Supporting Statement A
Part A_Message Platform_07112016_clean.docx
Message Testing for Tobacco Communication Activities
Supporting Statement A
OMB: 0920-0910
SUPPORTING STATEMENT FOR THE
National Tobacco Education Campaign
Message Platform Testing for
Regular Cigarettes and Dual Use
(OMB No. 0920-0910, Exp. Date 03/31/2018)
PART A: JUSTIFICATION
July 11, 2016
Submitted by:
Office on Smoking and Health
National Center for Chronic Disease Prevention and Health Promotion
Centers for Disease Control and Prevention
Department of Health and Human Services
Refer questions to:
Michelle O’Hegarty
Office on Smoking and Health
National Center for Chronic Disease Prevention and Health Promotion
Centers for Disease Control and Prevention
4770 Buford Highway, NE MS F-79
Atlanta, Georgia 30341
770-488-5582
FAX:770-488-5939
E-mail: [email protected]
TABLE OF CONTENTS
ABSTRACT
JUSTIFICATION
1. Circumstances Making the Collection of Information Necessary
2. Purpose and Use of Information Collection
3. Use of Improved Information Technology and Burden Reduction
4. Efforts to Identify Duplication and Use of Similar Information
5. Impact on Small Businesses or Other Small Entities
6. Consequences of Collecting the Information Less Frequently
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
a. Federal Register Announcement
b. Consultations
9. Explanation of Any Payment or Gift to Respondents
10. Protection of the Privacy and Confidentiality of Information Provided by Respondents
11. Institutional Review Board (IRB) and Justification for Sensitive Questions
12. Estimates of Annualized Burden Hours and Costs
13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers
14. Annualized Cost to the Federal Government
15. Explanation for Program Changes or Adjustments
16. Plans for Tabulation and Publication and Project Time Schedule
17. Reason(s) Display of OMB Expiration Date is Inappropriate
18. Exceptions to Certification for Paperwork Reduction Act Submissions
LIST OF ATTACHMENTS
1A. Screen Shots of the Recruitment Screener
1B. Screen Shots of the Main Questionnaire for Regular Cigarette Smokers and Dual Users
2A. Moderator’s Guide for IDI – Dual Use
2B. Additional Information About the Message Platforms for Dual Users
3A. Moderator’s Guide for IDI – Regular Cigarette Smokers
3B. Additional Information About the Message Platforms for Cigarette Users
4. Email Invitation to Potential Respondents
5. Terms and Conditions
6. Privacy Policy
7. Battelle Institutional Review Board Approval
Notes on Excluded Attachments
In this Gen IC, CDC outlines a plan to test message platforms with
content that may be considered sensitive. The draft materials are not
included in the attachments for this Gen IC because:
The message platforms have not been approved for public distribution by HHS/Assistant Secretary for Public Affairs (ASPA).
The untested message platforms could be perceived by the public as ineffective or offensive (testing is designed to identify potential problems).
To support adequate review of this Gen IC by OMB, the Centers for Disease Control and Prevention requests permission to provide OMB with a secure link to the draft materials.
Goal of
the Study: To assess attitudes and perceptions of participants
after viewing evidence-based message platforms that focus on the
consequences of smoking and the benefits of quitting. The goal is
to test 23 message platforms with an average of 487 respondents for
each platform. In addition, given the uncertainties regarding the
long-term health consequences of e-cigarette use, the qualitative
component of this proposal further explores respondents’
perceptions about the health consequences of e-cigarette use.
Overall, data collected from this study will help inform the
creative brief that will be used to develop the creative concept
for the next National Tobacco Education Campaign.
Intended use
of the resulting data: The resulting data will ensure that
future campaign concepts will be developed in a way that is clear,
credible, and believable, and that persuades smokers to quit
smoking regular cigarettes completely.
Methods to
be used to collect data: Quantitative and qualitative methods
will be used to collect data. Quantitative data will be collected
through 15-minute online surveys of 11,200 respondents. The
quantitative study questions will collect information about
respondents’ reactions to the draft message platforms, as
well as basic demographic and tobacco use information to assess
determinants of individual responses to message platforms.
Qualitative data will be collected through online 30-minute
individual in-depth interviews (IDIs) using video conference, of 50
respondents who completed the online quantitative survey.
Respondents will answer questions about message platforms that were
most persuasive.
Populations to be studied: The study population will be
adult smokers 18-54 years old, categorized as either: (1) exclusive
smokers of regular cigarettes of low socio-economic status (SES),
or (2) concurrent users of regular cigarettes and e-cigarettes who
are not of low SES (dual users). These two different target
audiences will be used to test two sets of message platforms, each
tailored to its respective target audience. Exclusive e-cigarette
smokers will not be included in the study. How data
will be analyzed: The resulting quantitative data will be
analyzed using aggregate measures such as percentages and means.
The qualitative analysis will be analyzed using thematic analysis;
interviewee responses will be read thoroughly and initial codes
created manually, identifying themes and patterns of response. The
qualitative data will provide rich detail, depth and context to the
quantitative results.

A.1 Circumstances Making the Collection of Information Necessary
Since 2012, the tobacco prevention and control landscape in the United States has changed. While significant improvements have been made in reducing the smoking rate in the United States, cigarette smoking is still the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths every year, or 1 of every 5 deaths (U.S. Department of Health and Human Services (DHHS, 2014). In addition, more than 16 million Americans live with a smoking-related disease (DHHS, 2014).
In 2012, for the first time, the DHHS, through the Centers for Disease Control and Prevention (CDC), aired an adult-focused national tobacco education campaign called Tips From Former Smokers (Tips). The goals of the Tips campaign are to:
Build public awareness of the immediate health damage caused by smoking and exposure to secondhand smoke;
Encourage smokers to quit, and make free help available, and;
Encourage smokers not to smoke around others, and encourage non-smokers to protect themselves and their families from exposure to secondhand smoke.
However, in recent years, the use of electronic nicotine delivery systems (ENDS) or e-cigarettes has rapidly increased; with most adult e-cigarette users (76.8%) also reporting current use of regular cigarettes (Agaku, 2014; King, 2015). Research on e-cigarettes and cessation is inconclusive as long-term longitudinal studies are not available to conclude that ENDS are a proven cessation product or to establish what long-term effect e-cigarettes have on users who might otherwise quit but instead engage in dual use of ENDS and other tobacco products (81 FR 28973, 2016). The United States Preventive Services Task Force (USPSTF) concluded that the current evidence is insufficient to recommend ENDS for tobacco cessation, and gave an “I” (Inconclusive) recommendation. Findings from the outcome evaluation of this campaign show that smokers who only smoke regular cigarettes are behaviorally and attitudinally different from smokers who use regular cigarettes in combination with e-cigarettes (dual users). These smokers may relate differently to public health messages about smoking. Thus, creating messages that also target dual tobacco users may be helpful in promoting quit attempts.
Message platforms are fact-based statements that underlie health communication messages. The objective of the proposed study is to test 23 message platforms among smokers of different socio-economic groups (low and non-low), and tobacco use behaviors (exclusive regular cigarette smokers and dual users of cigarettes and e-cigarettes). Exclusive e-cigarette smokers will not be included in the study. Each message platform will be tested with approximately 420-800 respondents to help identify the facts that are considered to be most relevant and motivating to the target audience. This information will help in developing appropriate public health messages that help smokers quit.
The 23 message platforms will focus on the following categories:
Low SES exclusive Regular Cigarette Smokers (15 message platforms):
Health consequences (Seven message platforms)
Impact on lifestyle (Three message platforms)
Secondhand smoke consequences (Four message platforms)
Impact on young adults (One message platform)
Non-low SES Dual Users of Regular Cigarettes and E-Cigarettes (8 message platforms):
Cutting down is not enough (Three message platforms)
Effective ways to quit (One message platform)
Guidance for parents and adults (Four message platforms)
Quantitative and qualitative methods will be used to collect data. Respondents will be recruited from an established, online panel system. All survey information collection will be conducted electronically on this platform. There is also a provision for in-depth interviews (IDI) to be conducted via a Skype-like interview format. Screening will be conducted to identify respondents using the “Recruitment Screener for Regular Cigarette Smokers and Dual Users, which will be called the screener in this ICR (Attachment 1A). Persons meeting the eligibility criteria will be administered the “Main Questionnaire for Regular Cigarette Smokers and Dual Users,” subsequently referred to as “the main questionnaire” in this ICR (Attachment 1B).
IDI’s will also be performed to collect qualitative data from about 50 respondents who completed the main questionnaire, including ~ 25 non-low SES adult dual users (“Moderator’s Guide for IDI- Dual Use,” Attachment 2A), and ~25 low SES exclusive regular cigarette smokers (“Moderator’s Guide for IDI – Regular Cigarette Smokers,” Attachment 3A). A flowchart of the sampling of participants is shown in Figure 1 below.
Figure 1: Flowchart of the sampling of participants for quantitative and qualitative aspects of the study
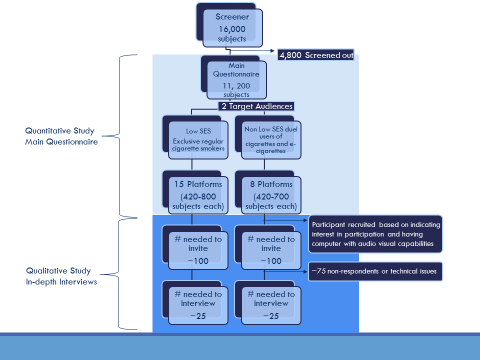
A.2 Purpose and Use of Information Collection
The overall purpose of the project is to assess whether the message platforms under test are likely to be perceived as credible, comprehensible, and persuasive among adult low SES exclusive regular cigarette smokers and non-low SES adult dual users. Although the effects of cigarette smoking on public health have been well-studied, data to determine the long-term effects of dual use of cigarettes and e-cigarettes on public health at the population level are insufficient (81 FR 28973, 2016). Given the uncertainties regarding the long-term health consequences of e-cigarette use, the qualitative component of this proposal further explores respondents’ perceptions about the health consequences of e-cigarette use. Attachments 2B and 3B were included to highlight which themes were evidence-based and which were exploratory. We deemed certain messages as "exploratory" if they were not supported by conclusive science and overall HHS messaging. Questions that are exploratory in nature are identified in Attachment 2B (Additional Information About the Message Platforms for Dual Users) and Attachment 3B (Additional Information About the Message Platforms for Cigarette Users). The information to be collected will help guide the development of creative briefs and future creative concepts for a new National Tobacco Education Campaign. Creative briefs will include the goal, background information, target audience, messages and tone to guide the creation of concepts for advertisements. If this data collection is not performed, CDC will not know whether these message platforms communicate credibly and effectively across varying audience segments. Additionally, message platform testing is a way to measure any unanticipated confusion, ambiguity, or lack of understanding of the facts.
As outlined in the preceding flowchart, information will be collected in three stages. First, CDC’s contractor will screen potential participants who are members of an existing online panel. Although the panel provider maintains demographic information about panelists in its proprietary database (so that the invitation to participate in this project will target only individuals who are likely to be eligible; Attachment 4), CDC will administer an online, project-specific screener to identify any changes in demographics that could affect eligibility for this study. CDC’s screening instrument will assess respondent characteristics such as age, smoking and e-cigarette behavior. In addition to confirming to eligibility, the screening information will be used to (a) assign respondents to the platforms to be tested, and (b) assist in the interpretation of study findings. In the second stage of information collection, respondents will complete the main online questionnaire. The purpose of the main questionnaire is to show respondents the message platforms under test and collect quantitative information on the credibility, clarity, and persuasiveness of the message platforms. In the third stage of information collection, in-depth interviews (IDI) will be conducted with a subset of respondents who completed the main questionnaire. The purpose of the IDIs is to obtain additional detailed information about two or three message platforms that seem to resonate best with each target audience.
Please note that the main online questionnaire identifies the behavioral characteristics of each target audience by asking the respondents fixed, quantitative questions. The purpose of many of the questions at the beginning of the main online questionnaire is to define these characteristics. In previous research, it is noted that the receptivity of messages are in part influenced by how open the respondent is to quitting smoking cigarettes as well as other attitudes and behaviors. These attitudinal and behavioral measures are required to properly interpret the receptivity of the qualitative information for each target audience.
A.3 Use of Improved Information Technology and Burden Reduction
All information will be collected electronically by utilizing an integrated Web-based software platform. Screening and response information will be collected electronically through online, Web-based surveys. Web-based surveys are an especially convenient option for eliciting feedback on textual stimuli such as the message platforms to be tested. In addition, we are using electronic technology to facilitate IDIs (similar to a CATI system or conversation over Skype).
The Web-based software system includes embedded logic that will route respondents efficiently through the 3 stages of information collection: the online screener, the online main questionnaire, and the IDI. The software assigns respondents to specific platform tests. After the first 100 responses per message platform are collected, the software randomly invites a subset of respondents to participate in IDIs. The software then displays a customized set of screen shots for each respondent, so that the respondent perceives the entire experience as seamless and continuous. For example, if a respondent is ineligible or declines to participate after screening, she/he is routed to the appropriate thank-you screen. If a respondent is eligible and completes the main questionnaire, he/she is routed to the appropriate thank-you screen. If the software determines that the respondent should be invited to participate in an IDI, the software will display the relevant invitation and routing screens, and the respondent can schedule the IDI at a time of their choosing. Overall, the software supports a very efficient assignment and routing process, and a smooth user experience that would be difficult to attain in other modes of data collection.
The Web-based platform has 2 additional advantages for managing the project.
First, use of an existing online panel system will allow CDC to obtain information quickly so that needed adjustments to health messaging can be made expeditiously and campaign development can progress rapidly from planning to implementation. Convenience panels managed by Qualtrics/Toluna will be used for the subpopulations under test. The panel used for this testing is very large, allowing quick selection from the overall pool and rapid identification of several potential respondents from extremely small subgroups of the population. Samples from this panel are not designed to generate nationally representative samples or precise population parameters but rather are used as a highly efficient, low cost, and low burden method of data collection for formative copy testing.
Second, when a respondent enters the screener for this project, the link to his or her identifiable information is severed (i.e., the link to the identifiable information maintained by the panel provider). None of the information collected through screening, the main questionnaire, or the IDI is identifiable, thus providing a secure environment for participants.
Please note that the information gathered in this package is not meant to generate any prevalence or generalizable statistics concerning users’ smoking or dual use habits, and no claims in results will be made about anyone other than those in the targeted population and only for the purposes listed herein, that is, to help shape future creative development for this specific campaign.
A.4 Efforts to Identify Duplication and Use of Similar Information
This Information Collection Request (ICR) is designed to test draft message platforms to support the development efforts of a new National Tobacco Education Campaign. As it does prior to conducting any data collection effort, CDC reviewed existing published literature, FDA’s Deeming Rule, and unpublished qualitative pretesting reports when they were available. Additionally, CDC consulted with outside experts to identify information that could facilitate message development.
There are no similar data available specific to the message platforms proposed for testing in this ICR. CDC’s Office on Smoking and Health (OSH) conducts quantitative research among adults to perform formative testing of Tips ad concepts. CDC/OSH collaborates with other federal government agencies that sponsor or endorse health communication projects, such as FDA’s Center for Tobacco Products (CTP). These affiliations serve as information channels, help prevent redundancy, and promote use of consistent measures of effectiveness. Coordination activities include the review of proposed messages; review of questionnaire and other support material for testing purposes; and sharing of data. FDA is not currently developing message platforms based on cessation to support the development efforts of the National Tobacco Education Campaign.
CDC will share the findings from this collection of information with FDA CTP. CDC and FDA are developing complementary but distinct messages to educate the public about the harmful effects of tobacco products. Staff members in OSH’s Health Communications Branch work closely with staff in FDA’s Health Communication and Education unit. Regularly scheduled conference calls are held to review plans and share research findings of mutual interest. Staff members in OSH’s Health Communications Branch are thus working closely with staff in FDA’s Health Communication and Education unit as appropriate. It was determined that message platform testing proposed in this ICR does not duplicate FDA efforts.
Points of contact for this coordination are:
CDC: Diane Beistle, Chief, Health Communication Branch, telephone (770) 488-5066, email [email protected]
CDC: Michelle O’Hegarty, Health Communications Specialist, Campaign Development, Health Communication Branch, telephone (770) 488-5582, email [email protected]
FDA: Tesfa Alexander, Health Communication Specialist, Office of Health Communication and Education, telephone (301) 796-9335, email [email protected]
A.5 Impact on Small Business or Other Small Entities
This data collection will not involve small businesses or other small entities.
A.6 Consequences of Collecting the Information Less Frequently
This is a one-time information collection request.
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
There are no special circumstances that require data collection to be conducted in a manner inconsistent with 5 CFR 1320.5 (d) (2). The information collection fully complies with the guidelines in 5 CFR 1320.5.
A.8 Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A.8.a Federal Register Announcement
A Notice was published in the Federal Register on August 11, 2014, volume 79, number 154, pp. 46829-46830). One public comment was received and addressed.
A.8.b Consultations
CDC’s National Tobacco Education Campaign has been funded primarily with funds from the Affordable Care Act/Public Health Fund designated for smoking education since 2010. CDC did not consult outside of the agency on the message platforms.
A.9 Explanation of Any Payments or Gift to Respondents
Respondents will be drawn from the established Toluna panel system which provides points to panelists as a reward for participation (see Toluna’s Terms and Conditions, Attachment 5). Immediately upon completion of the survey, each respondent will be provided with a certain number of points that are equivalent to $.50. These points are accrued with other points when the panelist takes part in other surveys. At any time, the panelist is able to redeem their points for different products, such as gift cards.
A.10.
Protection of the Privacy and Confidentiality of Information Provided
by Respondents
This submission has been reviewed by staff in
CDC’s National Center for Chronic Disease Prevention and Health
Promotion, who determined that the Privacy Act does not apply. The
identifiable information about Toluna panelists is maintained in a
proprietary records system and is not released (see Toluna’s
Privacy Policy, Attachment 6).
When the respondent begins the survey, all identifiable links
to the existing system of records are severed.
CDC has contracted with The PlowShare Group for this information collection. The PlowShare Group’s data collection and formative research sub-contractors are Qualtrics and Battelle. Qualtrics will recruit respondents by interfacing with an existing panel system managed by Toluna. CDC, Qualtrics, and Battelle participated in planning the information collection and will interpret de-identified response information and will not receive any PII on the respondents.
All information for the self-administered screening process and the self-administered main questionnaire will be collected electronically in a secure, online, Web-based data collection system. CDC will not have direct contact with nor access to any personal identifying information (PII) about participants. CDC will only have access to the audio-visuals of the IDIs via a password-protected Web stream.
No individually identifiable information or PII is being collected. Although demographic information (e.g., age) will be confirmed through screening, no direct personal identifiers (e.g., name, phone number, social security number, etc.) will be collected or maintained as part of the screener or main questionnaire (Attachment 1A/Attachment 1B). As such, because it does not exist, no directly identifying information will be transmitted to CDC. While Toluna has access to the email address of panel subscribers, no match back is possible between the survey response data and Toluna nor is this information available to Qualtrics. No link between the respondent’s email and the specific survey is made after the potential respondent clicks on the link to view the consent and potentially starts the survey. No information is collected that will tie the respondent back to the email or any other personal identifying information. In addition, the information at the observation level is identified through use only of sample unit identifiers. Neither Qualtrics, Battelle, nor CDC employees working on the project will have access to any identifying information.
After completing the main questionnaire, respondents will have the opportunity to schedule a follow-up IDI (Attachment 1B – page 100). Respondents who express interest in participating in an IDI and have the necessary computer equipment will be routed to a secure site where they can schedule the IDI at their convenience. No information that could potentially identify the respondent is collected for scheduling the IDI or during the IDI.
Data
Security
All findings will be
reported in the aggregate only. All information will be stored on
password-protected databases to which only Qualtrics employees
working on this project have access. Qualtrics will keep the data in
non-aggregate form for six months after information collection has
been completed, and then the observation-level data will be deleted
from the password-protected databases. No desktop or laptop computer
will contain any personal identifying information. To prevent
unauthorized access to their data servers (such as that which would
be done by “hacking”), Qualtrics is currently certified
and has achieved the distinguished ISO 27001 accreditation. With this
achievement, Qualtrics’ data systems have assurance that all
data will be managed in a secure environment. This means that
Qualtrics has been formally audited and has been certified compliant
with the standard ISO 27001 accreditation. CDC will retain and
destroy records in accordance with the applicable CDC Records Control
Schedule.
A.10.1 Access Controls
Technical Controls |
Physical Controls |
Administrative Controls |
|
|
|
A.11 Institutional Review Board (IRB) and Justification for Sensitive Questions.
IRB Approval
All procedures have been developed in accordance with federal, state, and local guidelines to ensure that the rights and privacy of participants are protected and maintained. Battelle’s Institutional Review Board (IRB) has review and approved all instruments, informed consent materials, and data collection and management procedures (see approval notice in Attachment 7).
Sensitive Questions
The majority of questions asked will not be of a sensitive nature (Attachment 1A/1B). There will be no requests for a respondent’s Social Security Number (SSN). Questions asked during the screening about regular cigarette or e-cigarette use, and some demographic information (e.g., age) could be considered sensitive, although these items would not generally be considered highly sensitive. It will also be necessary to ask some questions considered to be sensitive in order to assess individuals’ attitudes and behaviors or to test messages about the specific health behavior of regular cigarette smoking. These items are not generally considered highly sensitive. Respondents will be informed of the applicable privacy safeguards. Sensitive information will only be requested when necessary among specific subpopulations of interest, and is not identifiable. Smoking rates vary by subpopulations, and subpopulations of disproportional burden are racial and ethnic minorities and lesbian, gay, bisexual, and transgender (LGBT) individuals. Therefore, the survey includes questions about race, ethnicity, and sexual orientation, so that results of the current research can suggest whether message platforms may be more or less effective for individuals of racial and ethnic minorities and LGBT versus non-LGBT individuals. For questions requesting sensitive information, the question will include a “decline to answer” option. Respondents can refuse to answer questions or leave the interview at any time if they feel uncomfortable.
A.12 Estimates of Annualized Burden Hours and Costs
This message testing Gen IC reflects a stepwise screening, survey and CATI like IDI. The screening, main questionnaire, and invitation to participate in the IDI are conducted in a continuous process. The goal is to test 23 message platforms with approximately 420-800 respondents for each platform. Participants for the IDI will be selected based on their behavioral criteria and exposure to message platforms that are resonating well with their specific target audience in the quantitative portion of the current project. Through the online Web-based system, they will be informed that they qualify to participate in the IDI. Respondents who decline the IDI will see a screen that thanks them for participating in the survey. Respondents who agree to participate in the IDI will see a different screen that helps them schedule the IDI for a later time at the respondent’s convenience. The flowchart for this process is illustrated in Figure 1. Details of the invitation for the IDI are in B.1 and Screen Shots.
Approximately 16,000 respondents are anticipated to complete the screener (Attachment 1A). The burden per respondent for completing the screener is 4 minutes. The total estimated burden for completing the screener is 1,067 hours (16,000 respondents * 4/60 hours = 1,067 hours).
Based on projections from previous surveys, an estimated 4,800 respondents who complete the screener will be deemed ineligible for the study because of not meeting inclusion criteria by age (18-54 years), and tobacco use status.
The remaining 11,200 who meet the inclusion criteria will be administered the main questionnaire (Attachment 1B). The burden per respondent for completing the main questionnaire is 11 minutes. The total estimated burden for completing the main questionnaire is 2,053 hours (11,200 respondents * 11/60 hours = 2,053 hours).
Approximately 50 persons who completed the main questionnaire will be selected to participate in an in-depth interview done over video and audio software through the browser on the respondent’s computer (~25 low SES exclusive regular cigarette smokers [Attachment 3A and 3B] and ~25 non-low SES adult dual users [Attachment 2A and 2B]). The burden per respondent for completing the IDI is 30 minutes. The total estimated burden for completing an IDI is 26 hours [(25 non-low SES adult dual user respondents * 30/60 hours) + (25 low SES exclusive regular cigarette smoker respondents * 30/60 hours) = 26 hours].
The estimated burden per respondent will vary from 4 minutes for those who complete only the screener to 15 minutes for those who complete both the screener and the main questionnaire (4 minutes for screener + 11 minutes for main questionnaire) to 45 minutes for those who complete the screener, main questionnaire, and IDI (4 minutes for screener + 11 minutes for main questionnaire + 30 minutes for Moderator’s Guide for IDI). The adjusted average burden per respondent is approximately 11.8 minutes. The total estimated burden for the entire project is 3,146 hours [(16,000 respondents *4/60 hours) + (11,200 respondents *11/60 hours) + (25 non-low SES adult dual user respondents * 30/60 hours) + (25 low SES exclusive regular cigarette smoker respondents * 30/60 hours) (pre-screening participants, post-screening participants taking the main questionnaire, and IDI participants respectively) = 3,146 hours].
Table A.12.A. Estimated Annualized Burden to Respondents
Type of Respondent |
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Average Burden per Response (in hours) |
Total Burden (in hours) |
Adult smokers who are 18-54 years old (low SES exclusive smokers of regular cigarettes; non-low SES dual users) |
Recruitment Screener for Regular Cigarette Smokers and Dual Users (Att. 1A) |
16,000 |
1 |
4/60 |
1,067 |
Main Questionnaire for Regular Cigarette Smokers and Dual Users (Att. 1B) |
11,200 |
1 |
11/60 |
2,053 |
|
Adult smokers who are 18-54 years old (non-low SES dual users) |
Moderator’s Guide for IDI: Dual Use (Att. 2A) |
25 |
1 |
30/60 |
13 |
Adult smokers who are 18-54 years old (low SES exclusive smokers of regular cigarettes |
Moderator’s Guide for IDI: Regular Cigarette Smokers (Att. 3A) |
25 |
1 |
30/60 |
13 |
Total |
3,146 |
||||
The estimated cost of the time devoted to this information collection by respondents is $72,358 as summarized in Table A.12.B. To calculate this cost, we used the mean hourly wage of $23, which represents the Department of Labor estimated mean for state, local, and private industry earnings (U.S. Bureau of Labor Statistics, 2015). There are no direct costs to respondents associated with participation in this information collection.
Table A.12.B Estimated Annualized Cost to Respondents
Type of Respondents |
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Total Burden (in hours) |
Hour Wage Rate |
Total Cost |
Adult smokers who are 18-54 years old (low SES exclusive smokers of regular cigarettes; non-low SES dual users) |
Recruitment Screener for Regular Cigarette Smokers and Dual Users (Att. 1A) |
16,000 |
1 |
1,067 |
$23 |
$24,541 |
Main Questionnaire for Regular Cigarette Smokers and Dual Users (Att. 1B) |
11,200 |
1 |
2,053 |
$23 |
$47,219 |
|
Adult smokers who are 18-54 years old (non-low SES dual users) |
Moderator’s Guide for IDI: Dual Use (Att. 2A) |
25 |
1 |
13 |
$23 |
$299 |
Adult smokers who are 18-54 years old (low SES exclusive smokers of regular cigarettes) |
Moderator’s Guide for IDI: Regular Cigarette Smokers (Att. 3A) |
25 |
1 |
13 |
$23 |
$299 |
Total |
$72,358 |
|||||
A.13 Estimates of Other Annual Cost Burden to Respondents and Record Keepers
There will be no respondent capital and maintenance costs.
A.14 Annualized Cost to the Government
Approximately 5% of one full-time equivalent (FTE) and 1% of one senior manager will be required to oversee the information collection activities for one month. Responsibilities will include internal coordination and review of materials and reports and maintaining proper accounting of burden hours. The agency estimates that it will take a GS-13, at a wage rate of $54.33/hour, approximately 10 hours to manage the project, totaling about $543.00. It is estimated to take a GS-15, at a wage rate of $64.54 /hour, approximately three hours to oversee the total project, totaling $194.00. The total average annualized cost to the government for CDC oversight is $737.
Government Personnel |
Time Commitment |
Hourly Basic Rate |
Total |
GS-13 |
5% |
$54.33 |
$543 |
GS-15 |
1% |
$64.54 |
$194 |
Subtotal, Government Personnel |
$737 |
||
Contract Costs |
$97,000 |
||
Total Costs |
$97,737 |
||
Contractors will conduct the majority of information collection and management activities on CDC’s behalf. The total cost of the data collection contractors is $97,000 which includes consultation, instrument design and development, respondent incentive, data collection, and top line analyses. This cost does not include the actual cost of the recruitment of the respondents to answer the survey. Qualtrics will collect the information from the respondents. Activities are coordinated through a contract with the PlowShare Group, a specialist in media campaigns. The grand total cost for the project, including government and contractor cost, is $97,737.
A.15 Explanation for Program Changes or Adjustments
This is a new data collection.
A.16 Plans for Tabulation and Publication and Project Time Schedule
Data Tabulation Plans
The information will be used to inform the development of a new National Tobacco Education Campaign. The estimated OMB approval date of June 28, 2016, with an anticipated information collection to begin on June 29, 2016. The resulting quantitative data will be analyzed using conventional tabulation techniques and the resulting qualitative data will be analyzed using thematic analysis. The data will be read thoroughly and initial codes will be created manually, identifying themes and patterns of responses. These dates may adjust depending on the approval process of this package.
Publication and Dissemination Plans
This information will be used to inform the development of creative briefs, resulting in new messages and advertisements, for adult low SES smokers of regular cigarettes and adult dual users. Creative briefs are used to guide the creation of concepts for advertisements that will be used in future National Tobacco Education Campaigns and other communication strategies.
Project Time Schedule
Task |
Approximate |
CDC submits OMB Package to OMB for approval |
6/21/2016 |
Milestone: OMB approves Request |
6/28/2016 |
Message Platform Testing Field Period begins |
6/29/2016 |
Message Platform Testing Field Period complete |
7/28/2016 |
A.17 Reason(s) Display of OMB Expiration is Inappropriate
The expiration date of OMB approval will be displayed on all information collection instruments.
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
References
Agaku IT, King, BA, Husten CG, Bunnell R, Ambrose BK, Hu SS, Holder-Hayes E, Day HR; Centers for Disease Control and Prevention (CDC). Tobacco Product Use Among Adults— United States, 2012–2013. MMWR 63(25); 542-547.
King BA, Patel R, Nguyen KH, Dube SR. Trends in Awareness and Use of Electronic Cigarettes among U.S. Adults, 2010 -2013 Nicotine Tob Res, 2015;17(2):219-227.
Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products, 81 FR. 28973 (May 10, 2016). Federal Register: The Daily Journal of the United States. Web. 10 May 2016.
U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General.(http://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm) Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014 [accessed 2015 Dec 11].
U.S. Preventive Services Task Force. Tobacco Smoking Cessation in Adults and Pregnant Women: Behavioral and Pharmacotherapy Interventions. Available at http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement147/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed 05/01/2016.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | bkf4 |
| File Modified | 0000-00-00 |
| File Created | 2021-01-26 |
© 2026 OMB.report | Privacy Policy