Scrapie SS 20190225
Scrapie SS 20190225.docx
Scrapie in Sheep and Goats; Flock Certification, Interstate Movement, and Indemnity Revisions
OMB: 0579-0469
SUPPORTING STATEMENT - OMB NO. 0579-xxxx
SCRAPIE IN SHEEP AND GOATS; FLOCK CERTIFICATION, INTERSTATE MOVEMENT, AND INDEMNITY REVISIONS
A. Justification
1. Explain the circumstances that make the collection of information necessary. Identify any legal or administrative requirements that necessitate the collection. Attach a copy of the appropriate section of each statute and regulation mandating or authorizing the collection of information.
Under the Farm Security and Rural Investment Act of 2002, PL 107-71, subtitle E, Animal Health Protection, Section 10401-10418, the Secretary of Agriculture, to protect the agriculture, environment, economy, and health and welfare of the people of the United States by preventing, detecting, controlling, and eradicating diseases and pests of animals, is authorized to cooperate with foreign countries, States, and other jurisdictions, or other persons, to prevent and eliminate burdens on interstate commerce and foreign commerce, and to regulate effectively interstate commerce and foreign commerce. This authority permits the Secretary to prevent, control, and eliminate domestic disease such as scrapie and brucellosis, as well as to take actions to prevent and to manage exotic diseases.
More specifically, the act authorizes the Secretary of Agriculture to take such measures as he or she may deem proper to prevent the introduction or spread of any contagious or communicable disease of animals or live poultry from a foreign country into the United States or from one State to another. Disease prevention is the most effective method for maintaining a healthy animal population and enhancing the ability of the United States to complete in the world market of animal and animal product trade. The U.S. Department of Agriculture (USDA) is responsible for preventing the spread of contagious, infectious, or communicable animal diseases. The Veterinary Services (VS) division of USDA’s Animal and Plant Health Inspection Service (APHIS) is responsible for carrying out this disease prevention mission.
Scrapie is a progressive, degenerative, and eventually fatal disease affecting the central nervous system of sheep and goats. Its control is complicated because the disease has an extremely long incubation period without clinical signs of disease and because there is no test that can detect the disease early in the incubation period and there is no known treatment. APHIS restricts the interstate movement of certain sheep and goats to control the spread of scrapie within the United States. APHIS interstate movement and animal identification regulations for scrapie are contained in title 9 of the Code of Federal Regulations, part 79. Other regulations for the control of scrapie including indemnity, flock cleanup, and the Scrapie Free Flock Certification Program, are in Part 54.
APHIS interstate movement requirements are designed to minimize any contact between high-risk animals and healthy animals, while the animal identification requirements help with investigations of scrapie outbreaks. Implementing these regulations necessitates the use of information collection activities, such as requiring market operators, dealers, accredited veterinarians, and other persons who apply official identification to sheep and goats to submit information on the official identification applied to sheep and goats.
APHIS is asking OMB to approve, for 3 years, its use of this information collection activity associated with its efforts to contain and eventually eradicate scrapie in the United States. Much information is being collected for these purposes as described in 0579-0101. Rulemaking has changed the number of respondents and record keepers and responses in the existing information collection. Changes will also be made to some forms. This collection further tracks burden items added by the rulemaking.
2. Indicate how, by whom, how frequently, and for what purpose the information is to be used. Except for a new collection, indicate the actual use the agency has made of the information received from the current collection.
APHIS proposes to use the following information collection activities to contain and eventually eradicate scrapie in the United States.
9 CFR 79.2(b)(1), 9 CFR 79.2(b)(3): Data Assigning Official Identification to Flocks (VS Forms 5-11, 5-11A, 5-12, and 5-12A or equivalent (Draft)) – Private Sector
Currently, APHIS requires markets, dealers, accredited veterinarians, and other persons who apply official identification to sheep and goats to maintain certain information. Under the new scrapie rule, APHIS requires market operators, dealers, accredited veterinarians, and other persons who apply official identification to sheep and goats they don’t own to submit the same information to APHIS when requested. APHIS can then readily access it if needed in a disease investigation and can determine if adequate surveillance levels are being achieved based on the origin of the animals sampled. To facilitate data entry, APHIS is creating a new standardized format that will ask for information such as the name and address of the owner, and flock identification numbers for the flocks of origin or birth herd from market operators, dealers, accredited veterinarians, and other persons who apply official identification to sheep and goats who elect not to provide the data through a USDA Web site. APHIS will submit a Change Worksheet when it completes the form.
9 CFR 79.2(f)(6), 9 CFR 79.3(a), 9 CFR 79.3(g): Owner/Hauler Statement and Bill of Sale (VS Form 5-13 or equivalent (Draft))– Private Sector
Currently an owner statement may be used as an additional or alternative means of identifying regulated sheep and goats to their flock of origin. When these animals are moved in interstate commerce, it is vital that APHIS has documentation that lets it trace animals back to their flock of origin. The flock owner uses the owner statement to provide the owner’s name, signature, address, telephone number, the date the animals left the flock of origin, the number of animals involved in the movement, the premises identification number assigned to the premises, the group/lot identification number (a combination of the flock or premises ID, the date the lot was assembled and if needed to uniquely identify a lot a serial number) and other identifying information. This collection would change the name of this document to owner/hauler statement, and the document could be completed by the owner or hauler. The newly redesignated document would also require the name and address of point of origin, if different from the owner’s address, and the destination; the hauler’s contact information if different; the species, breed/type, and class of animal to be recorded; and, in the case of animals in slaughter channels, that they are in slaughter channels (through a separate bill of sale if necessary). APHIS anticipates more of these statements will be used due to a new requirement for them to accompany all animals in slaughter channels. An existing document that includes the same information that is signed by the owner or the hauler meets this definition. APHIS will also provide an optional form for those who wish to use it.
9 CFR 79.2(k)(2)(iii)(D): Data Entry of Official Identification Devices Produced and Assigned – Private Sector
Currently tag manufacturers enter the sequences of tags shipped into the Animal Identification Management module of the National Scrapie Database through a Web-based interface. APHIS anticipates the number of responses will increase significantly the first 2 years following publication due to the requirement to officially identify more classes of goats and then drop off substantially since only 12 percent of producers typically request official tags each year.
9 CFR 79.2 (b): Application for and Assignment of Identification Numbers – Private Sector and State, Local, and Tribal Governments
Currently flock owners, dealers, accredited veterinarians, and market operators must apply to the appropriate State or Federal representative to be assigned USDA serial numbers that correspond to official animal identification devices. They must provide their name, address, phone number, number of animals, and type of operation so State or Federal representatives can place their orders and create records. They generally make these applications by calling the State or Federal representative. If flock owners, dealers, accredited veterinarians, and market operators give this information to their State representatives, the State representatives record the application data. APHIS anticipates the number of respondents and responses will increase significantly the first 2 years after the rule is published due to the requirement to officially identify more classes of goats, and then drop off substantially as only 12 percent of producers typically request official tags each year.
Assignment of Official ID Numbers
Official identification numbers for use on animals not in slaughter channels may be assigned as follows:
• Directly to the owner of a breeding flock;
• In the case of official serial numbers or serial number devices, to APHIS or State representatives or accredited veterinarians or other responsible individuals (such as 4-H leaders) for reassignment to owners of breeding flocks, if the State animal health official and the District Director agree that such assignments will improve scrapie control and eradication within the State; or
• To any federally recognized tribe that maintains sheep or goats on tribal lands (Tribal codes).
APHIS or State representatives or accredited veterinarians that reissue official serial numbers or devices must provide data associating assigned serial sequences to the flock of origin and, when required, the flock of birth. This can be done by entering the data into the Animal Identification Management module of the National Scrapie Database.
Persons assigned serial numbers may either directly apply eartags to animals, or may reassign eartag numbers to producers. Such persons must maintain records that permit traceback of animals to their flock of origin, or flock of birth when required, and must either reassign the tags in the National Scrapie Database or, if permitted by the VS District office, provide a written record of the reassignment when requested to the District Office or the State Office for entry into the National Scrapie Database.
APHIS may also assign sets of unique individual identification numbers to breed registries that agree to reassign the sequences to the flock of origin and, and when required, the flock of birth; and to provide associated registry identifiers such as registry tattoo numbers to APHIS when requested in the Animal Identification Management module of the National Scrapie Database.
Livestock facilities may identify animals after sale if the facility maintains unidentified animals from different flocks of origin or, when required, birth in separate enclosures until officially identified.
Assignment of Unique Identification Numbers to Persons Who Do Not Own Breeding Flocks (9 CFR 79.3(a)(5))
Sets of unique individual identification serial numbers may be assigned to persons who handle sheep and goats, but who do not own breeding flocks, if they apply to and are approved by the State animal health official or the District Director in the State in which the person maintains his or her business location. The assigning entity should be the one responsible for issuing official identification devices or numbers in the State where the applicant maintains business and for assigning flock identification numbers and premises identification numbers in that State in the National Scrapie Database. Such persons must, if requested, provide data associating assigned serial sequences to the flock of origin and, when required, the flock of birth. This can be done by entering the data into the Animal Identification Management module of the National Scrapie Database.
9 CFR 79.1, 9 CFR 79.2 (b)(1 to 4): Application for Premises Identification Numbers and Flock Identification Numbers and Request to Change Information Associated with these Numbers – Private Sector and State, Local, and Tribal Governments
Currently, APHIS also allows sheep and goats moving in interstate commerce to be identified with an approved eartag, backtag, or tattoo bearing a premises or flock identification number assigned by a State, Tribal, or Federal animal health official to the premises or flock on which or from which the sheep or goats originated. Flock owners can obtain and apply these identification numbers in lieu of official USDA serial numbers. To apply for these numbers, the flock owners contact the State, Tribal, or Federal representative by telephone or other means and give their name, address, phone number, number of animals, and type of operation so the State, Tribal, or Federal representative can create a record. If the flock owners give this information to the State or Tribal representative, the State or Tribe records the information. These premises identification numbers help APHIS and States monitor sheep and goats moving in interstate commerce and provide APHIS and States with critical information if a trace investigation is necessary and to monitor the effectiveness of scrapie surveillance activities.
APHIS anticipates the number of respondents and responses will increase during the first 2 years after publication of the proposed rule due to the requirement to officially identify more classes of goats, and then drop back to near current levels as these numbers do not need to be renewed. Further, if the information provided changes such as adding a new premises to a flock this information is updated in the same manner as for requesting a premises or flock number. A request to APHIS to enter additional flock premises in the National Scrapie Database is required before animals are first moved to the premises. Notification is not required for each subsequent movement of animals to that premises. Neither group lot ID nor an owner/hauler statement is required for movements of a flock or its members for flock management purposes within a contiguous premises spanning two or more States.
Tag Transfer – 9 CFR 79.2(d)
Official identification devices assigned to a premises or flock are not to be transferred or sold unless transferred with sheep or goats to which they have been applied as official ID or as directed in writing by an APHIS or State representative. Such transfers are mostly likely to occur when a flock or premises is sold and will be part of updating the premises or flock record as covered above.
9 CFR 54.8(b), 9 CFR 79.2 (a)(3), 9 CFR 79.2(f): Recordkeeping, Identification – Private Sector
Flock owners, market operators, dealers, slaughter plant owners, tag manufacturers, and accredited veterinarians using official identification devices or methods must record all serial numbers and other identification numbers affixed to the sheep and goats, as well as the location where the identification was applied and the name, address, and when available, telephone number of the animal’s owner and the owner of the flock of origin if different. This information must be maintained for at least 5 years, and could provide invaluable information to State or Federal personnel conducting a trace investigation. APHIS does not prescribe a form for recording this information but plans to provide a form record keepers can use. APHIS anticipates the amount of recordkeeping will increase for some respondents due to the requirement to officially identify more classes of goats and decrease for other respondents due to the removal of the requirement to record existing official ID on animals not in slaughter channels.
9 CFR 79.3(k): Amendment of a Livestock Facility Agreement – Private Sector
APHIS plans to allow an approved livestock facility agreement to be amended to establish alternative methods to maintain the traceability of animals in slaughter channels to their flock of origin. APHIS may also waive the requirement for individual official identification of animals in slaughter channels if adequate surveillance has been conducted on the flock of origin or an alternative plan is in place to conduct surveillance on animals from the flock of origin. The Administrator and the State animal health official must agree that the application of an allowed official identification device or method is unsuitable for a specific circumstance to waive the ID requirement.
9 CFR 54.7(e)(2)(iv), 9 CFR 54.8(j)(1): Flock Plan or Laboratory Disinfection Worksheet – Private Sector and State, Local, and Tribal Governments
When producers enter into a flock plan, they must agree to follow a disinfection protocol requiring use of a product registered by the U.S. Environmental Protection Agency (EPA) specifically for reduction of prion infectivity in accordance with the label or with an emergency exemption issued by EPA for reduction of prion infectivity. On April 29, 2013, the EPA amended 40 CFR 152 to include prions as a “pest” under the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA). Accordingly, only products registered with the EPA specifically for the reduction of prion infectivity can be used to disinfect premises, laboratories, and research facilities after prion exposure. Currently there are no EPA registered products available; EPA therefore granted APHIS an exemption for the use of chlorine and sodium hydroxide (lye) in its prion control and eradication programs. The exemption requires APHIS to submit an annual report to EPA detailing the total amount of bleach and lye used by APHIS, our partners (State laboratories), and TSE-infected premises. For bleach, respondents must report the date of use, the concentration used, the amount used, and any adverse reactions noted in people, livestock, wildlife, or the environment. For lye, respondents must report the date of use, formulation (powder or liquid) used, the amount used, and any adverse reactions noted in people, livestock, wildlife or the environment.
9 CFR 54.1, 9 CFR 79: Terminal Feedlot Request for Approval/Agreement and Recordkeeping, Terminal Feedlots (VS Form 5-10 or equivalent (Draft)) – Private Sector
APHIS requires terminal feedlots to be approved. To be approved owners of feedlots will need to request approval by entering the address, owner, and contact information for the terminal feedlot on a form that lists the requirements for being approved and that states the owner understands and will comply with the requirements. The owner must sign the form. APHIS further requires terminal feedlot owners to maintain records of all animals entering and leaving a terminal feedlot for
5 years after the animal leaves the feedlot, including either a copy of the required owner/hauler statements for animals entering and leaving the facility or the information required to be on the statements. Records must be made available for inspection and copying by an APHIS or State representative on request.
9 CFR 79.1: Concurrence with APHIS/State Animal Designations - (definition of low-risk exposed animal) – State, Local, and Tribal Governments
In designating an exposed animal as low risk, there must be concurrence between the State and APHIS that the animal in fact presents a low risk of infection owing to factors such as the animal’s place of residence or the time period the animal resided in the flock. Notification of such occurrence can be done by phone or email.
9 CFR 54.10: Approval of New Tests or Test Methods – Private Sector
Laboratories or test kit manufactures may apply to APHIS for approval of new tests or test methods for diagnosing scrapie for use in the Scrapie Eradication Program or the Scrapie Free Flock Certification Program. APHIS requires the following information to evaluate the test:
A standardized test protocol including a description of the test; a description of the reagents, materials, and equipment used for the test; the test methodology; and any control or quality assurance procedures.
Data to support repeatability; that is, the ability to reproduce the same result repeatedly on a given sample.
Data to support reproducibility; that is, data to show that similar results can be produced when the test is run at other laboratories.
Data to support the diagnostic and in the case of assays the analytical sensitivity and specificity of the test.
Any other data or information APHIS requests to determine the suitability of the test for program use. This may include but is not limited to past performance, cost of test materials and equipment, ease of test performance, generation of waste, and potential use of existing equipment.
9 CFR 79.2(a)(2): Approval of Alternative ID Devices or Methods – Private Sector
Requests for approval of sheep or goat identification device types or methods not listed by APHIS as official ID must be presented in writing to APHIS. If APHIS determines that an identification device or method will provide an effective means of tracing sheep and goats in interstate commerce, APHIS will provide public notice that the device type or method, along with any restrictions on its use, has been added to the list of approved devices and methods of official sheep and goat identification.
9 CFR 79.2(k): Approval/Renewal of Official ID Devices – Private Sector
APHIS may approve companies to produce official identification devices for use on sheep or goats or for sheep and goat owners to use new methods of identification. To be approved, devices must be able to legibly accommodate the required alphanumeric sequences, must resist removal, and be difficult to place on another animal once removed unless the construction of the device makes such tampering evident. Devices must be readily distinguishable as USDA official sheep and goat identification devices; must carry the alphanumeric sequences, symbols, or logos specified by APHIS; must be an allowed color for the intended use, and must have a means of discouraging counterfeiting, such as use of a unique copyrighted logo or trademark. Devices for use only on animals in slaughter channels must be medium blue and marked with the words “Meat” or “Slaughter Only”. Devices that use radio frequency identification (RFID) must conform to ISO 11784 and ISO 11785 standards unless otherwise approved. Manufacturers must request approval in writing; the request must include:
• The materials used in the device and, in the case of RFID, the transponder type and data regarding the lifespan and read range.
• Any available data regarding the durability of the device, durability and legibility of the identification numbers, rate of adverse reactions such as ear infections, and retention rates of the devices in animals, preferably sheep and/or goats.
• A signed statement agreeing to:
o Send official identification devices only to a State or APHIS representative, to the owner of a premises, or to the contact person for a premises at the address listed in the National Scrapie Database, or as directed by APHIS;
o When requested by APHIS, provide a report by State of all tags produced, including the tag sequences produced and the name and address of the person to whom the tags were shipped, and provide supplemental reports of this information when requested by APHIS;
o Maintain the security and confidentiality of all tag recipient information acquired as a result of being an approved tag manufacturer and use the information only to provide official identification tags; and
o Enter the sequences of tags shipped into the online AIN management system module of the National Scrapie Database through an Internet Web page interface or other means specified by APHIS before shipping the identification device.
Approval to produce official identification devices will be valid for 1 year and must be renewed annually. The Administrator may grant provisional approval to produce devices for periods of less than 1 year in cases where there is limited or incomplete data. The Administrator may approve a new identification method after determining that it will provide an effective means of tracing sheep and goats in interstate commerce.
9 CFR 79.3(k): Compliance Agreement and Responses Required by the Agreement -- Private Sector
APHIS may enter into compliance agreements with dealers and owners of slaughter establishments and markets so they can receive unidentified animals or animals moved without an owner/hauler statement even if they cannot be identified to their flock of birth or origin because they were moved or commingled while unidentified. The movement and/or noncompliant shipment must be reported by the compliance agreement holder to the District Director so corrective action can be taken against the principal violator. In such cases the animal must be identified with a slaughter only tag, and moved only in slaughter channels or, in the case of sheep, moved for other purposes if the animal is inspected by an accredited veterinarian, found free of evidence of infectious or contagious disease, and officially genotyped as AA QR or AA RR where Q and R refer to codon 171 and A refers to codon 136. APHIS may also enter into compliance agreements with persons or, in the case of approved livestock facilities, may amend an approved livestock facility agreement to establish alternative methods to maintain the traceability of animals in slaughter channels to their flock of origin. APHIS may also waive the requirement for individual official identification of animals in slaughter channels if adequate surveillance has been conducted on the flock of origin or an alternative plan is in place to conduct surveillance on animals from the flock of origin. The APHIS Administrator and the State animal health official must agree that the application of an allowed official identification device or method is unsuitable for a specific circumstance.
9 CFR 54.10(h), 9 CFR 54.11(c), 9 CFR 79.4(c)(3): Appeal Process – Private Sector
The owner of an animal may appeal the designation of an animal as a scrapie-positive animal, high-risk animal, exposed animal, genetically susceptible exposed animal, genetically resistant exposed sheep, genetically less susceptible exposed sheep, low-risk exposed animal, or a suspect animal. The owner of a flock may appeal the designation of the flock as an exposed flock, an infected flock, a source flock, a flock under investigation, or a non-compliant flock. The owner of a laboratory or test manufacturing facility may appeal the suspension or withdrawal of approval for a laboratory or a test (APHIS may withdraw or suspend approval of any test or test method if the test or method does not perform at an acceptable level or if a more effective test or test method is subsequently approved). The appellant must appeal by writing to APHIS within 10 days after being informed of the reasons for the proposed action. The appeal must include all of the facts and reasons on which the appellant relies to show that the reasons for the proposed action are incorrect or do not support the action. The APHIS Administrator will grant or deny the appeal in writing as promptly as circumstances permit, stating the reason for his or her decision. If there is a conflict as to any material fact, a hearing will be held to resolve the conflict.
Similarly, a person not in compliance with the requirements for obtaining official ID numbers who has already been assigned such numbers may lose the assignment; such withdrawal or failure to assign official identification numbers may also be appealed. Finally, the Administrator may decline to renew a company's approval or suspend or withdraw approval if the devices do not show adequate retention and durability or cause injury in field use or if the tag company does not meet any of the requirements of 9 CFR 79.4(c)(3). Companies will get 60 days’ written notice of intent to withdraw approval and can appeal.
9 CFR 79.5(a)(4): Interstate Certificate of Veterinary Inspection, Signature – Private Sector and State, Local, and Tribal Governments
APHIS added the interstate certificate of veterinary inspection (ICVI) to its existing scrapie information collection on the last renewal; however, APHIS is now adding a signature requirement to its scrapie regulations. The ICVI must be signed by the issuing State, Federal, Tribal or accredited veterinarian. The signature must be legible on all copies.
3. Describe whether, and to what extent, the collection of information involves the use of automated, electronic, mechanical, or other technological collection techniques or other forms of information technology, e.g., permitting electronic submission of responses, and the basis for the decision for adopting this means of collection. Also, describe any consideration of using information technology to reduce burden.
Owner/Hauler Statement and Bill of Sale
Owners will typically use hard copies but may use other methods if acceptable to the person receiving the animals. Any method must accompany the animals and be shown to regulatory officials when requested. These documents can be submitted electronically as long as the technology includes a legally acceptable electronic signature.
Data Assigning Official Identification to Flocks (includes Data Entry of Official Identification Devices Produced; Application for Official and Unofficial ID, PINs, and Flock Identification Numbers)
Market operators, dealers, accredited veterinarians, and other persons who apply official identification to sheep and goats can submit the information in hard copy, by email, electronically through the animal identification number management system module of the National Scrapie Database at http://nais.aphis.usda.gov/ainmngt/, or in another manner acceptable to APHIS.
Concurrence with APHIS/State Animal Designations
Notification of such concurrence can be done via a phone call or email.
Flock Plan Disinfection Worksheet
APHIS has specific Excel spreadsheets for completing these worksheets; these may be submitted by email or in hard copy.
Applications for Approval of New Test Methods, Official ID, Alternate Forms of ID, and Assignment of ID
These may be done in hard copy or by emailing pdf files or other electronic file formats acceptable to APHIS.
Appeals of Withdrawal or Suspension of Approval of Test Method, ID Number Assignment and Approval
These may be done in hard copy or by emailing pdf files or other electronic file formats acceptable to APHIS.
4. Describe efforts to identify duplication. Show specifically why any similar information already available cannot be used or modified for use for the purpose described in item 2 above.
The information APHIS collects in connection with this effort is not available from any other source. APHIS is the only Agency responsible for controlling the interstate spread of domestic animal diseases.
5. If the collection of information impacts small businesses or other small entities, describe any methods used to minimize burden.
Only the information needed to conduct a successful program is being collected. APHIS minimizes the burden on respondents by providing them alternate ways to submit the information. APHIS estimates that 100 percent of the private sector respondents are small entities.
6. Describe the consequence to Federal program or policy activities if the collection is not conducted or is conducted less frequently, as well as any technical or legal obstacles to reducing burden.
If the information was collected less frequently or not collected, APHIS’ efforts to identify the remaining infected flocks and aggressively prevent the spread of scrapie would be severely hindered. APHIS would be unable to fulfill its mission of eradicating this economically damaging disease from the United States.
7. Explain any special circumstances that require the collection to be conducted in a manner inconsistent with the general information collection guidelines in 5 CFR 1320.5.
requiring respondents to report information to the agency more often than quarterly;
requiring respondents to prepare a written response to a collection of information in fewer than 30 days after receipt of it;
requiring respondents to submit more than an original and two copies of any document;
requiring respondents to retain records, other than health, medical, government contract, grant-in-aid, or tax records for more than 3 years;
The retention of records associated with this rulemaking is required for 5 years. This requirement is based on the fact that livestock animals typically live to be more than 3 years old and animal diseases can affect all ages and classes of livestock. Further, the incubation period for scrapie on average is 44 months (roughly 3 and a half years) and is often longer. Therefore, information that fully supports disease control, eradication, and surveillance needs to be maintained for longer than 3 years. APHIS also requires 5-year retention of records associated with animal movement kept by producers and operators of feedlots, markets, buying stations, and slaughter plants. The 5-year requirement brings consistency throughout APHIS regulations.
in connection with a statistical survey, that is not designed to produce valid and reliable results that can be generalized to the universe of study;
requiring the use of a statistical data classification that has not been reviewed and approved by OMB;
that includes a pledge of confidentiality that is not supported by authority established in statute or regulation, that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or
requiring respondents to submit proprietary trade secret, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information's confidentiality to the extent permitted by law.
There are no other special circumstances requiring that the collection of information be conducted in a manner inconsistent with the guidelines established in 5 CFR 1320.5.
8. Describe efforts to consult with persons outside the agency to obtain their views on the availability of data, frequency of collection, the clarity of instructions and recordkeeping, disclosure, or reporting form, and on the data elements to be recorded, disclosed, or reported. If applicable, provide a copy and identify the date and page number of publication in the Federal Register of the agency's notice, soliciting comments on the information collection prior to submission to OMB.
APHIS contacted Mr. Rodgers, Mr. Boyer, and Dr. Rowe by email and phone to discuss the information APHIS collects to administer its scrapie control and eradication programs. We discussed with them how we and they obtain the necessary data and how frequently; how much data is available; the convenience and clarity of reporting formats and other collection instruments; and the clarity of, and necessity for, any recordkeeping requirements. The respondents stated via email or phone that they had no concerns with any of these items and had no further recommendations.
Mr. Paul Rodgers
American Sheep Industry Association
Route 2, Box 94
Ronceverte, WV 24970
(304) 647-9981
Mr. Tom Boyer
American Goat Federation
2200 Chalk Creek
Coalville, UT 84017
(801) 376-4685
Joan Dean Rowe, DVM
American Association of Small Ruminant Practitioners
P.O. Box 3614
Montgomery, AL 36109
515-306-1129
APHIS’ proposed rule (APHIS-2007-0127-0001; 80 FR 54660) described its information gathering requirements, and also provided a 60-day comment period. During that time, interested members of the public had the opportunity to provide APHIS with their input concerning the usefulness, legitimacy, and merit of the information collection activities is proposing.
9. Explain any decision to provide any payment or gift to respondents, other than remuneration of contractors or grantees.
This information collection activity involves no payments or gifts to respondents.
10. Describe any assurance of confidentiality provided to respondents and the basis for the assurance in statute, regulation, or agency policy.
APHIS may require approved laboratories to reimburse it for part or all of the costs associated with the approval and monitoring of the laboratory. No additional assurance of confidentiality is provided with this information collection. However, the confidentiality of information is protected under 5 U.S.C. 552a.
11. Provide additional justification for any questions of a sensitive nature, such as sexual behavior or attitudes, religious beliefs, and other matters that are commonly considered private. This justification should include the reasons why the agency considers the questions necessary, the specific uses to be made of the information, the explanation to be given to persons from whom the information is requested, and any steps to be taken to obtain their consent.
This information collection activity will ask no questions of a personal or sensitive nature.
12. Provide estimates of the hour burden of the collection of information. Indicate the number of respondents, frequency of response, annual hour burden, and an explanation of how the burden was estimated.
• Indicate the number of respondents, frequency of response, annual hour burden, and an explanation of how the burden was estimated. If this request for approval covers more than one form, provide separate hour burden estimates for each form and aggregate the hour burdens in Item 13 of OMB Form 83-I.
See APHIS Form 71. Burden estimates were developed from National Agricultural Statistics Service data on sheep and goat farm numbers, APHIS records, and discussions with market operators, dealers, accredited veterinarians, flock owners, slaughter plant owners, tag manufacturers, feedlot managers, and laboratory personnel.
• Provide estimates of annualized cost to respondents for the hour burdens for collections of information, identifying and using appropriate wage rate categories.
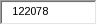
APHIS estimates the total annual cost to these respondents to be $5,533,050. APHIS arrived at this figure by multiplying the total burden hours (122,078) by the estimated average hourly wage of the above respondents ($30.82), and then multiplying the result by 1.4706 to capture benefit cots. .
Animal scientists: $33.10
Veterinarians: $48.81
Animal breeders: $20.89
First-line supervisors of production and operating workers: $30.13
Slaughters and meat packers: $13.38
Farmers, ranchers, and other agricultural managers: $38.62
The average hourly rate is derived from the U.S Department of Labor; Bureau of Labor Statistics May 2017 Report – National Compensation Survey: Occupational Employment and Wages. See http://www.bls.gov/oes/#tables. According to DOL BLS news release USDL-18-1499, dated September 18, 2018 (see https://www.bls.gov/news.release/pdf/ecec.pdf), benefits account for 32% of employee costs, and wages account for the remaining 68%. Mathematically, total costs can be calculated as a function of wages using a multiplier of 1.4706.
13. Provide estimates of the total annual cost burden to respondents or recordkeepers resulting from the collection of information (do not include the cost of any hour burden shown in items 12 and 14). The cost estimates should be split into two components: (a) a total capital and start-up cost component annualized over its expected useful life; and (b) a total operation and maintenance and purchase of services component.
There is zero annual cost burden is associated with capital and startup costs, operation and maintenance expenditures, and purchase of services.
14. Provide estimates of annualized cost to the Federal government. Provide a description of the method used to estimate cost and any other expense that would not have been incurred without this collection of information.
Annual cost to the Federal government is estimated at $615,658 (see APHIS Form 79).
15. Explain the reasons for any program changes or adjustments reported in Items 13 or 14 of the OMB Form 83-1.
ICR Summary of Burden:

|
Requested |
Program Change Due to New Statute |
Program Change Due to Agency Discretion |
Change Due to Adjustment in Agency Estimate |
Change Due to Potential Violation of the PRA |
Previously Approved |
Annual Number of Responses |
568,252 |
0 |
568,252 |
0 |
0 |
0 |
Annual Time Burden (Hr) |
122,078 |
0 |
122,078 |
0 |
0 |
0 |
Annual Cost Burden ($) |
0 |
0 |
0 |
0 |
0 |
0 |
This is a new information collection tied to a rule amending the scrapie and related indemnity regulations resulting in 122,078 total burden hours.
16. For collections of information whose results are planned to be published, outline plans for tabulation and publication.
APHIS has no plans to publish the information it collects in connection with this program.
17. If seeking approval to not display the expiration date for OMB approval of the information collection, explain the reasons that display would be inappropriate.
Not applicable. APHIS will display expiration date.
18. Explain each exception to the certification statement identified in the "Certification for Paperwork Reduction Act."
APHIS can certify compliance with all provisions in the Act.
B. Collections of Information Employing Statistical Methods
Statistical methods are not employed in this information collection.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Khbrown |
| File Modified | 0000-00-00 |
| File Created | 2021-01-15 |
© 2026 OMB.report | Privacy Policy