CMS-10276 - Supporting Statement A [rev 07-15-2015 by OSORA PRA]
CMS-10276 - Supporting Statement A [rev 07-15-2015 by OSORA PRA].docx
Physician Quality Reporting System (PQRS) (CMS-10276)
OMB: 0938-1059
Supporting Statement – Part A:
The Physician Quality Reporting System
CMS-10276 (OMB 0938-1059)
This package is associated with a July 15, 2015, NPRM (CMS-1631-P; RIN 0938-AS40).
Background
The Physician Quality Reporting System or PQRS (formerly known as the Physician Quality Reporting Initiative, or PQRI) was established by section 101(b) of Division B of the Tax Relief and Health Care Act of 2006 – Medicare Improvements and Extension Act of 2006 (MIEA-TRHCA) and is codified in sections 1848(a), (k), and (m) of the Social Security Act (the Act). Changes to the PQRS also resulted from the Medicare, Medicaid, and SCHIP Extension Act of 2007 (MMSEA), the Medicare Improvements for Patients and Providers Act of 2008 (MIPPA), the Affordable Care Act (ACA), and the American Taxpayer Relief Act (ATRA) of 2012. The program provided incentive payments to eligible professionals and group practices who satisfactory reported data on Quality measures or satisfactory participated until 2014. In 2015, eligible professionals and group practices became subject to payment adjustments for those who did not meet the criteria to satisfactory report or satisfactory participate in PQRS. This will be equal to the applicable quality percent of the Secretary’s estimate of allowed Part B charges for covered professional services furnished by the eligible professional or group practice during a specified year (for 2016 and subsequent years, the applicable quality percent is 2.0%).
In accordance with section 1848(k)(2) of the Act, an eligible professional or group practice who satisfactorily submits data on Quality measures for covered professional services furnished for an applicable year can (1) qualify to receive an incentive payment and/or (2) be relieved from the application of a payment adjustment. In lieu of satisfactory reporting, eligible professionals may (1) qualify to receive an incentive payment and/or (2) be relieved from the application of a payment adjustment if the eligible professional satisfactorily participates in a qualified clinical data registry (QCDR). The proposed criteria for satisfactory reporting (or, in lieu of satisfactory reporting, satisfactory participation in a Qualified Clinical Data Registry [QCDR]) for the 2018 PQRS payment adjustment are set forth in the CY 2016 Medicare Physician Fee Schedule (PFS) proposed rule. The ACA made changes to the PQRS, authorizing incentive payment adjustments until 2014 and requiring payment adjustments beginning in 2015 for eligible professionals or group practices that do not satisfactorily report data on quality measures during the applicable period for the year.
A. Justification
1. Need and Legal Basis
With respect to the 2018 PQRS payment adjustment, collection of this information applies to all eligible professionals and group practices. Therefore, since the reporting periods for the 2018 payment adjustment coincide in CY 2016, the collection of this information will apply to all eligible professionals and group practices.
Eligible professionals or group practices who do not satisfactorily report data on quality measures for covered professional services furnished during the applicable 2018 PQRS payment adjustment reporting period will be subject to a payment adjustment equal to 2.0 percent of the total estimated allowed charges. The proposed criteria for satisfactory reporting of data on individual quality measures and measures groups (or, lieu of satisfactory reporting, satisfactory participation in a QCDR) for the 2018 PQRS payment adjustment are set forth in the CY 2016 PFS proposed rule.
Individual eligible professionals do not need to sign up or pre-register with the PQRS program to begin participating in the PQRS. Eligible professionals wishing to report data on quality measures may do so via five reporting mechanisms: claims, qualified registry, EHR (which includes submitting quality measures data via a direct EHR product and an EHR data submission vendor’s product that is certified by the Office of National Coordinator (ONC) as “Certified EHR Technology” (CEHRT)), and QCDR.
While individual eligible professionals do not need to sign up or pre-register to begin participating in the PQRS, eligible professionals in group practices interested in participating in the PQRS as a group practice must follow the guidelines for the reporting mechanism for which they choose. For those group practices, that choose the group practice reporting option using web interface (GPRO) reporting option they must indicate their intent to participate for an applicable reporting period by registering. Group practices participating via GPRO wishing to report data on quality measures may do so via four reporting mechanisms – the web interface, qualified registry, EHR (which includes submitting quality measures data via a direct EHR product and an EHR data submission vendor’s product that is certified by ONC as CEHRT), and the CMS-certified survey vendor (in conjunction with registry, direct EHR product and an EHR data submission vendor’s product that is certified by ONC as CEHRT, or GPRO Web Interface).
Note: For qualified registries and QCDRs to submit quality measure results with numerator and denominator data on individual quality measures or measures groups on behalf of eligible professionals (and, in the case of qualified registries, and group practices), a qualified registry or QCDR will need to self-nominate to become “qualified. If a qualified registry was qualified for a prior year and successfully submitted quality measure results with numerator and denominator data on quality measures on behalf of their participants, the qualified registry would not need to become re-qualified for PQRS purposes. Qualified registries previously qualified to submit PQRS quality measures data may be disqualified for submitting grossly inaccurate data to CMS. In order to participate in PQRS the following year, registries that have been disqualified would be required to self-nominate and undergo the qualification process again in order to become re-qualified to submit PQRS quality measures data. However, a QCDR must become qualified every year in which it seeks to submit quality measure results on behalf of its eligible professionals.
Information Users
The data on quality measures collected from eligible professionals or group practices will be used by CMS to:
Determine whether an eligible professional or group practice meets the criteria for satisfactory reporting of quality measures data (or, in lieu of satisfactory reporting, satisfactory participation in a QCDR) for the 2018 PQRS payment adjustment.
To calculate and make payment adjustments to eligible professionals and group practices for the 2018 PQRS payment adjustment.
Publicly post the names of eligible professionals and group practices who satisfactorily report quality measures data (or, in lieu of satisfactory reporting, satisfactory participation in a QCDR) on the CMS Physician Compare Web site.
To determine a group practice’s quality score under the Physician Value-based Payment Modifier.
Only registry vendors and QCDRs that are interested in participating in PQRS will self-nominate to be a respective qualified registry and/or QCDR. The information collected from the self-nomination process for qualified registries and QCDRs will be used by CMS to determine whether the registry or QCDR meets the PQRS respective qualified registry or QCDR requirements and is qualified to submit quality measures results with numerator and denominator data on PQRS individual quality measures and/or measures groups on behalf of eligible professionals and/or group practices.
Improved Information Technology
Reporting via claims-based reporting mechanism: For claims-based reporting, the normal Medicare Part B claims submission process is used to collect data on quality measures from eligible professionals. Individual eligible professionals are not asked to provide any documentation by CD or hardcopy.
Reporting via qualified registry-based reporting mechanism or QCDR: For qualified registry-based and QCDR-based reporting, qualified registries and QCDRs submit quality measures results with numerator and denominator data on PQRS measures or other measures to CMS on behalf of its eligible professional and group practice members electronically.
There is no application for qualified registries that wish to self-nominate to become a qualified PQRS qualified registry. Qualified registries are asked to submit a self-nomination letter requesting inclusion in PQRS for each program year in which the qualified registry seeks to be qualified to submit quality measures data on behalf of its participants.
Likewise, there is no application for clinical data qualified registries that wish to self-nominate to become a PQRS QCDR. QCDRs must meet established requirements for being qualified to participate in the PQRS as a QCDR and are asked to submit a self-nomination statement requesting inclusion in PQRS for each program year in which the clinical data qualified registry seeks to be qualified to submit quality measures data on behalf of its participants.
Reporting via a direct EHR product that is CEHRT or EHR data submission vendor who’s product is CEHRT: For EHR-based reporting, eligible professionals and group practices submit data on quality measures to CMS electronically through a direct EHR product or via an EHR data submission vendor’s product that is CEHRT.
Reporting via GPRO Web Interface: This group practice reporting mechanism stems from a previously OMB-approved data collection web interface (see OMB Control Number (OCN) 0938-1059). This web interface is an automated, electronic tool developed by CMS and refined with industry input. In prior years, this web interface was the “PAT,” or Performance Assessment Tool. It was developed explicitly for specific Medicare demonstrations and has been used successfully over the past 4 years for these demonstrations. Although we moved away from use of the PAT, we note that the GPRO Web Interface that will be used is similar in terms of burden to using the PAT.
Reporting via CMS-certified survey vendor: A CMS-certified survey vendor is certified by CMS for a particular program year to transmit survey measures data to CMS. Please note that discussion of this certified survey vendor option is discussed in a separate PRA package CMS-10450 (OMB control number: 0938-1222,). Therefore, we do not address this reporting option in this PRA package.
Duplication of Similar Information
To minimize duplication of similar information being reported amongst PQRS and other similar programs, CMS is proposing efforts to align PQRS reporting requirements with the requirements of other quality reporting programs, such as the EHR Incentive Program, the Value-based Payment Modifier, the Medicare Shared Savings Program, and Pioneer ACOs. For example, with respect to reporting as an individual eligible professional, CMS has put forth a number of requirements related to aligning the criteria for satisfactory reporting under PQRS with the requirements for meeting the clinical quality measure (CQM) component of achieving meaningful use under the EHR Incentive Program. Specifically, beginning in 2014, the measures available for reporting under the EHR-based reporting mechanisms under PQRS aligns with the EHR measures available for reporting under the EHR Incentive Program. Beginning in 2014, we have aligned the criteria for satisfactory reporting using the EHR-based reporting mechanisms under PQRS with the reporting criteria for meeting the CQM component of achieving meaningful use under the EHR Incentive Program. In addition, the Value-based Payment Modifier continues to use quality measures data reported in the PQRS to measure a physician’s performance in their program.
With respect to participating in PQRS using the group practice reporting option (GPRO), CMS sets forth requirements that align with the requirements for Accountable Care Organizations (ACOs) participating under the Medicare Shared Savings Program and Pioneer ACOs. Under the Medicare Shared Savings Program, group practices had statutory authority to earn a PQRS incentive through their participation in the Medicare Shared Savings Program. Similarly, group practices could have earned a PQRS incentive through their participation in a Pioneer ACO. By doing so, we were rewarding those practices that voluntarily agreed to participate and reduced their reporting burden they would otherwise have had if they had to submit duplicate clinical quality data using two different systems. CMS has also set forth requirements that would allow group practices to avoid the downward-based adjustment under the Value-based Payment Modifier.
Small Businesses
The collection of information will primarily affect small entities (e.g., individual eligible professionals). We have attempted to minimize the burden on eligible professionals by providing eligible professionals with multiple reporting options for submitting quality measures data.
6. Less Frequent Collection
If data on the quality measures are not collected from individual eligible professionals or group practices, CMS will have no mechanism to: (1) determine whether an eligible professional or group practice meets the criteria for satisfactory reporting of quality measures data for PQRS, or, in lieu of satisfactory reporting, whether an eligible professional satisfactorily participates in a QCDR, (2) to calculate for payment adjustments to eligible professionals or group practices, and (3) publicly post the names of eligible professionals and group practices who satisfactorily report quality measures data or satisfactorily participate in a QCDR on the CMS Web site.
If qualified registries and QCDRs are not required to submit a self-nomination statement, CMS will have no mechanism to determine which qualified registries and clinical data qualified registries will participate in submitting quality measures data. As such, CMS would not be able to post the annual list of qualified registries that eligible professionals use to select qualified registries and QCDRs to use to report quality measures data to CMS. Similarly, if group practices participating in PQRS GPRO are not required to indicate their intent to participate, CMS will have no mechanism to determine that group practices be assessed differently (at the TIN level) than eligible professionals (who are assessed at the TIN/NPI level).
7. Special Circumstances
There are no special circumstances that would require an information collection to be conducted in a manner that requires respondents to:
Report information to the agency more often than quarterly;
Prepare a written response to a collection of information in fewer than 30 days after receipt of it;
Submit more than an original and two copies of any document;
Retain records, other than health, medical, government contract, grant-in-aid, or tax records for more than three years;
Collect data in connection with a statistical survey that is not designed to produce valid and reliable results that can be generalized to the universe of study,
Use a statistical data classification that has not been reviewed and approved by OMB;
Include a pledge of confidentiality that is not supported by authority established in statute or regulation that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or
Submit proprietary trade secret, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information's confidentiality to the extent permitted by law.
8. Federal Register Notice/Outside Consultation
The NPRM is serving as the 60-day Federal Register notice which published on July 15, 2015 (80 FR 41685). The NPRM was placed on public inspection on July 8 whereby comments are due Sept 8.
9. Payment/Gift To Respondent
As authorized under section 1848(m)(1)(A) of the Act, eligible professionals or group practices who satisfactorily report data on quality measures earned incentive payments from 2007 through 2014 based on an eligible professional’s total estimated allowed charges for all covered professional services. Eligible professionals, including eligible professionals in a group practice participating in the PQRS GPRO, who satisfactorily report quality measures data during 2012, 2013, and 2014 also qualified for an additional 0.5 percent incentive by both participating in a Maintenance of Certification Program and successfully completing a Maintenance of Certification Program practice assessment more frequently than is required to qualify for or maintain board certification status. These incentive payments ceased in 2014.
The ACA) made changes to the PQRS, authorizing incentive payments until 2014 and requiring payment adjustments beginning in 2015 for eligible professionals or group practices who do not satisfactorily report data on quality measures during the applicable reporting period for the year. In 2015, eligible professionals or group practices who do not satisfactorily report data on quality measures for covered professional services furnished during the applicable PQRS payment adjustment reporting period will be subject to a payment adjustment equal to 1.5 percent in 2015 and 2.0 percent in 2016 and subsequent years of the eligible professional’s total estimated allowed charges for the respective year.
Confidentiality
Consistent with federal government and CMS policies, CMS will protect the confidentiality of the requested proprietary information. Specifically, any confidential information (as such terms are interpreted under the Freedom of Information Act, the Privacy Act of 1974, and other applicable Federal government rules and regulations) will be protected from release by CMS under 5 U.S.C. § 552a(b).
11. Sensitive Questions
Other than the labeled information noted above in section 10, there are no sensitive questions included in the information request.
12. Burden Estimate (Total Hours & Wages)
Wage Estimates
To derive average costs, we used data from the U.S. Bureau of Labor Statistics’ May 2014 National Occupational Employment and Wage Estimates for all salary estimates (www.bls.gov/oes/current/oes_nat.htm ). In this regard, the following table presents the mean hourly wage, the cost of fringe benefits, and the adjusted hourly wage.
Occupation Title |
Occupation Code |
Mean Hourly Wage ($/hr) |
Fringe Benefit ($/hr) |
Adjusted Hourly Wage ($/hr) |
Billing and Posting Clerks |
43-3021 |
17.10 |
9.58* |
26.68 |
Computer Systems Analysts |
15-1121 |
41.98 |
41.98 |
83.96 |
*For fringe benefits, we are using the December 2014 Employer Costs for Employee Compensation (http://www.bls.gov/news.release/archives/ecec_03112015.pdf).
Except where noted, we are adjusting our employee hourly wage estimates by a factor of 100 percent. This is necessarily a rough adjustment, both because fringe benefits and overhead costs vary significantly from employer to employer, and because methods of estimating these costs vary widely from study to study. Nonetheless, there is no practical alternative and we believe that doubling the hourly wage to estimate total cost is a reasonably accurate estimation method.
Burden
The annual burden estimate is calculated separately for the 2014 PQRS for (1) individual eligible professionals and group practices using the claims (for eligible professionals only), (2) qualified registry and QCDR, (3) EHR-based reporting mechanisms, and (4) group practices using the GPRO. There is also a separate annual burden estimate for qualified registry and QCDR vendors who wish to be qualified to submit quality measures data. Please note that we are grouping group practices using the qualified registry and EHR-based reporting mechanisms with the burden estimate for individual eligible professionals using the qualified registry and EHR-based reporting mechanisms because we believe the criteria for satisfactory reporting for group practices using these 2 reporting mechanisms under the GPRO are similar to the satisfactory reporting criteria for eligible professionals using these reporting mechanisms.
Burden Estimates for the PQRS: (CY 2016)
Burden Estimate for PQRS Reporting by Individual Eligible Professionals: Reporting in General
According to the 2013 Reporting Experience, “more than 1.25 million eligible professionals were eligible to participate in the 2013 PQRS, Medicare Shared Savings Program, and Pioneer ACO Model.”1 In this burden estimate, we assume that 1.25 million eligible professionals, the same number of eligible professionals eligible to participate in the PQRS in 2012, will be eligible to participate in the PQRS. Since all eligible professionals are subject to the 2018 PQRS payment adjustment, we estimate that ALL 1.25 million eligible professionals will participate in the PQRS in 2016 for purposes of meeting the criteria for satisfactory reporting (or, in lieu of satisfactory reporting, satisfactory participation in a QCDR) for the 2018 PQRS payment adjustment.
Historically, the PQRS has never experienced 100% participation in reporting for the PQRS. In the 2013 PQRS and eRx Reporting Experience Report more than 1.25 million professionals were eligible to participate in the 2013 PQRS (including group practices reporting under the GPRO, Medicare Shared Savings Program, and Pioneer ACO Model).Therefore, we believe that although 1.25 million eligible professionals will be subject to the 2018 PQRS payment adjustment, not all eligible participants will actually report quality measures data for purposes of the 2018 PQRS payment adjustment. In this burden estimate, we will only provide burden estimates for the eligible professionals and group practices who attempt to submit quality measures data for purposes of the 2018 PQRS payment adjustment.
In 2013, 641,654 eligible professionals (51 percent) eligible professionals (including those who belonged to group practices that reported under the GPRO and eligible professionals within an ACO that participated in the PQRS via the GPRO) participated in the PQRS, Medicare Shared Savings Program, or Pioneer ACO Model.2 We expect to see a steady increase in participation in reporting for the PQRS in 2016 than 2013. Eligible professionals have become more familiar with the PQRS payment adjustments since eligible professionals are currently experiencing the implementation of the first PQRS payment adjustment the 2015 PQRS payment adjustment. Therefore, we estimate that we will see a 70 percent participation rate in 2016. Therefore, we estimate that 70 percent of eligible professionals (or approximately 840,000 eligible professionals) will report quality measures data for purposes of the 2018 PQRS payment adjustment.
With respect to the PQRS, the burden associated with the requirements of this voluntary reporting initiative is the time and effort associated with individual eligible professionals and group practices identifying applicable quality measures for which they can report the necessary information, selecting a reporting option, and reporting the information on their selected measures or measures group to CMS using their selected reporting option. We assume that most eligible professionals participating in the PQRS will attempt to meet the criteria for satisfactory reporting for the 2018 PQRS payment adjustment.
We believe the labor associated with eligible professionals and group practices reporting quality measures data in the PQRS is primarily handled by an eligible professional’s or group practice’s billing clerk or computer analyst trained to report quality measures data. Therefore, we will consider the hourly wage of a billing clerk and computer analyst in our estimates. For purposes of this burden estimate, we will assume that a billing clerk at $26.68/hr will handle the administrative duties associated with participating in the PQRS. In addition, we assume that a computer analyst at $83.96/hr will engage in the duties associated with the reporting of quality measures.
For individual eligible professionals, the burden associated with the requirements of this reporting initiative is the time and effort associated with eligible professionals identifying applicable quality measures for which they can report the necessary information, collecting the necessary information, and reporting the information needed to report the eligible professional’s measures. We believe it is difficult to accurately quantify the burden because eligible professionals may have different processes for integrating the PQRS into their practice’s work flows. Moreover, the time needed for an eligible professional to review the quality measures and other information, select measures applicable to his or her patients and the services he or she furnishes to them, and incorporate the use of quality data codes into the office work flows is expected to vary along with the number of measures that are potentially applicable to a given professional’s practice. Since eligible professionals are generally required to report on at least nine measures covering at least three National Quality Strategy domains criteria for satisfactory reporting (or, in lieu of satisfactory reporting, satisfactory participation in a QCDR) for the 2018 PQRS payment adjustment, we will assume that each eligible professional reports on an average of nine measures for this burden analysis.
For eligible professionals who are participating in PQRS, we will assign five total hours as the amount of time needed for an eligible professional’s billing clerk to review the PQRS Measures List, review the various reporting options, select the most appropriate reporting option, identify the applicable measures or measures groups for which they can report the necessary information, review the measure specifications for the selected measures or measures groups, and incorporate reporting of the selected measures or measures groups into the office work flows. The measures list contains the measure title and brief summary information for the eligible professional to review. Assuming the eligible professional has received no training from his/her specialty society, we estimate it will take an eligible professional’s billing clerk up to two hours to review this list, review the reporting options, and select a reporting option and measures on which to report. If an eligible professional has received training, then we believe this would take less time. CMS believes three hours is plenty of time for an eligible professional to review the measure specifications of nine measures or one measures group they select to report for purposes of participating in PQRS and to develop a mechanism for incorporating reporting of the selected measures or measures groups into the office work flows. Therefore, we believe that the start-up cost for an eligible professional to report PQRS quality measures data is five hours x $26.68/hour = $133.40.
We continue to expect the ongoing costs associated with PQRS participation to decline based on an eligible professional’s familiarity with and understanding of the PQRS, experience with participating in the PQRS, and increased efforts by CMS and stakeholders to disseminate useful educational resources and best practices.
We believe the burden associated with actually reporting the quality measures will vary depending on the reporting mechanism selected by the eligible professional. As such, we break down the burden estimates by eligible professionals and group practices participating in the GPRO according to the reporting mechanism used.
Burden Estimate for PQRS Reporting by Individual Eligible Professionals and Group Practices: Claims-Based Reporting Mechanism
According to the 2011 PQRS and eRx Experience Report, in 2011, 229,282 of the 320,422 eligible professionals (or 72 percent) of eligible professionals used the claims-based reporting mechanism. According to the 2012 Reporting Experience, 248,206 eligible professionals participated in the PQRS using the claims-based reporting mechanism in 2012.3 According to the 2013 PQRS and eRx Experience Report, 641,654 eligible professionals participated as individuals or group practices through one of the PQRS reporting mechanism, a 47 percent increase from those that participated in 2012 (435,931). Through the individual claims-based reporting mechanism, 331,668 of those eligible professionals (or 52 percent) reported using this mechanism. Increased claims based reporting to 350,000 (approximately five percent increase over 2013). Though claims reporting was declining, we did see an increase in 2013 once the payment adjustment was applied to all participants, so we assume a slight increase in 2016.
According to the historical data cited above, while the claims-based reporting mechanism is still the most widely-used reporting mechanism, we were seeing a decline in the use of the claims-based reporting mechanism in the PQRS. There was a slight increase in 2013, which may be reflected by the use of administrative claims-based reporting mechanism by individual eligible professionals and group practices only for the 2015 PQRS payment adjustment (in CY2013).
While these eligible professionals continue to participate in the PQRS, these eligible professionals have started to shift towards the use of other reporting mechanisms – mainly the Group Practice Web Interface (whether used by a PQRS GPRO or an ACO participating in the PQRS via the Medicare Shared Savings Program), registry, or the EHR-based reporting mechanisms. For purposes of this burden estimate, based on PQRS participation using the claims-based reporting mechanism in 2012 and 2013, we will assume that approximately 350,000 eligible professionals will participate in the PQRS using the claims-based reporting mechanism.
Under the claims‑based reporting option, eligible professionals must gather the required information, select the appropriate quality data codes (QDCs), and include the appropriate QDCs on the claims they submit for payment. The PQRS collects QDCs as additional (optional) line items on the the CMS-1500 claim form or electronic equivalent HIPAA transaction 837‑P, approved under OMB Control number 0938-0999. This rule does not propose any changes to these forms. Beginning in 2014, CMS made changes on how Critical access hospitals (CAHs) were billed under Medicare which made it possible for eligible professionals in CAH method II payment to participate in PQRS. CMS Form 1450 or UB04 will be used.
Based on our experience with the PVRP, we continue to estimate that the time needed to perform all the steps necessary to report each measure (that is, reporting the relevant quality data code(s) for nine measures measure) would range from 15 seconds (0.25 minutes) to over 12 minutes for complicated cases and/or measures, with the median time being 1.75 minutes. To report nine measures, we estimate that it would take approximately 2.25 minutes (0.25 min x 9) to 108 minutes (12 min x 9) to perform all the steps necessary to report nine measures.
Per measure, at an adjusted labor cost of $83.96/hour per practice, for a computer systems analyst, the cost per measure associated with this burden will range from $0.35 [($83.96/hr / 60) x 0.25 minutes] to $16.79 [(83.96/hr / 60) x 12 minutes], with a median cost of $2.45 [($83.96/hr / 60) x 1.75 minutes].. To report nine measures, using an average labor cost of $83.96/hour, we estimated that the time cost of reporting for an eligible professional via claims would range from $3.15 ($0.35 x 9) to $151.11 ($16.79 x 9), with a median cost of $22.05 ($2.45 x 9).
The total estimated annual burden for this requirement will also vary along with the volume of claims on which quality data is reported. In previous years, when we required reporting on 80 percent of eligible cases for claims‑based reporting, we found that on average, the median number of reporting instances for each of the PQRS measures was nine. Since we reduced the required reporting rate by over one‑third to 50 percent, then for purposes of this burden analysis we will assume that an eligible professional or eligible professional in a group practice will need to report each selected measure for six reporting instances. The actual number of cases on which an eligible professional or group practice is required to report quality measures data will vary, however, with the eligible professional's or group practice’s patient population and the types of measures on which the eligible professional or group practice chooses to report (each measure's specifications includes a required reporting frequency). For the 2018 payment adjustment, EPs will also report on one cross-cutting measure if they see at least one Medicare patient. However, we do not see any additional burden impact as they are still reporting on the same number of measures.
Based on the assumptions discussed previously, we estimate the total annual reporting burden per individual eligible professional associated with claims‑based reporting will range from 13.5 minutes (0.25 minutes per measure x nine measures x six cases per measure) to 648 minutes (12 minutes per measure x nine measures x six cases per measure), with the burden to the median practice being 94.5 minutes (1.75 minutes per measure x nine measures x six cases). We estimate the total annual reporting cost per eligible professional in a group practice associated with claims‑based reporting will range from $18.90 ($0.35 per measure x nine measures x six cases per measure) to $906.66 ($16.79 per measure x nine measures x six cases per measure), with the cost to the median practice being $132.30per eligible professional ($2.45 per measure x nine measures x six cases per measure).
Based on the assumptions discussed above and in Part B of this supporting statement, Table 1 provides an estimate of the range of total annual burden hours and total annual cost burden associated with eligible professionals using the claims-based reporting mechanism.
Table 1: Summary of Burden Estimates for Eligible Professionals using the Claims-based Reporting Mechanism
|
Minimum Burden Estimate |
Median Burden Estimate |
Maximum Burden Estimate |
Estimated # of Participating Eligible Professionals (a) |
350,000 |
350,000 |
350,000 |
Estimated # of Measures Per Eligible Professional Per Year (b) |
9 |
9 |
9 |
Estimated # of Cases Per Measure Per Eligible Professional Per Year (c) |
6 |
6 |
6 |
Total Estimated # of Cases Per Eligible Professional Per Year (d) = (b)*(c) |
54 |
54 |
54 |
Estimated Burden Hours Per Case (e) |
0.00415 |
0.02917 |
0.19992 |
Estimated Total Burden Hours For Measures Per Eligible Professional Per Year (f) = (d)*(e) |
0.2241 |
1.57518 |
10.79568 |
Estimated Burden Hours Per Eligible Professional to Prepare for PQRS Participation (g) |
5 |
5 |
5 |
Estimated Total Annual Burden Hours Per Eligible Professional (h) = (f)+(g) |
5.2241 |
6.57518 |
15.79568 |
Estimated Total Annual Burden Hours (i) = (a)*(h) |
1,828,435 |
2,301,313 |
5,528,488 |
Estimated Cost Per Case (j) |
$0.35 |
$2.45 |
$16.79 |
Total Estimated Cost of Cases Per Eligible Professional Per Year (k) = (d)*(j) |
$18.90 |
$132.30 |
$906.66 |
Estimated Cost Per Eligible Professional to Prepare for PQRS Participation (l) |
$133.40 |
$133.40 |
$133.40 |
Estimated Total Annual Cost Per Eligible Professional (m) = (k) + (l) |
$152.30 |
$265.70 |
$1,040.06 |
Estimated Total Annual Burden Cost (n) = (a)*(m) |
$53,305,000 |
$92,995,000 |
$364,021,000 |
Burden Estimate for PQRS Reporting by Individual Eligible Professionals and Group Practices: Qualified Registry-based and QCDR-based Reporting Mechanisms
In 2011, approximately 50,215 (or 16 percent) of the 320,422 eligible professionals participating in PQRS used the qualified registry-based reporting mechanism. In 2012, 36,473 eligible professionals reported individual measures via the registry-based reporting mechanism, and 10,478 eligible professionals reporting measures groups via the registry-based reporting mechanism in 2012.4 According to the 2013 Reporting Experience, approximately 67,896 eligible professionals participated in the PQRS using the registry-based reporting mechanism (51,473 for individual measures and 16,423 for measures groups). Please note that we currently have no data on participation in the PQRS via a QCDR as 2014 is the first year in which an eligible professional may participate in the PQRS via a QCDR.
We believe that the rest of the eligible professionals not participating in other PQRS reporting mechanisms will use either the registry or QCDR reporting mechanisms for the following reasons:
The PQRS measures set is moving away from use of claims-based measures and moving towards the use of registry-based measures
We believe the number of QCDR vendors will increase as the QCDR reporting mechanism evolves.
Therefore, based on these assumptions, we expect to see a significant jump from 47,000 eligible professionals to approximately 212,000 eligible professionals using either the registry-based reporting mechanism or QCDR in 2016. We believe the majority of these eligible professionals will participate in the PQRS using a QCDR, as we presume QCDRs will be larger entities with more members.
For qualified registry‑based and QCDR-based reporting, there will be no additional time burden for eligible professionals or group practices to report data to a qualified registry as eligible professionals and group practices opting for qualified registry‑based reporting or use of a QCDR will more than likely already be reporting data to the qualified registry for other purposes and the qualified registry will merely be re‑packaging the data for use in the PQRS. Little, if any, additional data will need to be reported to the qualified registry or QCDR solely for purposes of participation in the PQRS. However, eligible professionals and group practices will need to authorize or instruct the qualified registry or QCDR to submit quality measures results and numerator and denominator data on quality measures to CMS on their behalf. We estimate that the time and effort associated with this will be approximately five minutes per eligible professional or eligible professional within a group practice.
Based on the assumptions discussed above and in Part B of this supporting statement, Table 2 provides an estimate of the total annual burden hours and total annual cost burden associated with eligible professionals using the qualified registry-based or QCDR-based reporting mechanism. Please note that, unlike the claims-based reporting mechanism that would require an eligible professional to report data to CMS on quality measures on multiple occasions, an eligible professional would not be required to submit this data to CMS, as the qualified registry or QCDR would perform this function on the eligible professional’s behalf.
Table 2: Summary of Burden Estimates for Eligible Professionals (Participating Individually or as Part of a Group Practice) using the Qualified registry-based and QCDR-based Reporting Mechanisms
|
Burden Estimate |
Estimated # of Participating Eligible Professionals (a) |
212,000 |
Estimated Burden Hours Per Eligible Professional to Authorize the Qualified registry or QCDR to Report on Eligible Professional’s Behalf (b) |
0.083 |
Estimated Burden Hours Per Eligible Professional to Report PQRS Data to Qualified registry or QCDR (c) |
3 |
Estimated Burden Hours Per Eligible Professional to Prepare for PQRS Participation (d) |
5 |
Estimated Total Annual Burden Hours Per Eligible Professional (e) = (b)+(c)+(d) |
8.083 |
Estimated Total Annual Burden Hours (f) = (a)*(e) |
1,713,596 |
Estimated Cost Per Eligible Professional to Authorize Qualified registry or QCDR to Report on Eligible Professional’s Behalf (g) |
$6.97 |
Estimated Cost Per Eligible Professional to Report PQRS Data to Qualified registry or QCDR (h) |
$251.88 |
Estimated Cost Per Eligible Professional to Prepare for PQRS Participation (i) |
$133.40 |
Estimated Total Annual Cost Per Eligible Professional (j) = (g)+(h)+(i) |
$392.25 |
Estimated Total Annual Burden Cost (k) = (a)*(j) |
$83,157,000 |
For CY 2014, 90 qualified registries and 50 QCDRs were qualified to report quality measures data to CMS for purposes of the PQRS.5 Therefore, a total of 140 entities are currently classified as qualified registries and/or QCDRs under the PQRS. Although we believe the number of qualified registries will remain the same in 2015, we believe we will see a slight increase in the number of entities that become a QCDR in 2015. We estimate that an additional 10 entities (bringing the total number of QCDRs to 60 in 2015) will become QCDRs in 2015. We attribute this slight increase to entities that wish to become QCDRs but, for some reason (lack of information regarding the QCDR option, rejected during the qualification process, the inability to get its self-nomination info provided in time, etc.), were not selected to be QCDRs in 2014. Therefore, we estimate that a total of 150 entities will become qualified registries and/or QCDRs under the PQRS in 2015.
Qualified registries or QCDRs interested in submitting quality measures results and numerator and denominator data on quality measures to CMS on their participants' behalf will need to complete a self‑nomination process in order to be considered qualified to submit on behalf of eligible professionals or group practices unless the qualified registry or clinical data qualified registry was qualified to submit on behalf of eligible professionals or group practices for prior program years and did so successfully. We estimate that the self‑nomination process for qualifying additional qualified registries or clinical data qualified registries to submit on behalf of eligible professionals or group practices for the PQRS will involve approximately one hour per qualified registry or clinical data qualified registry to draft the letter of intent for self‑nomination.
Please note that the self-nomination statement is an online form that entities will use to provide information on their business. The self-nomination statement will be available on the following website address: https://jira.oncprojectracking.org/login.jsp. Below is a screen shot of the website that will contain the self-nomination form.
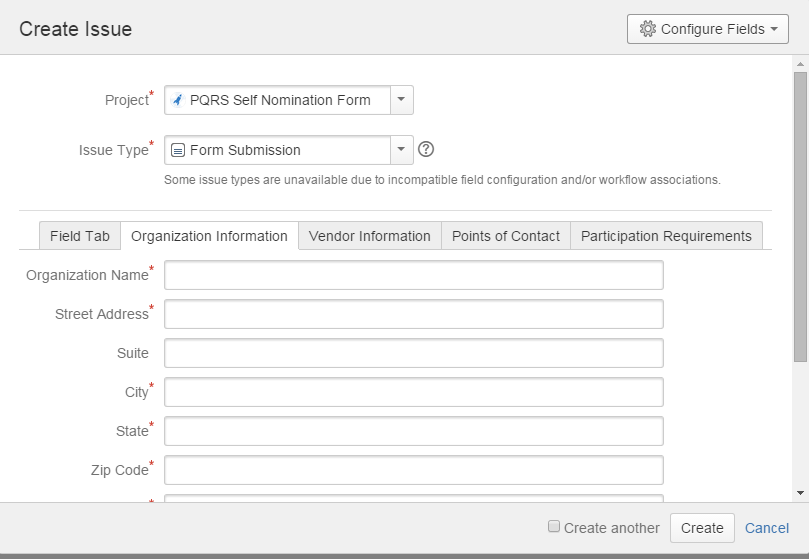
In addition to completing a self-nomination statement, qualified registries and QCDRs will need to perform various other functions, such as develop a measures flow and meet with CMS officials when additional information is needed. In addition, QCDRs must perform other functions, such as benchmarking and calculating their measure results. We note, however, that many of these capabilities may already be performed by QCDRs for purposes other than to submit data to CMS for the PQRS. The time it takes to perform these functions may vary depending on the sophistication of the entity, but we estimate that a qualified registry or QCDR will spend an additional 9 hours performing various other functions related to being a PQRS qualified entity.
We estimate that the staff involved in the qualified registry or QCDR self‑nomination process will have an average labor cost of $42/hour. Therefore, assuming the total burden hours per qualified registry or QCDR associated with the self‑nomination process is 10 hours, we estimate that the total cost to a qualified registry or QCDR associated with the self‑nomination process will be approximately $839.60 ($83.96 per hour x 10 hours per qualified registry).
The burden associated with the qualified registry‑based and QCDR reporting requirements of the PQRS will be the time and effort associated with the qualified registry calculating quality measures results from the data submitted to the qualified registry or QCDR by its participants and submitting the quality measures results and numerator and denominator data on quality measures to CMS on behalf of their participants. We expect that the time needed for a qualified registry or QCDR to review the quality measures and other information, calculate the measures results, and submit the measures results and numerator and denominator data on the quality measures on their participants' behalf will vary along with the number of eligible professionals reporting data to the qualified registry or QCDR and the number of applicable measures. However, we believe that qualified registries and QCDRs already perform many of these activities for their participants. Therefore, there may not necessarily be a burden on a particular qualified registry or QCDR associated with calculating the measure results and submitting the measures results and numerator and denominator data on the quality measures to CMS on behalf of their participants. Whether there is any additional burden to the qualified registry or QCDR as a result of the qualified registry's or QCDR’s participation in the PQRS will depend on the number of measures that the qualified registry or QCDR intends to report to CMS and how similar the qualified registry's measures are to CMS' PQRS measures.
In this proposed rule, we are proposing that group practices of 25or more eligible professionals must report on CAHPS for PQRS. Therefore, a group practice of 25or more eligible professionals would be required to report on the CAHPS for PQRS, six or more measures covering two domains of their choosing. At this point, we do not believe the requirement to report CAHPS for PQRS adds or reduces the burden to the group practices, as we consider reporting the CAHPS for PQRS survey as reporting three measures covering one domain.
Based on the assumptions discussed above, Table 3 provides an estimate of total annual burden hours and total annual cost burden associated with a qualified registry or QCDR self-nominating in order to be considered “qualified” for the purpose of submitting quality measures results and numerator and denominator data on PQRS individual quality measures or measures groups on behalf of individual eligible professionals.
Table 3: Summary of Burden Estimates for Qualified registry or QCDR Vendors to Report Quality Measures Data on Behalf of Eligible Professionals and/or Group Practices to CMS
|
Burden Estimate |
Estimated # of Qualified registries or QCDRs Self-Nominating for the PQRS (a) |
150 |
Estimated Total Annual Burden Hours Per Qualified registry or QCDR (b) |
10 |
Estimated Total Annual Burden Hours For Qualified registries or QCDRs (c) = (a)*(b) |
1,500 |
Estimated Cost Per Qualified registry or QCDR (d) |
$839.60 |
Estimated Total Annual Burden Cost For Qualified registries or QCDRs (e) = (a)*(d) |
$125,940 |
Burden Estimate for PQRS Reporting by Individual Eligible Professionals and Group Practices: EHR-Based Reporting Mechanism
According to the 2011 PQRS and eRx Experience Report, in 2011, 560 (or less than 1%) of the 320,422 eligible professionals participating in PQRS used the EHR-based reporting mechanism. 2012 saw a sharp increase in reporting via the EHR-based reporting mechanism. Specifically, according to the 2012 Reporting Experience, in 2012, 19,817 eligible professionals submitted quality data for the PQRS through a qualified EHR.6 According to the 2013 PQRS and eRx Experience Report, in 2013, 23,194 (3.6 percent) eligible professionals participating in PQRS used the EHR-based reporting mechanism.
As can be seen in from the 2013 Experience Report the number of eligible professionals and group practices using the EHR-based reporting mechanism are steadily increasing as eligible professionals become more familiar with EHR products and more eligible professionals participate in programs encouraging use of an EHR, such as the EHR Incentive Program. In particular, we believe eligible professionals will transition from using the claims-based to the EHR-based reporting mechanisms. To account for this anticipated increase, we continue to estimate that approximately 50,000 eligible professionals, whether participating as an individual or part of a group practice under the GPRO, would use the EHR-based reporting mechanism in CY 2016.
For EHR-based reporting, which includes EHR reporting via a direct EHR product and an EHR data submission vendor’s product, the eligible professional or group practice must review the quality measures on which we will be accepting PQRS data extracted from EHRs, select the appropriate quality measures, extract the necessary clinical data from his or her EHR, and submit the necessary data to the CMS-designated clinical data warehouse.
For EHR‑based reporting for the PQRS, the individual eligible professional or group practice may either submit the quality measures data directly to CMS from their EHR or utilize an EHR data submission vendor to submit the data to CMS on the eligible professional’s or group practice’s behalf. To submit data to CMS directly from their EHR, the eligible professional or eligible professional in a group practice must have access to a CMS‑specified identity management system, such as IACS, which we believe takes less than one hour to obtain. Once an eligible professional or eligible professional in a group practice has an account for this CMS‑specified identity management system, he or she will need to extract the necessary clinical data from his or her EHR, and submit the necessary data to the CMS‑designated clinical data warehouse. With respect to submitting the actual data file for the respective reporting period, we believe that this will take an eligible professional or group practice no more than two hours, depending on the number of patients on which the eligible professional or group practice is submitting. We believe that once the EHR is programmed by the vendor to allow data submission to CMS, the burden to the eligible professional or group practice associated with submission of data on quality measures should be minimal as all of the information required to report the measure should already reside in the eligible professional's or group practice’s EHR.
In this proposed rule, we are proposing that group practices of 25or more eligible professionals must report on CAHPS for PQRS. Therefore, a group practice of 25or more eligible professionals would be required to report on the CAHPS for PQRS, six or more measures covering two domains of their choosing. At this point, we do not believe the requirement to report CAHPS for PQRS adds or reduces the burden to the group practices, as we consider reporting the CAHPS for PQRS survey as reporting three measures covering one domain.
Based on the assumptions discussed above and in Part B of this supporting statement, Table 4 provides an estimate of the total annual burden hours and total annual cost burden associated with EHR-based reporting for individual eligible professionals or group practices. Please note that, unlike the claims-based reporting mechanism that would require an eligible professional to report data to CMS on quality measures on multiple occasions, an eligible professional would not be required to submit this data to CMS, as the EHR product would perform this function on the eligible professional’s behalf.
Table 4: Summary of Burden Estimates for Eligible Professionals (Participating Individually or as Part of a Group Practice) using the EHR-based Reporting Mechanism
|
Burden Estimate |
Estimated # of Participating Eligible Professionals (a) |
50,000 |
Estimated Burden Hours Per Eligible Professional to Obtain IACS Account (b) |
1 |
Estimated Burden Hours Per Eligible Professional to Submit Test Data File to CMS (c) |
1 |
Estimated Burden Hours Per Eligible Professional to Submit PQRS Data File to CMS (d) |
2 |
Estimated Burden Hours Per Eligible Professional to Prepare for PQRS Participation (e) |
5 |
Estimated Total Annual Burden Hours Per Eligible Professional (f) = (b)+(c)+(d)+(e) |
9 |
Estimated Total Annual Burden Hours (g) = (a)*(f) |
450,000 |
Estimated Cost Per Eligible Professional to Obtain IACS Account (h) |
$83.96 |
Estimated Cost Per Eligible Professional to Submit PQRS Data File to CMS (includes 1hr for submitting test file, which is optional) (i) |
$251.88 |
Estimated Cost Per Eligible Professional to Prepare for PQRS Participation (j) |
$133.40 |
Estimated Total Annual Burden Cost Per Eligible Professional (k) = (h)+(i)+(j) |
$469.24 |
Estimated Total Annual Burden Cost (m) = (a)*(k) |
$23,462,000 |
Burden Estimate for PQRS Reporting by Group Practices Using the GPRO Web Interface
As noted in the 2011 Experience Report, approximately 200 group practices participated in the GPRO in 2011. According to the 2012 Reporting Experience, 66 practices participated in the PQRS GPRO.7 In addition, 144 ACOs earned participated in the PQRS GPRO through either the Medicare Shared Savings Program (112 ACOs) or Pioneer ACO Model (32 practices).8 These group practices encompass 134,510 eligible professionals (or approximately 140,000 eligible professionals).9 According to the 2013 PQRS and eRx Experience Report, 677 group practices self-nominated to participate via the PQRS GPRO (compared to 68 total that self-nominated in 2012), 550 moved on to become PQRS group practices, another 220 practices were approved by CMS to participate as Medicare MSSP ACOs, and 23 were eligible under the Pioneer ACO model. The number of eligible professionals (from the 2013 Experience Report) participating in one of these reporting methods include: 131,690 in PQRS group practices, 21,678 in Pioneer ACO, and 85,059 in MSSP ACO. Group practices participating in PQRS GPRO are increasing each year, from roughly 200 group practices in 2011 and 2012, to 860 eligible practices in 2013 (including all GPRO, Pioneer ACO, and MSSP ACO. However, not all group practices use the Web Interface to report. We will assume, based on these numbers that 500 group practices (accounting for approximately 228,000 eligible professional) will continue to participate in the PQRS using the GPRO Web Interface in 2016.
With respect to the process for group practices to be treated as satisfactorily submitting quality measures data under the PQRS, group practices interested in participating in the PQRS through the group practice reporting option (GPRO) must complete a self-nomination process similar to the self-nomination process required of qualified registries. However, since a group practice using the GPRO web interface would not need to determine which measures to report under PQRS, we believe that the self-nomination process is handled by a group practice’s administrative staff. Therefore, we estimate that the self-nomination process for the group practices for the PQRS involves approximately two hours per group practice to review the PQRS GPRO and make the decision to participate as a group rather than individually and an additional two hours per group practice to draft the letter of intent for self-nomination, gather the requested TIN and NPI information, and provide this requested information. It is estimated that each self-nominated entity will also spend two hours undergoing the vetting process with CMS officials. We assume that the group practice staff involved in the group practice self-nomination process has an average practice labor cost of $25.45 per hour. Therefore, assuming the total burden hours per group practice associated with the group practice self-nomination process is six hours, we estimate the total cost to a group practice associated with the group practice self-nomination process to be approximately $152.70 ($25.45 per hour x six hours per group practice).
The burden associated with the group practice reporting requirements under the GPRO is the time and effort associated with the group practice submitting the quality measures data. For physician group practices, this would be the time associated with the physician group completing the web interface. We estimate that the time and effort associated with using the GPRO web interface will be comparable to the time and effort associated to using the PAT. As stated above, the information collection components of the PAT have been reviewed by OMB and was approved under OMB control number 0938-0941- Form 10136, with an expiration date of December 31, 2011 for use in the PGP, MCMP, and EHR demonstrations. As the GPRO was only recently implemented in 2010, it is difficult to determine the time and effort associated with the group practice submitting the quality measures data. As such, we will use the same burden estimate for group practices participating in the GPRO as we use for group practices participating in the PGP, MCMP, and EHR demonstrations. Since these changes will not have any impact on the information collection requirements associated with the PAT and we will be using the same data submission process used in the PGP demonstration, we estimate that the burden associated with a group practice completing data for PQRS under the web interface will be the same as for the group practice to complete the PAT for the PGP demonstration. In other words, we estimate that, on average, it will take each group practice 79 hours to submit quality measures data via the GPRO web interface at a cost of $83.96 per hour. Therefore, the total estimated annual cost per group practice is estimated to be approximately $6,632.84.
Based on the assumptions discussed above, Table 5 provides an estimate of the range of total annual burden hours and total annual cost burden associated with the group practice reporting of quality measures.
Table 5: Summary of Burden Estimates for Group Practices using the GPRO Web Interface Reporting Mechanism
|
Burden Estimate |
Estimated # of Eligible Group Practices in 2013/2014 (a) |
500 |
Estimated # of Burden Hours Per Group Practice to Self-Nominate to Participate in PQRS Under the Group Practice Reporting Option (b) |
6 |
Estimated # of Burden Hours Per Group Practice to Report (c) |
79 |
Estimated Total Annual Burden Hours Per Group Practice (d) = (b)+(c) |
85 |
Estimated Total Annual Burden Hours (e) = (a)*(d) |
42,500 |
Estimated Cost Per Group Practice to Self-Nominate to Participate in PQRS Under the Group Practice Reporting Option (at a labor rate of $26.28/hour) (f) |
$160.08 |
Estimated Cost Per Group Practice to Report(g) |
$6,632.84 |
Estimated Total Annual Cost Per Group Practice (h) = (f) + (g) |
$6,792.92 |
Estimated Total Annual Burden Cost (i) = (a)*(h) |
$ 3,396,460 |
Please note that, beginning in 2013, we are requiring group practices that use the GPRO web interface reporting mechanism to administer a CAHPS survey. Please note that the burden estimates of implementing this survey is provided in a separate PRA package submission.
Total Estimated Burden of this Information Collection Requirement for 2013 and 2014
It is difficult to accurately estimate the total annual burden hours and total annual burden costs associated with the submission of the quality measures data for the PQRS. For example, there are a number of reporting mechanisms available that eligible professionals can choose to use to report the PQRS measures. It may be more burdensome for some practices to use some reporting mechanisms to report the PQRS measures and/or electronic prescribing measure than others. This will vary with each practice. We have no way of determining which reporting mechanism an individual eligible professional will use in a given year, especially since EHR reporting and group practice reporting were new options for the 2010 PQRS and the QCDR option is new for the 2014 PQRS. Therefore, Table 6 provides a range of estimates for individual eligible professionals or group practices using the claims, qualified registry, or EHR-based reporting mechanisms. The lower range of the estimate assumes that eligible professionals will only participate in PQRS to avoid the PQRS payment adjustments that begin in 2015. The upper range assumes that eligible professionals participate in PQRS for purposes of earning an incentive as well as avoiding the PQRS payment adjustments. This upper range represents the sum of the estimated maximum burden hours and burden cost per eligible professional from Tables 1, 2, and 4 above. We are requesting approval for the upper range of the estimates provided in Table 6.
Table 6: Summary of Burden Estimates for Eligible Professionals and/or Group Practices using the Claims, Qualified registry, and EHR-based Reporting Mechanisms
|
Minimum Burden Estimate |
Maximum Burden Estimate |
Estimated Annual Burden Hours for Claims-based Reporting (for individual eligible professionals only) |
1,828,435 |
5,528,488 |
Estimated Annual Burden for Qualified registry-based or QCDR-based Reporting |
1,713,596 |
1,713,596 |
Estimated Annual Burden Hours for EHR-based Reporting |
450,000 |
450,000 |
Estimated Total Annual Burden Hours for Eligible Professionals or Eligible Professionals in a Group Practice |
3,992,031 |
7,692,084 |
Estimated Cost for Claims-based Reporting (for individual eligible professionals only) |
$53,305,000
|
$364,021,000
|
Estimated Cost for Qualified registry-based Reporting |
$83,157,000
|
$83,157,000
|
Estimated Cost for EHR-based Reporting |
$23,462,000
|
$23,462,000
|
Estimated Total Annual Cost for Eligible Professionals or Eligible Professionals in a Group Practice |
$159,924,000
|
$470,64,000
|
For purposes of estimating the reporting burden for group practices, table 7 provides a summary of an estimate for group practices to participate in PQRS under the group practice reporting option using the GPRO web interface during 2015 (that is, Table 5).
Table 7: Summary of Burden Estimates for Group Practices using the GPRO Web Interface Reporting Mechanism
|
Maximum Burden Estimate |
Estimated # of Participating Group Practices |
500 |
Estimated # of Burden Hours Per Group Practice to Self-Nominate to Participate in PQRS and the Electronic Prescribing Incentive Program Under the Group Practice Reporting Option |
6 |
Estimated # of Burden Hours Per Group Practice to Report Quality Measures |
79 |
Estimated Total Annual Burden Hours Per Group Practice |
85 |
Estimated Total Annual Burden Hours for Group Practices |
42,500 |
Estimated Cost Per Group Practice to Self-Nominate to Participate in PQRS for the Group Practice Reporting Option |
$160.08 |
Estimated Cost Per Group Practice to Report Quality Measures |
$6,632.84 |
Estimated Total Annual Cost Per Group Practice |
$6,792.12 |
Annual Burden Cost for Group Practices |
$3,396,460 |
Capital Costs (Maintenance of Capital Costs)
CMS requirements do not require the acquisition of new systems or the development of new technology to participate in the PQRS. However, to the extent an eligible professional decides to participate in the PQRS through the EHR-based reporting mechanism and he or she does not already have an EHR, he or she will need to purchase one. The cost of purchasing an EHR product can range anywhere from $25,000 to $54,000 with ongoing maintenance costs averaging up to $18,000 per year. We believe, however, that it is unlikely than an eligible professional would purchase an EHR solely for the purpose of participating in the PQRS Instead, we believe that having the option to use their EHR to participate in the PQRS is simply an added benefit for eligible professionals who already have an EHR product.
Cost to Federal Government
Aside from program administration and implementation costs, as, beginning in 2016 the PQRS will no longer provide incentives for satisfactory reporting (or, in lieu of satisfactory reporting, satisfactory participation in a QCDR), there will be no cost to the federal government with respect to the application of the PQRS payment adjustments.
Program or Burden Changes
The changes in the estimated burden in this PRA application for CY 2016 from the original submission are due to the following:
A change in participation estimates for eligible professionals using the qualified registry, QCDR, and EHR-based reporting mechanisms due to the release of data from the 2013 PQRS and eRx Experience Report.
The movement away from the distribution of PQRS incentives to the sole application of the PQRS payment adjustments.
A change in reporting requirements in the PQRS for the 2018 PQRS payment adjustment.
Publication and Tabulation Dates
As required by the MIPPA, the names of eligible professionals and group practices who satisfactorily report data on quality measures and who are successful electronic prescribers for 2015 will be posted on the CMS website at www.medicare.gov in 2016 following completion of the reporting periods occurring in 2015. Performance information on group practices will also be posted on the Physician Compare website.
17. Expiration Date
CMS would like approval for this information collection for a period of three years from the expiration of the current PQRS approval. There are no paper forms involved in this data collection activity.
18. Certification Statement
There are no exceptions to the certification statement.
1 Centers for Medicare and Medicaid Services, 2012 Reporting Experience Including Trends (2007—2013): Physician Quality Reporting System and Electronic Prescribing (eRx) Incentive Program, March 14, 2014, at xiii.
2 Id. at XV.
3 Id. at xvi. See Figure 4.
4 Id. at xvi. See Figure 4.
5 The full list of qualified registries for 2014 is available at http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/Downloads/2014QualifiedRegistryVendors.pdf.
\ Id. at XV.
7 Id. at xv.
8 Id. at xvi.
9 Id. at 18.
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Supporting Statement for the Physician Quality Reporting System and the Electronic Prescribing Incentive Program |
| File Modified | 0000-00-00 |
| File Created | 2021-01-24 |
© 2026 OMB.report | Privacy Policy