Word Version of the Information Collection Instrument (for reference)
Attachment A- Instrument_Word Version.docx
Information Collections to Advance State, Tribal, Local and Territorial (STLT) Governmental Agency System Performance, Capacity, and Program Delivery
Word Version of the Information Collection Instrument (for reference)
OMB: 0920-0879

Welcome! This information collection is meant for state Medicaid Medical Directors, or their designee, with knowledge of state Medicaid policies related to pediatric attention-deficit/hyperactivity disorder (ADHD) treatment. The Policy Surveillance Program at Temple University Beasley School of Law (PSP) and the Centers for Disease Control and Prevention (CDC) have already begun collecting information about your state’s Medicaid policies from publicly available sources. The purpose of this questionnaire is to verify that we collected the most up-to-date and accurate information from your state.
Your feedback is important to us. Your participation will help us identify where and why there are gaps in Medicaid reimbursement related to ADHD treatment, which will allow us to conduct more precise analyses on the impacts of these policies.
Completing the questionnaire is voluntary and takes approximately 20 minutes. Please note that any personally identifiable information about respondents related to their official duties (name, position, agency, phone, and email) will be removed when the results of this questionnaire are aggregated for analysis. Responses will be kept secure and will not be shared with other respondents or other entities. There are no known risks or direct benefits to you from participating or choosing not to participate, but your answers will help CDC improve its understanding of Medicaid policies that influence pediatric ADHD treatment and might help other states develop or improve their policies.
If you have any questions or concerns about this questionnaire, please email CDC at [email protected].
To begin, please proceed to page 2.
When you have completed the questionnaire, please click the SUBMIT button on page 1 or email the survey as an attachment to [email protected].

CDC estimates the average public reporting burden for this collection of information as 20 minutes per response, including the time for reviewing instructions, searching existing data/information sources, gathering and maintaining the data/information needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (0920-0879).

The Temple PSP and CDC have compiled information on ADHD prior authorization policies across all states and the District of Columbia. For the purposes of responding to all questions below, a prior-authorization policy is defined as any policy that requires prior authorization, additional review, or other additional prescriber involvement for obtaining approval and payment for ADHD medications prescribed to children younger than age 18 years.
The policy information we have compiled should reflect what was accurate for your jurisdiction as of November 1, 2015.
Please review Appendix A to verify the prior authorization policy information we collected for your state. Is the information listed in Appendix A the most up-to-date policy in your state?
 Yes
Yes
 No,
we have
updated our
policy since
November 1,
2015
No,
we have
updated our
policy since
November 1,
2015


 No,
we did
not have
a policy
on November
1, 2015,
but we
now have
a policy No,
the policy
you have
provided is
incorrect
No,
we did
not have
a policy
on November
1, 2015,
but we
now have
a policy No,
the policy
you have
provided is
incorrect
 Other
Other
If your state Medicaid program does NOT currently have any prior-authorization policy limiting ADHD medication prescriptions to children younger than age 18 years, PLEASE SKIP AHEAD TO SECTION B.
If you answered “No” to question 1, please email a copy of the most recent version of your state’s policy to [email protected], and include the policy’s effective date.
Please review Appendix B to verify the table listing characteristics of your state’s policy. Are all of the responses correct as of November 1, 2015?
 Yes
Yes

 No,
at least
one response
is not
correct as
of November
1, 2015
No,
at least
one response
is not
correct as
of November
1, 2015
Unsure whether all responses are correct as of November 1, 2015
If you answered “Yes” to question 2, please skip question 3 and continue to question 4.
Please indicate below which questions you believe we did not correctly answer in Appendix B. Then, please add an explanation in the box to the right.
 Questions
from Appendix
B
Questions
from Appendix
B
Does your state Medicaid program have a policy that requires prior authorization for ADHD medications prescribed to children younger than age 18 years?
The answer to this question in Appendix B is not correct.
 Please
explain.
Please
explain.
 Which
ages require
prior authorization? The
answer to
this question
in Appendix
B is not
correct.
Which
ages require
prior authorization? The
answer to
this question
in Appendix
B is not
correct.
 Please
explain.
Please
explain.
Does the policy automatically deny authorization for certain ages?
The answer to this question in Appendix B is not correct.

 Please
explain.
Please
explain.
 Does
the policy
specify ADHD
medications that
require prior
authorization?
Does
the policy
specify ADHD
medications that
require prior
authorization?
The answer to this question in Appendix B is not correct.
 Please
explain.
Please
explain.
 Are
stimulants included? The
answer to
this question
in Appendix
B is not
correct.
Are
stimulants included? The
answer to
this question
in Appendix
B is not
correct.
 Please
explain.
Please
explain.
Questions from Appendix B (continued)
Reminder: If you answered “Yes” to question 2, please skip question 3 and continue to question 4.
 Are
non-stimulants
included? The answer
to this
question in
Appendix B
is not correct.
Are
non-stimulants
included? The answer
to this
question in
Appendix B
is not correct.
 Please
explain.
Please
explain.
 Does
the policy
list criteria
for approval? The
answer to
this question
in Appendix
B is not
correct.
Does
the policy
list criteria
for approval? The
answer to
this question
in Appendix
B is not
correct.
 Please
explain.
Please
explain.
 What
criteria are
listed to
receive approval? The
information in
Appendix B
is not
correct.
What
criteria are
listed to
receive approval? The
information in
Appendix B
is not
correct.
 Please
explain.
Please
explain.
How is the prior authorization approval process triggered in your state?
 At
the medication’s
point-of-sale
At
the medication’s
point-of-sale


 When
the prescriber
submits a
prior authorization
form to
a reviewing
body Other (please
explain)
When
the prescriber
submits a
prior authorization
form to
a reviewing
body Other (please
explain)
Is the prior authorization request peer-reviewed by medical experts?

 Yes
No
Yes
No
 Don’t
know
Don’t
know

 Other
(please explain)
Other
(please explain)
When was the very first prior authorization policy implemented in your state for children younger than age 18 years? (Please indicate "unsure" if you do not know the date.)

Has your program ever evaluated this prior authorization policy’s effect on prescription rates for ADHD medications?

 Yes
No
Yes
No
 Don’t
know
Don’t
know
If yes, please explain below.


SECTION B
Please answer the following additional questions regarding behavioral health carve-outs for services provided to children (< age 18 years) in your state Medicaid program.
Does your state Medicaid program currently carve out behavioral or mental health services provided to children?

 Yes
No
Yes
No
 Don’t
know
Don’t
know
If you answered “No” to question 8, please skip ahead to question 11.
If your Medicaid program currently carves out any behavioral or mental health services for children, which services are carved out?


 Outpatient
behavioral/mental health
services Inpatient
behavioral/mental health
services Prescription
drugs
Outpatient
behavioral/mental health
services Inpatient
behavioral/mental health
services Prescription
drugs


 Substance
abuse services
Other (please
explain)
Substance
abuse services
Other (please
explain)
How are they carved out?
 Carved
out to
a fee-for-service delivery system
Carved
out to
a fee-for-service delivery system

 Carved
out to a behavioral health
organization
Carved
out to a behavioral health
organization
 Other,
please explain
Other,
please explain
Please complete the following matrix to indicate whether your state has ever carved out children’s behavioral health services in the past 5 years.
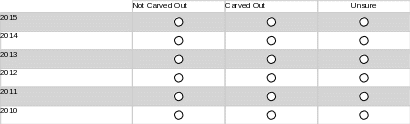
12. Please use this space to provide additional comments about the dates of your state’s carved out behavioral health services in the past five years, if necessary.

Please contact CDC with questions or to discuss your state’s Medicaid program in greater detail.
Contact Information

 13.
Please provide
the
following
information
about the
person who
completed
this
survey:
13.
Please provide
the
following
information
about the
person who
completed
this
survey:
First name: Last name:
Job title: Description of your position:


State: Department:


E-mai: Phone:


14. Has this survey been delegated to you by the Medicaid Director or Medicaid Medical Director in your state?

 Yes
No
Yes
No
 Don’t
know
Don’t
know
15. Please use this section to provide any additional comments or information about your state Medicaid program’s policies related to ADHD medication prescription to children under age 18 years and/or behavioral health services carve outs. You may also email CDC to share any additional information.

When you have completed this survey, please click the SUBMIT button on page 1 or email the survey as an attachment to [email protected].
Click here to return to question 1.
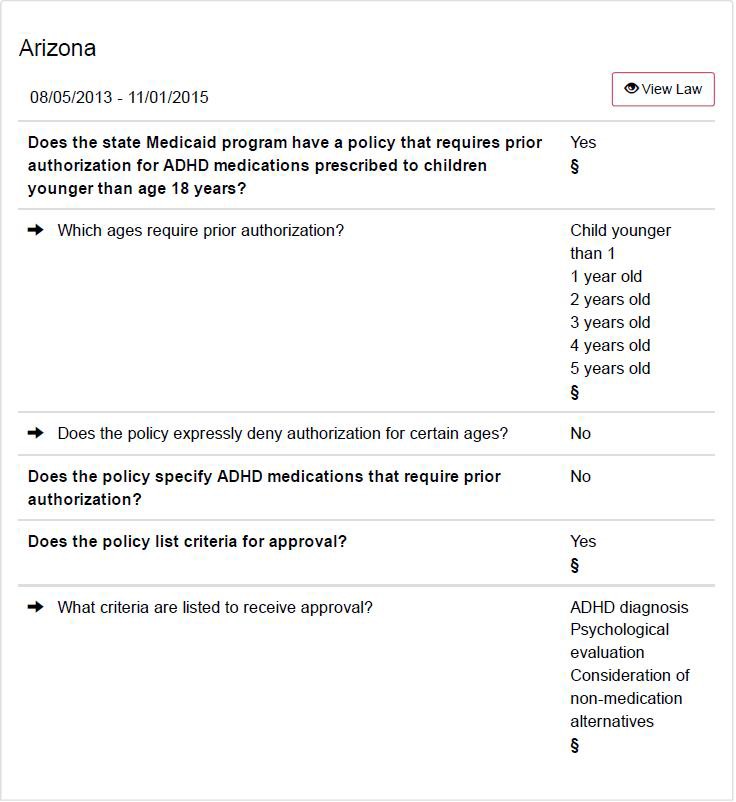
Prior Authorization Guidelines
ADHD Medications in Children Under 6 Years Old
FDA Approved Indication:
Treatment of Attention Deficit Hyperactivity Disorder (ADHD)
Guidelines for Approval:
The requesting clinician has documented that the child has a diagnosis of ADHD
Psychosocial issues and non-medical interventions are being addressed by the clinical team.
Documentation of psychosocial evaluation occurring before request for ADHD medications.
Documentation of non-medication alternatives that have been attempted before request for ADHD medications.
Additional Requirements:
Children under 6 years old will be monitored in accordance with the ADHS/DBHS Clinical Practice Protocol on Psychiatric Best Practice Guidelines for Children: Birth to Five Years of Age.
Coverage is Not Authorized for:
Indications other than ADHD.
Doses greater than FDA recommended maximum daily dosage.
References:
ADHS/DBHS: Provider Manual Section 3.15: Psychotropic Medication: Prescribing and Monitoring
Manufacturer Product Information
Pliska SR, Greenhill LL, Crismon ML, et al. The Texas children’s medication algorithm project: report of the Texas census conference panel on medication treatment of childhood deficit/ hyperactivity disorder. Part 1. J Am Academy Child Adolescent Psychology.
200;39(7):920-927
Finalized 8/5/2013
Click here to return to question 2.
Click here to return to question 3.

| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Attachment A- Instrument_Word Version |
| Author | Penn, Matthew S. (CDC/OSTLTS/OD) |
| File Modified | 0000-00-00 |
| File Created | 2021-01-26 |
© 2026 OMB.report | Privacy Policy