient would sue the clinician when a poor outcome occurs.
Unfortunately, there are many problems with the informed consent process in hospitals today.
Both clinicians and patients often treat informed consent as a nuisance, a formality, and an obstacle on the way to care.
This is a problem, because even after signing a consent form, many patients don’t understand basic information about the benefits, harms, and risks of their proposed treatment, including the possibility of poor outcomes; and some patients may not understand that they can say no.
As a result, informed consent is one of the top 10 most common reasons for medical malpractice suits.
|
Slide 8: When “informed” consent isn’t informed |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
JAMIE: VISUAL
Show last 1:45 minutes of video clip of Toni talking: https://www.youtube.com/watch?v=ubPkdpGHWAQ. This is not a good quality clip, but it can be extracted from the AMA health literacy video.
When audio on Art starts, add picture of Art (use a stock photo, or we can ask the person who contributed this story, Audrey Riffenburg, if she would share a real picture). When the quote from Art begins (i.e., What the hell do you mean…), add conversation balloon.
When audio on Dai starts, add his picture (use a stock photo) and the buttons.
|
Section 1: Principles of Informed Consent
When “informed” consent isn’t informed
Picture of Toni with link to video. Caption: Toni Cordell had a hysterectomy without realizing it.
Picture of older White male in a hospital bed with doctor standing next to him. Conversation bubble, “What do you mean! I’m not going to be able to talk?”
Picture of young Vietnamese man with injured arm. Caption:
|
Examples of failures in informed consent include the story of Toni Cordell. Toni had a hysterectomy without realizing the procedure recommended to solve her “woman’s problem” was the removal of her uterus.
Click on the picture of Toni to hear her describe what happened.
While Toni’s experience was not recent, failures in informed consent happen in hospitals every day.
Take Art, for example. He agreed to have surgery to remove throat cancer after his doctor explained it using terms like “laryngectomy,” “palliative trach,” “ventilator problems,” “bronchiecstasis,” and “purulent bronchitis.” Then his adult daughter explained, “Dad, what the doctor is saying is that with the surgery, you would have your voice box taken out. You wouldn’t be able to talk anymore. You’d have a breathing hole through the front of your throat for the rest of your life. You’d have to keep the hole protected so germs couldn’t go straight into your lungs. And you’d have a tube in your breathing pipe that you’d have to take care of every day.” Art was surprised and got angry. He asked, “What the hell do you mean! I won’t be able to talk?!”
Let’s look at one more case. Dai is a young agricultural worker who speaks only Vietnamese. He arrived at the hospital with a badly injured arm. The hospital wanted to perform an invasive diagnostic test and gave Dai a poorly translated consent form to sign. Dai signed it, because he thought that if he didn’t, he wouldn’t be given pain reliever.
Since these patients weren’t truly informed, we can’t say that they gave informed consent. |
Slide 9: Ethical Principles and Legal Standards |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
|
Section 1: Principles of Informed Consent
Principle of Autonomy
P
Patients’ Rights to Informed Consent:
|
The ethical principle of autonomy gives patients the right to decide what happens to their bodies.
The legal doctrine on informed consent in health care has evolved over time and varies from state to state. But in every state, by law, patients have the right to:
|
Slide 10: Ethical Principles and Legal Standards |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
|
Section 1: Principles of Informed Consent
Legal Standard for “Adequate Disclosure”:
|
State law defines what constitutes adequate disclosure – what you are required to tell patients.
In most states, adequate disclosure is the duty of the clinician who is providing the treatment. It can’t be delegated to another person. The information to be disclosed must include:
Many states have additional requirements. |
Slide 11: It’s Not About the Form |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
Digital Ignite to explore opportunities for interactive learning for this slide |
Section 1: Principles of Informed Consent
Signed Form ≠ Informed consent
L
S Risk
Liability
[Picture of MD talking to patient with question marks over patient’s head?]
“The statute requires a physician to "explain" the treatment, alternatives, and risks to his or her patient. ‘Explain’ means ‘to make plain or understandable: clear of complexities or obscurity’…. Explanation implies more than a mere correct statement of the facts. An explanation clarifies an issue or makes it understandable to the recipient …. For example, a physician can mouth words to an infant, or to a comatose person, or to a person who does not speak his or her language, but unless and until such patients are capable of understanding the physician’s point, the physician cannot be said to have explained anything to any such person.”
Macy v. Blatchford case, Oregon Supreme Court, 2000) |
In the previous slide we described what clinicians have to tell patients as part of obtaining their consent. But telling patients isn’t enough for consent to be informed, even if patients sign the form.
The consent form exists to document that the patient has been provided information, understood the information and agreed to a particular treatment or procedure. A signed consent form actually implies that prior to the patient’s signing, a process of adequately informing the patient and ensuring understanding has taken place. Yet, many patients sign informed consent forms even when they don’t understand the procedure, its benefits, harms, risks, or alternatives to treatment.
If the patient didn’t understand the information presented, it’s a patient safety problem, and you may be sued.
For example, in the Macy versus Blatchford case the Oregon Supreme Court, discussing whether a physician failed to obtain a patient’s informed consent for surgery, made the point that informing without understanding does not constitute informed consent. The court stated, “The statute requires a physician to "explain" the treatment, alternatives, and risks to his or her patient. ‘Explain’ means ‘to make plain or understandable: clear of complexities or obscurity’…. Explanation implies more than a mere correct statement of the facts. An explanation clarifies an issue or makes it understandable to the recipient …. For example, a physician can mouth words to an infant, or to a comatose person, or to a person who does not speak his or her language, but unless and until such patients are capable of understanding the physician’s point, the physician cannot be said to have explained anything to any such person.” |
Slide 12: Recognizing patient capacity for decision-making |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
JAMIE: consider bringing in bullets 1 by 1 with audio guidance – these are key points that need to be emphasized on-screen. I’m also open to other options to achieve that goal.
JAMIE: Let us explore alternative ways to stress the key points in this slide without using bullet points (graphics, images etc).
Consider making the 3rd and fourth bullets interactive (show the story, offer “yes/no” buttons, feedback to learner whether they got it right, then show the right answer
Add to the resources section this document on minors’ right to consent: https://www.guttmacher.org/statecenter/spibs/spib_OMCL.pdf
Link to Resources: FAQs for patients that lack decision making capacity.
|
Section 1: Principles of Informed Consent
Patient capacity for decision-making
Most patients have capacity for decisions about medical treatment.
Key Criteria for patient capacity:
- Their condition - Options - Benefits, harms, and risks
Capacity can change over time and can vary depending on the decision to be made.
What’s not incapacity:
|
Patient capacity for decision-making
To uphold a patient’s right to participate in decisions about their care, it is important to recognize their capacity for decision-making.
The main thing to remember is that most patients have capacity for decision-making about their medical care and treatment.
Sometimes a patient is perceived as not having the ability to make an informed decision due to signs of intoxication, mental illness, cognitive impairment, or other factors.
In some cases, that perception is right, but in many cases, it is not.
The key criteria in assessing the patient’s capacity are the following. The patient has capacity if he or she:
Capacity is both the ability and the right to make a decision. It can change over time, and can depend on the decision to be made.
Patients don’t automatically lack capacity just because they disagree with the care team’s treatment plan. This is true even if members of the care team strongly disagree with the patient’s choice and think they know what’s best for the patient. Patients may refuse treatment even if it puts their lives in jeopardy.
Also, just because some patients can’t speak, have an intellectual or physical disability, mental illness, or cognitive impairment, or are under the influence of alcohol or pain medications, that does not automatically mean they lack capacity to make a decision. These conditions can make it harder to communicate and make decisions, though, so later in this course, we’ll share some communication strategies that can help. |
Slide 13: When to consult an authorized representative |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
JAMIE: When narrator says, “Click on the label ‘Authorized representative, show this text:
For minors: the authorized representative is a parent or legal guardian (show a picture of a mom next to a hospital bed with a young child in it with arrow pointing to mom saying “Authorized representative (Mom)” [or use a picture of a Dad next to hospital bed with young child, with the label “Authorized representative (Dad)”]
For adults: an authorized representative can either be designated by the patient (health proxy) or designated by someone other than the patient who has authority (for example the hospital policy can establish a hierarchy of authorized representatives in the absence of a proxy, typically spouse first, then adult children, then siblings, then other relatives).
The Cecile story is a true story of Cindy’s. She can record it. |
Section 1: Principles of Informed Consent
Except:
Consult an authorized representative
Picture of Cecile [Caption: Click here to hear Cecile’s real life story on informed consent in an emergency situation.]
|
The patient’s family and friends often play an important role in the decision-making process, but in most cases, the final decision rests with the patient.
There are some exceptions to this rule, namely:
In these cases, you will need to consult with someone who is legally authorized to make a decision on the patient’s behalf. Click on the label “Authorized Representative,” to learn more about who can serve as an authorized representative.
Even when you’re working with an authorized representative, sharing information with the patient can help them to feel included, respected, and more comfortable with the care they are receiving.
A last exception is a life- or health-threatening emergency leaving no time to identify or speak with an authorized representative. In that case, the clinician can make a decision in the patient’s best interests. But often there’s still time to hold a consent discussion in emergency situations. Click on Cecile to hear her story about informed consent in an emergency.
Cecile: My father was recovering from minor surgery when I noticed he was trying to say something but was having trouble coming up with the words. I called in the nurse practitioner, and he decided to call the stroke team. Well, the stroke team arrived, performed an assessment, and started to wheel my father out the door. “Where are you taking him?” I asked. “To give him medicine to break up the blood clot,” they said. I said, “But you haven’t gotten consent.” “It’s an emergency!” they called, halfway out the door. But I was my father’s health proxy and I called after them, “You can’t give him anything until I consent.” That caught them short. “You’re right,” they agreed. “Can you walk with us while we tell you about this medicine?” And I did. I understand they were in a rush – they had to give him the medicine within 3 hours of his first symptoms, but that didn’t mean they didn’t have time to get consent.
Informed consent rules vary state-by-state and hospital-by-hospital, so check your hospital policy or state laws for further guidance.
|
Slide 14: Making Informed Consent an Informed Choice |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
JAMIE: During first paragraph have the word “consent” morph into the word “choice |
Section 1: Principles of Informed Consent
Informed Consent Informed Choice
Informed choice requires:
Of course, the information must be presented in a way the patient can understand. [We will contact the author of this cartoon for permission. http://www.cagle.com/tag/informed-consent/]
|
The goal of this course is to help you mobilize resources and improve your hospital’s systems to make informed consent an informed choice for your hospital’s patients. Let’s talk about what we mean by “informed choice”.
What we often see in informed consent is that a clinician will recommend a treatment, explain the treatment, and then get the patient’s consent to deliver the treatment.
This may satisfy the minimum requirements for informed consent, but to truly make an informed choice, patients need clear, unbiased medical information they can understand about all their treatment options, including what happens if they decide to do nothing.
This is challenging, because clinicians may not always be in a position to provide information about all the options. It’s important to recognize that, and to know that patients may factor into their decision knowledge they’ve obtained through sources other than the clinician.
In addition to considering all the options, to make an informed choice, patients factor their values and preferences into the decision. Of course, in order for a patient to make an informed choice, information about the choices must be presented in a way that the patient can understand.
|
Slide 15: Section 2: Crafting and Disseminating Your Informed Consent Policy
|
||||
Content to the designer |
On-Screen Content |
Audio Guidance |
||
|
Section 2: Crafting and Disseminating Your Informed Consent Policy
Why focus on hospital informed consent policy? An analysis of The Joint Commission accreditation data:
Common problems:
Frequently asked questions:
|
Section 2: Crafting and Disseminating Your Informed Consent Policy
This section will help you to assess your hospital’s current informed consent policy, improve it if need be, and better disseminate it.
You may be asking yourself, “Why should my hospital focus on improving its informed consent policy?
A recent analysis of The Joint Commission accreditation data suggests that many hospitals could benefit from improving their informed consent policies. Some hospitals were found out of compliance with accreditation standards because they did not have a formal written informed consent policy. The most frequent area of concern was failure to obtain informed consent in accordance with the hospital’s policy and processes. The analysis also revealed that many policies were overly broad and lacked the detail necessary for clinicians to be able to implement the policy.
Judging from the questions asked of The Joint Commission, clinicians need more detailed guidance from their hospital policies on informed consent. Examples of commonly asked questions include:
|
||
Slide 16: Informed Consent Policy Worksheet |
||||
Content to the designer |
On-Screen Content |
Audio Guidance |
||
Upon completion of this track, the completed worksheet should be savable electronically/printable for reference as the learner continues to improve their informed consent policy.
In the resources section, include:
Resources on partnering with patients and families:
http://www.ipfcc.org/resources/guidance/index.html
[include the header and link. Publications are not free, so we can only make the link part of the resources, not the publications].
|
Gather your materials
[thumbnail of the informed consent policy worksheet]
Click here for Worksheet |
The next few slides will walk you through the essential elements of an informed consent policy. To get the most out of this section, please get a copy of your hospital’s informed consent policy. If you believe no policy is available, double-check that this is the case. Most accredited hospitals have a written informed consent policy. If your hospital truly does not have an informed consent policy, this section can help you to create one. In addition to obtaining your informed consent policy, please open the worksheet shown on this slide. You may print it or save it and work on it electronically. We’ll refer back to this worksheet in Section 4 of this course.
Policy examples given here are offered for illustrative purposes only, and this exercise is only a starting point. If your assessment shows any deficiencies in your policy, consider working to improve the policy with a Task Force that includes representatives of your health care facility’s legal, risk management, and medical teams, as well as patients. If you are not sure how to engage patients and families, the resources section of this module includes links to reports from the Institute of Patient- and Family-Centered Care on engaging patients and families in quality improvement. |
||
Slide 17: Statement of Purpose |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
Instruction to designer: provide link to the following resource: Guidelines from the Office of Human Subjects Protection and the Code of Federal Regulations (Title 45 CFR Part 46)
Jamie – consider highlighting the “Note” to catch the eye of the learner. |
Section 2: Crafting and Disseminating Your Informed Consent Policy
Statement of Purpose
Example Wellness Hospital Informed Consent Policy [Text box] Purpose: To ensure that every patient receiving invasive tests or procedures or other medical treatments at Wellness Hospital will be fully informed as to all benefits, harms, risks, and alternatives prior to choosing whether to consent.
Note: Policy examples given here are offered for illustrative purposes only.
Please fill out your worksheet for this slide.
|
Hospitals’ informed consent policies generally start with a statement of purpose.
Here is an example of a statement of purpose from a fictional hospital we’ll call Wellness Hospital.
Purpose: To ensure that every patient receiving invasive tests or procedures or other medical treatments at Wellness Hospital will be fully informed as to all benefits, harms, risks, and alternatives prior to choosing whether to consent.
Note that this example, and the other policy examples provided in this training, are just for illustrative purposes. Your hospital’s policy should be tailored to your hospital’s needs.
Please take a moment to fill out your worksheet for this slide, before moving on to the next slide.
|
Slide 18: General Policy |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
Instruction to designer: provide link to the following resource: Guidelines from the Office of Human Subjects Protection and the Code of Federal Regulations (Title 45 CFR Part 46) |
Section 2: Crafting and Disseminating Your Informed Consent Policy
Policy
Example – Wellness Hospital [Text box] Policy: The physician or Licensed Independent Practitioner (LIP) in charge will ask for consent from the patient or the patient’s authorized representative for all surgeries, invasive procedures or treatments involving risk, such as cardiac catherizations, lumbar punctures, biopsies, and administration of medicines.
Patients have the right to:
Note: This policy focuses on informed consent for medical procedures and treatments. Participation in research is governed by guidelines from the Office of Human Subjects Protection and the Code of Federal Regulations (Title 45 CFR Part 46).
Please fill out your worksheet for this slide.
|
In addition to the statement of purpose, a general policy may also be provided to outline the key principles of informed consent at the hospital.
Here is an example from our fictional hospital, Wellness Hospital.
The physician or Licensed Independent Practitioner in charge (also known as an LIP) will ask for consent from the patient or the patient’s authorized representative for all surgeries, invasive procedures, or treatments involving risk, such as cardiac catherizations, lumbar punctures, biopsies, and administration of medicines.
Patients have the right to:
The Wellness Hospital’s policy continues: Note: This policy focuses on informed consent for medical procedures and treatments. Participation in research is governed by guidelines from the Office of Human Subjects Protection and the Code of Federal Regulations (Title 45 CFR Part 46).
(Remember, this is just a fictional example of a policy).
Please take a moment to fill out your worksheet for this slide, before moving on to the next slide.
|
Slide 19: Who can obtain informed consent |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
|
Who can obtain informed consent
Example – Wellness Hospital [Text box] For all tests, treatments, and procedures offered at Wellness Hospital:
Please fill out your worksheet for this slide.
|
Your policy should include a section stating who is responsible for obtaining informed consent.
In many states, the physician or licensed independent practitioner who orders or orders a test, prescribes a treatment, or performs a procedure is responsible for the informed consent process. In some states, informed consent tasks can be delegated. The Joint Commission has received many questions from hospital staff regarding the appropriate roles of physicians, nurses and other staff members in the informed consent process. In particular, persons who are delegated by the physician in charge to perform informed consent tasks are unsure of whether it is appropriate for the physician to delegate these tasks, and are unclear about how to execute their role in relation to the physician responsible.
If other staff members are playing a support role in your hospital’s informed consent process, your policy can clarify who should play what support roles. For example, a nurse can conduct patient education, and support staff can verify that a signed informed consent form is on file before a procedure takes place.
Here is an example from Wellness Hospital: For all tests, treatments, and procedures offered at Wellness Hospital:
Please take a moment to fill out your worksheet for this slide, before moving on to the next slide.
|
Slide 20: Procedures that require explicit consent |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
|
Section 2: Crafting and Disseminating Your Informed Consent Policy
Procedures that require explicit consent
Example – Wellness Hospital [text box]
All surgeries, invasive procedures or treatments involving risk, such as cardiac catherizations, lumbar punctures, biopsies, and administration of medicines
Please fill out your worksheet for this slide.
|
Procedures that require explicit consent
This section of a hospital informed consent policy defines what procedures require explicit consent.
In the example of Wellness Hospital, the general policy, shown earlier, is that all surgeries, invasive procedures or treatments involving risk, such as cardiac catherizations, lumbar punctures, biopsies, and administration of medicines, require explicit consent.
Explicit consent doesn’t always a patient’s signature. For many procedures and treatments, oral consent can be sufficient. Later on, we’ll discuss hospital policies regarding how informed consent should be documented.
Please take a moment to fill out your worksheet for this slide, before moving on to the next slide.
|
Slide 21: Timing of informed consent discussion |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
|
Section 2: Crafting and Disseminating Your Informed Consent Policy
When to Hold the Informed Consent Discussion
Example – Wellness Hospital [text box, both paragraphs below]
Timing of Informed Consent Discussions
Informed consent discussions must be held before tests, treatments, and procedures are carried out. Except in emergency situations, discussions should be held well in advance to give patients an opportunity to process the information. Obtaining informed consent when the patient is not in a position to readily say “No” does not give the patient a choice.
For example, having the informed consent discussion with a colonoscopy patient after the patient has completed the colon prep is not considered adequate timing.
Please fill out your worksheet for this slide.
|
When to Hold the Informed Consent Discussion
This section of your policy defines when informed consent should be obtained. At a minimum, the policy should state that consent must be obtained before the test, treatment, or procedure is given. Sometimes it helps to state the obvious.
In addition, you may want to have a statement of principle about the importance of giving patients enough time to process the information, and to not wait until it’s too late to say no.
For example, your policy could say:
Timing of Informed Consent Discussions
Informed consent discussions must be held before tests, treatments, and procedures are carried out. Except in emergency situations, discussions should be held well in advance to give patients an opportunity to process the information. Obtaining informed consent when the patient is not in a position to readily say “No” does not give the patient a choice.
For example, having the informed consent discussion with a colonoscopy patient after the patient has completed the colon prep is not considered adequate timing.
Please take a moment to fill out your worksheet for this slide, before moving on to the next slide.
|
Slide 22: Content of an Informed Consent Discussion |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
Interactive exercise: informed consent policy worksheet, continued |
Section 2: Crafting and Disseminating Your Informed Consent Policy
Content of an Informed Consent Discussion
Varies based on state laws, and should include at least:
Please fill out your worksheet for this slide.
|
You’ll want your informed consent policy to address what information should be covered in the informed consent discussion. In many states, the content of informed consent communications is mandated by law. At a minimum, a hospital policy should require informed consent discussions to include:
Please take a moment to fill out your worksheet for this slide, before moving on to the next slide.
|
Slide 23: Documentation of consent |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
Interactive exercise: informed consent policy worksheet, continued |
Section 2: Crafting and Disseminating Your Informed Consent Policy
Documentation of consent
Example – Wellness Hospital [Text box] Patients at Wellness Hospital sign a blanket consent form for treatment prior to admission. This form documents that the patient has been admitted to the hospital of his or her own accord, and covers non-invasive, routine, minimal risk procedures such as taking the patient’s blood pressure and asking intake questions.
Oral consent is required for routine treatments and procedures with very low, but not minimal risk, such as the administration of most drugs, vaccines, blood draws and minor procedures, such as routine X-rays.
A signed written consent is required prior to all surgery, and for any treatments and procedures that involve a significant risk of harm, pain or discomfort, and/or require sedation or anesthesia. For recurring treatments such as radiation or chemotherapy, a single form can be used to cover multiple sessions.
Qualified interpreters who interpreted an informed consent discussion and/or sight translated the informed consent form must also sign the form. In the case of telephone interpreters, the clinician conducting the discussion may write the interpreters name on the form.
Both oral and written consent must be documented in the patient’s electronic health record. If the informed consent discussion took place outside Wellness Hospital, consent must be verified by the physician and documented in the patient’s Wellness Hospital Electronic Health record before treatment occurs.
Please fill out your worksheet for this slide.
|
The Joint Commission receives frequent queries about how to document informed consent, suggesting that hospital policies are often insufficiently detailed on this topic.
Your policy should specifically identify the procedures and treatments that are covered by the blanket “consent to treatment” that patients sign upon admission to the hospital, and which procedures and treatments require separate explicit consent.
It should specify which ones require only oral consent, which ones require a signed consent form, including signed by interpreters when used, and how consent should be documented.
Joint Commission standards require a signed informed consent prior to surgery, except in emergencies, such as an unconscious patient requiring life-saving surgery when surrogate decision makers (such as a family member) cannot be consulted in time.
It can also be helpful to specify that recurring treatments such as radiation or chemotherapy can be covered by a single form. Since informed consent discussions often take place before the patient gets to the hospital, you’ll want your policy to address how to document informed consent in those instances.
Here is an example of a policy on documentation of consent from the fictional Wellness Hospital:
Patients at Wellness Hospital sign a blanket consent form for treatment prior to admission. This form documents that the patient is present of his or her own accord, and covers non-invasive, routine, minimal risk procedures such as taking the patient’s blood pressure and asking intake questions.
Oral consent is required for routine treatments and procedures with very low, but not minimal risk, such as the administration of most drugs, vaccines, blood draws and minor procedures, such as routine X-rays.
A signed written consent is required prior to all surgery, and for any treatments and procedures that involve a significant risk of harm, pain or discomfort, and/or require sedation or anesthesia. For recurring treatments such as radiation or chemotherapy, a single form can be used to cover multiple sessions.
Qualified interpreters who interpreted an informed consent discussion and/or sight translated the informed consent form must also sign the form. In the case of telephone interpreters, the clinician conducting the discussion may write the interpreters name on the form.
Both oral and written consent must be documented in the patient’s electronic health record. If the informed consent discussion took place outside Wellness Hospital, consent must be verified by the physician and documented in the patient’s Wellness Hospital Electronic Health record before treatment occurs.
Please take a moment to fill out your worksheet for this slide, before moving on to the next slide.
|
Slide 24: Exceptions to informed consent |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
Interactive exercise: informed consent policy worksheet, continued
Cite this book in the resources section:
|
Section 2: Crafting and Disseminating Your Informed Consent Policy
Exceptions to informed consent
What do to in an exception
|
Once your policy has outlined the general rules regarding informed consent, it should also note the exceptions.
In brief, the exceptions include certain emergencies, cases where the patient is incapacitated, most minors, patients whose treatment is required by law or court-order, and cases where a patient asks not to be informed.
Your policy should provide more details on each of these exceptions. The resources section cites a legal reference book by Fay Rozovsky that provides extensive information on this and other informed consent topics.
Your organization’s informed consent policy might first address what constitutes an emergency, such as if irreparable harm will result if immediate action isn’t taken. It should also provide clear guidance on what to do when exceptions arise. If time allows and treatment is not mandated by law or court-order, it may be possible to identify a surrogate decision-maker. Laws vary from state to state regarding who can be the patient’s duly authorized legal representative. Priority should be given to persons named in health care proxy or power of attorney documents, and the hospital may establish a hierarchy of decision-makers in the event that a health care proxy or power of attorney is not available. For example, the spouse or same-sex partner may be the first in line, followed by adult children, then siblings, and so forth, with the medical team making decisions as a last resort. This level of detail can help to reduce conflict when the patient is unable to make or express decisions and relatives disagree on the course of treatment.
You may also want to include in your hospital policy that clinicians should communicate with patients about their treatment even if the patient can’t communicate or consent to care, unless the patient has asked not to be informed. Communicating with the patient can help to alleviate feelings of anxiety and improve cooperation with treatment. |
Slide 25: Informed consent for minors |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
Interactive exercise: informed consent policy worksheet, continued
Read the story of the teen girl in a different voice from that of the main narrator. We could ask the person who contributed the story, Jana Towne, to read and record it for us. |
Section 2: Crafting and Disseminating Your Informed Consent Policy
Informed consent for minors
General policy
[PHOTO OF TEEN GIRL IN HOSPITAL – caption: click here for a real-life story from a nurse illustrating the importance of seeking assent from minors]
|
The hospital’s policy should include a section about consent for minors. In most cases, minors can’t legally consent to treatment, and parental consent is required. Nonetheless, in addition to gaining parental consent, you may want to encourage clinical staff to engage minor patients in their care when possible by providing information about their treatment and, for older children, seeking their assent. Assent is an agreement that does not have legal power.
When seeking assent, a commonly used rule of thumb is that teenagers, typically over the age of 14, can process similar information to what is given to their parents or guardian, and younger children, typically above the age of 7, can process information about what the experience will be, how it may help, how long it will take, and whether it might involve any pain or discomfort.
Click here for a real-life story from a nurse illustrating the importance of seeking assent from minors.
“We received a 14-year-old on our inpatient unit who had a PICC line in place. When talking to her mother regarding her medicines, she learned that the line terminated in her heart and was quite distressed by this. Later that day, the RN went to administer antibiotics and discovered that the patient had pulled the PICC out on her own and hidden it in her gown. That issue might have been avoided with a consenting process that more actively involved the patient, given her age.”
|
Slide 26: Informed consent for minors contd. |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
Interactive exercise: informed consent policy worksheet, continued
|
Section 2: Crafting and Disseminating Your Informed Consent Policy
Exceptions to the rule that minors can’t consent
|
There can be exceptions to the rule that minors can’t consent, and if these apply in your hospital, they should be noted in your policy.
First, some states allow mature minors to consent to treatment without their parents’ involvement. Definitions of mature minors vary. If your state allows it (or does not forbid it), your hospital policy should spell out who can be considered a mature minor. Some state laws define mature minors or the conditions under which a minor can consent. Absent guidance from the law, your definition of a mature minor can be based on age (for example, 14+), whether the minor is married or has children, or based on the minor’s ability to make a decision based on information about possible treatments and their benefits, harms, and risks.
Second, it is generally recognized that minors who are parents have the right to consent to care on behalf of their children.
Third, some states allow minors to consent to certain services without involvement from their parents, such as reproductive health care and substance abuse treatment. |
Slide 27: Clear communication policies |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
When the learner clicks on the picture of Magda, play the story provided in the comment. |
Section 2: Crafting and Disseminating Your Informed Consent Policy
Clear communication policies
Picture of Magda with caption “Magda’s real life close call”
Example – Wellness Hospital [Text box] Wellness Hospital is committed to clear communication. To ensure that patient consent is truly informed, we strive to use plain language, clear and simple forms, and high-quality educational materials and decision aids. We also use teach-back to ensure that patients have understood the information that has been presented to them.
For patients with limited English proficiency, clinicians should conduct informed consent discussions with the assistance of a qualified interpreter. (See Wellness Hospital’s Language Access Plan for details on our interpreter services).
Clinicians should offer assistive devices, such magnifying readers and audio amplifiers, and ask patients if they would like forms read aloud to them. |
Clear communication policies
Regardless of what patients say or sign, patients haven’t consented unless they understand the information provided.
To foster a culture of clear communications with patients, consider including in your informed policy a statement to describe how clinicians can ensure that patient consent is informed. For example you can highlight the importance of plain language, clear and simple forms, the use of high-quality decision aids, graphics, and other educational materials, and teach-back. Teach-back is asking the patient to explain in their own words what they need to know or do, so clinicians can make sure they’ve explained things well.
Click on Magda to hear what a difference teach-back can make.
A clear communication policy should also address how to make reasonable accommodations to help patients participate in the informed consent process. Accommodations include providing professionally translated forms and language assistance for patients with limited English proficiency, using large print forms as well as magnifier reading glasses for patients with limited vision, and offering to read the form to all patients in case they are embarrassed to admit difficulties with reading.
Coming back to Wellness Hospital, here is an example of a clear communication policy:
Wellness Hospital is committed to clear communication. To ensure that patient consent is truly informed, we strive to use plain language, clear and simple forms, and high-quality decision aids, graphics, and other educational materials. We also use teach-back to ensure that clinicians have explained the information in a way that patients can understand.
For patients with limited English proficiency, clinicians should conduct informed consent discussions with the assistance of a qualified interpreter. (See Wellness Hospital’s Language Access Plan for details on our interpreter services.)
Clinicians should offer assistive devices, such magnifying readers and audio amplifiers, and ask patients if they would like forms read aloud to them. |
Slide 28: Compliance |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
Compliance |
Section 2: Crafting and Disseminating Your Informed Consent Policy
Compliance
Check compliance of your policy:
Offer Contact information for concerns/ complaints
Example – Wellness Hospital. [Text box] Compliance: If you have questions or concerns about this policy, or if you would like to report a violation of this policy, please call 1.800.xxx.xxxx or visit www.wellness.org/complianceline
|
Compliance
Before you share your policy, check with your legal, quality and safety teams to make sure it complies with Federal, State and local laws, regulations, such as Medicare and Medicaid rules, and accreditation standards.
A final part of your policy is offering a point of contact at the hospital to report concerns about or violations of the policy. The point of contact should have a clear process for referring complaints for quality improvement or disciplinary action, as appropriate. Here is an example: Compliance: If you have questions or concerns about this policy, or if you would like to report a violation of this policy, please call our compliance hotline at 1.800.xxx.xxxx or visit |
Slide 29: Disseminating the hospital’s policy on informed consent |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
For resources section, include examples of brochures/posters informing patients of their rights: http://www.aha.org/advocacy-issues/communicatingpts/pt-care-partnership.shtml
|
Section 2: Crafting and Disseminating Your Informed Consent Policy
Disseminating the hospital’s policy on informed consent
|
To ensure that your hospital’s policy is implemented, both patients and clinicians should be aware of patients’ rights with regard to informed consent.
Consider several modes of dissemination to inform patients and clinicians about patients’ rights. Common modes of dissemination include posting the informed consent policy on your hospital’s Web site, placing posters on walls, and training clinicians and staff both during orientation and in-service. Plain language brochures in multiple languages can be distributed to patients upon admission, and the policy can also be disseminated through any patient- and family-centered care networks or online patient social networks your hospital may have. |
Slide 30: Periodic review of informed consent policy |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
|
Section 2: Crafting and Disseminating Your Informed Consent Policy
Plan for periodic review of the hospital’s informed consent policy
|
As part of keeping your policy current, establish a time-frame for periodic review, for example, at least every two years. Conducting a periodic review should be part of one of the hospital leader’s responsibilities. Noting on the policy document the date when it was last updated can help to ensure that policies are kept current.
The policy should be evaluated in light of new legal or ethical doctrines, new evidence that changes which procedures are considered risky, and hospital experiences that suggest the policy should be clarified or changed. |
Slide 31: Section 3: Building Systems to Improve The Informed Consent Process |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
In resources section, please link to Temple Health’s “a Practical Guide for Informed Consent”: http://www.templehealth.org/ICTOOLKIT/html/ictoolkitpage1.html
|
Section 3: Building Systems to Improve the Informed Consent Process
System Supports
[thumbnail of the informed consent systems worksheet]
Click here for Worksheet
|
Clinical staff, however well intentioned, cannot improve informed consent on their own. Systems need to be put in place to support them in making informed consent an informed choice.
In this section, we describe the systems that can set the stage for an improved informed consent process. These include:
Please open the worksheet shown on this slide. You may print it or save it and work on it electronically. We’ll refer back to this worksheet in Section 4 of this course.
|
Slide 32: Compile a library of clear and simple forms |
||||
Content to the designer |
On-Screen Content |
Audio Guidance |
||
For the “before” and “After” forms, use Mary Ann Abrams’s forms from here: http://www.iom.edu/~/media/Files/Activity%20Files/PublicHealth/HealthLiteracy/2013-APR-11/Abrams.pdf
Mary Ann has given informal permission (as part of her feedback) and we will ask for formal permission.
|
Section 3: Building Systems to Improve the Informed Consent Process
System Support #1: Compile a library of clear and simple informed consent forms
Choose forms that:
Test forms
Click here to see an example of an informed consent form before and after it was converted to a reader-friendly plain language format. Thumbnails of “before” and “after” forms; full forms pop up when learners click on the thumbnails |
Let’s start by describing the resources you need.
Your hospital should have a library of clear and simple informed consent forms for all the tests, treatments, and procedures that require a signed consent form according to your hospital’s informed consent policy. A signature on a form that the patient doesn’t understand doesn’t serve its purpose, which is to document the patient’s understanding from the informed consent discussion. Nor does it protect your hospital from liability.
Choose forms that are written using health literacy principles to maximize reading ease and comprehension. This includes writing in plain language and avoiding technical terms.
Clear and simple forms sequence information logically, breaking the information into chunks with informative headings. Layout also matters – lots of white space, large easy-to-ready fonts, and short line lengths all contribute to readability.
Don’t forget to include in your library forms that have been professionally translated in languages commonly spoken by your patients.
The best way to make sure the forms meet the needs of your patient population is to test both English-language and translated forms with their intended audience. Ask for feedback from a sample of diverse groups of patients within your patient community.
Click on the thumbnails of sample forms before and after simplification.
|
||
Slide 33: Where to obtain clear and simple forms |
||||
Content to the designer |
On-Screen Content |
Audio Guidance |
||
Put in resources section:
Link to Queensland Health’s online database: http://www.health.qld.gov.au/consent/html/for_clinicians.asp
Resources on plain language: Pdf of “A Practical Guide to Informed Consent”, available here: http://www.rwjf.org/content/dam/web-assets/2009/04/a-practical-guide-to-informed-consent
Link to: www.plainlanguage.gov
Link to: Toolkit for Making Written Material Clear and Effective: http://www.cms.gov/Outreach-and-Education/Outreach/WrittenMaterialsToolkit/index.html?redirect=/WrittenMaterialsToolkit |
Section 3: Building Systems to Improve the Informed Consent Process
Support #1: Compile a library of clear and simple informed consent forms
Where to obtain informed consent forms
[Picture of Mary Ann Abrams, or picture selected by Mary Abrams to represent the Iowa Health system’s health literacy initiative to develop reader-friendly informed consent forms.] |
To build a library of informed consent forms, you can either use or customize a pre-packaged library, or develop your own consent forms.
Pre-packaged solutions include free online databases of informed consent forms, such as Queensland Health’s online database. A link to this database is provided in the resources section of this module. There are also commercial products, available for a fee, and some are designed to integrate with electronic health records. Be sure to assess pre-packaged solutions both before and after implementing them, to make sure they meet your clinicians’ and patients’ needs. You may be able to build on an existing library of forms and modify or customize it to meet your hospital’s needs.
If you are creating your own informed consent forms, you’ll want to make sure that your forms follow the health literacy principles that we just discussed. In addition to consulting plain language writing guides, try to enlist the help of health literacy experts. Be prepared to educate and collaborate with lawyers or risk managers to produce clear and simple forms that meet everyone’s needs.
In addition to serving as documentation, a clear and simple form can help clinicians structure their informed consent discussion and give them simple ways of explaining complex concepts. Involve clinicians in the development of forms so the forms match the flow of the informed consent discussion and to obtain buy-in for the new forms.
You’ll also want to provide forms in the key languages spoken by your patients. Make sure you use professional translators. Untrained translators are more likely to make mistakes, which can expose your hospital to liability.
Before you roll out your new forms to the entire hospital, pilot them with a few clinicians or in a few units. Get feedback from both clinicians and patients and revise accordingly. Finally, make sure you update your forms on a regular basis. You’ll want to modify the forms in your library as you learn of new treatment options or the expected outcomes or risks change.
Click on the picture to learn how the Iowa Health System developed reader-friendly informed consent forms.
For tips on developing clear and simple informed consent forms, see the “resources” section of this course. |
||
Slide 34: Maintain a library of high quality decision aids and patient education materials |
|
|||
Content to the designer |
On-Screen Content |
Audio Guidance |
||
For the resources section, offer this resource to learn more about the standards for high-quality decision aids: Volk RJ, Llewelyn-Thomas H, Stacey D, Elwyn G (2013). Ten years of the International Patient Decision Aid Standards Collaboration: evolution of the core dimensions for assessing the quality of patient decision aids.
http://www.biomedcentral.com/1472-6947/13/S2/S1 what constitutes a high-quality decision aid: |
Section 3: Building Systems to Improve the Informed Consent Process
System Support #2 – Maintain a library of high-quality decision aids and patient education materials
Decision aids provide unbiased information. They can be:
Decision aids provide information about:
Decision aids are NOT a substitute for the informed consent discussion.
Using decision aids:
[Reference: Kinnersley et al 2013; Legare et al. 2014.]
|
While plain language informed consent forms can help patients to understand what they are consenting to, many patients need additional materials to help them make an informed choice. It can be very helpful for your clinical staff to have your hospital maintain a library of high-quality decision aids and other educational materials for common tests, treatments, and procedures offered in your hospital.
A decision aid presents options in an unbiased way to patients so that they can make an informed choice. Decision aids can be paper-based, audio-visual, multimedia, web-based, or interactive. Some decision aids are meant for patients to use on their own, while other decision aids are to be used jointly, with the clinician helping the patient process the information and highlight important points.
Decision aids provide information about:
Decision aids are designed to be part of, rather than replace, the informed consent discussion. For example, after a patient has viewed a decision aid, the clinician can use teach-back to make sure the patient understood the information, personalize the information for that patient, encourage and answer questions, and discuss the information in the context of the patient’s goals and values.
Clinicians often find that using decision aids helps them structure conversations about choices with patients. Research suggests using decision aids improves patients’ knowledge of the options available to them. Patients who use decision aids also have more accurate expectations of possible benefits, harms, and risks of their options. Most importantly, decision aids help patients clarify what matters most to them, makes them more likely to participate in the decision-making process and communicate effectively with their providers, and makes them more likely to reach decisions consistent with their goals and values.
And finally, patients whose decisions are fully informed through the use of decision aids are better able to cope with treatment outcomes and adverse events. An added advantage is that the use of decision aids can sometimes be used as evidence that consent was informed. In fact, Washington State passed a law in 2007 that established that the use of certified patient decision aids was evidence of patients’ informed consent.
|
||
Slide 35: 7Assessing the Quality of Decision Aids |
|
|||
Content to the designer |
On-Screen Content |
Audio Guidance |
||
|
How do you know if you have a high-quality decision aid?
|
There are a lot of decision aids available. Not all of them are high quality. Here are some questions to consider when assessing the quality of decision aids:
Finally, a high quality decision aid will help patients be clear about what matters the most to them and factor those goals and values into their decision. |
||
Slide 36: Other patient education materials, finding high-quality aids, and maintaining your library |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
In resources section, please include links to:
Free databases of decision aids and other patient education materials: the Informed Medical Decisions Foundation, the Ottawa Hospital Research Institute, and the Mayo clinic.
International Patient Decision Aid Standards Collaboration: http://ipdas.ohri.ca/
this resource for assessing decision aids (under development):
and this resource for evaluating patient education materials (including decision aids): |
Section 3: Building Systems to Improve the Informed Consent Process
Support #2 – Maintain a library of high-quality decision aids and other patient education materials
Other patient education materials:
Finding high-quality decision aids and education materials
Maintaining your library
|
Other patient education materials can also be helpful for patients to understand and remember information their clinician shares about their condition and the available options for tests, treatments or procedures. Unlike decision aids, these materials do not provide comparisons of the options or facilitate decision-making. But high quality decision aids are not always available, and some patients may find single subject materials easier to absorb.
To help you assess and select decision aids, see the standards developed by the International Patient Decision Aid Standards Collaboration; a link to the standards is provided in the resources section of this module.
Once you have assembled a library of high quality decision aids, someone will need to maintain it. This includes obtaining feedback from patients and clinicians about how useful and practical they are. It also involves making sure that the decision aids reflect the most up-to-date clinical information. Decision aids that are too difficult to understand or use or that are no longer accurate should be removed from the library.
|
Slide 37: Support #3: Remove communication barriers
|
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
Add to resources: https://hclsig.thinkculturalhealth.hhs.gov/
Include this document in the resources section (evidence sheet on LEP and patient safety) |
Section 3: Building Systems to Improve the Informed Consent Process
Support #3: Remove communication barriers
[Text box?] In the Tran case, a 9-year old Vietnamese girl died from a reaction to the drug Reglan. Her parents primarily spoke Vietnamese, yet no competent interpreter was used throughout the girl’s encounters with the medical system. Instead, the 9-year-old patient and her 16-year-old brother served as interpreters.
Without an interpreter present, the physician could not inform the parents about Reglan's side effects or warnings, or that it was not approved for pediatric use. The parents also could not understand his instructions to bring their daughter back to the emergency room if side effects arose.
The family received $200,000 from the physician and hospital, and the medical malpractice insurance carrier paid legal fees of $140,000. (Quan 2010)
Reference ICON Quan K. (2010). The High Costs of Language Barriers in Medical Malpractice: University of California, Berkeley. School of Public Health. National Health Law Program. Available at: http://www.healthlaw.org/images/stories/High_Costs_of_Language_Barriers_in_Malpractice.pdf |
One of the common communication challenges patients face is language barriers. Patients with limited English proficiency are at greater risk of not understanding what is in informed consent forms that they have signed. Hospitals need to develop a language assistance plan to address the needs of patients with limited English proficiency. While developing an entire language access plan is beyond the scope of this course, we will discuss a few of the highlights. In the resource section you’ll find a Health Care Language Services Implementation Guide that can take you through all the steps for meeting the needs of your patients with limited English proficiency.
If your hospital participates in Medicare or Medicaid, you are required to take reasonable steps to ensure that you are providing equal access to patients with limited English proficiency. Failure to provide language assistance is risky for patients and can serve as the basis for lawsuits.
Take for example the Tran case. A 9-year old Vietnamese girl died from a reaction to the drug Reglan. Her parents primarily spoke Vietnamese, yet no competent interpreter was used throughout the girl’s encounters with the medical system. Instead, the 9-year-old patient and her 16-year-old brother served as interpreters. Without an interpreter present, the physician could not inform the parents about Reglan's side effects or warnings, or that it was not indicated for pediatric use. The parents also could not understand the doctor’s instructions to bring their daughter back to the emergency room if side effects arose.
The family received a $200,000 settlement from the physician and hospital, and the medical malpractice insurance carrier paid legal fees of $140,000. |
Slide 38:Systems to provide language assistance |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
In resources section, include links to:
Joint Commission Roadmap on Advancing Effective Communication, Cultural Competence, and Patient- and Family-Centered Care http://www.jointcommission.org/assets/1/6/aroadmapforhospitalsfinalversion727.pdf
Robert Wood Johnson Speaking Together program: http://www.rwjf.org/en/research-publications/find-rwjf-research/2011/04/speaking-together.html
National certification programs for medical interpreters: http://www.certifiedmedicalinterpreters.org/ http://www.cchicertification.org/
|
Section 3: Building Systems to Improve the Informed Consent Process Support #3: Remove communication barriers Systems to identify patient language
Systems to provide language assistance
|
To address your patients’ language assistance needs, you will need a system to identify the languages your patients speak. As part of this system, all patients should be informed of their right to a free interpreter, and patients should be asked in which language they would prefer to receive care. These questions can be asked during registration, when scheduling an appointment, and/or upon admission to the hospital. If the patient can’t understand the question, your hospital can use “I speak” cards or touch-screen registration menus allowing patients to point to their language. In addition, some over-the-phone interpretation services can help to identify the patients’ language. Once you have identified patient language needs, you’ll need a system to meet their needs.
|
Slide 39: Support #3: Remove communication barriers |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
Provide thumbnail to definition of a cultural broker |
Section 3: Building Systems to Improve the Informed Consent Process
Support #3: Remove communication barriers
Systems to stock assistive devices
|
Another common communication challenge is partial hearing or vision loss. For persons with these conditions, it may be helpful and cost-effective to have on hand assistive devices, including sound amplification devices and magnifying readers.
You’ll need someone to keep an inventory of the devices, make sure they are in good working order, and purchase replacements as needed.
You’ll also need places to store the devices. Keeping assistive devices in unit supply closets will ensure that they will be easy to access when needed.
Finally, you’ll need to notify clinicians about the availability of assistive devices and encourage both their use and their return to the storage area.
|
Slide 39: Improving workflows |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
Link to Module 5 of the practice facilitator’s handbook on creating workflow maps:
|
Section 3: Building Systems to Improve the Informed Consent Process
Improving Workflows
Key points:
|
The Joint Commission’s Standards Interpretation Group receives many questions each year from hospital staff expressing confusion about who should be responsible for what in the informed consent process.
A workflow map or flowchart can help to clarify and improve the informed consent process. A high-level flowchart can be developed as part of the hospital’s informed consent policy to outline the major steps in the informed consent process. Detailed flowcharts can be developed at the unit level to clarify how the process steps fit together and who is responsible for what.
Swim lane flowcharts can be particularly helpful, because they clarify not only the steps in the process, but also how every team member fits into the process. Click on the thumbnail for an example of a swim lane flowchart.
Developing a flowchart is most beneficial when you do it as a group exercise with the people who are involved in the process. As you develop your initial flowchart, map the process as it is, not as you think it should be. This will help you to see what needs to be improved. Once you have a flowchart developed, observe the real process and check that the flowchart matches what actually happens; edit the flowchart until it matches what actually happens. Once you’ve done this, you can work with your clinical team to improve the workflow. |
Slide 40: Improving workflows – swim lane (offline team exercise ) |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
In the resources section, include the document found at this site on swim lane diagrams: http://www.ahrq.gov/professionals/systems/hospital/red/swimlane.html
|
Section 3: Building Systems to Improve the Informed Consent Process
Swim lane exercise:
Boxes – for process steps.
Diamonds – for decisions.
Ovals – for start and end steps.
Arrows – To connect activities, decisions, or start and end steps. |
If you are a unit lead, consider leading your team through a swim lane process map exercise to clarify and improve your workflow for informed consent. This exercise can extend over two team meetings.
Include up to 12 people involved in the informed consent process, plus at least one person who is not familiar with the process who can ask questions to clarify the process, and a neutral facilitator who can guide participants through the exercise. Ideally, a patient advocate should be included to ensure that the process is patient-centered.
At the first team meeting, have each person map the part of the process they are responsible for, and connect their piece of the diagram to the pieces of other team members.
Use squares to show process steps, diamonds to show decision points, and ovals to mark start and end steps. Use the columns or swim lanes to show who is responsible for what.
Map the process as it is, not as you think it should be.
Between meetings, observe the informed consent process in your unit; correct the current process map if needed to reflect actual practice.
Then, bring your team back together for a second meeting, review the process map, and work together to design a better workflow. Click on the link for a list of questions you can ask to improve a workflow. |
Slide 41: Training |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Content to the designer |
On-Screen Content |
Audio Guidance |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In the resources section, link out to the following training programs:
- The health care professional module
Teach-back training:
LEP Patient Safety training program: http://www.ahrq.gov/professionals/education/curriculum-tools/teamstepps/lep/
We are reviewing the training program on doctor-patient communication developed by Drs. Miller and Fleisher as a possible resource to be listed in the resources section. Melanie Wasserman has the CD.
Coaching: http://www.ahrq.gov/professionals/education/curriculum-tools/teamstepps/instructor/fundamentals/module9/igcoaching.html#coaching
|
Section 3: Building Systems to Improve the Informed Consent Process
Address staff training needs
Key MD = Physician LIP = Licensed Independent Practitioner Other clinical staff = Nurses (including nurse educators) allied health professionals (e.g. medical assistants, technicians, therapists) Administrative staff (e.g., registration, billing) Hospital leaders = Q-suite, risk managers, patient safety and quality officers, heads of departments/units
Training Options
|
Address staff training needs.
To be successful, hospital staff at all levels will have to be trained for their roles in the informed consent process.
As shown on this table, everyone– from the highest level of management to the administrative staff in the registration and billing offices – can benefit from training on the principles of informed consent and hospital policy. Hospital leaders should take this entire training so they know how to develop the supportive systems needed to make informed consent an informed choice.
Certain skills, such as how best to offer choices and explain the benefits, harms, and risks of all options, are only critical for the clinicians who have the main responsibility for informed consent. But other hospital staff – everyone who interacts with patients, including interpreters – can benefit from training in many of the other strategies for clear communication and presenting options. The course you are now taking has a companion course for health care professionals that covers all of those topics.
More in-depth training on your hospital’s policies, and how to access the resources that you have put in place to support them, will be required. As part of the rollout of your informed consent improvement initiative, you’ll probably want to use multiple avenues to train staff.
In addition to developing in-service training and conducting Grand Rounds, think about how the strategies to make informed consent an informed choice can fit into other training you sponsor, such as patient safety or anti-discrimination training.
Think creatively. Could a lunchtime session be devoted to building skills, or could informed choice be on the agenda of a departmental or unit meeting?
If you’re a teaching hospital, be sure that you train your residents, and don’t forget to make sure that new staff members get the trainings too as part of their orientation.
In addition, new behaviors can be reinforced through coaching activities such as being a role model for new behaviors, motivating team members to implement the new behaviors, observing performance and providing feedback and, providing opportunities to practice and improve performance.
The resources section for this module provides links to several useful trainings.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Slide 42. Section 4: Developing and Implementing an Action Plan |
||
Content to the designer |
|
Audio Guidance |
For the Sherman Hospital example: we could ask the person who contributed this story, Barbara Giardino, to read the script.
For the Military Health System example: we could ask the person who contributed this story, Jamie Oberman, to read the script
Note to designer: Can you make it so they can’t advance to the next slide until the listen to both stories
We will ask Barb Giardino and Jamie Oberman if they have any specific statistics on the results of implementing the new systems (e.g. fewer complaints? Fewer surgeries rescheduled or cancelled?) |
Section 4: Developing and Implementing an Action Plan
With careful planning, you can improve informed consent in your hospital.
On-screen images of Sherman Hospital and a military hospital. Caption: click on each picture to hear stories of how two hospitals changed their informed consent process.
|
Section 4: Developing and Implementing an Action Plan
With careful planning, you can improve the communication, quality and safety of the informed consent process in your hospital.
Click on each picture to hear stories of how two hospitals changed their informed consent process.
Sherman Hospital: [we could ask the person who contributed this story, Barbara Giardino, to read the script] When Sherman Hospital introduced the teach-back process into its ORs, it revealed many discrepancies between what the clinician thought was presented versus what the patient understood. It wasn’t easy to make that change: introducing time for teach-back was a big culture change, and the need to use qualified interpreters at the bedside took additional time. There was extensive nursing and physician education about the teach-back process and the use of qualified interpreters. In addition, the interpreters were educated in the role of patient advocacy as well as their role in patient safety. Nurse leaders spearheaded the initiative, and interpreters were assigned exclusively to the OR areas and they came to know the process well. At the start the consent process was slower, it also slowed down the flow in the holding room and OR implementing teach-back, but eventually it became routine. In the end, there was significant improvement in patient understanding.
Military Health System: [we could ask the person who contributed this story, Jamie Oberman, to read the script] As a member of the military health System and as a surgeon, I have found in my 17 year military career many disparate approaches to informed consent. There is a drive now to standardize the approach throughout the military health system. A significant amount of the U.S. Navy component has implemented an electronic informed consent process as an adjunct to the Electronic Medical Record, and providers have received training to become better communicators. These changes have been well-received by end-users. |
Slide 43: Plan for Success |
|
|
Content to the designer |
|
Audio Guidance |
In the resources or references section, include this reference: Warrick DD. Developing Organization Change Champions: A High Payoff Investment! OD Practitioner 41(1): 14-19. url: http://www.polytechnic.edu.na/centres/docs/coll/ODChange/ContentServer2.pdf We will need to get permission from the author to quote the article and/or include it in the resources section.
Will need permission for cartoon as well, if you decide to use it. |
Section 4: Developing and Implementing an Action Plan
Plan for Success
70% of significant organizational change efforts fail. [Picture representing failure or challenges of organizational changes, such as picture of someone trying to drag at the end of a rope an anthropomorphic building that’s kicking and screaming; or insert this picture. It’s picturing that organizational change is hard rather than the failure. Or this picture from: http://onmyfrontporch.com/2014/01/12/kicking-and-screaming/?
A major reason that change efforts fail is a lack of knowledge on how to bring about change.
In this section:
|
This module has thus far examined why we need to improve the process of informed consent for health care and how we can assess and improve policies and systems that would support a good informed consent process in our hospitals. That being said, the road to change is not easy.
Organizational development research suggests that 70% or more of significant organization changes either fail to achieve the desired results, fail altogether, or make things worse. A major reason that change efforts fail is lack of knowledge on how to bring about change.
This section offers action steps, planning tools, and insights from the fields of organizational development and business to help you bring about meaningful change. |
Slide 44: Championing change |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
In the resources section, cite these two books: Kotter J, Rathgeber H. (2006). Our Iceberg is Melting: Changing and Succeeding under Any Conditions. St Martin’s Press.
Kotter J. (2012.) Leading Change. Harvard Business Review Press. |
Section 4: Developing and Implementing an Action Plan
Championing Change
“Never doubt that a small group of thoughtful, and committed citizens can change the world; indeed, it's the only thing that ever has.” – Margaret Mead
Kotter’s 8 steps for organizational change: 1. Establish a sense of urgency 2. Create the guiding coalition 3. Develop a change vision 4. Communicate the vision 5. Empower broad-based action [remove obstacles, facilitate risk-taking/new ideas and activities] 6. Generate short-term wins/communicate about success 7. Never let up 8. Incorporate changes into the culture
|
You can play an instrumental role in changing the informed consent process in your hospital. In the words of Margaret Mead, “Never doubt that a small group of thoughtful and committed citizens can change the world; indeed, it's the only thing that ever has.”
In this section we’ll take you through the 8 steps for organizational change outlined by Harvard Business School Professor John Kotter, and help you to develop an action plan for each of these steps. These steps are: 1. Establish a sense of urgency 2. Create the guiding coalition 3. Develop a change vision 4. Communicate the vision for buy-in 5. Empower broad-based action[remove obstacles, facilitate risk-taking/new ideas and activities] 6. Generate short-term wins/communicate about success 7. Never let up 8. Incorporate changes into the culture
|
Slide 45: Gather your materials |
|
||
Content to the designer |
On-Screen Content |
Audio Guidance |
|
In the resources section, cite these two books: Kotter J, Rathgeber H. (2006). Our Iceberg is Melting: Changing and Succeeding under Any Conditions. St Martin’s Press.
Kotter J. (2012.) Leading Change. Harvard Business Review Press. |
Section 4: Developing and Implementing an Action Plan
Gather your materials:
[thumbnail of the Championing Change/Action Plan worksheet]
Click here for Worksheet
|
We’ll walk you through each of the 8 steps and help you to develop an action plan for each one.
These steps may look different depending on your position within the hospital. For example, for step 5, if you lead a hospital unit, you can empower staff throughout your unit to come up with ideas, whereas a clinician working on the frontlines might simply focus on providing encouragement, support or coaching to a colleague who is incorporating teach-backs into his or her practice.
Before moving forward, gather your materials. You’ll need:
|
|
Slide 46: Establish a sense of urgency |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
|
Section 4: Developing and Implementing an Action Plan
Step 1: Establish a sense of urgency
Failure to obtain informed consent:
[When narrator gets to this part, conversation bubbles materialize that say]:
“During this hospital stay, how often did doctors explain things in a way you could understand?”
“Before giving you any new medicine, how often did hospital staff describe possible side effects in a way you could understand?”
Fade bubbles when narrator gets to next paragraph.
|
The first step to organizational change is to establish a sense of urgency.
General facts that can help you to create a sense of urgency include the following:
Ensuring informed choice during the informed consent process is a patient safety step
[Use different voices for the conversation bubbles] “During this hospital stay, how often did doctors explain things in a way you could understand?”
“Before giving you any new medicine, how often did hospital staff describe possible side effects in a way you could understand?” |
Slide 47: Establish a sense of urgency (cont’d) |
|
||
Content to the designer |
On-Screen Content |
Audio Guidance |
|
For the UVA example, have the second and third audio clips play when the learner clicks to learn more.
|
Section 4: Developing and Implementing an Action Plan
Step 1: Establish a sense of urgency (Cont’d)
Get data about your hospital:
Example [Text box] What motivated the University of Virginia Health System to implement teach-back? [click here to find out]
8% of surgeries were delayed or cancelled Delays cost the hospital $70 per minute, given wasted staff time, preparation of equipment, and other issues. 95% of delays and cancellations were due to patients misunderstanding pre-op instructions. [Click here to learn the outcome of the teach-back intervention]
Four months after adopting Teach-Back within the Preanesthesia Evaluation and Testing Center at UVA, which sees approximately 80 to 100 patients/day, the surgery cancellation/delay rate dropped to 0.8 percent of visits, resulting in significant cost savings for UVA. |
In addition to the general facts, it’s helpful to gather some facts specific to your hospital, including:
Think about what information you can readily access that will be most effective in establishing a sense of urgency.
For example, what motivated the University of Virginia Health System to implement teach-back? [click here to find out]. 8% of surgeries at UVA were delayed or cancelled. Delays cost the hospital $70 per minute, given wasted staff time, preparation of equipment, and other issues. 95% of delays and cancellations due to patients misunderstanding pre-op instructions. [Click here to learn the outcome of the teach-back intervention] . Four months after adopting Teach-Back within the Preanesthesia Evaluation and Testing Center at UVA, which sees approximately 80 to 100 patients/day, the surgery cancellation and delay rate dropped to 0.8 percent of visits, resulting in significant cost savings for UVA.
Please take a moment now to fill out your worksheet for Step 1. When you’re ready, go to Step 2. |
|
Slide 48: Step 2. Creating a powerful guiding coalition / gain organizational consensus |
|||
Content to the designer |
On-Screen Content |
Audio Guidance |
|
|
Section 4: Developing and Implementing an Action Plan
Step 2. Create a powerful guiding coalition/ gain organizational consensus
Recruit hospital leaders
Strategically recruit allies
Broad-based coalition (5-50). Include:
Don’t seek out:
|
Step 2 in managing change is to create a powerful guiding coalition.
Changing a hospital’s informed consent processes is not a one-person job. You’ll need to find other hospital leaders who will join you, and work with you to gain consensus within your organization.
When selecting hospital leaders to engage, think broadly. Include leaders in quality and safety, physician and nursing leadership, including unit and team leaders, and managers from legal/risk management, interpreter services, IT, and any other leaders who play a role in the informed consent process.
Start by reaching out to those who will be most receptive. If they are not the top leaders of their groups, ask for their help to recruit their bosses. You’ll need powerful leaders with senior-level job titles on your side - people who are at the top of the hierarchy in your organization.
Think about those outside your hospital who could be helpful to advancing informed consent as an important patient safety, care quality and legal issue. Perhaps there are patient advisory committees or patient advocacy organizations you could activate. Or maybe some members of the hospital board would be sympathetic – either because they’re aligned with the ethical principles of informed consent or because they’re aware of that an inadequate informed consent process makes the hospital vulnerable. But be careful not to overstep your position. Don’t risk alienating your superiors by doing an end run around them – check with them first.
Once your leaders are on board, you’ll want to form a broader coalition of people who are committed to improving the informed consent process in your hospital. The size can vary from about 5 to 50 people. What’s important is that you include the leaders who are owners of the informed consent process, motivated clinicians who can serve as clinical champions, and other influential health care professionals who are opinion leaders.
Don’t seek out people who will undermine your efforts. They will find you soon enough. Avoid people who dominate proceedings and leave little room for others’ initiatives, as well as people who tend to encourage distrust among colleagues. |
|
Slide 49: Step 2. Creating a powerful guiding coalition / gain organizational consensus (Cont’d) |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
|
Section 4: Developing and Implementing an Action Plan
Persuading others to join your efforts:
Two-way dialogue
Click on each question below for responses to common concerns.
Question 1. With all the other quality initiatives we have going on, why should we focus on informed consent?
Question 2. All of our patients sign informed consent forms before major procedures and surgery, so what’s the problem?
Question 3. Won’t the changes you recommend take too much time? |
Step 2 in managing change is to create a powerful guiding coalition.
Persuading others in your hospital to join your efforts should be a two-way dialogue. While you show the reasons for improving the informed consent process, your colleagues may raise legitimate objections and point out challenges. Hear them out.
We can help you to respond to some common concerns. See below for some common questions, and click on each questions for a possible response.
For more specific questions and concerns, work with your colleagues to think though how you might solve the problems together.
Please take a moment now to fill out your worksheet for Step 2. When you’re ready, go to Step 3. |
Slide 50: Step 3. Develop a change vision |
|
|||
Content to the designer |
On-Screen Content |
Audio Guidance |
||
Light up each box as the audio talks about that step in the process. |
Section 4: Developing and Implementing an Action Plan
Step 3. Develop a change vision
A vision statement:
A good vision should be:
C
Share with key stakeholders
Revise
Final Vision State-ment
Share with Guiding Coalition
Test with staff
[BUTTON: TO WORKSHEET] |
Vision plays a key role in producing useful change. A sound vision statement helps people to picture a worthwhile future that they can help to achieve, and helps to direct, align, and inspire actions on the part of large numbers of people. You’ll see an example of a vision statement when you go to your worksheet.
A good vision will be motivating. Keep the vision focused. If you go too broad it will be seen as too vague to act on. Your vision should also be achievable, flexible enough for people to achieve it in a variety of ways, and easy to understand. Finally, the vision statement should be short. Leave the details to your action plan.
Creating a vision for informed consent transformation will be an iterative process involving many people. Here you see a possible process, starting with a draft that your share with several key stakeholders – your inner circle. Once you refine it based on the feedback you get, you’re ready to share it with your guiding coalition. This might be done most effectively in a few meetings that focus on building consensus.
Once you finalize the first draft of the vision statement with your guiding coalition, test it with staff within your hospital through focus groups or town hall meetings. Then revise it again based on the feedback you receive.
Click on the Final Vision Statement box to see examples of a short vision statement and a longer one. Please take a moment now to fill out your worksheet for Step 3. When you’re ready, go to Step 4.
|
||
Slide 51: Step 4. Communicate the vision |
||||
Content to the designer |
On-Screen Content |
Audio Guidance |
||
|
Section 4: Developing and Implementing an Action Plan
Step 4. Communicate the vision
Still photo or very brief video – 1 older attending clinician talking to a young resident. Resident: I consented the patient. Attending: Consent is something the patient does, not something we do. Did you explain the treatment and alternatives and make sure that the patient understood, or did you just get a signature on a consent form?
|
Step 4. Communicate the change vision
It’s not enough to have a vision. To bring the vision to life, senior leaders must communicate often with everyone in the hospital about the vision. You will need to win the hearts and minds of your hospital staff, convincing them of the urgency and rightness of improving the informed consent process.
Your informed consent improvement effort should be launched in a formal, ceremonial way, for example through announcements at a meeting of the hospital’s governing board, in a hospital publication, at a leadership retreat, and through posters placed around the hospital announcing the new vision.
Communication should happen through many channels and at all levels of the hierarchy: at board meetings, senior leadership meetings, patient safety meetings, morbidity and mortality conferences, unit-level team meetings, speakers invited to do Grand Rounds on informed consent, Lunch and Learn sessions, and informally in email and one-on-one conversations. Communication should happen not only through words, but also through actions that are consistent with the vision. Above all, your hospital’s top leadership must speak and act in a way that is consistent with the vision.
Click on the photo to hear an Attending encourage a Resident to use language that affirms the patient’s autonomy. [play video or run audio associated with photo]: Resident: I consented the patient. Attending: Consent is something the patient does, not something we do. Did you explain the treatment and alternatives and make sure that the patient understood, or did you just get a signature on a consent form?
The communication should be repeated many times to make the vision stick. Please take a moment now to fill out your worksheet for Step 4. When you’re ready, go to Step 5. |
||
Slide 52: Step 5. Empower Broad-Based Action |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
In the resources section, include these links to resources on quality improvement methods:
http://www.ihi.org/resources/Pages/default.aspx
http://www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx
http://www.ahrq.gov/professionals/prevention-chronic-care/improve/system/pfhandbook/index.html
|
Section 4: Developing and Implementing an Action Plan
Step 5. Empower broad-based action
Example Wellness Hospital goal: increase from 71% to 85% the percentage of patients who answer “always” to HCAHPS Question 7: “During this hospital stay, how often did doctors explain things in a way you could understand?” Key driver diagram [thumbnail of diagram; click here to view larger diagram]
Health care professional training:
QI initiatives/PDSA cycles:
|
5. Empower broad-based action
Once you have communicated the vision, it’s time to empower people to make changes aligned with the vision.
Leaders set the expectations, so they should be empowered to set goals and mobilize resources, for example to begin staff training, improve informed consent forms, and improve access to interpreter services.
Health care professionals can be empowered too – for example, by forming quality improvement teams and initiating plan-do-study-act cycles to apply what they learn through informed consent training.
Here’s an example from our fictional Wellness Hospital. The Hospital’s Coalition for Informed Choice has set a goal of increasing from 71% to 85% the percentage of patients who answer “always” to HCAHPS Question 7: “During this hospital stay, how often did doctors explain things in a way you could understand?”
The Coalition decided to implement changes first in the surgery and labor and delivery units. It drew up a key driver diagram to focus its efforts. Click on the thumbnail for a larger view.
Based on this diagram, one of the actions taken was to roll out informed consent training to all clinicians in the surgical and labor and delivery units. Unit leads, who were part of the Coalition for Informed Choice, encouraged their staff members to come up with ideas to best apply what they learned. The labor and delivery unit, which had many Spanish-speaking patients, focused on a workflow redesign: clinicians agreed to work with a phone interpreter when in-person interpreters weren’t available at the time of the informed consent discussion. In the surgical unit, clinical staff focused on applying “teach-back” as an “always event” during the informed consent process.
Within three months of implementing these changes, Wellness Hospital is closer to reaching its goal. According to a small informal poll of patients discharged for the target units, 80% of them say doctors always explained things in a way they could understand. Wellness Hospital is ready to expand the informed consent improvement effort to other units.
Please take a moment now to fill out your worksheet for Step 5. When you’re ready, go to Step 6. |
Slide 54: Step 6. Generate Short-Term Wins |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
Mary Ann Abram supports including anecdotes/stories/testimonials of short-term successes. We will try to identify some. |
Section 4: Developing and Implementing an Action Plan
Step 6. Generate short-term wins; communicate about success
Plan short-term successes you can celebrate
Immediate successes (a month or two after rolling out the training)
Short-term wins (a few months after training)
|
Step 6. Generate short-term wins; communicate about success
It can be hard to motivate people toward a distant, future goal. Generate some short-term wins that you can celebrate, and share them widely in your hospital. This gives you a chance to recognize and reward people who have made positive changes, and increases momentum in the hospital. The wins should be visible, unambiguous and clearly related to your change effort.
Some immediate wins may include: completion of training by a large number or proportion of staff, high satisfaction ratings or positive comments from trainees, and a description of new initiatives undertaken as a result of the training.
Examples of short-term wins that could be achieved within a few months after training is completed, as a result of new initiatives, could include:
Please take a moment now to fill out your worksheet for Step 6. When you’re ready, go to Step 7.
|
Slide 55: Never Let Up |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
Include in the resources section this resource on coaching:
|
Section 4: Developing and Implementing an Action Plan
Step 7. Never let up
|
Step 7. Never let up
A common reason that changes don’t take hold is that organizations stop moving forward after the initial wins. If you want to make permanent, meaningful change, your early wins should be your signal to accelerate the effort.
To accelerate the effort, expand the initiative to more units in your hospital, involve more people at lower hierarchical levels to lead initiatives, and continue identifying and removing obstacles to an improved informed consent process. Once you have identified one or more clinicians who regularly make informed consent an informed choice, you can also do “see one, do one, teach one” cycles where you allow others to observe, let them try it, give them feedback, and once they’ve mastered it, encourage them to teach the others.
Staff members can and often do revert to old ways of gathering patient consent. Change champions can use a variety of techniques to help everyone keep moving in the right direction. One method is coaching to help staff be successful by enhancing their skills in moving away from long-standing habits and integrating new habits. Coaches can identify “teachable moments,” when a person is particularly receptive to learning, perhaps right after a miscommunication has occurred. They can also provide detailed, useful feedback, so staff know how to approach a similarly situation next time. Coaches can also re-enforce changes by pointing out when they done a good job, a technique called “catch them being good.” A presentation summarizing key elements of coaching is provided in the “Resources” section of this training.
Please take a moment now to fill out your worksheet for Step 7. When you’re ready, go to Step 8. |
q |
|
|
Slide 56: Step 8. Incorporate Changes into the Culture |
|
|
Content to the designer |
On-Screen Content |
Audio Guidance |
|
Section 4: Developing and Implementing an Action Plan
Step 8. Incorporate changes into the culture
“Culture eats strategy for breakfast” – Peter Drucker
“Cultural Change Comes Last, Not First” – John Kotter
To anchor changes in the culture:
|
Once you have generated some good momentum and informed consent is being improved throughout your hospital, you’ll want to consolidate those changes by incorporating them into the hospital culture.
In the words of Peter Drucker, “culture eats strategy for breakfast”: unless your culture supports patient informed choice, care teams may revert back to the meaningless ritual of having patients sign informed consent forms they don’t understand.
John Kotter’s insight on culture change is that, while it is an essential step, it should be the last step in a change strategy. Furthermore, change in culture is slow. People need to see that the new processes and ideas work better, before the culture can change.
Anchoring change in the hospital’s culture requires communication and incentives.
Hospital leaders will need to communicate a lot about:
In addition, hospital leaders will need to properly align incentives to support culture change. Clinicians who do an exemplary job of making informed consent an informed choice should be rewarded through recognition, pay and promotion; and in extreme cases, there should be consequences , such as disciplinary hearings, warnings, or even revocation of privileges for clinicians willfully disregard the hospital’s informed consent policy.
Please take a moment now to fill out your worksheet for Step 8. |
Slide 57: Course Summary |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
|
Course Summary:
|
Before you go, let us quickly recap. We have learnt that:
|
Slide 58: Thank you and Next Steps |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
|
Thank you!
Next steps?
|
THANK YOU for taking the time to work through this training module for health care leaders on making informed consent an informed choice.
What will be your next steps to make informed consent an informed choice in your hospital? Please take one last moment as part of this training to note your next steps in your worksheet. |
Slide 59: Post-Test |
||
Content to the designer |
On-Screen Content |
Audio Guidance |
|
|
|
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Melanie Wasserman |
| File Modified | 0000-00-00 |
| File Created | 2021-01-26 |
© 2026 OMB.report | Privacy Policy

 atients’
Right to Decide what Happens to their Bodies
atients’
Right to Decide what Happens to their Bodies ack
of patient understanding Patient
ack
of patient understanding Patient
 afety
afety atient
requests not to be informed
atient
requests not to be informed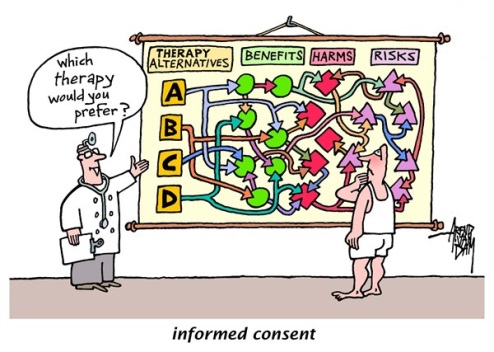


 nder
training and education, perhaps just list the bullets (far right)
with a “check mark’ as they are spoken
nder
training and education, perhaps just list the bullets (far right)
with a “check mark’ as they are spoken