Section I - Vaccination Planning - WORD
G1. Section I_Vaccination Planning.doc
Information Collections to Advance State, Tribal, Local and Territorial (STLT) Governmental Agency System Performance, Capacity, and Program Delivery
Section I - Vaccination Planning - WORD
OMB: 0920-0879
Attachment G1 Section I: Vaccination Planning_TEST - Final
Form Approved
OMB Number: 0920-0879
Expiration Date: 03/31/2018
Introduction
Background
The
2009 H1N1 influenza pandemic underscored the importance of
communities being prepared for potential threats to public health
security. Because of its unique abilities to respond to infectious,
occupational, or environmental incidents, the Centers for Disease
Control and Prevention (CDC) plays a pivotal role in ensuring that
state and local public health systems are prepared for these and
other public health incidents.
The
identification of the novel influenza A (H7N9) virus illnesses in
China in 2013 highlights the importance of influenza pandemic
preparedness. To date, the reported case fatality ratio from human
H7N9 infections is more than 30%. Should the H7N9 virus mutate to
allow for sustained human-to-human transmission, it appears capable
of causing severe disease in all ages. To better prepare for such a
scenario, it is important to understand the collective
ability of our nation to prepare for and respond to a pandemic of
substantially different epidemiology than the 2009 H1N1
pandemic.
State and local public health
departments are first responders for public health incidents. To
better prepare these agencies to respond, CDC provides funding and
technical assistance for state, local, and territorial public health
departments through the Public Health Emergency Preparedness (PHEP)
cooperative agreement. CDC’s Public Health Preparedness
Capabilities: National Standards for State and Local Planning provide
national standards that help state and local public health
departments strengthen their ability to respond to all hazards,
including influenza pandemics, and build more resilient communities.
Consistent with this approach, the following Pandemic Preparedness
Readiness Assessment for State and Local Public Health Planners
specifically aligns with 11 public health preparedness capabilities
and administrative preparedness planning goals.
Overview
The
Pandemic Preparedness Readiness Assessment for State and Local Public
Health Planners promotes state, local, and territorial public health
preparedness and immunization program collaboration through the
administration of a self-assessment designed to measure
jurisdictional readiness to respond to an influenza pandemic.
Although the content of this assessment does not encompass every
contingency or element necessary to effectively respond to an
influenza pandemic, CDC technical experts in differing programs have
helped to arrange content within the following seven priority
planning areas:
1.
Vaccination Planning
2.
Epidemiology and laboratory
3.
Medical Care and Countermeasures
4.
Healthcare Systems
5.
Community Mitigation
6.
Public Information and Communication
7.
Public Health and Immunization Workforce
Information
collected from the assessment will not be used to score or
competitively rank public health emergency preparedness or
immunization programs. Rather, this assessment is designed to
identify preparedness gaps, as well as promising state, local, and
territorial preparedness practices. Assessment results will be used
by the CDC to inform technical assistance and future program
improvement initiatives.
Definitions
Allocation:
Amount of pandemic influenza vaccine available for
ordering.
Allocating:
Process of dividing available vaccine among CDC’s PHEP awardees
or among registered pandemic influenza vaccine providers and
facilities within an awardee’s jurisdiction.
Critical
infrastructure personnel (CIP):
The full list of CIP is defined in Guidance on Allocating and
Targeting Pandemic Influenza Vaccine; U.S. Department of Health and
Human Services (HHS)/U.S. Department of Homeland Security (DHS);
2008 Guidance
on Allocating and Targeting Pandemic Influenza Vaccine
Distribution:
The process of transporting pandemic influenza vaccine from one
location to another.
Enrollment:
The process of enabling registered healthcare providers and
facilities to legally provide pandemic influenza vaccine.
Ordering:
Process of requesting pandemic influenza vaccine from either the
federal, state, city, or local government. Orders can be placed
against an allocation or independent of
allocation.
Non-pharmaceutical
interventions (NPIs): Those
interventions that can mitigate transmission of influenza and do not
involve medical countermeasures. NPIs include voluntary home
isolation, school closures, respiratory etiquette, hand hygiene, and
routine cleaning of frequently touched surfaces and objects.
Peak
vaccine administration capacity:
The highest rate at which a jurisdiction is able to provide pandemic
influenza vaccine to its population; CDC recommends a peak vaccine
administration capacity of at least 10% of the population per
week.
Point
of dispensing (POD) / mass vaccination clinic:
Location for dispensing medical countermeasures, specifically for
vaccine, during an influenza pandemic response. Located in a
public or private space, this clinic is designed to vaccinate a large
group of persons over a short time period. The POD or clinic might
target the entire population or people in specific priority or
high-risk groups. Public and/or private entities can manage a POD or
clinic.
Closed
POD: Point of
dispensing/vaccination clinic closed to the general public and open
only to a specific group (e.g., staff of a participating business or
healthcare personnel in a specific hospital).
Open
POD: Point of
dispensing/vaccination clinic open to the general public,
specifically to provide vaccine, during an influenza pandemic
response.
Recruitment:
The process of soliciting healthcare providers and facilities
interested in and willing to provide pandemic influenza
vaccine.
Registration:
The submission of required information, similar to an application, by
healthcare providers or facilities interested in providing pandemic
influenza vaccinations.
Retail-based
clinics: Non-pharmacy
businesses that sell retail products (e.g., Walmart, Target) and
serve as PODs/mass vaccination clinics.
School-located
vaccination clinics:
Vaccination clinics that target students and are typically held on
school grounds.
Public reporting burden of this collection of information is estimated to average 120 minutes per response, including time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid Office of Management and Budget control number. Send comments regarding this burden estimate, or any other aspect of this information collection, including suggestions for reducing this burden to CDC/Agency for Toxic Substance and Disease Registry Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; Attention: PRA (0920-0879).
(End of Page 1)
![]()
Section I: Vaccination Planning
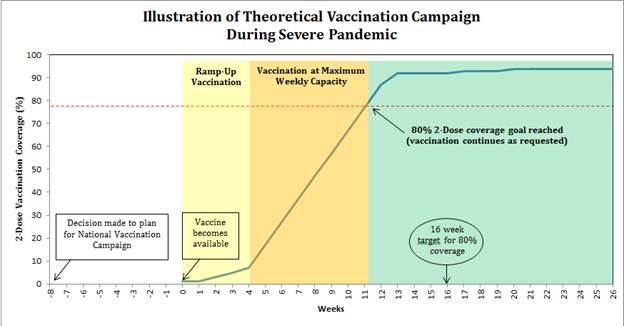
The
identification of novel influenza A(H7N9) virus illnesses among
persons in China in 2013 highlighted the importance of planning and
preparedness for a potential vaccination response during a severe
pandemic. During the 2009 H1N1 pandemic, state and local public
health programs had four to five months to prepare for their 2009
H1N1 vaccination campaigns. Nationally, the maximum number of persons
vaccinated at the peak week of vaccination was approximately 5-6
million in that week. In a severe pandemic, nationally, at peak
capacity, approximately 30 million doses of pandemic vaccine could be
available for distribution each week. This increased capacity is due
to improvements in vaccine manufacturing and distribution capacity in
the United States since 2009 and other potential dose sparing
strategies. CDC’s theoretical modeling of a possible severe
influenza pandemic, where all ages are susceptible, suggests that
rapid administration of much larger numbers of pandemic vaccine doses
than available during the 2009 H1N1 pandemic and much earlier in a
response is vital to reducing the number of hospitalizations and
deaths associated with a severe pandemic. Intense planning is needed
for broad vaccination campaigns to be initiated as soon as vaccine
becomes available at the state and local level in order to
substantially reduce the potential morbidity and mortality during a
severe pandemic.
Public health programs should aim to
vaccinate 80% of their jurisdiction’s population with two doses
(separated by 21 days) of a pandemic influenza vaccine in less than
16 weeks.
With this national goal in mind, the purpose of
this influenza pandemic plan assessment is to:
•
Assess public health emergency preparedness programs’ ability
to achieve the goal of vaccinating 80% of their jurisdiction’s
population with two doses of a pandemic influenza vaccine separated
by 21 days in less than 16 weeks.
• Assess gaps and
challenges to meeting the national goal
Severe
Pandemic Vaccine Preparedness Goal:
Given an 8 week notification to begin a national pandemic
vaccination campaign, state and local programs will achieve at least
an 80% pandemic vaccination coverage for two doses of the pandemic
vaccine (separated by 21 days) for their jurisdiction’s
population, within 16 weeks from when the vaccine becomes available.
(Note: In this scenario, time zero would be a declaration of the
start of a national vaccination program. Vaccine would become
available two months (8 weeks) after that notification.)
Severe Pandemic
Influenza: Preparedness Assumptions
• Disease will be severe, and all ages will be
susceptible.
• Demand for the pandemic influenza vaccine
will be high throughout the response; 80% of the population will want
to be vaccinated.
• Seasonal influenza vaccine production
and campaign could be halted.
• Two doses of pandemic
influenza vaccine, separated by at least 21 days will be recommended
for all people ages 6 months and older.
• Most pandemic
influenza vaccine will be inactivated and include an adjuvant (some
pre-mixed) and be supplied in multi-dose vials. Some live attenuated
inactivated vaccine (nasal spray) will be available.
•
Adequate federal funding will be available to implement a large-scale
vaccination response.
• Federally-supplied pandemic
influenza vaccine and ancillary supplies will be supplied under an
Emergency Use Authorization (EUA) in amounts needed to vaccinate at
least 10% of the U.S. population per week at the onset of
distribution.
• CDC will provide standard communication
materials on the EUA for the general public, similar to the Vaccine
Information Statement (VIS), and specific communication to vaccine
providers on the EUA.
• Pandemic influenza vaccine
distribution based on awardee population will begin approximately 60
days after notification of a national vaccination campaign.
•
Vaccine prioritization may be recommended for the first two to three
weeks for young children, pregnant women, high-risk adults,
healthcare workers, and certain other critical infrastructure
personnel (CIP) (see Guidance on Allocating and Targeting Pandemic
Influenza Vaccine U.S.; Department of Health and Human Services
(HHS)/U.S. Department of Homeland Security (DHS); 2008. Guidance
on Allocating and Targeting Pandemic Influenza Vaccine)
•
Awardees will be required to legally enroll pandemic influenza
vaccine providers (similar to the 2009 H1N1 response)
•
Awardees will be allocated vaccine, as it becomes available, based on
their jurisdiction’s population size (similar to the 2009 H1N1
response)
Please select your jurisdiction:
Alabama
Alaska
American Samoa
Arizona
Arkansas
California
Chicago
Colorado
Commonwealth of the Northern Mariana Islands
Connecticut
Delaware
Federated States of Micronesia
Florida
Georgia
Guam
Hawaii
Idaho
Illinois
Indiana
Iowa
Kansas
Kentucky
Los Angeles County
Louisiana
Maine
Maryland
Massachusetts
Michigan
Minnesota
Mississippi
Missouri
Montana
Nebraska
Nevada
New Hampshire
New Jersey
New Mexico
New York
New York City
North Carolina
North Dakota
Ohio
Oklahoma
Oregon
Pennsylvania
Puerto Rico
Republic of Palau
Republic of the Marshall Islands
Rhode Island
South Carolina
South Dakota
Tennessee
Texas
U.S. Virgin Islands
Utah
Vermont
Virginia
Washington
Washington, DC
West Virginia
Wisconsin
Wyoming
Please select your position:
PHEP Director
Immunization Coordinator
Epidemiologist
Other (please specify) ____________________
(End of Page 2)
![]()
Vaccine Planning, Management, and Administration
During the most recent H7N9 pandemic vaccine assessment conducted in June 2013, H7N9 vaccination response leads in PHEP awardee jurisdictions were asked to estimate their jurisdiction’s maximum weekly capacity to administer H7N9 vaccine given a set of assumptions. H7N9 response leads were also asked to estimate how many weeks this maximum weekly capacity could be sustained. The first few questions refer specifically to your prior answers to the June 2013 assessment, which we provided in the e-mail with the link to this survey.
(End of Page 3)
![]()
Vaccine Administration / Capacity
The following set of questions asks you to think again about your maximum weekly vaccination capacity, including any revisions to your previous estimates.
(End of Page 4)
![]()
Vaccine Administration / Capacity
1. Given any changes in your plan since the June 2013 H7N9 pandemic influenza vaccine assessment, do you wish to revise your jurisdiction’s estimate of maximum weekly vaccination capacity?
Yes >>>> Skip to Page 6: 2. What is your revised estimate?
No >>>>
Skip to Page 7: 3. Do you wish to revise your estimate of the maximum
number of weeks that you believe your jurisdiction could sustain
vaccination at maximum capacity?
(Note: This question does not include any time your jurisdiction
would be vaccinating at less than maximum capacity).
(End of Page 5)
![]()
Vaccine Administration / Capacity
2. What is your revised estimate?
(Please enter a whole number) ____________________
(End of Page 6)
![]()
Vaccine Administration / Capacity
3.
Do you wish to revise your estimate of the maximum number of weeks
that you believe your jurisdiction could sustain vaccination at
maximum capacity? (Note:
This question does not include any time your jurisdiction would be
vaccinating at less than maximum capacity).
Yes >>>> Skip to Page 8: 4. What is your revised estimate?
No >>>> Skip to Page 9: 5. How many weeks do you estimate that it would take for your jurisdiction to accelerate and reach your maximum weekly vaccination capacity once vaccination begins?
(End of Page 7)
![]()
Vaccine Administration / Capacity
4. What is your revised estimate?
(Please enter a whole number) ____________________
(End of Page 8)
![]()
Vaccine Administration / Capacity
5. How many weeks do you estimate that it would take for your jurisdiction to accelerate and reach your maximum weekly vaccination capacity once vaccination begins?
(Please enter a whole number) ____________________
6. Please estimate the percent of your population that could receive one dose of vaccine during the time it would take for your jurisdiction to reach your maximum weekly vaccination capacity:
(Please enter a percentage) ____________________
7. Please estimate the percent of your population that would receive two doses of vaccine separated by at least three weeks during the time it would take for your jurisdiction to reach your maximum weekly vaccination capacity. (Please note that this question will assume that CDC has matched your jurisdictions’ vaccine capability and that you have received proper advanced notice on vaccine readiness before a widespread epidemic)
(Please enter a percentage) ____________________
The following questions refer specifically to the newly stated goal of 80% two-dose pandemic vaccination coverage within 16 weeks of vaccination initiation.
(End of Page 9)
![]()
Vaccine Administration / Capacity
8. Do you expect that your program will be able to vaccinate 80% of your jurisdiction’s population within 16 weeks of vaccination initiation?
Yes >>>>
Skip to Page 11: 9. How did you calculate the number of weeks your
program would need to reach 80% two-dose pandemic vaccination
coverage?
•
For example, one way of outlining your thinking would be to estimate
the number of PODs your jurisdiction can simultaneously conduct based
on staffing and resources, how many hours a day and numbers of days
per week and number of total weeks that these PODs can be sustained.
Programs would also need to estimate the number of doses administered
per hour per POD.
• Some programs may also
outline the estimated number of vaccine providers which could be
enrolled, trained, and would be willing to administer pandemic
vaccines within 60 days and then use any data on the number of doses
per day each provider could reasonably administer.
•
Please keep in mind the health department staffing and vaccine
provider enrollment and training requirements for any of your plans.
No (Please specify the number of weeks you estimate that your program will need to vaccinate 80% of your jurisdiction’s population ) ____________________ >>>> Skip to Page 12: 10. Please list up to five barriers you would need to address to reach this newly stated goal of 80% two-dose pandemic vaccination coverage in 16 weeks. (Please note that this should allow, at a minimum, 3 weeks between doses).
(End of Page 10)
![]()
Vaccine Administration / Capacity
9.
How did you calculate the number of weeks your program would need to
reach 80% two-dose pandemic vaccination coverage?
•
For example, one way of outlining your thinking would be to estimate
the number of PODs your jurisdiction can simultaneously conduct based
on staffing and resources, how many hours a day and numbers of days
per week and number of total weeks that these PODs can be sustained.
Programs would also need to estimate the number of doses administered
per hour per POD.
• Some programs may also
outline the estimated number of vaccine providers which could be
enrolled, trained, and would be willing to administer pandemic
vaccines within 60 days and then use any data on the number of doses
per day each provider could reasonably administer.
•
Please keep in mind the health department staffing and vaccine
provider enrollment and training requirements for any of your plans.
______________________________________________________________
______________________________________________________________
______________________________________________________________
(End of Page 11)
![]()
Vaccine Administration / Capacity
10. Please list up to five barriers you would need to address to reach this newly stated goal of 80% two-dose pandemic vaccination coverage in 16 weeks. (Please note that this should allow, at a minimum, 3 weeks between doses).
Barrier 1 ____________________
Barrier 2 ____________________
Barrier 3 ____________________
Barrier 4 ____________________
Barrier 5 ____________________
(End of Page 12)
![]()
Vaccination of Critical Infrastructure
11.
In an earlier assessment, you were asked whether your jurisdiction
had an operational plan for identifying and vaccinating critical
infrastructure personnel (CIP) as described in Tier 1 and 2 of the
2008 HHS/ DHS Guidance on Allocating and Prioritization of Pandemic
Vaccine (see: Guidance
on Allocating and Targeting Pandemic Influenza Vaccine)
Does
your jurisdiction currently have such an operational plan?
Yes, to identify CIP
Yes, to vaccinate CIP
Yes, to identify and vaccinate CIP
This is a local health responsibility
Not sure
No >>>> Skip to Page 15: 16. Do you plan to develop an operational plan for identifying and vaccinating CIP?
(End of Page 13)
![]()
Vaccination of Critical Infrastructure
12. Des your plan include using the regular seasonal influenza vaccinators to vaccinate CIP during a pandemic?
Yes
No
Not sure
N/A
13. Des your plan include coordination with occupational health providers, to include closed PODs, to vaccinate CIP during a pandemic?
Yes
No
Not sure
N/A
14. Does your plan incorporate the use of closed PODs, separate from existing seasonal influenza clinics, for vaccinating CIP during a pandemic?
Yes
No
Not sure
N/A
15. What other specific characteristics of your plans to identify and vaccinate CIP during a pandemic can you share with CDC?
______________________________________________________________
______________________________________________________________
______________________________________________________________
(End of Page 14)
![]()
Vaccination of Critical Infrastructure
16. Do you plan to develop an operational plan for identifying and vaccinating CIP?
Yes
No >>>> Skip to Page 18: 18. Which of the following statements BEST describes the process you will use to divide your pandemic influenza vaccine among providers and facilities within your jurisdiction? (Note: this question refers to both the ‘ramp-up’ and ‘maximum capacity’ phases)
(End of Page 15)
![]()
Vaccination of Critical Infrastructure
17. Please describe how you might develop and exercise such a plan and the timeline for doing so:
______________________________________________________________
______________________________________________________________
______________________________________________________________
(End of Page 16)
![]()
Vaccine Allocation
The next set of questions focus on the processes your jurisdiction will use to distribute pandemic influenza vaccine from your allocation. Distribution procedures may change over time; please answer the following questions with reference to the first three months after pandemic influenza vaccine becomes available for administration.
(End of Page 17)
![]()
Vaccine Allocation
18. Which of the following statements BEST describes the process you will use to divide your pandemic influenza vaccine among providers and facilities within your jurisdiction? (Note: this question refers to both the ‘ramp-up’ and ‘maximum capacity’ phases)
We (i.e., PHEP awardee) plan to divide all or most of our allocation (PHEP awardee) equally among all of our local jurisdictions by the total population of each (e.g., if one local health department serves 10% of our state's population, we will send them 10% of the vaccine allocated to us by CDC). Our local jurisdictions will be responsible for allocating their proportion to providers and facilities within their jurisdictions >>>> Skip to Page 19: 19. Do you provide general guidance to local jurisdictions for how to allocate vaccine to registered providers and facilities?
We (i.e., PHEP awardee) will decide, at the awardee level, how to allocate vaccine among providers and facilities located within all our jurisdictions. >>>> Skip to Page 22: 22. What factors or principles will you use to decide how to allocate vaccine to registered providers and facilities within your jurisdiction? (For example, a state’s general principle might be with each batch of vaccine the state is allocated from CDC, they would spread vaccine “wide and thin” – allocating to many providers, but each would get many small shipments over the entire response. Another state might choose to focus initial vaccine supplies on mass vaccination clinics to target priority groups).
Both A and B >>>> Skip to Page 19: 19. Do you provide general guidance to local jurisdictions for how to allocate vaccine to registered providers and facilities?
Neither A nor B >>>> Skip to Page 25: 25. What processes will you use to divide the jurisdiction’s pandemic influenza vaccine allocation?
(End of Page 18)
![]()
Vaccine Allocation
19. Do you provide general guidance to local jurisdictions for how to allocate vaccine to registered providers and facilities?
Yes
No
(End of Page 19)
![]()
Vaccine Allocation
20. Do most of your local jurisdictions have plans to identify and adjust vaccine allocation levels (if necessary) due to changes in the target population, spot shortages, uneven demand for vaccine, etc.?
Yes
No >>>>
Skip to Page 26: 26. Using the allocation tool provided by CDC, and
based on your pandemic plan and knowledge about your jurisdiction,
please estimate the proportion of your jurisdiction’s weekly
vaccine allocation that you plan to allocate to each of the following
type of provider group or venue when there is ample vaccine supply.
(You may need to
work directly with local jurisdictions to understand their plans to
provide this breakdown for your entire state or jurisdiction.)
(End of Page 20)
![]()
Vaccine Allocation
21. Do your local jurisdictions’ plans describe the processes, procedures, and logistics necessary to reallocate pandemic influenza vaccine promptly?
Yes
No >>>>
Skip to Page 26: 26. Using the allocation tool provided by CDC, and
based on your pandemic plan and knowledge about your jurisdiction,
please estimate the proportion of your jurisdiction’s weekly
vaccine allocation that you plan to allocate to each of the following
type of provider group or venue when there is ample vaccine supply.
(You may need to
work directly with local jurisdictions to understand their plans to
provide this breakdown for your entire state or jurisdiction.)
(End of Page 21)
![]()
Vaccine Allocation
22. What factors or principles will you use to decide how to allocate vaccine to registered providers and facilities within your jurisdiction? (For example, a state’s general principle might be with each batch of vaccine the state is allocated from CDC, they would spread vaccine “wide and thin” – allocating to many providers, but each would get many small shipments over the entire response. Another state might choose to focus initial vaccine supplies on mass vaccination clinics to target priority groups).
______________________________________________________________
______________________________________________________________
______________________________________________________________
(End of Page 22)
![]()
Vaccine Allocation
23. Do you have processes in place to identify and adjust vaccine allocation levels (if necessary) due to changes in the target population, spot shortages, uneven demand for pandemic influenza vaccine, etc.?
Yes
No >>>>
Skip to Page 26: 26. Using the allocation tool provided by CDC, and
based on your pandemic plan and knowledge about your jurisdiction,
please estimate the proportion of your jurisdiction’s weekly
vaccine allocation that you plan to allocate to each of the following
type of provider group or venue when there is ample vaccine supply.
(You may need to
work directly with local jurisdictions to understand their plans to
provide this breakdown for your entire state or jurisdiction.)
(End of Page 23)
![]()
Vaccine Allocation
24. Does your plan describe the processes, procedures, and logistics necessary to reallocate pandemic influenza vaccine promptly?
Yes
No
(End of Page 24)
![]()
Vaccine Allocation
25. What processes will you use to divide the jurisdiction’s pandemic influenza vaccine allocation?
______________________________________________________________
______________________________________________________________
______________________________________________________________
(End of Page 25)
![]()
Vaccine Allocation
26.
Using the allocation tool provided by CDC, and based on your pandemic
plan and knowledge about your jurisdiction, please estimate the
proportion of your jurisdiction’s weekly vaccine allocation
that you plan to allocate to each of the following type of provider
group or venue when there is ample vaccine supply.
(You
may need to work directly with local jurisdictions to understand
their plans to provide this breakdown for your entire state or
jurisdiction.)
Closed PODs (i.e., those PODs designed only to administer vaccines to certain target groups, such as CIP) ____________________
Open PODs - to vaccinate general public ____________________
School-located vaccination clinics ____________________
Providers already enrolled as Vaccines for Children (VFC) providers ____________________
Non-VFC pediatric providers ____________________
Non-VFC adult providers ____________________
Community vaccinators such as visiting nurse associations ____________________
Large retail-based stores or grocery stores ____________________
Large national or regional chain pharmacies ____________________
Independent, local pharmacies ____________________
Local health departments (LHDs) ____________________
Other ____________________
(End of Page 26)
![]()
Vaccine Provider Enrollment and Training
27. Please estimate the proportion of your jurisdiction’s population that you expect will be vaccinated through the public health system.
(Please round to the nearest whole percent) ____________________
(End of Page 27)
![]()
Vaccine Provider Enrollment and Training
28. Does your jurisdiction plan to provide more than 20% of your vaccine allocation through PODs?
Yes
No >>>> Skip to Page 30: 33. Has your jurisdiction conducted jurisdiction-wide multi-POD exercises (greater than 1 POD conducted simultaneously at different venues) to test POD capacities?
(End of Page 28)
![]()
Vaccine Provider Enrollment and Training
29. Has your jurisdiction pre-identified enough personnel to staff such events?
Yes
Yes, but funding / support not available
No
In progress
30. Has the pre-identified POD staff received training in POD (open and closed) operations?
Yes
No
In progress
31. Has the pre-identified staff received training in mass vaccination clinic operations?
Yes
No
In progress
32. Does your jurisdiction plan to conduct multiple, simultaneous PODs (open and closed) or other mass vaccination clinics during an influenza pandemic response?
Yes
No
(End of Page 29)
![]()
Vaccine Provider Enrollment and Training
IF JURISDICTION PLANS TO CONDUCT MULTIPLE, SIMULTANEOUS PODS AND/OR MASS VACCINATION CLINICS
33. Has your jurisdiction conducted jurisdiction-wide multi-POD exercises (greater than 1 POD conducted simultaneously at different venues) to test POD capacities?
Yes
No >>>> Skip to Page 32: 35. Does your jurisdiction plan to conduct jurisdiction-wide multi-POD exercises to test your POD capacities?
(End of Page 30)
![]()
Vaccine Provider Enrollment and Training
34. Please describe your POD exercises:
______________________________________________________________
______________________________________________________________
______________________________________________________________
(End of Page 31)
![]()
Vaccine Provider Enrollment and Training
35. Does your jurisdiction plan to conduct jurisdiction-wide multi-POD exercises to test your POD capacities?
Yes
No
(End of Page 32)
![]()
Vaccine Provider Enrollment and Training
36. Does your jurisdiction have laws or regulations in place permitting expanded authority for additional types of providers, including but not limited to, pharmacists, pharmacist assistants, medical/nursing students, dentists, medical provider retiree, and others, to vaccinate during a public health emergency, outside of those providers typically permitted to vaccinate during regular influenza seasons and non-emergencies, and state of emergencies?
Yes
No >>>> Skip to Page 35: 38. Does your jurisdiction have laws or regulations in place permitting expanded authority for existing vaccinators, including pharmacists and other nontraditional providers, to vaccinate persons of all ages during a public health emergency, if they are typically not permitted to do so during non-emergencies?
In progress
(End of Page 33)
![]()
Vaccine Provider Enrollment and Training
37. Please cite the code of the regulation or law and provide relevant URL or website here:
______________________________________________________________
(End of Page 34)
![]()
Vaccine Provider Enrollment and Training
38. Does your jurisdiction have laws or regulations in place permitting expanded authority for existing vaccinators, including pharmacists and other nontraditional providers, to vaccinate persons of all ages during a public health emergency, if they are typically not permitted to do so during non-emergencies?
Yes
No >>>> Skip to Page 37: 40. Does your jurisdiction have laws or regulations in place permitting out-of-state providers to provide vaccinations in your jurisdiction during a public health emergency?
In progress
(End of Page 35)
![]()
Vaccine Provider Enrollment and Training
39. Please cite the code of the regulation or law and provide relevant URL or website here:
______________________________________________________________
(End of Page 36)
![]()
Vaccine Provider Enrollment and Training
40. Does your jurisdiction have laws or regulations in place permitting out-of-state providers to provide vaccinations in your jurisdiction during a public health emergency?
Yes
No >>>> Skip to Page 39: 42. Please provide any other information, comments, or concerns about these provisions, especially if these provisions differ by provider type (e.g. pharmacy versus other community vaccinator), including any information about requirements or guidance to these providers on reporting vaccination data to immunization information systems:
In progress
(End of Page 37)
![]()
Vaccine Provider Enrollment and Training
41. Please cite the code of the regulation or law and provide relevant URL or website here:
______________________________________________________________
(End of Page 38)
![]()
Vaccine Provider Enrollment and Training
42. Please provide any other information, comments, or concerns about these provisions, especially if these provisions differ by provider type (e.g. pharmacy versus other community vaccinator), including any information about requirements or guidance to these providers on reporting vaccination data to immunization information systems:
______________________________________________________________
______________________________________________________________
______________________________________________________________
(End of Page 39)
![]()
Vaccine Provider Enrollment and Training
43. Which entity within your jurisdiction would have primary responsibility for activities related to registering and enrolling healthcare providers and facilities to become pandemic influenza vaccine providers?
Awardee health department
Local health department(s)
Regional health department(s)
Other
44. Please choose the statement below that most accurately reflects your jurisdiction’s plans to recruit non-Vaccines for Children (VFC) providers serving primarily adults.
Directly using contact information contained in a current list maintained by public health
Either directly or indirectly by using contact information collected by an entity or entities other than public health (e.g., professional organizations)
By making a general call for volunteers (e.g., via media)
Other ____________________
45. If using contact information provided by entity other than public health, please specify which entity:
______________________________________________________________
______________________________________________________________
______________________________________________________________
IF JURISDICTION PLANS TO RECRUIT NON-VFC PROVIDERS SERVING PRIMARILY ADULTS
46. Please choose the statement below that most accurately reflects when your jurisdiction plans to begin registering and enrolling non-VFC providers serving primarily adults to become pandemic influenza vaccine providers.
Active registration (or preregistration) is currently ongoing
Pre-declaration
When a pandemic is declared
When a decision is made to initiate a national vaccination program
Other ____________________
47. Given the increased number of providers you expect to enroll as pandemic influenza vaccine providers to reach the 80% two-dose pandemic vaccination coverage in 16 weeks, for which of these areas does your jurisdiction have the ability to train all newly enrolled providers (check all that apply)?
Vaccine provider ordering requirements
Appropriate vaccine storage and handling
Adverse events reporting
Gaining approval for access to the Immunization Information System (IIS)
Submitting data to the IIS or similar administration reporting systems
Other ____________________
(End of Page 40)
![]()
Vaccine Ordering and Management
48. Given the increased number of providers and vaccine orders during a severe pandemic, does your jurisdiction have a staffing and budget plan in place to manage vaccine orders?
Yes
No
49. Please briefly describe how provider vaccine orders will be placed and managed in your jurisdiction. For example, some jurisdiction may have providers place orders directly in VTrckS or another system linked to VTrckS, while other programs may have individual providers submit vaccine requests in some other manner for the jurisdictions to manage these orders.
______________________________________________________________
______________________________________________________________
______________________________________________________________
(End of Page 41)
![]()
Immunization Information System (IIS)
50. Given that 80% of your jurisdiction’s population may have vaccine information on each of their two pandemic doses entered into your jurisdiction’s IIS, can your IIS process this increased amount of data?
Yes >>>> Skip to Page 44: 52. Does your IIS have the capacity to track use of adjuvant and match adjuvant and vaccine type by person?
No
Not sure
(End of Page 42)
![]()
Immunization Information System (IIS)
51. Do you have a plan and projected budget needs to increase its capacity?
Yes
No
Not sure
(End of Page 43)
![]()
Immunization Information System (IIS)
52. Does your IIS have the capacity to track use of adjuvant and match adjuvant and vaccine type by person?
Yes >>>> Skip to Page 46: 54. Given the increased number of vaccine providers and amount of vaccine data, do you have a staffing plan to provide technical assistance to this number of providers and manage data in your IIS?
No
Not sure
(End of Page 44)
![]()
Immunization Information System (IIS)
53. Will you develop a plan and projected budget needs for your IIS to have this capacity?
Yes
No
Not sure
(End of Page 45)
![]()
Immunization Information System (IIS)
54. Given the increased number of vaccine providers and amount of vaccine data, do you have a staffing plan to provide technical assistance to this number of providers and manage data in your IIS?
Yes >>>> Skip to Page 48: 56. In the April 2013 IIS survey, many programs noted that they have pandemic/mass vaccination/preparedness modules or functionality in their IIS or other system. Does your jurisdiction have such a module?
No
Not sure
(End of Page 46)
![]()
Immunization Information System (IIS)
55. If no, will you develop a staffing plan to do so?
Yes
No
Not sure
(End of Page 47)
![]()
Immunization Information System (IIS)
56. In the April 2013 IIS survey, many programs noted that they have pandemic/mass vaccination/preparedness modules or functionality in their IIS or other system. Does your jurisdiction have such a module?
Yes
No >>>> Skip to Page 51: 60. How are these functions currently used?
(End of Page 48)
![]()
Immunization Information System (IIS)
57. Has this module or functionality been used in any capacity (e.g., seasonal flu clinic) in your jurisdiction?
Yes
No >>>> Skip to Page 52: 61. If your jurisdiction’s IIS or other system does not have a separate module or functionality, please describe how you would support a mass vaccination clinic in terms of collecting vaccine administration data?
(End of Page 49)
![]()
Immunization Information System (IIS)
IF JURISDICTION HAS A SEPARATE PANDEMIC / MASS VACCINATION MODULE
58. When was this module or functionality last used?
______________________________________________________________
______________________________________________________________
______________________________________________________________
59. Please describe the primary functions of the module or system. For example, do the primary functions of your module or IIS include rapid data entry modes or specialized reports?
______________________________________________________________
______________________________________________________________
______________________________________________________________
(End of Page 50)
![]()
Immunization Information System (IIS)
60. How are these functions currently used?
______________________________________________________________
______________________________________________________________
______________________________________________________________
(End of Page 51)
![]()
Immunization Information System (IIS)
IF JURIDISDICTION DOES NOT HAVE A SEPARATE PANDEMIC/MASS VACCINATION MODULE
61. If your jurisdiction’s IIS or other system does not have a separate module or functionality, please describe how you would support a mass vaccination clinic in terms of collecting vaccine administration data?
______________________________________________________________
______________________________________________________________
______________________________________________________________
(End of Page 52)
![]()
62. In the April 2013 IIS survey, many jurisdictions noted that they would require pandemic vaccine providers to report doses administered to the IIS. To the best of your knowledge, please estimate the timeframe, on average, in which your jurisdiction would expect providers administering pandemic vaccine to submit data on the first dose of pandemic vaccine antigen and adjuvant to the IIS after the vaccination encounter? (See the CY2012 Immunization Information System Annual Report for current timeliness intervals and the 2013-2017 IIS Functional Standards)
Awardee health department
Local health department(s)
Regional health department(s)
Other
(End of Page 53)
![]()
| File Type | application/msword |
| File Title | G1. Section I_Vaccination Planning |
| Author | Nacalaban, Olga |
| Last Modified By | CDC User |
| File Modified | 2015-03-25 |
| File Created | 2015-03-20 |
© 2026 OMB.report | Privacy Policy