Supporting Statement Combo Final
Supporting Statement Combo Final.docx
Revision of the Hawaiian and Territorial Fruits and Vegetables Regulations
OMB: 0579-0346
June 26, 2015
Supporting Statement
Movement of Plants and Plant Products
From Hawaii and the Territories
OMB No. 0579-0346
NOTE: The Animal and Plant Health Inspection Service (APHIS) has changed the name of this Information Collection from “Hawaiian and Territorial Fruits and Vegetables Regulations” to “Movement of Plants and Plant Products from Hawaii and the Territories” to better reflect what is included. This collection is merging burden hours from OMB Nos. 0579-0198 (Quarantine for Hawaii and the U.S. Territories) and 0579-0281 (Treatment for Fruits and Vegetables). Upon approval of this renewal package, APHIS will retire 0579-0198 and 0579-0281.
A. Justification
1. Explain the circumstances that make the collection of information necessary. Identify any legal or administrative requirements that necessitate the collection.
The United States Department of Agriculture (USDA), APHIS, is responsible for preventing plant diseases or insect pests from entering the United States, preventing the spread of pests and noxious weeds not widely distributed in the United States, and eradicating those imported pests when eradication is feasible. The Plant Protection Act authorizes the Department to carry out this mission.
Under the Plant Protection Act (7 U.S.C. 7701 – et. seq.), the Secretary of Agriculture is authorized to prohibit or restrict the importation, entry, or movement of fruits, vegetables, plants, and plant products to prevent the introduction of pests or diseases into the
United States, or the dissemination of pests or diseases within the United States. The Plant Protection Act authorizes the Department to carry out this mission.
Plant Protection and Quarantine (PPQ), a program within APHIS, is responsible for implementing this Act and does so through the enforcement of its Hawaiian and territorial quarantine regulations, contained in Part 318 of Title 7, Code of Federal Regulations (CFR).
Under the Hawaii and territorial regulated article regulations (7 CFR 318.13-1 through 318.13-26, referred to as the regulations), PPQ prohibits or restricts the interstate movement of fruits, vegetables, plants, and plant products from Hawaii, Puerto Rico, the U.S. Virgin Islands, the Commonwealth of the Northern Mariana Islands, and Guam to the continental United States to prevent the spread of dangerous plant diseases and plant pests that occur in Hawaii and the territories, including the Mediterranean fruit fly, the melon fly, the Oriental fruit fly, green coffee scale, the bean pod borer, and other plant pests which are new to or not known to be widely prevalent or distributed within and throughout other States.
Additionally, the phytosanitary treatment regulations contained in Title 7 CFR Part 305, set out standards and schedules for treatments required for articles whose importation could introduce plant pests or noxious weeds into the United States or whose interstate movement could spread plant pests or noxious weeds within the United States. Within
Title 7 CFR Part 305, the irradiation treatment regulations in Subpart 305.9 (referred to as the regulations) set out standards and minimum doses for irradiation treatments for imported fruits, vegetables, and regulated articles moved interstate from quarantined areas within the United States, along with other requirements for performing irradiation treatments.
APHIS established criteria within the regulations that, if met, allows APHIS to approve certain new fruits, vegetables, and other regulated articles for interstate movement in the United States and to acknowledge pest-free areas in Hawaii and U.S. territories expeditiously, doing away with the practice of listing in the regulations specific commodities as regulated articles. These changes simplify and expedite our processes for approving certain regulated articles for interstate movement and pest-free areas.
Implementing APHIS’ quarantines often requires APHIS to collect information from a variety of individuals who are involved in growing, packing, handling, transporting, and exporting plants and plant products. The information APHIS collects serves as the supporting documentation required for the issuance of PPQ forms and documents that authorize the movement of regulated articles, and they are vital in helping APHIS ensure that injurious plant diseases and insect pests do not spread within the United States.
APHIS is asking the Office of Management and Budget (OMB) to approve, for an additional 3 years, the use of these information collection activities, associated with its effort to prevent the spread of plant pests and diseases into the continental United States.
2. Indicate how, by whom, and for what purpose the information is to be used. Except for a new collection, indicate the actual use the agency has made of the information received from the current collection.
APHIS uses the following information collection activities to prevent the spread of plant pests and diseases in the United States:
Limited Permit (PPQ Form 530) (business) (previously in 0346, 0281, and 0198) – Fruits or vegetables shipped from Hawaii, Puerto Rico, Guam, the Commonwealth of
the Northern Mariana Islands, or the U.S. Virgin Islands through the continental
United States must be accompanied by a limited permit, a copy of which must be presented to an inspector at the port of arrival and the port of export in the United States, and at any other location in the United States where an air consignment is authorized to stop or where overland consignments change means of conveyance. These limited permits are used to authorize movement of regulated articles that are not certifiable to specified destinations for processing, treatment, or utilization. These permits must be endorsed by an APHIS- approved destination officer in order to be valid.
Application for Permit toTransit Plants and/or Plant Products, Plant Pests, and/or Associated Soil through the United States (PPQ Form 586) (business) (previously in 0346) – A transit permit is required for the arrival, unloading, and movement through the continental United States of fruits and vegetables otherwise prohibited by this subpart
(§ 318.13-6) from being moved through the continental United States from Hawaii, Puerto Rico, Guam, the Commonwealth of the Northern Mariana Islands, or the U.S. Virgin Islands. Businesses apply to APHIS to receive this transit permit so that their commodities can safely and legally transit the country. This also gives APHIS the ability to do any tracebacks in the event of a pest or disease occurrence.
Labeling of Boxes for Pest Free Areas; Package, Marking, and Identify (business) (previously in 0346 and 0281) - Each box of fruits or vegetables that is moved interstate from a pest-free area must be clearly labeled with: (1) the name of the orchard or grove of origin, or the name of the grower; (2) the name of the municipality and State or territory in which the fruits or vegetables were produced; and (3) the type and amount of fruits or vegetables the box contains.
Sweet potatoes that are treated in Hawaii must be packaged in cartons that have no opening that will allow the entry of fruit flies and must be sealed with seals that will visually indicate if the cartons have been opened. Cartons may be constructed of any material that prevents the entry of fruit flies and prevents oviposition by fruit flies into the fruit in the carton. Packaging must be labeled with treatment lot numbers, packing and treatment facility identification and location, and dates of packing and treatment. This requirement is needed to ensure safe and secure movement of regulated articles providing information for oviposition by pest or disease occurrences, and other information needed for inspectors in keeping pests out.
Inspection and Certification; Inspection of Production Areas; and Inspection of Sweet Potatoes; and Irradiation Treatment and Inspection (State) (previously in 0346, 0281, and 0198) – States do not have individual NPPOs. Inspectors are State agricultural inspectors or individuals authorized by APHIS or the Department of Homeland Security.
Grower production areas must be inspected annually by inspectors and found free of green scale. If green scale is found during an inspection, a 2-month ban will be placed on the interstate movement of cut plants from that production area. Near the end of the
2 months, an inspector will reinspect the grower's production area to determine whether green scale is present. If reinspection determines that the production area is free of green scale, shipping may resume. If reinspection determines that green scale is still present in the production area, another 2-month ban on shipping will be placed on the interstate movement of cut plants from that production area. Each ban will be followed by reinspection in the manner specified, and the production area must be found free of green scale prior to interstate movement.
Hawaiian agricultural inspectors are also responsible for inspecting sweet potatoes, affirming that the following is accomplished: (1) The sweet potatoes must be sampled, cut, and inspected and found to be free of the ginger weevil (Elytrotreinus subtruncatus). Sampling, cutting, and inspection must be performed under conditions that will prevent any pests that may emerge from the sampled sweet potatoes from infesting any other sweet potatoes intended for interstate movement. (2) The sweet potatoes must be inspected and found to be free of the gray pineapple mealybug (Dysmicoccus neobrevipes) and the Kona coffee-root knot nematode (Meloidogyne konaensis).
(3) Sweet potatoes that are not treated with an irradiation dose approved to neutralize the ginger weevil (Elytrotreinus subtruncatus) must be sampled, cut, and inspected and found to be free of the ginger weevil by an inspector in Hawaii. Sampling, cutting, and inspection must be performed under conditions that will prevent any pests that may emerge from the sampled sweet potatoes from infesting any other sweet potatoes intended for interstate movement in accordance with the regulations.
Any sweet potatoes that are to be treated with irradiation are to be irradiated by certified irradiation facilities. Inspections are needed to ensure sufficient irradiation is being performed. The sweet potatoes must be treated with irradiation in accordance with
Part 305 of the regulations.
Compliance Agreement (PPQ Form 519) (business) (previously in 0346 and 0198) – Businesses wishing to move fruits and vegetables from a pest-free area in Hawaii,
Puerto Rico, Guam, the Commonwealth of the Northern Mariana Islands, or the
U.S. Virgin Islands must enter into a compliance agreement with APHIS in accordance with §318.13-3(d), and the fruits and vegetables must otherwise meet the requirements of paragraphs (a) and (b) of this section. Any person whose Limited Permit or Compliance Agreement has been withdrawn may appeal the decision, in writing, to the APHIS Administrator within 10 days after receiving the written notification of the withdrawal.
Trapping and Surveillance (State) (previously in 0346) – APHIS determines if an area’s pest-free status is based on information provided by the State/territory. The information used to make this determination includes trapping and surveillance data, survey protocols, and protocols for actions to be performed upon detection of a pest.
Contingency Plans, approved by APHIS (business) (new/violation) – Irradiation facilities must have contingency plans in place and have them approved by APHIS to ensure the proper and safe treatment of regulated articles.
Certificate (PPQ 540) (State) (previously in 0281) – Certification is issued by a Hawaiian inspector for the movement of sweet potatoes from Hawaii that have been treated in accordance with Part 305 of this chapter and handled in Hawaii in accordance with §§ 318.13-25 and 305.9. This certificate accompanies the associated shipment and must be surrendered to the consignee at the destination of the shipment. Information from this certificate is also needed to determine if a permit can be issued or is needed.
Facility must maintain and provide APHIS with an updated map identifying places where horticultural or other crops are grown (business) (new/violation) – Irradiation facilities must maintain and provide APHIS with updated maps identifying places where horticultural or other crops are grown within a 4-mile radius of the facility. Knowledge of the proximity of host material to the facility may necessitate trapping or other pest monitoring activities to help prevent unwanted occurrences.
Written Request for Facility Approval, and Recertification (business) (previously in 0281) – Irradiation treatment facilities requesting certification must submit the request for approval, in writing, to APHIS, PPQ, Center for Plant Health Science and Technology, 1730 Varsity Drive, Suite 400, Raleigh, NC 27606. The initial request must identify the owner, location, and radiation source of the facility, and the applicant must supply additional information about the facility construction, treatment protocols, and operations upon request by APHIS, only if APHIS requires additional information to evaluate the request.
The irradiation treatment facility must be certified by APHIS. Recertification is required in the event of: (1) an increase in the amount of radioisotope, (2) a decrease in the amount of radioisotope for a reason other than natural decay, (3) a major modification to equipment that affects the delivered dose, or (4) a change in the owner or managing entity of the facility. Recertification also may be required in cases where a significant variance in dose delivery has been measured by the dosimetry system.
Recordkeeping (business) (new/violation) - Irradiation facilities must maintain records of each treated lot for 1 year following the treatment date, and must make these records available for inspection by an inspector during normal business hours (8 a.m. to 4:30 p.m., Monday through Friday, except holidays). These records must include the lot identification, scheduled process, evidence of compliance with the scheduled process, ionizing energy source, source calibration, dosimetry, dose distribution in the product, and the date of irradiation.
Decertification of Pest-free Areas; Reinstatement; Certification (State) (previously in 0346) - If a pest is detected in an area that is designated as free of that pest, APHIS will publish a notice in the Federal Register announcing that the pest-free status of the area in question has been withdrawn and that interstate movement of host crops for the pest in question is subject to application of an approved treatment for the pest. If a treatment for the pest is not available, interstate movement of the host crops would be prohibited. In order for a decertified pest-free area to be reinstated, it would have to meet the criteria for a pest-free area by trapping and surveillance, survey protocols, and a Federal Register notice. It is the responsibility of the State or territory of the area to meet the criteria and provide any trapping and surveillance data, survey protocols, etc., to APHIS.
3. Describe whether, and to what extent, the collection of information involves the use of automated, electronic, mechanical, or other forms of information technology, e.g., permitting electronic submission of responses, and the basis for the decision for adopting this means of collection. Also describe any consideration of using information technology to reduce burden.
PPQ Form 530 (Limited Permit) - This form is an accountable form that must be issued by a PPQ employee or a person under Compliance Agreement with PPQ. Strict control is needed for the issuance of this form, as it allows the movement of regulated products that are subject to restrictions, and can only be issued after an inspection proves that the shipment meets the requirements for movement. Movement may also require a treatment, which has to be determined by an inspector. The form must accompany the shipment throughout transport from the inspection until destination.
PPQ Form 519 (Compliance Agreement) – This form is downloadable, fillable, and posted at: www.aphis.usda.gov/library/forms/pdf/ppq519.dot
PPQ Form 586 (Application for Permit to Transit Plants and/or Plant Products, Plant Pests, and/or Associated Soil through the United States) - This form is downloadable, fillable, and posted at:
http://www.aphis.usda.gov/permits/ppq_epermits.shtml
PPQ Form 540 (Certificate) - This form is not automated for several reasons. This form has a unique identifier (serial number) and it is an accountable form that must be issued by a PPQ employee. APHIS needs to have strict control over the issuance of this form since they allow the movement of regulated products that are subject to restrictions. They can only be issued after an inspection proves that the shipment meets the requirements for movement. An inspector has to determine if a treatment is required before movement. Finally, the forms must accompany the shipment throughout transport from the inspection until destination.
Letter of Request for Facility Approval - A letter of request for facility approval can be automated by the respondent using a computer.
Forms in this information collection are part of the International Trade Data System (ITDS) via the Automated Commercial Environment (ACE) initiative. Being a part of this system/initiative will change how the information is collected; this system will eliminate actual forms and collect the information as raw data. This will be accomplished in a fully electronic manner. This system will use components of Certification, Accreditation, Registration, Permitting, and Other Licensing (CARPOL) and spans/shares data with 48 other Government agencies.
4. Describe efforts to identify duplication. Show specifically why any similar information already available cannot be used or modified for use for the purpose described in item 2 above.
The information APHIS collects is exclusive to its mission of preventing the incursion or interstate spread of plant pests, diseases, and noxious weeds and is not available from any other source.
5. If the collection of information impacts small businesses or other small entities, describe any methods used to minimize burden.
The information APHIS collects in this information collection is the minimum needed to protect the United States from destructive plants and plant products. APHIS estimates 60 percent of the respondents are small entities.
6. Describe the consequences to Federal program or policy activities if the collection is not conducted or is conducted less frequently, as well as any technical or legal obstacles to reducing burden.
If APHIS did not collect this information or if APHIS collected this information less frequently, the spread of dangerous plant diseases and pests that occur in Hawaii and the territories could spread to the continental United States causing millions of dollars in damage.
7. Explain any special circumstances that require the collection to be conducted in a manner inconsistent with the general information collection guidelines in 5 CFR 1320.5.
requiring respondents to report information to the agency more often than quarterly;
requiring respondents to prepare a written response to a collection of information in fewer than 30 days after receipt of it;
Facilities that carry out continual irradiation operations must notify an inspector at least 24 hours before the date of operations.
Any person whose Limited Permit or Compliance Agreement has been withdrawn may appeal the decision, in writing, to the APHIS Administrator within 10 days after receiving the written notification of the withdrawal.
requiring respondents to submit more than an original and two copies of any document;
requiring respondents to retain records, other than health, medical, government contract, grant-in-aid, or tax records for more than three years;
in connection with a statistical survey, that is not designed to produce valid and reliable results that can be generalized to the universe of study;
requiring the use of a statistical data classification that has not been reviewed and approved by OMB;
that includes a pledge of confidentiality that is not supported by authority established in statute or regulation, that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or
requiring respondents to submit proprietary trade secret, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information's confidentiality to the extent permitted by law.
No other special circumstances exist that would require this collection to be conducted
in a manner inconsistent with the general information collection guidelines in
5 CFR 1320.5.
8. Describe efforts to consult with persons outside the agency to obtain their views on the availability of data, frequency of collection, the clarity of instructions and recordkeeping, disclosure, or reporting form, and on the data elements to be recorded, disclosed, or reported. If applicable, provide a copy and identify the date and page number of publication in the Federal Register of the agency’s notice, soliciting comments on the information collection prior to submission to OMB.
The following individuals were consulted regarding this information collection:
Hawaii Tropical Fruit Growers
Liz Ito, Vice President
P.O.
Box 603
Anahola,
HI 96703
Phone: 808-828-1514
Amos Hill Associates/Koppensteiner Veneer
Susanne Renner
112 Shelby Avenue
Edinburgh, Indiana 46124
Phone: 812-526-2671
Tom Gitzlafft
Director of Field Operations
Copesan
W175 N5711 Technology Drive
Menomonee Falls, WI 53051
Phone: 800-267-3726
On Tuesday, March 24, 2015, pages 15548-15549, APHIS published in the Federal Register, a 60-day notice seeking public comments on its plans to request a 3-year renewal of this information collection and also on its plans to combine it with OMB Nos. 0579-0281 and 0579-0198. No comments from the public were received.
9. Explain any decision to provide any payment or gift to respondents, other than reenumeration of contractors or grantees.
This information collection activity involves no payments or gifts to respondents.
10. Describe any assurance of confidentiality provided to respondents and the basis for the assurance in stature, regulation, or agency policy.
No additional assurance of confidentiality is provided with this information collection. Any and all information obtained in this collection shall not be disclosed except in accordance with 5 U.S.C. 552a.
11. Provide additional justification for any questions of a sensitive nature, such as sexual behavior and attitudes, religious beliefs, and others that are considered private. This justification should include the reasons why the agency considers the questions necessary, the specific uses to be made of the information, the explanation to be given to persons from whom the information is requested, and any steps to be taken to obtain their consent.
This information collection activity asks no questions of a personal or sensitive nature.
12. Provide estimates of the hour burden of the collection of information. Indicate the number of respondents, frequency of response, annual hour burden, and an explanation of how the burden was estimated.
. Indicate the number of respondents, frequency of response, annual hour burden, and an explanation of how the burden was estimated. If this request for approval covers more than one form, provide separate hour burden estimates for each form and aggregate the hour burdens in Item 13 of OMB Form 83-I.
See APHIS Form 71 for hour burden estimates.
. Provide estimates of annualized cost to respondents for the hour burdens for collections of information, identifying and using appropriate wage rate categories.
The estimated annualized cost to respondents is $259,520.80. APHIS arrived at this figure by multiplying the total burden hours by the estimated hourly rate of businesses.
7,660 total burden hours X $33.88 estimated hourly rate = $259,520.80
The estimated hourly rate of $33.88 is based on salaries of Hawaii businesses derived from the U.S. Department of Labor, Bureau of Labor Statistics May 2014 Report: Occupational Wages in the U.S., at http://www.bls.gov/oes/current/oes_hi.htm#45-0000
13. Provide estimates of the total annual cost burden to respondents or recordkeepers resulting from the collection of information, (do not include the cost of any hour burden shown in items 12 and 14). The cost estimates should be split into two components: (a) a total capital and start-up cost component annualized over its expected useful life; and (b) a total operation and maintenance and purchase of services component.
There is zero annual cost burden associated with capital and start-up, operation and maintenance, and purchase of services in connection with this program.
14. Provide estimates of annualized cost to the Federal government. Provide a description of the method used to estimate cost and any other expense that would not have been incurred without this collection of information.
The estimated cost to the Federal Government is $201,471 (See APHIS Form 79.)
15. Explain the reasons for any program changes or adjustments reported in Items 13 or 14 of the OMB Form 83-I.
|
Requested |
Program Change Due to New Statute |
Program Change Due to Agency Discretion |
Change Due to Adjustment in Agency Estimate |
Change Due to Potential Violation of the PRA |
Previously Approved |
Annual Number of Responses |
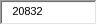
20,832 |
0 |
632 |
0 |
0 |
20,200 |
Annual Time Burden (Hr) |
7,660 |
0 |
-986 |
0 |
0 |
8,646 |
|
|
|
|
|
|
|
With this renewal submission, the Agency is merging the burden from 0579-0198 and 0579-0281 into this collection package. Also, when reviewing the collection for renewal, the Agency discovered it had omitted accounting for burden in the previous submission, yet had been collecting this information in violation. These actions resulted in a program change increase as shown below.
Responses Burden Hours
Merging of – 0596-0198 – 4,200 3,096
Merging of – 0596-0281 – 225 6
Subtotal: 4,425 3,102
Violations:
Contingency Plans – 10 40
Updated Map – 10 5
Recordkeeping 10 10
Subtotal: 30 55
Total program increase: 4,455 3,157
A group of respondents was counted several times inflating the true number of respondents in the last submission. This error is corrected in this submission with a decrease from 600 to 178 respondents. This renewal also has an adjustment decrease
of -3,823 responses and -4,143 burden hours. The decreases are a result of a more accurate account of, and fluctuation in, the responses per respondent since the last submission. With the combined increase in program change and decrease in adjustments, there is an overall increase of 632 responses and a decrease of -986 burden hours.
16. For collections of information whose results are planned to be published, outline plans for tabulation and publication.
APHIS has no plans to tabulate or publish the information collected.
17. If seeking approval to not display the expiration date for OMB approval of the information collection, explain the reasons that display would be inappropriate.
PPQ Form 519 is used in 14 collections, PPQ Form 530 is used in 10 collections, PPQ Form 540 is used in 10 collections, and PPQ Form 586 is used in 3 collections; therefore, it is not practical to include an OMB expiration date on these forms because of the various expiration dates for each collection. APHIS is seeking approval to not display the OMB expiration date on these PPQ forms.
18. Explain each exception to the certification statement identified in the “Certification for Paperwork Reduction Act.”
APHIS certifies compliance with all the provisions under the Act.
B. Collections of Information Employing Statistical Methods.
Statistical methods are not used in this information collection.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Supporting Statement |
| Author | lctoran |
| File Modified | 0000-00-00 |
| File Created | 2021-01-25 |
© 2026 OMB.report | Privacy Policy