2 Clinical Professional Consent Form and Script
Generic Clearance for the Collection of Qualitative Feedback on Agency Service Delivery (NIH)
Attachment_2_Clinical Trial Professional Consent Script 3_25_15
Educational Game Focus Group
OMB: 0925-0648
ATTACHMENT 2: Educational Game Focus Group: CLINICAL TRIAL PROFESSIONAL
Form Approved
OMB No. 0925-0648
Exp. Date 3/31/2018
CLINICAL TRIAL PROFESSIONAL: CONSENT
You have been asked to participate in a focus group webinar to discuss an educational game that is being developed by NOVA Research Company, under funding provided by the National Heart, Lung, and Blood Institute. The game is intended to teach elementary-school-aged children about pediatric clinical research.
This focus group is considered research. We have provided information below about focus group procedures, steps we will take to preserve your privacy, benefits and possible risks, and your rights as a participant in this research study.
Procedures
During the focus group webinar, we will describe the game, show you mockups of the game, and you for feedback. The webinar is expected to last 30–45 minutes.
Each participant will receive a $50 gift card for participating in the group discussion.
NOVA staff will observe the focus group webinar to listen and take notes. We will audio record the focus group and use the recording to help us remember what people said. NOVA will use these notes to summarize your experience and opinions.
Collection of this information is authorized by The Public Health Service Act, Section 411 (42 USC 285a). Rights of study participants are protected by The Privacy Act of 1974. Participation is voluntary, and there are no penalties for not participating or withdrawing from the study at any time. Refusal to participate will not affect your benefits in any way. The information collected in this study will be kept private under the Privacy Act. Names and other identifiers will not appear in any report of the study. Information provided will be combined for all study participants and reported as summaries. You are being contacted by telephone to complete this instrument so that we can determine whether you qualify to participate in a website usability study.
Public reporting burden for this collection of information is estimated to average 55 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to: NIH, Project Clearance Branch, 6705 Rockledge Drive, MSC 7974, Bethesda, MD 20892-7974, ATTN: PRA (0925-0648). Do not return the completed form to this address.
Privacy and Confidentiality
We will report results of this focus group to NHLBI, but the report will not include participants’ names or any information that would identify participants. The focus group recording and all other study data will be stored on a password-protected secure server. All data in reports will be combined by focus group, and nothing will be tied to an individual. NHLBI will retain ownership of all data collected.
Possible Benefits and Risks
You may not benefit directly from being in this study. However, the information gained from this study will help improve how information about pediatric clinical research is shared with children and their families.
Some of the statements and questions in the focus group may make you feel uncomfortable. You do not have to respond to these statements if you do not wish to do so.
Participation is voluntary
You should not feel any pressure to participate in the focus group. If you decide not to participate, that is fine. You may change your mind and withdraw your approval at any time; if the focus group is already in progress, you may ask to be excused or sit quietly until the focus group session has concluded. You will still receive the gift for your time.
Your Questions
If you have questions about this research, including questions about scheduling the focus group or about incentives for participating, you can contact Kathy Sedgwick or Dan Eckstein, NOVA Research Company, 801 Roeder Road, Suite 700, Silver Spring, MD 20910, 301-986-1891, [email protected], [email protected].
Your Rights
If have questions about your rights as a research participant or wish to discuss any concerns about this study with someone other than the researcher(s), please contact Paul Young, NOVA IRB Liaison, NOVA Research Company, 801 Roeder Road, Suite 700, Silver Spring, MD 20910, 301-986-1891, [email protected].
You will receive a copy of this document for your records, and one copy will be kept with the study records.
Clinical Trials Professional Consent
By signing below, I am agreeing to participate in the Educational Game Focus Group. My participation in this focus group is completely voluntary. I may change my mind and stop participating at any time.
I agree to participate in this focus group.
_____________________________________ ____________________
Signature Date
Clinical Trials Professionals Webinar
Welcome
Thank you for agreeing to be a part of this study.
I’d like you to know that [observer name(s)] are on the call today to take notes. They will observe the webinar and listen to the discussion while we work together. We will also record our session together, just to make sure that we don’t miss anything you say. Is that okay? WAIT FOR RESPONSE
As was mentioned when we scheduled this webinar, we expect the process will take 30–45 minutes.
Webinar Housekeeping – Adobe Connect
CONFIRM THAT THEY CAN SEE THE WELCOME ANNOUNCEMENT AND THAT EVERYONE IS LOGGED IN.
Introduction and Overview
Today we’ll be looking at mockups of a web-based educational game that we are developing for the National Heart, Lung, and Blood Institute (NHLBI), which is part of the National Institutes of Health. I’m going to explain the game to you, show you the mockups, and then ask you some questions.
As we go through this process, please keep in mind that we are showing you a very early mockup of the game, which describes some but not all of the features we hope to include if additional funding is awarded to us.
Your responses are entirely voluntary. If you don’t want to answer a particular question, you do not need to respond.
NOVA is developing the web-based game and its content with the help of subject matter experts. Don’t worry that anything you say might hurt my feelings or offend in any way. Any comments you have, either positive or negative, will be useful, so please feel free to tell me what you think.
After we’ve finished the discussion questions, I’ll give you some time to ask me anything you’d like. Do you have any questions for me before we begin?
OK. Before we move on, please introduce yourself and your role in pediatric clinical trials.
NAME OF FIRST PARTICIPANT? REPEAT UNTIL INTRODUCTIONS ARE COMPLETE.
About the Game
NHLBI is working toward improving public understanding of clinical research, and particularly pediatric clinical trials. The NHLBI website has dedicated “kids pages” that include videos of children and their parents talking about clinical trials. There is also a computer game that is designed to address fears about participating in clinical trials.
The web-based educational game we’re developing has a different purpose. It is intended to increase awareness of, knowledge about, empathy for, and acceptance of pediatric clinical research among elementary-school-age children. To make sure the game works well, we are talking to children, their parents, teachers, and, of course, to pediatric clinical research professionals.
In this game, the player—that’s a child in grades 4–6—is joining a team of researchers at Heartland Pediatric Clinic. The player starts as a Clinical Research Trainee. As they go from room to room in the clinic, they meet different members of the research team and learn what those people do.
DISPLAY MAP.
Each room has a minigame they can play—for example, a match game, a find-the-hidden-objects game, or a jigsaw puzzle. To advance to the next room, they answer questions about what they just learned.
Questions
In your experience, is a game a good way to present this type of information in a clinical setting?
In your experience, what aspects of clinical trials do children most often struggle with?
Mockups/Initial Impression
Now that I’ve given you a brief description of the game, I’d like to walk through several of the rooms that will be included in our Phase 1 prototype.
DISPLAY SPLASH SCREEN TEXT.
This is the text that will display on the opening splash screen of the game. Can everyone read that?
DISPLAY CLINIC MAP MOCKUP. [See attached illustration.]
This is a mockup of the clinic map.
DISPLAY LOBBY MOCKUP. [See attached illustration.]
This is a mockup of the Lobby, the first room players will see. They will meet the Receptionist here and receive a phone with apps to help them in the game.
DISPLAY LEARNING OBJECTIVES FOR STAFF TRAINING OFFICE.
The second room is the Staff Training Office where they will meet the Training Director. These are the learning objectives for this room. In this room, two apps will be added to their phones: a pictorial staff directory and a glossary.
DISPLAY LABORATORY MOCKUP. [See attached illustration.]
This is the laboratory. In this room, players meet the Clinical Laboratory Scientist and the Laboratory Assistant, who introduce them to the scientific method.
DISPLAY LEARNING OBJECTIVES FOR EXAM ROOM.
After the lab, players meet the Clinical Researcher in the exam room. These are the learning objectives for this room. Players learn about the different types of clinical trials and phases of clinical trials.
DISPLAY CLINIC MAP. [See attached illustration.]
When you think about the rooms we’ve described, are the furnishings and equipment what you would expect to see in a real clinic? Was anything missing that you would expect to see?
What can we do to improve any room?
We chose generic CT team titles. Are these titles appropriate? What titles do your team members have?
What other things do CT team members do that would be interesting to children and contribute to their understanding of pediatric clinical research?
When you think about the game concept, what is your overall impression?
What did you like most about the game?
What did you like least about the game?
Do you feel this game will be successful in teaching these concepts to children? Why/why not?
Wrapup
OK, we’ve finished the questions I had for you. Do you have any further comments you’d like to add?
Thank you again for your participation. We will mail your incentives to you within the next 2 weeks.
Game Map
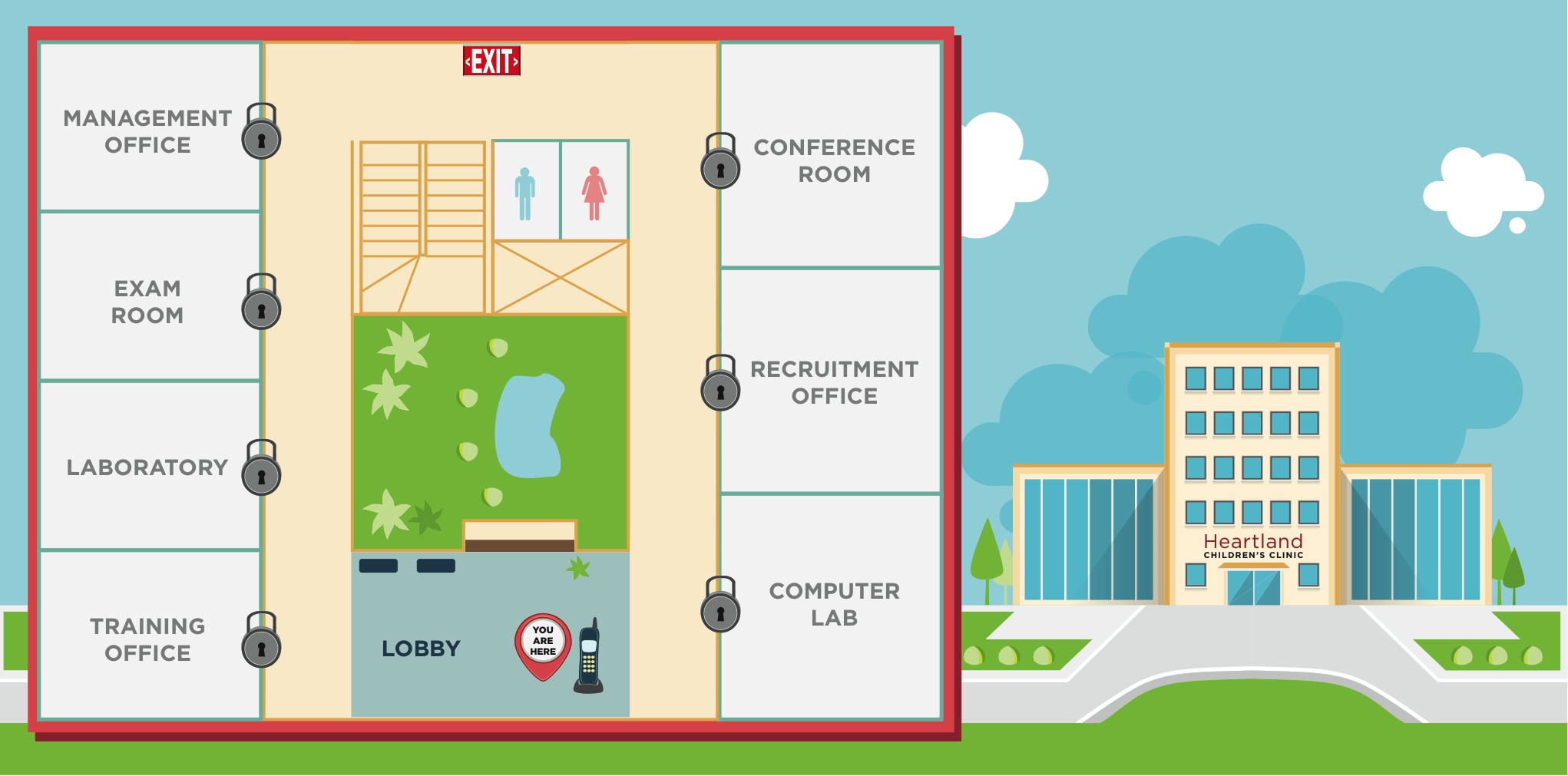
Lobby

Laboratory

Page
| File Type | application/msword |
| File Title | CONSENT TO PARTICIPATE IN A RESEARCH STUDY |
| Author | Rachel Nosowsky |
| Last Modified By | Perryman, Seleda |
| File Modified | 2015-03-25 |
| File Created | 2015-03-25 |
© 2026 OMB.report | Privacy Policy