20150518 SupptngStmntA-BodyFluidsSL_clean
20150518 SupptngStmntA-BodyFluidsSL_clean.docx
Persistence of Ebola Virus in Body Fluids of Ebola Virus Disease Survivors in Sierra Leone
OMB: 0920-1064
Persistence of Ebola Virus in Body Fluids of Ebola Virus Disease Survivors in Sierra Leone
Request for OMB Approval for an
Emergency Information Collection Request
Supporting Statement A
Based on Protocol Version May 6, 2015
Submitted: May 18, 2015
Program Official/Project Officer
Barbara Knust, DVM, MPH, DACVPM
CDR, US Public Health Service
Viral Special Pathogens Branch
US Centers for Disease Control
404-639-1104 phone
404-639-1118 fax
404-218-9626 mobile
Table of Contents
1. Circumstances Making the Collection of Information Necessary Background 4
2. Purpose and Use of the Information Collection 5
3. Use of Improved Information Technology and Burden Reduction 6
4. Efforts to Identify Duplication and Use of Similar Information 6
5. Impact on Small Businesses or Other Small Entities 6
6. Consequences of Collecting the Information Less Frequently 6
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 7
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency 7
9. Explanation of Any Payment or Gift to Respondents 8
10. Assurance of Confidentiality Provided to Respondents 8
10.1. Privacy Impact Assessment Information 8
11. Justification for Sensitive Questions 14
12. Estimates of Annualized Burden Hours and Costs 14
13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers 17
14. Annualized Cost to the Government 17
15. Explanation for Program Changes or Adjustments 18
16. Plans for Tabulation and Publication and Project Time Schedule 18
17. Reason(s) Display of OMB Expiration Date is Inappropriate 20
18. Exceptions to Certification for Paperwork Reduction Act Submissions 20
Persistence of Ebola Virus in Body Fluids of Ebola Virus Disease Survivors in Sierra Leone:
Emergency Request for OMB Approval
Goal of the research study:
Assess the presence and duration of infectious Ebola virus (EBOV)
in semen, vaginal fluids, and other body fluids of Ebola Virus
Disease (EVD) survivors in Sierra Leone, which will help inform
EBOV transmission prevention strategies during EVD outbreaks.
Intended use of the resulting
data: Provide health care and public health practitioners working
on the Ebola response with insight into how long EBOV persists in
body fluids other than blood. This information is needed in order
to understand transmission risk of EBOV to others in the community
from survivors for whom the virus is no longer active in blood. Methods to be used to collect:
This is an observational cohort study that will collect
socio-demographic and health data from EVD survivors using
information collection instruments and body fluid specimens tested
using standard laboratory techniques. Information will be collected
until a participant’s specimens from all relevant body fluids
are EBOV negative, defined as having two consecutive negative
reverse
transcription polymerase chain reaction
results.
Population to be studied: EVD
survivors ages 18 and older in Sierra Leone. How data will be analyzed:
Descriptive data will be followed by cross tabulations to
understand transmission patterns and potentially associated
factors. If adequate numbers of viral transmission are identified,
multivariate techniques that include logistic regression will also
be used.

Part A — Justification
CDC is requesting a review of the following information collection pursuant to the Office of Management and Budget (OMB) procedures established at 4 CFR 1320, Controlling Paperwork Burdens on the Public, in accordance with Section 1320.13 Emergency Processing. CDC cannot reasonably comply with the normal clearance procedures because delays in this proposed information collection are likely to result in continued public harm, illness, and death. Even a single case of Ebola Virus Disease (EVD) caused by an unknown transmission source, if not rapidly identified and controlled, could set off another chain of transmission in the country. Thus reaching the goal of zero new cases is essential for CDC to fulfill its public health mission to assist the government of Sierra Leone end the Ebola outbreak as quickly and as completely as possible.
This information collection is authorized by Section 301 of the Public Health Service Act (42 U.S.C. 241) (Appendix A). CDC requests that OMB approval for 180 days is granted by May 20, 2015. CDC anticipates that this information collection will continue for a year; therefore, an extension information collection request (ICR) will be submitted after emergency OMB approval to allow completion of this research study.
1. Circumstances Making the Collection of Information Necessary Background
The epidemic of EVD that began in 2014 in West Africa has been unprecedented in scale and duration. As of May 7, 2015, there have been over 26,600 reported cases and over 11,000 deaths, mostly in three highly affected countries: Sierra Leone, Guinea, and Liberia. To date, the case fatality rate is estimated to be between 53% and 64% in these three countries. The precise number of survivors is unknown, but is expected to be in the thousands. The ongoing Ebola outbreak remains a public health emergency with devastating socioeconomic and humanitarian consequences. To reach the goal of zero new cases, all possible routes of transmission must be investigated and understood in order to prevent another chain of transmission.
Survivors are released from Ebola treatment units (ETUs) with a discharge certificate after two tests for Ebola virus (EBOV) in the blood have been negative by reverse transcription polymerase chain reaction (RT-PCR). Anecdotal reports suggest that some new cases might be occurring from sexual transmission of the virus between EVD survivors and their sexual partners. In addition to concerns about semen and vaginal fluid, concerns exist regarding the unknown persistence of EBOV in other body fluids of survivors (e.g., saliva, sweat, tears, urine, rectal swabs, and breast milk), and their potential as a source of ongoing transmission; the literature does not rule out these possibilities.
After clinical improvement and elimination of viremia, limited data exist concerning EBOV clearance, persistence, and shedding during convalescence. It has been reported that EBOV can be detected in seminal fluids of a convalescent man at 82 days after onset of symptoms. Limited evidence suggests that live EBOV can persist in urine for 26 days following symptom onset. Evidence regarding EBOV in vaginal secretions is also limited. EBOV has been detected in vaginal secretions at 33 days after symptom onset; due to the type of diagnostic test used (RT-PCR), it was not clear whether these traces represented live virus. Similarly, traces of EBOV were detected in sweat at 40 days after symptom onset, but no live virus was isolated. In addition, rectal swabs and conjunctival fluid have been found by RT-PCR to contain EBOV at 29 and 22 days after illness onset, respectively. A limited number of saliva specimens have been tested by RT-PCR, all yielding negative results. Sexual transmission has never been definitively documented for EVD, although there have been a small number of reports of suspected sexual transmission during the current outbreak.
The risk of sexual transmission of EBOV is unknown. Current guidance from the World Health Organization (WHO) recommends that Ebola survivors should abstain from sexual intercourse for 6 months (180 days) after the onset of symptoms, or use condoms if abstinence is not possible. CDC recommends abstinence or condom use until more information becomes available through scientific research.
Additionally, there is limited data on EBOV in breast milk. In one published case series, two mothers each provided a single breast milk specimen after EBOV was no longer detectable in their blood, which were tested by virus isolation. One mother’s specimen had detectable virus at 7 days after disease onset and the second mother’s specimen had detectable virus at 15 days after disease onset. Another study reported that three breastfed infants of mothers with EVD and one neonate with an unknown method of feeding died shortly after their mothers. In general, infants breastfeeding from women who had laboratory-confirmed EVD are considered to be at high risk of developing EVD themselves.
Given limited knowledge related to the presence and potential risk of transmission of EBOV via breast milk, the current WHO and CDC recommendation is that women with and recovering from EVD, who are caring for an infant who does not have EVD, should discontinue breastfeeding when safe replacements for breastfeeding and infant care exist. Another organization, UNICEF, recommends that breastfeeding be discontinued for at least eight weeks following recovery from EVD.
The potential public health impact of EBOV transmission by contact with body fluids other than blood from convalescent EVD survivors is substantial. Given large numbers of survivors from the current outbreak in West Africa, even a small percentage of survivors who transmit EBOV to their sex partners, children, or other contacts could prolong clusters of infection or spread EBOV to new communities.
2. Purpose and Use of the Information Collection
The purpose of this study is to assess the presence and duration of infectious EBOV in semen, vaginal fluids, and other body fluids of EVD survivors in Sierra Leone, which is critically important in understanding potential risk of EBOV transmission from survivors. Given the large number of survivors and the lack of evidence for virus persistence in various body fluids, the urgency to collect this needed information is of upmost importance in controlling future cases and potential clusters, and refining recommendations and public health practices. Describing persistence of EBOV in semen, vaginal secretions, and other body fluids (e.g., saliva, sweat, tears, urine, rectal swabs, and breast milk) among male and female EVD survivors in Sierra Leone is needed in order to eliminate transmission in ways currently not being addressed.
Information collected will be used to address two objectives:
To analyze EBOV persistence in body fluids (semen or vaginal secretions, breast milk, rectal swabs, saliva, sweat, urine, tears) using RT-PCR for all collected specimens, and virus isolation for RT-PCR positive specimens
To assess concordance between RT-PCR and virus isolation test results
Results and analysis will be used to update public health interventions and messaging, relevant counselling messages, and other recommendations from CDC, WHO, and the Sierra Leone Ministry of Health and Sanitation (MoHS).
3. Use of Improved Information Technology and Burden Reduction
A CDC data manager will oversee data management and data integration activities in close partnership with WHO and MoHS collaborators. All questionnaire data will be collected via secure laptop computers using in-person interviews. Collected data will be directly recorded on computers or tablets to minimize data recording and entry errors and minimize delays in data availability. Interview and laboratory data will be organized in databases stored on secure local servers within the Sierra Leone MoHS and WHO and will be backed-up regularly. Given that the study will be conducted in Sierra Leone under emergency conditions, the ability to utilize more advanced technology in data collection approaches will be limited. Information collection instruments were each designed to collect the minimum information necessary for the purposes of this project.
4. Efforts to Identify Duplication and Use of Similar Information
This will be the first systematic study of the persistence of EBOV in various body fluids other than blood; therefore, information being collected for this study is unique.
5. Impact on Small Businesses or Other Small Entities
No small businesses will be involved in this information collection.
6. Consequences of Collecting the Information Less Frequently
This request is for emergency information collection for a one-time data collection. There are no legal obstacles to reduce the burden.
If no data are collected, CDC, WHO, and MoHS will be unable to:
Understand the potential for EBOV transmission though body fluids other than blood (e.g., semen or vaginal secretions, breast milk, rectal swabs, saliva, sweat, urine, tears)
Completely control EBOV transmission in Sierra Leone
Develop recommendations for public health and clinical partners responding to the Ebola outbreak
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
There are no special circumstances with this information collection package. This request fully complies with the regulation 5 CFR 1320.5 and will be voluntary.
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A. A broad 60-day Federal Register Notice was published on May 6, 2015, Vol. 80, No. 87; pp.26053–55.
B. Consultations: For this project, the Sierra Leone MoHS and WHO are integrally involved in the concept, design, planning, staff hiring, data collection, analysis, reporting, and data storage together with CDC. The Sierra Leone MoHS Lead for the Ebola Response is serving as the principal investigator. CDC study roles, to date and future, are described below:
Initiated the idea for the project. The information obtained will assist in creating and targeting public health interventions to decrease spread of EVD in Sierra Leone.
Designed and had significant input or control into the design of the data collection instruments.
Will provide monetary resources for the collection of information.
Will analyze laboratory specimens and will report jointly with MoHS and WHO staff.
Will assist in the training of locally hired staff to conduct interviews and maintain the ethical protection of human subjects.
Joint data analysis will be collaboratively performed by the MoHS, WHO, and CDC staff.
The CDC will be co-authors on manuscripts from this research study.
CDC staff have worked with the following research partners on all aspects of the research protocol and project management:
Sierra Leone MoHS; Ministry of Social Welfare, Gender, Children’s Affairs (MSWGCA) |
PI: Dr. Gibrilla Fadlu Deen ([email protected]) |
Co-PIs: Dr. Amara Jambai ([email protected]), Dr. Alie Wurie ([email protected]) |
Additional staff: Abdul Kamara, Tina Davies ([email protected]) |
WHO |
Coordination HQ: sexually transmitted infection, epidemiology subject matter expert: Dr. Nathalie Broutet ([email protected]) |
Coordination SL: Dr. Faiqa Ebrahim ([email protected]) |
Epidemiologist infectious diseases subject matter experts: Dr. Anna Thorson ([email protected]), Dr. MarkAlain Dery |
Research coordinator, SL: Theo Grace ([email protected]) |
WHO representative – Ebola response: Dr. Anshu Banerjee |
Laboratory Virology: Dr. Pierre Formenty |
Reproductive health experts: Dr. Tim O’Dempsey, Dr. Lisa Thomas |
9. Explanation of Any Payment or Gift to Respondents
At each study visit, participants will receive a token of appreciation of 120,000 Leones (approximately $28 US dollars), as well as a 5-week supply of condoms, counselling, and linkages to health services as needed. This token of appreciation is in recognition of the effort required for participants to remain in the study potentially long-term, and for travel to study sites. Participant referral will be provided to EVD survivor services for post-recovery complications, as needed, and human immunodeficiency virus (HIV) testing will be offered to all participants. HIV-positive study participants will be referred to national HIV services. Counselling on potential breastfeeding transmission among lactating female participants will be executed by trained counsellors and alternative feeding will be provided.
Participants will receive these tokens of appreciation for attending a study visit even if they do not complete the interview or specimen collection. Participants who do not complete multiple study visits may be deemed ineligible from participating further.
10. Assurance of Confidentiality Provided to Respondents
This ICR has been reviewed by the OMB PRA Advisor for the CDC emergency response who determined that the Privacy Act does not apply. See justification in Section A.10.1.8.
In addition, the Human Subjects Regulatory Advisor for the CDC emergency response has reviewed the proposed information collection, which is determined to be research. The CDC has entered into a reliance agreement with the Sierra Leone MoHS Institutional Review Board, which has reviewed and approved the research protocol (Appendix B).
10.1. Privacy Impact Assessment Information
10.1.1. Overview of the data collection system
The Sierra Leone MoHS will ensure coordination of project activities with routine MoHS response activities. Data from routine MoHS response activities (case investigation and contact tracing) will be shared for project purposes as needed. The Sierra Leone MoHS will advise in hiring local clinical and data entry staff. Clinical staff will interact with participants; clinical and data entry staff will have access to individually identifiable data. CDC and WHO will provide technical assistance in all project aspects and will supervise clinical staff and lead data analysis. As supervisory staff, CDC and WHO field staff will interact with participants and have access to individually identifiable data.
The MoHS identified four study sites in three high EVD burden districts (Western, Bombali, and Port Loko) for the study:
M34 hospital, Freetown (pilot site location)
PTS1 (Police Training Station) Hospital
Makeni District Hospital
Port Loko Government Hospital
The specific objectives of the study components are:
Pilot Study (Persistence of Ebola virus in semen among EVD survivors)
Inform the process and implementation of the study
Provide rapid results on the prevalence of EBOV by RT-PCR in semen of survivors
Assess the prevalence of live Ebola RNA detected by culture/viral isolation from RT-PCR semen
Module A (Persistence of Ebola virus in semen, vaginal secretions, and other body fluids among EVD survivors)
Assess the prevalence and duration after EVD recovery of any Ebola ribonucleic acid (RNA) (live or dead) detected by RT-PCR in: i) semen, ii) vaginal swab, iii) rectal swab, iv) sweat, v) urine, vi) saliva, and vii) tears.
Assess the prevalence after EVD recovery of live Ebola RNA detected by culture/viral isolation from i) semen, ii) vaginal swab, iii) rectal swab, iv) sweat, v) urine, vi) saliva, and vii) tears.
Evaluate the time to EBOV clearance using RT-PCR and viral isolation assays among persons with specimens that are positive by RT-PCR at the baseline visit.
Describe concordance between RT-PCR and viral culture results for EBOV in these body fluids.
Module B (Persistence of Ebola virus in breast milk and sweat among lactating women surviving Ebola virus disease)
Assess the prevalence and duration after EVD recovery any Ebola RNA (live or dead) detected by RT-PCR in i) breast milk, and ii) sweat from lactating women.
Assess the prevalence after EVD recovery of live Ebola RNA detected by culture/viral isolation from breast milk and sweat in lactating females.
Evaluate the time to EBOV clearance using RT-PCR and viral isolation assays among persons with specimens that are positive by RT-PCR.
Describe concordance between RT-PCR and viral culture results for EBOV in breast milk and sweat to inform infant feeding recommendations in the context of this epidemic.
For the pilot study and both modules, the study site(s) will be the setting for all initial and follow-up visits of survivor participants. These study sites will be the location for interviews, specimen collection, and counselling regarding test results. Study participants can be referred to HIV and survivor services within the hospitals where the study sites are located.
For the study:
Eligible study participants must demonstrate an EVD discharge certificate and a national identification card in order to be recruited; to ensure survivor status and eligibility for the study, collaborators may cross-check laboratory information as available; this information will be recorded in the Intake Form (Attachment 1) and a unique ID will be assigned.
Entry will then begin with the informed Consent Form (Attachment 2 or Attachment 3; for pilot study/Module A or Module B, respectively).
A Participant Study ID Card (Attachment 4) will be issued to the participant, and a photo will be taken for verification of identity at subsequent study visits.
The participant will then complete a Survivor Questionnaire (Attachment 5) and initial specimen collection.
Specimen(s) will be tested for EBOV RNA by RT-PCR in Sierra Leone at the CDC laboratory facility in Bo; any specimen positive for EBOV by RT-PCR will be frozen and shipped to CDC for culture/virus isolation in a BSL-4 laboratory facility in Atlanta, GA.
Participants with a RT-PCR positive result in any of the initial specimens will be invited to complete a Survivor Follow-up Questionnaire (Attachment 6) at every subsequent study visit and to provide prospective specimens (i.e., repeat specimen collection) of each RT-PCR positive body fluid according to the following schedule:
Semen collection every 2 weeks for the first three visits and then monthly until the RT-PCR is negative (pilot).
Specimen (i.e., semen or vaginal secretions, rectal swabs, sweat, urine, saliva, tears) collection every 2 weeks for the first three visits and then monthly until the RT-PCR is negative (Module A).
Specimen (i.e., sweat and breast milk) collection every 3 days until the RT-PCR is negative (Module B).
All laboratory results will be entered into a Laboratory Results Form (Attachment 7) marked with the participant’s study ID and kept in confidential storage for entering of follow-up results.
At follow up visits, laboratory results will be shared with the participant, and transmission prevention and potentially other counselling messages will be provided via a Counselling Script (Attachment 8).
Confirmatory virus culture results will be reported back to participants when available.
Once a participant has had two consecutive negative test results for all body fluid specimens collected in this study, there will be no further follow-up.
Study instruments will be translated into Krio and other regional languages as necessary by qualified translators. Data collectors will be trained extensively in conducting interviews and the ethical protection of human subjects. Clinic staff will enter participant questionnaire responses into laptop computers which will have the ability to connect to the internet and upload data into a secure questionnaire database. They will also be skilled in specimen collection and ways to communicate instructions for specimen collection to participants. A trained data manager will complete the laboratory results form (Attachment 7) each time a participant submits a specimen for testing. Data for laboratory specimen results will be stored in a secure specimen database after being recorded in computers at the laboratories conducting testing.
Data Flow Diagram
10.1.2. Items of information to be collected
Several items containing personally identifiable patient information will be collected:
Name
Date of Birth
Photographic Identifiers
Mailing Address
Phone Numbers
Email Address
Medical Information and Notes
Medical Records Numbers
Biological Specimens
Certificates
Legal Documents
Employment Status
Other
The personal identifiers collected through the Intake Form (Attachment 1) and the assigned unique ID will be stored in a secure database. A Participant Study ID Card (Attachment 4) will be given to the patient with his/her unique ID number.
10.1.3. How information will be shared and for what purpose
A letter of agreement between CDC and the Sierra Leone MoHS regarding sharing of data and laboratory specimens has been in place since September 2014; this agreement covers shipment of specimens from Sierra Leone to the United States. A study-specific data sharing/data use agreement will be developed in advance of the study between MoHS, WHO, and CDC in order to ensure collaboration and appropriate recognition of all contributing coauthors. Sierra Leone MoHS will determine how and with whom their research data will be shared. For this study, it has been agreed that data will belong to the Sierra Leone MoHS, and joint analysis will be collaboratively performed by MoHS, WHO, and CDC staff. The data will be stored in a Sierra Leone MoHS-owned database. While in the field, CDC will have access to identifiable information. Data delivered to the CDC for statistical analysis will be identified by unique study ID only.
10.1.4. Impact on the respondent’s privacy
Data are treated in a private manner, unless otherwise compelled by law. Highly sensitive information is being collected and would affect a respondent’s privacy if there were a breach. MoHS, CDC, and WHO research partners will make every effort to secure the information as described in Section A.10.1.7.
10.1.5. Whether individuals are informed that providing the information is voluntary or mandatory
Respondents are informed about the voluntary nature of their participation during the consent process (Attachments 2 & 3).
10.1.6. Opportunities to consent, if any, to sharing and submission of information
Consent scripts are included on the consent form attachments (Attachments 2 & 3). Project assistants will obtain written consent from respondents prior to conducting all questionnaires.
10.1.7. How the information will be secured
All MoHS-owned data related to a study participant will be assigned the participant’s unique study ID. No patient-identifying information (i.e., names) will leave Sierra Leone.
Interview data will be organized in databases stored on secure local servers within the MoHS in Sierra Leone and WHO and will be backed-up regularly. Collected data will be directly recorded on computers or tablets to minimize data recording and entry errors and minimize delays in data availability. If paper forms must be used, interview responses will be entered into the database either daily or as a group at the close of data collection; and 10% of entered forms will be re-checked to identify any problems with data entry accuracy that must be addressed.
Electronic equipment and files will be kept password-protected.
Paper forms and electronic devices will be kept locked when not in use.
All individual data identifying direct patient identifiers will be removed from the dataset before analysis and replaced with a unique participant code that can be linked back to individuals via a master key at a centralized secure server and database.
Individual records and the key linking the participant code number will be kept secure, accessible only to the local study team under the supervision of the study PI and MoHS in collaboration with WHO and CDC.
Paper interview forms, if used, will be destroyed within one year after all data are entered and verified.
Laboratory results will be batch processed and complete PCR results for all specimen types will be reported back within 1 week of specimen receipt to each site coordinator. A positive RT-PCR result on any specimen should be entered in the secured data base and reported within 8 hours to the study PI and WHO and CDC local coordinators as well as the site coordinator where the specimen was collected. Laboratory staff will identify specimens only by the labelled study ID, and will not have access to any personally identifying information.
Site coordinators will provide laboratory results only in person and only to participants who (1) return for a follow-up study visit; and (2) present their study ID card or confirm their identity and study ID number; and (3) request to receive their own results. Individual results will not be shared with anyone other than the study participant. Results will be presented according to the counselling script.
Viral isolation results from CDC Atlanta will be reported promptly to the study PI and entered in the secured data base as they arrive. These confirmatory results will not be available within a clinically relevant time frame (6 months) although positive results will be reported to participants.
10.1.8. Whether a system of records is being created under the Privacy Act.
The Privacy Act does not apply and no system of records is being created:1
The Privacy Act covers only records in the possession and control of Federal agencies.
A system of records consists of any item, collection, or grouping of information about an individual, where those records can be retrieved by the name of the individual or by some other type of identifier unique to the individual.
To qualify as a Privacy Act record, the information must identify an individual.
The Sierra Leone MoHS is the owner of the data and will not deliver data for storage on CDC servers except in coded or aggregate formats. These records will not be retrievable by any identifiers.
The Privacy Act applies to records maintained on a living individual who is “a citizen of the United States or an alien lawfully admitted for permanent residence.” All participants will be from Sierra Leone; therefore, their records will not be afforded privacy protections under the Act.
Regardless of the Privacy Act requirements, CDC will take all efforts to protect the privacy of study participants’ records.
11. Justification for Sensitive Questions
The project is dependent on willingness from participants to share intimate information on sexual behavior, and to provide biological specimens. For the individual, there is a risk of the intimate interview and sampling procedure creating anxiety for the interviewee and for his/her family members. Questions will be asked about EVD symptoms experienced (vomiting, diarrhea, etc.), current health (health status, co-morbidities), and sex-related items (using condoms, sore in genital area, type of sexual practice, etc.) that might be embarrassing.
It is also anticipated that study participants receiving their specimen test results may experience psychological and social stress, especially when results are of unknown clinical utility and may be subject to misinterpretation. At the community level, there is a risk of stigma or discrimination enacted towards study participants. However, this information is essential to understanding EBOV persistence in different body fluids and efficiently detecting a public health threat from contact with EVD survivors. This information will allow public health officials, including CDC, to rapidly implement appropriate public health control measures to prevent the introduction and spread of further EVD in communities.
Several survivors’ support groups are actively working to provide counselling and support of various qualities to EVD survivors in Sierra Leone, in collaboration with UNICEF and the national survivors’ association, which study collaborators anticipate will mitigate community concerns.
Benefits of the study also include possibilities for participants to gain information about EBOV persistence in body fluids, and for the community to receive accurate recommendations on how to stop transmission chains.
12. Estimates of Annualized Burden Hours and Costs
The total estimated annualized time burden to Sierra Leone respondents is 2,091 hours at a total cost burden in respondent wages of $7,079 in US Dollars (USD). We anticipate that one-half of this time and cost burden will be incurred by respondents during the 180-day emergency approval period, and the remaining one-half will be incurred during the remaining 180-days, for which an extension ICR will be requested (1,046 burden hours and $3,540, respectively).
Estimated Burden Hours
Using WHO funds only, the study team previously held a focus group with 15 male EVD survivors at the Military 34 hospital grounds; CDC personnel observed, but did not conduct, this focus group. The study team provided an overview of the study and explained the rationale, followed by a review of the informed consent and questionnaire with the group. The group provided feedback on their comprehension of the informed consent, the acceptability of the subject matter, and questionnaire content. Revisions were made to the informed consent and questionnaire based on this feedback. The group did not find the informed consent or questionnaire process burdensome. From this process, it is estimated that the average time to complete the:
Survivor questionnaire (including time for reviewing instructions, gathering needed information and completing the instrument) is 30 minutes;
Follow-up questionnaire is 15 minutes; and
Patient laboratory report is 10 minutes.
The estimated number of responses per respondent is based on several criteria:
The intake form will be completed by the trained data manager once for all participants from the pilot and two Modules.
The initial survivor questionnaire will be administered once for all participants from the pilot and two Modules.
The frequency of the follow-up questionnaire in the pilot and two Modules is dependent on positive test results, which will vary among study participants. Follow-up visits will continue at specified intervals for the pilot and Modules until RT-PCR for pertinent specimens is negative.
Estimated number of follow-up visits, or the expected number of responses per respondent, is based on previously published literature. Male participants are expected to have the highest number of follow-up visits given the persistence of detectable EBOV (PCR and/or virus isolation) in semen several months post symptoms onset. Study collaborators do not expect participants to drop out of the study; however, if this occurs, the provided estimated burden hours would actually be overestimated.
Because laboratory tests are planned at each clinical visit, the estimated number of responses for the data manager entering laboratory results was calculated by multiplying the number of respondents by their estimated number of responses.
Table 12.A. Estimated Burden Hours
Type of Respondent |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Average Burden per Response (hours) |
Total Burden Hours |
Data manager |
Intake Form |
1 |
550 |
20/60 |
183 |
Pilot participants |
Survivor Questionnaire |
100 |
1 |
30/60 |
50 |
Pilot participants |
Survivor Follow-up Questionnaire |
100 |
5 |
15/60 |
125 |
Module A male participants |
Survivor Questionnaire |
175 |
1 |
30/60 |
88 |
Module A male participants |
Survivor Follow-up Questionnaire |
175 |
12 |
15/60 |
525 |
Module A female participants |
Survivor Questionnaire |
175 |
1 |
30/60 |
88 |
Module A female participants |
Survivor Follow-up Questionnaire |
175 |
4 |
15/60 |
175 |
Module B female participants |
Survivor Questionnaire |
100 |
1 |
30/60 |
50 |
Module B female participants |
Survivor Follow-up Questionnaire |
100 |
4 |
15/60 |
100 |
Data manager |
Laboratory Results Form |
1 |
4,250 |
10/60 |
708 |
Total |
|
2,091 |
|||
Estimated Annualized Burden Costs
For Sierra Leone, we based our wage estimate on internet news pages where increases in 2015 minimum wages are published (Appendix C).
To convert from monthly to hourly wages, we also assumed the following relationships: 8 hours/day; 40 hours/week; 4 weeks/month; 160 hours/month. We used the following equation:
Hourly
minimum wage rate = USD per hour =
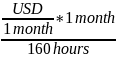 or
or
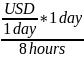
Table 12.B.1. Minimum Wage in Sierra Leone
West Africa |
Official Language |
2013 Population Estimate |
Minimum Wage Estimates |
Source |
||
Country Wage |
US Dollar2 |
|||||
Monthly |
Hourly |
|||||
Sierra Leone |
English |
6.09m |
500,000 SL leone/month |
112.65 |
0.70 |
Internet News 2015 |
Due to lack of documented information from existing sources, we assume that all survivor participants will be unskilled workers to which minimum wage applies, and that trained data managers will be skilled workers and have wages that are 10 times the minimum wage ($7.00/hour).
Table 12.B.2. Estimated Annualized Burden Costs
Type of Respondent |
Form Name |
Total Burden Hours |
Hourly Wage Rate |
Total Respondent Costs |
Data manager |
Intake Form |
183 |
$7.00 |
$1,281 |
Pilot participants |
Survivor Questionnaire |
50 |
$0.70 |
$35 |
Pilot participants |
Survivor Follow-up Questionnaire |
125 |
$0.70 |
$88 |
Module A male participants |
Survivor Questionnaire |
88 |
$0.70 |
$61 |
Module A male participants |
Survivor Follow-up Questionnaire |
525 |
$0.70 |
$368 |
Module A female participants |
Survivor Questionnaire |
88 |
$0.70 |
$61 |
Module A female participants |
Survivor Follow-up Questionnaire |
175 |
$0.70 |
$123 |
Module B female participants |
Survivor Questionnaire |
50 |
$0.70 |
$35 |
Module B female participants |
Survivor Follow-up Questionnaire |
100 |
$0.70 |
$70 |
Data manager |
Laboratory Results Form |
708 |
$7.00 |
$4,958 |
Total |
|
$7,079 |
||
13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
There will be no direct costs to the respondents other than their time to participate in each information collection.
14. Annualized Cost to the Government
The total estimated annualized cost of this research study is $905,083.30.
The CDC is funding this study under cooperative agreement for the total estimated amount of $176,933.50. The annualized wage and travel cost to the federal government is estimated at $728,149.80. This is based on doubling the 180-day estimate detailed in Table 14, which represents the amount of time for CDC staff to advise and design the data collection methodology, provide data management and statistical support, perform laboratory testing, and aggregate and summarize the study data both in the field and in Atlanta during the emergency approval period. Travel costs are included, although there are no equipment or overhead costs.
Therefore, over the initial 180-day emergency approval period, the cost to the government is one-half of the annualized amount, or $452,541.65.
Table 14. Estimated 180-day Federal Government Wages and Travel Costs
Staff (FTE) |
Est. Hours |
Average Hourly Rate |
Average Cost |
Atlanta-based Support Hourly Wage |
|||
Design of methods |
1 FTE 60 hours |
GS14 $49.38 |
$2,962.80 |
2 FTE 200 hours |
GS13 $41.79 |
$16,716.00 |
|
Laboratory testing: virus isolation |
3 FTE 200 hours |
GS12 $35.14 |
$21,084.00 |
Field-based Support Hourly Wage |
|||
Field operations support |
2 FTE 1920 hours |
GS13 $41.79 |
$160,473.60 |
Data management: aggregation and summary |
1 FTE 50 hours |
GS14 $49.38 |
$2,469.00 |
1 FTE 50 hours |
GS13 $41.79 |
$2,089.50 |
|
Laboratory testing: RT-PCR |
4 FTE 500 hours |
GS12 $35.14 |
$70,280.00 |
Travel expenses |
|||
Flights (2 FTEs, 4 flights each, estimated $2,000 per flight) |
$16,000.00 |
||
Per diem (estimated $300/day, 240 days) |
$72,000.00 |
||
Total Cost |
$364,074.90 |
||
http://www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/salary-tables/pdf/2015/ATL_h.pdf |
|||
15. Explanation for Program Changes or Adjustments
This is an emergency collection request of new information.
16. Plans for Tabulation and Publication and Project Time Schedule
Descriptive analysis of the study participants will be performed by the study PI and staff with the support of WHO and CDC. WHO and CDC technical assistance may be provided as needed for these and any additional analyses of the study data.
Pilot study data collection and analysis results will inform the full study (i.e., Modules A and B). Results and analysis (both interim and final) will be used to update and refine relevant counselling messages and recommendations from WHO and CDC. Potential products include scientific abstracts and manuscripts, presentations, guidance documents, and others. A data sharing/data use agreement will be developed in advance of the study between MoHS, WHO and CDC in order to ensure collaboration and appropriate recognition of all contributing coauthors. All parties (MoHS, WHO, and CDC) will share ownership of all data in the study; although, CDC will retain only de-identified data on federal servers. Publication policy will follow international guidelines for authorship applying Vancouver criteria, and all publications will be shared and reviewed by the three parties before submission for publication. Local partnerships are involved with the UNAIDS country office, with MSWGCA and local survivors’ organizations. These local partners will support the study process in different respects, during the data collection process and/or in relation to specific needs of study participants. Local partners will be offered co-authorship in relation to fulfilling the Vancouver criteria for authorship.
A more detailed 2015 study timeline is as follows:
Work Plan |
||||||||||||
ACTIVITIES - Lead agency (other agency involvement TBD) |
Study Timeline (2015) |
|||||||||||
Month 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|
Local ethics committee review - MoHS |
1 |
|
|
|
|
|
|
|
|
|
|
|
Investigators coordination meeting - MoHS |
|
2 |
|
|
|
|
|
|
|
|
|
|
Data and Safety Monitoring Board (DSMB) initial discussions - MoHS |
1 |
|
|
|
|
|
|
|
|
|
|
|
Questionnaire development & completion - WHO/CDC |
1 |
|
|
|
|
|
|
|
|
|
|
|
Training on participant recruitment – MoHS/WHO/CDC |
|
2 |
|
|
|
|
|
|
|
|
|
|
Training on informed consent process - MoHS/WHO/CDC |
|
2 |
|
|
|
|
|
|
|
|
|
|
Training on administering questionnaire - MoHS/WHO/CDC |
|
2 |
|
|
|
|
|
|
|
|
|
|
Months after OMB Approval |
||||||||||||
Recruitment for pilot - MoHS |
|
2 |
|
|
|
|
|
|
|
|
|
|
Data collection for pilot - MoHS/WHO/CDC |
|
|
3 |
4 |
5 |
|
|
|
|
|
|
|
Data analysis for pilot - WHO/CDC |
|
|
3 |
4 |
|
|
|
|
|
|
|
|
Laboratory analysis for pilot - CDC |
|
|
3 |
4 |
|
|
|
|
|
|
|
|
Recruitment for full study (Module A and B) - MoHS |
|
|
|
4 |
5 |
|
|
|
|
|
|
|
Data collection for full study (Module A and B) - MoHS / WHO/CDC |
|
|
|
4 |
5 |
6 |
7 |
8 |
9 |
|
|
|
Data Management (for pilot, Module A and B) - MoHS / WHO/CDC |
|
|
|
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
|
Data analysis - WHO/CDC |
|
|
|
|
|
|
|
|
|
10 |
11 |
12 |
Laboratory analysis (for pilot, Module A and B) - CDC |
|
|
|
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
Writing interim project report - WHO/CDC |
|
|
|
|
|
6 |
7 |
|
|
|
|
|
DSMB interim review - MoHS |
|
|
|
|
|
6 |
|
|
|
|
|
|
Writing final project reports - WHO/CDC |
|
|
|
|
|
|
|
|
|
10 |
11 |
12 |
DSMB final review - MoHS |
|
|
|
|
|
|
|
|
|
|
|
12 |
Final project review process - MoHS |
|
|
|
|
|
|
|
|
|
|
|
12 |
Dissemination of project outcomes - MoHS / WHO/CDC |
|
|
|
|
|
|
|
|
|
|
|
12 |
17. Reason(s) Display of OMB Expiration Date is Inappropriate
The display of the OMB expiration date is appropriate.
18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification. These activities comply with the requirements in 5 CFR 1320.9.
LIST OF APPENDICES
APPENDIX A. Authorizing Legislation
APPENDIX B. Human Subjects Review and Approvals
APPENDIX C. Sierra Leone News 2015 Minimum Wage
LIST OF ATTACHMENTS
ATTACHMENT 1. Intake Form
ATTACHMENT 2. Consent Form – Pilot and Module A
ATTACHMENT 3. Consent Form – Module B
ATTACHMENT 4. Participant Study ID Card
ATTACHMENT 5. Survivor Questionnaire
ATTACHMENT 6. Survivor Follow-up Questionnaire
ATTACHMENT 7. Laboratory Results Form
ATTACHMENT 8. Counselling Script
1 Source: "Overview of the Privacy Act of 1974," prepared by the Department of Justice. Accessed April 30, 2015 at http://www.justice.gov/sites/default/files/opcl/docs/1974privacyact-2012.pdf
2 USD conversion performed 02/12/2015 at eXchangeRate.com at http://www.exchangerate.com/currency-converter/XOF/USD/60,000%20/?XR-200Plus_Converter=convert&calc_short_from_iso=59&calc_short_to_iso=239
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | O2C2-Supporting-Statement-A-Template-CDCID-NICKNAME-SSA |
| Author | CDC User |
| File Modified | 0000-00-00 |
| File Created | 2021-01-25 |
© 2026 OMB.report | Privacy Policy