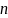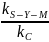SSB GHS Add-on 20160304
SSB GHS Add-on 20160304.docx
Factors Influencing Children's Potential Exposures to Indoor Contaminants
OMB: 0920-1107
Factors Influencing Children’s
Potential Exposures to Indoor Contaminants
March 3, 2016
Supporting Statement B
Collections of Information Using Statistical Methods
OMB Control No. 0920-NEW
Project Official:
Ginger L. Chew, ScD
Principal Investigator
National Center for Environmental Health
U.S. Centers for Disease Control and Prevention (CDC)
4770 Buford Hwy., N.E., MS-F60
Atlanta, GA 30341
Tel: (770) 488-3992
Fax: (770) 488-3635
Table of Contents
COLLECTIONS OF INFORMATION EMPLOYING STATISTICAL METHODS 3
B.1. Respondent Universe and Sampling Methods 3
B.2. Procedures for the Collection of Information 9
B.3. Methods to Maximize Response Rates and Deal with Nonresponse 10
List of Appendices
APPENDIX A - Authorizing Legislation and Other Relevant Laws
APPENDIX B - 60-Day Federal Register Notice (FRN)
APPENDIX C - Research Protocol
APPENDIX D - Questionnaires and Instruction & Record Book
APPENDIX D1 Participant Instruction and Record Book
APPENDIX D2 Biospecimen Collection from Children
APPENDIX D3 Preparation and Collection of Data Other than Biospecimens
APPENDIX D4 Questionnaire about Sibling of Index Child
APPENDIX D5 Questionnaire about Index Chi
APPENDIX D6 Household Inventory
APPENDIX E – Flow chart of recruitment and sample collection
APPENDIX F – Consent Form
APPENDIX G – Assent Form
APPENDIX H – IRB Approval Letters
COLLECTIONS OF INFORMATION EMPLOYING STATISTICAL METHODS
B.1. Respondent Universe and Sampling Methods
Information collection request (ICR), titled “Factors Influencing Children’s Potential Exposures to Indoor Contaminants (hereafter, the “Add-on Study”), adds research components to the third study site of the ongoing Green Housing Study (GHS) (OMB Control No. 0920-0906; expiration 10/31/2017). The Add-on Study is designed with two objectives: 1) to contribute to EPA’s interest in evaluating questionnaire-derived exposure estimates against those derived from measurement; and 2) to understand whether there is sufficient value in adopting alternative measurement methods for the GHS at future sites.
For the Add-on Study, the target sample size is 32 green renovated homes and 32 non-green homes. The Add-on Study respondents are a convenience sample from the New Orleans study site of the GHS. The respondent universe is the same number of families as are recruited to participate in the GHS. Participants in each family will include the index child and a sibling of the index child living in the same household, and the mother/caregiver. Siblings do not participate in the main GHS. Sixty-four younger siblings (only one sibling per household) will be the maximum number that could be enrolled as part of the Add-on Study. The index child is defined as the child recruited to participate in the GHS who ranges in age from 7-12 years with asthma. By including both the index child and a sibling, it is an opportunity to collect exposure information for two children living in the same household, allowing us to explore differences in exposure for different ages. The respondents for the questionnaires are the mother/primary caregivers of the children.
Eligibility will be limited to families who participate in the GHS. In addition to the index child, a sibling of the index child residing in the same home will be enrolled. The sibling age range of primary interest is newborn to 3 years because of the dearth of information for this age group. Since it may not be possible to recruit siblings in that age range from every family, eligibility will be based on the availability of the youngest sibling in the newborn to 12 year age range. Although an asthma diagnosis was a requirement for eligibility for the index child, it is not a requirement for the sibling of the index child.
Sample Size: This study is an add-on to the GHS. The sample size for the Add-on Study is the same as for the New Orleans GHS site; a maximum of n=64 households will be enrolled. We estimate that this fixed sample size (n=64) will be sufficient to test the Add-on Study’s primary objective (objective 1) of “evaluating questionnaire-derived exposure estimates against those derived from measurements.” Assuming this comparison will be accomplished through correlation analyses (Pearson and Spearman), using SAS PROC POWER (equation 1) (Hulley et al., 2013) we estimate that with this fixed sample size (n=64), α=0.05, and 80% power, we will be able to detect a correlation (r) of at least 0.345. Previous studies have shown that comparable correlations have been observed when comparing similar types of data (Denys et al., 2014; English et al., 2015; Villaneuva et al., 2015; Whitehead et al., 2015; Wilhelm et al., 2015).
Equation 1: Total sample size = N = [(Zα+Zβ)/C]2 + 3
Where:
The standard normal deviate for α = Zα
The standard normal deviate at power 1- β = 0.80: Z1-β; and
C = 0.5 * ln[(1+r)/(1-r)]
The questionnaire-based assessment of exposure will be derived from a combination of the home consumer product inventory and product use information captured in the questionnaire (Q21-23). We will cross-reference the specific consumer products found within the home with EPA databases identifying chemicals and amounts in consumer products (Goldsmith et al., 2014). Exposure will be determined by the number of consumer products within the home containing the chemical and product use frequency. For example, the information from Q21-23 combined with the consumer product use inventory can be used to evaluate the relationship(s) between frequency of consumer product use and concentrations of consumer product active ingredients in dust and air to categorize children’s potential exposures in the home as it relates to objective 1. Additional analyses to determine how, and to what extent, questionnaire data can predict measured environmental contaminant levels (e.g., questionnaire responses related to consumer product uses and dust/air concentrations in the home) will be explored with the available data.
U.S. EPA's Stochastic Human Exposure and Dose Simulation (SHEDS) model utilizes simulation to estimate the quantitative distribution of personal exposures for a target population, including the uncertainty of the model estimates. The SHEDS model is capable of estimating highly complex inhalation, dermal, and dietary exposures for populations like young children. A crucial input of the SHEDS model is information obtained from field studies (e.g., the Add-on Study) on observed distributions of factors affecting biomarkers and exposure dynamics. These studies also provide important evaluation and verification feedbacks for continual improvement of the model’s accuracy.
Generalizations about the accuracy of the SHEDS model are challenging because its results, such as those reported by Xue et al. (2014) depend on the inputs and parameterizations provided by the user. Moreover, the SHEDS model is aimed at estimating not only the most likely population exposure level, but also the variability of these exposures. Conceptually, this implies that the SHEDS model intentionally eschews estimation of central tendency in favor of accurately estimating an entire population distribution. The challenges of ascribing some notion of accuracy to SHEDS model results, which derive from complex simulations, are magnified considerably when attempting to compare its accuracy to that of an observational field measurement study.
Bearing in mind these caveats, consideration of fundamental theoretical results may shed light on the relative magnitude of a particular expression of accuracy contrasted between results from the SHEDS model and an observational field measurement study of finite sample size. Taking Xue et al. (2014) as an example, their Table 1 displays SHEDS model estimates of cumulative annual absorbed dose of seven pyrethroids. Among 3 – 5 year olds in the general population, the mean dose was estimated to be 3.1 nmol/day (std. dev. 5.8) based on a sample size of 5,733 persons. The accuracy of these estimates may be characterized in terms of the number of standard deviations that cover an interval at a given level of confidence, such as the routinely used 95 percent confidence level. More accurate samples may be said to enclose a given confidence interval with a fewer number of standard deviations than less accurate samples. Samples from the SHEDS model, however, are not “observed” in the same way as for an observational field measurement study like the Add-on Study. Still, it may be useful to suggest that comparing their confidence interval coverage is appropriate if no assumptions were necessary regarding the probability distributions of their samples. This would enable comparison of accuracy purely in terms of their computed standard deviations with respect to fundamental theoretical limits on their respective sample sizes.
The theoretical basis for comparison is Chebyshev’s Inequality, which is a distribution-free expression of the maximum interval covered for a given mean and standard deviation (Konijn, 1987). The large size of the SHEDS model sample supports estimation of this interval with Chebyshev’s Inequality, which represents the maximum for an entire population. In contrast, the Add-on Study has a finite population sample of 64 participants. For a finite sample, Chebyshev’s Inequality is expressed in a modified form as Saw-Yang-Mo’s Inequality (Saw et al., 1984).
Reiterating
the suggestion that accuracy can be judged in terms of the number of
standard deviations (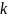 )
that cover a fixed 95 percent confidence interval, and that
Chebyshev’s Inequality can be reasonably assumed to represent
SHEDS model results that arise from thousands of observations, then
for a mean cumulative pyrethroids dose of 3.1 nmol/day and standard
deviation of 5.8, Chebyshev’s Inequality indicates that
)
that cover a fixed 95 percent confidence interval, and that
Chebyshev’s Inequality can be reasonably assumed to represent
SHEDS model results that arise from thousands of observations, then
for a mean cumulative pyrethroids dose of 3.1 nmol/day and standard
deviation of 5.8, Chebyshev’s Inequality indicates that
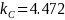 standard deviations would cover an interval about the mean comprising
95 percent of observations. Assuming the same mean cumulative
pyrethroids dose and standard deviation can be expected to be
observed in the Add-on Study, then for a finite sample size of 64
observations, Saw-Yang-Mo’s Inequality indicates that
standard deviations would cover an interval about the mean comprising
95 percent of observations. Assuming the same mean cumulative
pyrethroids dose and standard deviation can be expected to be
observed in the Add-on Study, then for a finite sample size of 64
observations, Saw-Yang-Mo’s Inequality indicates that
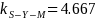 standard deviations would cover an interval comprising 95 percent of
observations. The ratio
standard deviations would cover an interval comprising 95 percent of
observations. The ratio
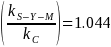
indicates
that a sample of 64 has 4.4 percent less accuracy than assessing the
entire population. These results are tabulated below, and for
additional comparison
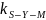 estimated for sample sizes of 60 and 70 observations.
estimated for sample sizes of 60 and 70 observations.
Inequality |
|
|
|
Chebyshev |
Inf |
4.472 |
Ref |
|
|
|
|
Saw–Yang–Mo |
60 |
4.521 |
1.011 |
|
64 |
4.667 |
1.044 |
|
70 |
4.876 |
1.090 |
In summary, ceteris paribus an observational field measurement study with a finite sample size of 64 observations has approximately 4.4 percent less accuracy than a simulation study like the SHEDS model with a sample size of ≥100 observations aimed at estimating the same mean and standard deviation.
From the activity survey results (questionnaire/accelerometer/GPS), descriptive statistics of reported time spent in microenvironments and activities will be calculated. In addition, GPS-based time spent in microenvironments will be calculated using EPA’s MicroTrac model (Breen et al., 2014) and compared with the survey results as in Breen et al. (2014).
For example, to evaluate objective 2, EPA and CDC will use the Add-on Study data to explore how the use of these metrics may enhance the understanding of relationships between environmental exposures, time activity/location information, and asthma in the main GHS. The Add-on Study metrics, including the additional environmental measures and activity information, could be additional explanatory variables for asthma or could modify relationships for variables already collected in the main GHS. A goal of this evaluation is to identify which metrics may be candidates for incorporation into the full protocol at future GHS sites. Data quality (e.g., percent completion or detected, accuracy, precision) will be assessed to help make decisions on more complex statistical analyses that can be conducted with the available data. A key limitation will be the relatively small sample size at the single study site. While this exploratory analysis may be suggestive of relationships, there may not be sufficient statistical power for desired levels of confidence. However, this exploratory analysis may inform decision-making regarding incorporation of alternative measurement methods (as described in Section B.4) into future sites of the main GHS.
In regards to the time-activity/location information being collected (via questionnaire, accelerometer, GPS), differences among age groups and genders for both survey and GPS results will be quantified and tested using appropriate parametric or non-parametric techniques. These comparisons will help EPA and CDC evaluate whether electronic time-activity/location information collection can supplant activity diaries. Specifically, an analysis of Q3-15 in combination with the accelerometer and GPS data will generate comparisons between questionnaire responses and electronic information capture.
Listed below are specific data analysis plans for five of the sub-objectives associated with overarching objective 1.
Assess factors affecting children’s exposures to chemical ingredients from consumer products found in their everyday environment in order to support the data and modeling needs of the exposure components of EPA’s national research programs
Descriptive statistics will be compiled for measurement and survey data to identify variables with sufficient measurable results and to evaluate variability. Spearman/Pearson correlation analyses will be performed to elucidate potential associations within the data. The power calculation described above is relevant for this objective (Equation 1).
Examine the relationships between consumer products in a residence, environmental concentrations, and exposure to active ingredients found in consumer product chemicals to support development and evaluation of models for predicting exposure to these chemicals
For each residence, a chemical inventory of the products in the home will be performed (based on available databases of chemicals in products), and compared to media measurements (chemical presence/absence and group comparison of concentrations of chemicals in the consumer products versus other measured chemicals not found in consumer products). This information will be used in more complex analyses.
EPA has recently developed new methods for predicting exposure to chemicals in consumer products. These methods are implemented in the Stochastic Human Exposure and Dose-Simulation– High Throughput (SHEDS-HT) human exposure model (Isaacs et al., 2014). In order to accommodate high-throughput chemical assessments, SHEDS-Multimedia has been numerically and operationally modified to reduce user burden and increases run speed. The SHEDS-HT model uses a dynamic fugacity-based source-to-concentration module to estimate indoor concentrations by media (air, dust, and surfaces) for chemicals with indirect exposure scenarios, while direct scenarios (exposure during product use) are addressed via appropriate exposure equations. The concentration estimates, relevant exposure factors, exposure predictions, and human activity data are then used by the SHEDS-HT model to rapidly generate population distributions of potential exposures via dermal, non-dietary ingestion, and inhalation pathways. Due to the small sample size of the Add-on Study, direct comparison of predicted population SHEDS exposures and those measured in the Add-on Study may not be entirely appropriate. However, the pilot study Add-on will provide valuable observations of product use matched to indoor media concentration, exposure (e.g., hand wipes), and biomarker measurements that refine algorithms and assumptions of the SHEDS-HT model.
Quantification of relationship between the dust, surface, and air concentrations will be performed for all chemicals. Correlations between media (air, surface, dust) concentrations and biomarker concentrations will be calculated in an attempt to determine a subset of chemicals for which chemical media concentrations are a useful surrogate for chemical exposures. Correlation power calculations and the accuracy of SHEDS model estimates compared theoretically to an observational field measurement study like the Add-On Study with fixed sample size (n=64) were described above.
Measure biomarkers of consumer product chemicals for young children in conjunction with environmental measurements to evaluate exposure and dose models
Biomarker data can enhance exposure assessment through modeling in both a forward (exposure to dose) and reverse (dose to exposure) direction. Biomarkers will be analyzed in blood and urine samples for a complementary suite of chemicals to the target analytes investigated in the environmental samples. Descriptive statistics will describe measurements for the population, households, and individuals. Correlations will compare concentrations between biomarkers, different biological media, the same biomarker over time, and biomarkers to parent compounds. The accuracy of SHEDS model estimates compared theoretically to an observational field measurement study like the Add-On Study with fixed sample size (n=64) were described above.
IV. Use low burden techniques and survey instruments to collect current information on children’s activities, locations, and dietary habits to support exposure models and databases
The information collected in the Add-on Study questionnaires will be analyzed to develop mean and variability metrics of exposure factors for the children's cohorts being studied, and to identify interactions or correlations among exposure factors that could be used to derive relationships for future assessments of children. Correlation power calculations were described above (Equation 1).
Activity and location data will be aggregated into an electronic database and further processed by EPA investigators into a format consistent with EPA's Consolidated Human Activity Database (CHAD; McCurdy et al., 2000, U.S. EPA, 2002). This format includes demographic, date, and housing information linked with a minute-by-minute diary of location and activity for the individual studied. It is anticipated that if the data quality from the questionnaires are adequate, these time activity data (de-identified) would be permanently entered in CHAD for use by EPA exposure models (and available to the public via download).
Understanding the type, magnitude, and variability of time spent in microenvironments across ages, geographic region, subculture, or socioeconomic status is critical in performing exposure assessments for different populations of children. Therefore, time spent in each microenvironment by each child will be summarized by standard methods (for example, Xue et al., 2004). Of specific interest will be differences in time spent in locations for children of different ages in the same household.
From the activity survey results, descriptive statistics of reported time spent in microenvironments and activities will be calculated. In addition, GPS-based time spent in microenvironments will be calculated using EPA’s MicroTrac model (Breen et al., 2014). This model takes as input a GPS time-series and a general location of the participant’s home and using a computational algorithm calculates time spent at home and in travel (in vehicles). The MicroTrac results will be compared with the survey results as in Breen et al. (2014). Differences among age groups and genders for both survey and GPS results will be quantified and tested using appropriate parametric or non-parametric techniques.
Children's activities are an important determinant of the types and amounts of chemicals encountered (McCurdy, 2000). Therefore, time spent in exposure-relevant activities (e.g., time spent with pet or exercising) will be characterized via standard methods (Xue et al., 2004). A primary analysis will evaluate age- and asthma-dependent differences in activity level in children living in the same household. The results from this analysis will aid in characterizing/elucidating the contributions of age, health, socioeconomic status, and other factors to describe the variability in activity levels. Similar to location, the interaction between housing and community factors (e.g., crime or noise pollution) and exposure relevant activities will be addressed. In addition, the location of high-dose rate activities (e.g., exercise) for these children will be compared to other, previously studied child cohorts to assess the influence of community or socioeconomic status-driven factors (such as distance from pollutant sources, or indoor versus outdoor exercise locations). Analysis of the activity information (questionnaires, accelerometer, GPS) will help EPA and CDC determine whether electronic data collection can supplant questionnaires for future GHS sites.
V. Evaluate the feasibility of using a simplified mass balance approach to estimate chemical exposure and dose rates incorporating children’s nail clippings, other multimedia measurements, and activity information
The collection of nail clippings is attractive because obtaining the sample is noninvasive and easily performed by the primary caregiver. Arsenic, cadmium, mercury, manganese, zinc and other elements may be sequestered in nails and hair following environmental exposures and have utility in determining exposure and dose rates and serve as simple, low cost metrics to supplant other biomarkers, such as blood or urine, particularly for children. This objective will evaluate the relationship of nail clippings with other environmental and biological measures and determine the feasibility of using nail clippings to estimate chemical exposure and dose rates for very young children in observational exposure measurement studies. Associations and correlations between renovation activities, sources, exposure pathways, and indoor/outdoor concentrations will be evaluated. Descriptive statistics will be calculated. Correlations (both Spearman and Pearson) will be conducted in order to evaluate relationships both within and between the measurement data to elucidate relationships and associations. Power calculations for correlations were described above (Equation 1).
References:
Breen MS, Long TC, Schultz BD, Crooks J, Breen M, Langstaff JE, Isaacs K, Tan C, Williams R, Cao Y, Devlin R, Batterman S, Buckley T. 2014. GPS-based microenvironment tracker (MicroTrac) model to estimate time-location of individuals for air pollution exposure assessments: Model evaluation in central North Carolina. Journal of Exposure Science and Environmental Epidemiology, 24(4):412-420.
Denys S, Fraize-Frontier S, Moussa O, Le Bizec B, Veyrand B, Volatier JL. 2014. Is the fresh water fish consumption a significant determinant of the internal exposure to perfluoroalkylated substances (PFAS)? Toxicology Letters, 231(2):233-238.
English K, Healy B, Jagals P, Sly PD. 2015. Assessing exposure of young children to common endocrine-disrupting chemicals in the home environment: a review and commentary of the questionnaire-based approach. Reviews on Environmental Health, 30(1):25-49.
Goldsmith MR, Grulke CM, Brooks RD, Transue TR, Tan YM, Frame A, Egeghy PP, Edwards R, Chang DT, Tornero-Velez R, Isaacs K, Wang A, Johnson J, Holm K, Reich M, Mitchell J, Vallero DA, Phillips L, Phillips M, Wambaugh JF, Judson RS, Buckley TJ, Dary CC. 2014. Development of a consumer product ingredient database for chemical exposure screening and prioritization. Food and Chemical Toxicology 65:269-279.
Hulley SB, Cummings SR, Browner WS, Grady D, Newman TB. Designing clinical research: an epidemiologic approach. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. Appendix 6C, page 79.
Konijn, HS. Distribution-Free and Other Prediction Intervals. The American Statistician, Vol. 41, No. 1 (Feb., 1987), pp. 11-15.
McCurdy T. 2000. Conceptual basis for multi-route intake dose modeling using an energy expenditure approach. Journal of Exposure Analysis and Environmental Epidemiology 10(1):86-97.
Saw, JG; Yang, MCK; Mo, TC. Chebyshev Inequality with Estimated Mean and Variance. The American Statistician, Vol. 38, No. 2 (May, 1984), pp. 130-132.
U.S. EPA. Consolidated Human Activity Database (CHAD). 2002. Available at http://www.epa.gov/chadnet1/.
Villanueva F, Tapia A, Amo-Salas M, Notario A, Cabañas B, Martínez E. 2015. Levels and sources of volatile organic compounds including carbonyls in indoor air of homes of Puertollano, the most industrialized city in central Iberian Peninsula. Estimation of health risk. International Journal of Hygiene and Environmental Health, 218(6):522-534.
Whitehead TP, Crispo Smith S, Park JS, Petreas MX, Rappaport SM, Metayer C. 2015. Concentrations of persistent organic pollutants in California women's serum and residential dust. Environmental Research, 136:57-66.
Wilhelm M, Wittsiepe J, Völkel W, Fromme H, Kasper-Sonnenberg M. 2015. Perfluoroalkyl acids in children and their mothers: Association with drinking water and time trends of inner exposures--Results of the Duisburg birth cohort and Bochum cohort studies. International Journal of Hygiene and Environmental Health, 218(7):645-655.
Xue J, McCurdy T, Spengler J, Ozkaynak H. 2004. Understanding variability in time spent in selected locations for 7-12-year old children. Journal of Exposure Analysis and Environmental Epidemiology 14(3):222-233.
Xue J, Zartarian V, Tornero-Velez R, Tulve NS. EPA's SHEDS-multimedia model: children's cumulative pyrethroid exposure estimates and evaluation against NHANES biomarker data. Environ Int. 2014 Dec;73: 304-11. doi: 10.1016/j.envint.2014.08.008.
B.2. Procedures for the Collection of Information
Briefly, information collection proceeds as follows: 1) trained study staff set up appointments for home visits; and 2) a team of two trained field technicians collect questionnaire data and environmental samples at the study participant’s home. At each home visit, one field technician will complete a field technician report. For more details on the data collection procedures see Appendix C.
To assess the environmental variables, the mother/primary caregiver will answer questionnaires at each of the four home visits. During each visit period, the study technicians will visit the home on Day 1 to collect samples/information and deploy equipment, and return on Day 5 to collect additional samples and retrieve equipment. Each mother/primary caregiver who will be queried about consumer products used in the home and information pertaining to each of the enrolled children: location throughout the day, transportation used, activities, and foods consumed. The questionnaires and estimated time burdens are listed in Section A.12. To ease the burden on the participants, the Add-on Study will employ the same schedule to ensure that additional visits are unnecessary. The housing and community information, duplicate diet, nail clippings, blood, and feces will be collected at a single visit.
While siblings are asked to provide biological samples and the mother /caregiver would provide their duplicate diets, a complete matched set of these four samples is necessary for mass-balance estimation (described in Appendix C): 1) feces; 2) duplicate diet, 3) blood (collected in EDTA tubes); and 4) nails.
Statistical analysis: Results from all types of media will be analyzed using descriptive statistics and compared between and among each media type (see Appendix C). Cross-sectional and longitudinal analyses will be conducted. Data quality measures such as survey percent completion, percent of homes where an analyte was detected, measurement variation will be assessed.
Descriptive statistics, regression analyses, and correlations will be employed to evaluate the Add-on Study data. Descriptive statistics will be developed for all variables to be used in data analyses that will be conducted as a means of addressing the research objectives. Categorical variables will be summarized by frequencies, while continuous variables will be summarized by mean, standard deviation, median, and range. Environmental and biological measurement variables, such as analyte concentrations in dust and urinary biomarker concentrations, will be characterized by mean and standard deviation, median, range, appropriate distribution percentile values, and percent of measurements above the detection limit.
For detection limit censored data distributions, appropriate approaches for reducing bias in distributional parameter estimates will be considered. Measurement distributions will be assessed for normality using the Shapiro-Wilks or other appropriate normality test. Depending on the distribution, measurement values may be log-transformed to compute geometric means and geometric standard deviations. Other types of transformations and/or non-parametric analysis methods will be considered if necessary.
B.3. Methods to Maximize Response Rates and Deal with Nonresponse
We employ a number of strategies in an attempt to maximize response rates. These include having a trained field staff technician: 1) make multiple phone calls/visits at different times of day and on different days of the week; 2) leave detailed messages with a call-back number; and 3) calling “alternate contacts.” “Alternate contact” information will be requested from each mother/caregiver to be used by the field staff in the event the mother/caregiver cannot be reached at the contact number(s) provided on the consent form.
B.4. Tests of Procedures or Methods to be Undertaken
The Add-on Study questionnaires are primarily based on questions from national health and housing surveys and different epidemiologic studies. Some questions were included verbatim; some were modified to fit our study framework; and some new questions were developed in consultation with subject matter experts to collect data specifically required for this study.
The Add-on Study originally had one questionnaire (CDC IRB approved and pilot-tested with n=9 participants in the Cincinnati cohort of the GHS). The average time for completion was estimated at 40 minutes based on the pilot testing in the Cincinnati cohort; 30 minutes was the maximum time for one participant and a 10 minute buffer was added on top of this time. This was for the entire questionnaire in its original form which was more than 31 questions and administered via laptop. Subsequent to the pilot testing, the questionnaire was reduced and broken into component questionnaires. The burden estimate was adjusted, accordingly.
We will determine if any of the Add-on Study methods will be incorporated into future GHS study sites by the following four factors: 1) a cooperation rate of ≥ 75% of mothers/primary caregivers allowing the collection of any given Add-on Study measurement (i.e., comprehensive time-activity and location questionnaire, GPS and accelerometer, duplicate diet, feces, nail clippings, blood, and urine); 2) data collection time for any given measurement is not significantly higher than the estimated burden listed in Supporting Statement A Section A12; 3) ≥ 50% of the measurements from any given sample type are within acceptable range limits (i.e., not below limit of detection); and 4) ≥ 75% of the measurements from any given sample type are not considered invalid because of integrity of sample during collection, storage, or transportation. All four objectives much be met in order to consider pursuing full implementation into future GHS study sites.
B.5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
Individuals Consulted on Statistical Aspects of the Design
CDC’s and EPA’s Add-on Study staff were consulted on the statistical aspects of the design or planned statistical analyses.
Table 1. Personnel Consulted on Statistical Design
Name |
Title |
Affiliation |
Phone |
|
CDC |
||||
B. Rey de Castro, PhD |
Biostatistician |
National Center of Environmental Health |
(770) 488-0162 |
|
EPA |
||||
Paul Jones, MS |
Biostatistician |
Office of Research and Development |
(919) 541-5767 |
|
Table 2. Personnel Responsible for Collection and Analysis of Information
Name |
Title |
Affiliation |
Phone |
|
Felicia Rabito, PhD |
Associate Professor |
Tulane University |
(504) 988-3479 |
|
Ginger Chew, ScD |
Health Scientist |
CDC |
(770) 488-3992 |
|
Nicolle Tulve, PhD |
Research Scientist |
EPA |
(919) 541-1077 |
Grantees Responsible for Collecting Information for the Agency
Data will be collected by Tulane University, which is the grantee for the third study site of the Green Housing Study (New Orleans).
Contractors Responsible for Analyzing Information for the Agency
Not applicable. CDC and EPA staff associated with the Add-on Study will analyze data from the study.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | INFORMATION COLLECTION REQUEST FOR OMB REVIEW |
| Subject | AHHS draft ICR |
| Author | Schwab, Margo |
| File Modified | 0000-00-00 |
| File Created | 2021-01-24 |
© 2026 OMB.report | Privacy Policy