Ss B
SS B.doc
Interventions to Reduce Shoulder MSDs in Overhead Assembly
OMB: 0920-0964
Request for Office of Management and Budget (OMB) Review and Approval
for a Federally Sponsored Data Collection
Section B
INTERVENTIONS TO REDUCE SHOULDER MSDS IN OVERHEAD ASSEMBLY
0920-0964 REINSTATEMENT
Brian D. Lowe, Ph.D.
Research Industrial Engineer
Project Officer
National Institute for Occupational Safety and Health
Division of Applied Research and Technology
4676 Columbia Parkway, Mail Stop C-24
Cincinnati, Ohio 45226
513-533-8161 (phone)
513-533-8596 (fax)
Table of Contents
B. COLLECTION OF INFORMATION EMPLOYING STATISTICAL METHODS 3
B1. Respondent Universe and Sampling Methods 3
B2. Procedures for the Collection of Information 5
B3. Methods to Maximize Response Rates and Deal with Nonresponse 12
B4. Tests of Procedures or Methods to be Undertaken 13
B5. Individuals Consulted on Statistical Aspects and/or Analyzing Data 14
B. COLLECTION OF INFORMATION EMPLOYING STATISTICAL METHODS
NIOSH and Toyota Motors Engineering and Manufacturing North America, Inc. (TEMA) will collaborate on a study to evaluate the efficacy of two intervention strategies aimed at reducing shoulder discomfort and MSD symptoms from overhead assembly work in automotive manufacturing. In overview, a workstation modification engineering intervention (articulating tool support arm) and a regionally specific shoulder exercise program will be tested for their effectiveness in reducing self-reported arm and shoulder pain among 125 employees performing overhead assembly work in automotive chassis assembly. The interventions are designated as TS (articulating tool support), E (exercise), and TSE (treatment group receiving both the tool support and exercise interventions). The study will be conducted using a prospective experimental design with multiple baselines across groups, a cluster randomization of groups to treatments, and a control group. The assignment of cluster groups to the four treatment conditions will keep the treatment groups physically isolated by using two distinct assembly lines in different areas of the manufacturing facility and two different work shifts (first and second shifts).
B1. Respondent Universe and Sampling Methods
Definitions of the Target Population, Sampling Frame, Study Sample and Sub-Sample
For this study, the target population (people, groups or workplaces which might benefit from the MSD interventions being tested) includes workers performing overhead work from a generally stationary work cell. This situation would most frequently be encountered in the manufacturing industry, however, other industry sectors exhibit examples of similar work characteristics and these industries will benefit from the knowledge gained in this study. The sampling frame (segment of the target population) includes employees at the Toyota Motors Manufacturing Kentucky (TMMK) manufacturing facility in Georgetown, KY. Specifically, employees will be recruited from the Chassis Assembly departments. The study sample includes team members employed in these departments who volunteer to participate. Any team member from these departments may participate provided that he/she meets the screening requirements for participation in the shoulder exercise program. All potential participants in the E and TSE groups will be screened for exercise participation readiness with the PAR-Q questionnaire (Attachment G1).
The sub-sample will be eligible employees at the Toyota TMMK assembly plant, working on two chassis assembly lines. These individuals are exposed to overhead work in which the upper arm is elevated for significant periods of time during the work cycle. Their tasks involved shoulder and arm elevation when fastening parts to the vehicle chassis. Prevalence of shoulder discomfort symptoms are higher among this group owing to the nature of the overhead work. It is believed that this group will derive the greatest benefit from the interventions.
Power Calculations for Main Outcomes
Sample size calculations were conducted based on data from a preventive exercise program study of Ludewig and Borstad (2003). Their study reported outcomes with the Shoulder Rating Questionnaire (SRQ), which will be adopted in the present study. The calculations assumed that dependent variables approximate a continuous outcome that can be modeled with a linear mixed model (Fitzmaurice et al., 2004). Power calculations were performed using PASS (NCSS, 2008) for a mixed model in which subject was a random factor, type of intervention was a between-subject fixed factor, and the pre-post intervention status was a within-subject fixed factor. The primary statistical test of interest is for differences in pre- and post-intervention scores depending on intervention (control, TS, E, or TSE). This will be based on the interactions between pre- and post-intervention status and intervention treatment. Power was assessed for assumptions of a between-subject standard deviation (sd) of 18.47. This s.d. was derived by converting the standard error reported by Ludewig and Bordstad (2003). An alpha error of 0.05 was used. An effect size, measured as the difference in SRQ scores, of approximately 8.5 can be detected with a power of 0.80. (See Figure B1.) Since the SRQ is scaled to 100, this represents an effect size of approximately 8.5%. Studies have suggested that the minimal clinically significant difference with similar questionnaire instruments is a change of 5-7% (Wyrwich et. al. 1999; Redelmeier and Lorig 1993; Beaton et. al. 2001). Thus, the study has the statistical power to detect a significant effect which is nearly as small as the minimum clinically meaningful effect.
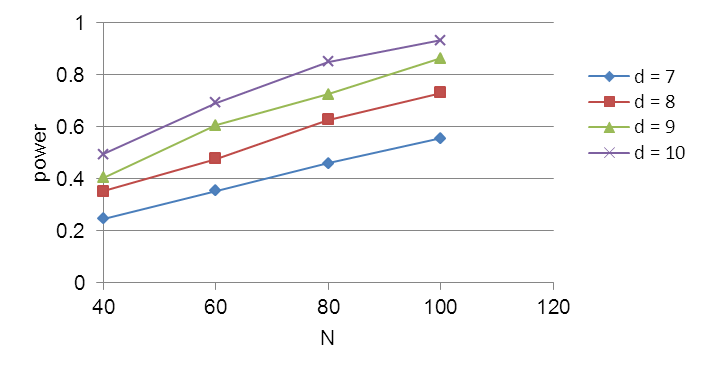
Figure B1. Sample size calculations for effect sizes (d) of 7,8,9, and 10 in the SRQ (differences in pre-/post-intervention). Calculation assumes a between subjects sd of 13.47 and an alpha error of 0.05.
B2. Procedures for the Collection of Information
Study Design
The intervention study will be conducted as a cluster randomized multiple time series design (DHHS/NIOSH, 2001). Delayed introduction of the intervention will allow for baseline symptom prevalence to be collected for three months (at 30 day intervals) prior to the intervention onset. Primary outcomes will be based on self report questionnaire for discomfort and upper limb symptoms and functional impairment using the instruments described below (Dependent Variables section). Symptom data will be collected for an additional three months following the four-month intervention period. These observations allow for more stable estimates of pre- and post- intervention symptom levels and reduce regression to the mean effects. Reports of musculoskeletal symptoms will be obtained at one month intervals throughout the study period using the instruments described below (dependent variables).
Randomization of the tool support intervention (XTS) group, exercise intervention (XE) group, the group receiving the tool support and exercise treatments (XTSE), and the control group will be by cluster, or work unit, rather than by individual (Sjogren et al., 2005, 2006). There will be four groups of 25-30 employees in groups according to two production shifts and two chassis assembly lines. Employees in each of these clusters will be eligible and invited to participate. Physical risk factors for MSDs (e.g. posture, force, and repetition) among these four groups have been shown to be similar in Toyota’s Early Symptom Investigation (ESI) screening of jobs for risk factors for shoulder disorders. The treatment groups will be separated by physical non-proximity in the plant or by differing work shift. This will reduce rivalry threats that might otherwise exist. The control group will be evaluated with the same metrics for symptomology.
Randomization of intervention treatment at the individual level is not feasible because of the nature of employees’ work schedules and the permanence of the tool support devices when they are installed on the assembly line. Additionally, individual-level randomization risks rivalry threats among individuals in physical proximity receiving different intervention treatments. The strategy of randomization by cluster isolates the treatment groups to prevent contamination, and there is precedent for a randomization at the level of the work unit rather than of the individual (e.g. Koespell et al., 1995; Daltroy et al., 1997; Volinn, 1999).
Independent Variables
Intervention (TS, E, TSE, control) – This variable represents the intervention condition, which is defined by four treatments: tool support arm intervention (TS), exercise intervention (E), and tool support arm plus intervention (TSE), in addition to a control group receiving no intervention. The TS and E interventions are described in Attachment K: Details of Interventions.
Time (pre-, and post- intervention) – the average pre intervention score will be calculated from the three monthly pre-intervention scores. The same will be done for average post intervention score in months eight, nine, and ten. Obtaining multiple observations pre- and post- intervention will reduce the potential for a regression to the mean effect.
Dependent Variables (Outcomes)
Shoulder Rating Questionnaire (SRQ). (Attachment G2). This is a 21-item questionnaire that includes six separately scored domains for global assessment, pain, daily living activities, recreational/athletic activities, work, and satisfaction. Validity, reliability, and sensitivity have been demonstrated for this instrument (L’Insalata et al., 1997).
DASH (Disability of the Arm Shoulder and Hand). (Attachment G3.) Self-reported arm and shoulder pain will be measured by the DASH Outcome Measure with Work Module Option. The DASH outcome has been found to have acceptability, high re-test reliability, internal reliability, and validity for shoulder/ arm pain and disability (Beaton et al., 2001; Hudak et. al., 1996; Adams et al., 2005; Atroshi et al., 2000; Gay et al., 2003).
Body part discomfort mapping of general musculoskeletal symptoms. This instrument will be based on the Standardized Nordic Questionnaire for Analysis of Musculoskeletal symptoms (Kuorinka et al., 1986). (Attachment G4) A Borg CR-10 Scale (Borg, 1982) rating of discomfort will be collected for each region reported as experiencing “…trouble at any time in the last 7 days.”
Work Organization and Non-Occupational Physical Activity. (The NIOSH Work Organization Questionnaire contains 218 questions that address a wide range of work organization issues that may affect musculoskeletal symptoms, job satisfaction, and the relationship between psychosocial work factors and the prevalence of symptoms. This information will be collected to determine whether changes in work environment have occurred during the study period that need to be considered when evaluating differences in musculoskeletal symptoms believed to be attributable to the intervention(s). The majority of this questionnaire has been previously administered to Toyota employees in an office facility in the NIOSH study of computer input devices. Some questions have been modified or deleted because they are specific to computer work and would not be relevant to employees working on the manufacturing lines. A brief set of questions is also included to assess changes in non-occupational physical activity during the intervention period.
Functional Capacity. Immediately prior to receiving the intervention, at day 90, and after four-months of intervention use, at day 210, participants will undergo a test battery for functional capacity of the shoulder. The specific content of this assessment will be determined through the job-specific FCE development approach of Frings-Dresen and Sluiter (2003), and will involve a Physical Therapist under contract with TMMK. The purpose of the FCE is to assess efficacy of the exercise program in improving shoulder strength and endurance. Results will not be used to make inferences about individual capability to tolerate physical demands of overhead work. It is proposed to include the following: (1) Isernhagen Work Systems overhead test for overhead work tolerance; (2) battery of strength tests using a work simulator/evaluator, similar to the BTE Technologies EvalTech™ System, and including shoulder flexion/extension, ab-/adduction, and internal/external rotation strength; (3) active shoulder range of motion (ROM); (4) test of shoulder elevation endurance with associated discomfort ratings (e.g. Lundblad et al., 1999). The FCE testing will be administered by an athletic training and work performance specialist under contract with TMMK. This individual will have certifications in strength and conditioning and corrective exercise.
Statistical Analysis
The statistical model will be a mixed model including independent variables for type of intervention (TS, E, TSE, and control) and for pre- or post-intervention status. Individual will be modeled as a random effects factor, type of intervention as a between-subjects fixed factor, and the pre-post intervention status as a within-subject fixed factor. The model will also control for the effects of other covariates, such as gender, age, height, weight, job tenure, years of total employment, work hours, work status, shift, pay, etc., with the aim of accounting for any impact that these variables could have on the outcome variables. The primary statistical test of interest is for differences in pre- and post-intervention scores on the dependent variable outcome measures (below) as a function of intervention (control, TS, E, or TSE). This will be based on the interactions between pre- and post-intervention status and intervention treatment. Analyses will be conducted using SAS 9.2 (SAS Institute, Inc., Cary, NC).
Cost-Benefit Analysis
A cost-benefit analysis (CBA) allows decision makers to directly compare the safety or health outcomes of different types of interventions (Carande-Kulis et al, 2009). There are a number of models for conducting CBAs of OSH interventions (e.g. Oxenburgh, 1991, Beevis, 2003, Hendrick, 2003). The majority of the prior CBAs referenced in the literature are cross-sectional without a control group. The prospective controlled trial in will measure several outcomes related to implementation of the TS and E interventions. Findings from this study will be used to estimate long term costs and benefits of the interventions if expanded at Toyota and contribute to the research gap on cost-effectiveness of MSD interventions.
Short Term Economic-Related Outcome Analysis: Specific economic-related outcome measures will be collected as part of this phase for participating employees (directly impacted by the TS, E interventions) at 30-day intervals for ten months (3 months prior to intervention onset, during the 4 month intervention period, and for 3 months post intervention):
First aid log reports: The rate (per 100 FTE) of MSD related first aid log reports within the work unit
Absenteeism: The rate of absenteeism (absent days among participants, already tracked at TMMK)
Quality: The target process assembly error rate (already tracked at TMMK)
Productivity: The target process assembly productivity rate (already tracked at TMMK)
It is hypothesized that the TS and E intervention groups will be associated with improvements in economic related outcomes when compared pre- and post- intervention.
Long Term Cost- Benefit Estimation: The detailed costs of the TS and E interventions will be tracked throughout the study. Utilizing this cost information and the potential benefit information gained by the short term economic-related outcome analysis described above, the long term costs and benefits of the interventions will be estimated. To do so, this study will follow the framework suggested by the CDC for developing a cost benefit analysis (CBA), which includes seven mains steps and constructing an a priori inventory of potential costs and benefits that one would expect from each intervention.
After estimating all benefits and costs, a number of economic and financial measures (including simple return on investment, ROI, net present value, NPV, among others) will be calculated using the ORC Worldwide, Inc. Return on Occupational Health Safety and Environmental Investments (ROHSEI) tool. A CBA will yield a positive NPV and ROI if the benefits exceed the costs. To complete the analysis using the ROHSEI tool, additional data will be collected including the method of depreciation, the predicted lifespan and salvage value of the intervention equipment, the discount rate, the corporate tax rate, and the savings related to successful interventions including estimated avoided workers’ comp and medical treatment costs, productivity losses (time off the job) and any quantified indirect costs. CDC guidelines suggest that sensitivity analyses be performed “to provide quantitative assessment of how variations in parameter estimates will affect the values of the business-case-merit measures. Sensitivity analysis usually is performed by repeated re-estimation of financial measures by changing values of underlying parameters” (Carande-Kulis et al, 2009).
Study Limitations
Limitations of the study are believed to include the following:
Single study site. This is considered a study limitation insofar as a multi-site study would improve generalizability of results. However, sampling from a single establishment, the Toyota, Georgetown, KY manufacturing facility, reduces variability that would otherwise exist in a multi-company study.
Randomization of assignment to treatment by work unit. The ideal study design would be completely randomized assignment to treatment at the individual level. This is not feasible, however, as it would involve extreme, and unacceptable, administrative changes to achieve random assignment. Keeping employees on their same work shift within the same work cell unit is a necessary compromise. There is precedent for the randomization by group approach in studies of workplace safety interventions.
Lack of a placebo condition. Ideally, a controlled trial would include a placebo group in addition to a control group. Studies of ergonomic interventions are difficult to establish a placebo because the placebo condition relies on the participant being uninformed about the characteristics of the treatment.
Recruitment
NIOSH will recruit employees using a simple written description of the study on an informational flyer distributed at the work site and by word of mouth with assistance from the environmental safety specialist at TMMK. The safety specialist will also be asked to provide a contact list for eligible individuals working on the chassis assembly lines. The recruitment flyer will be distributed directly to eligible recruits. Personal contact will be made with potential study participants. There will be no coercion for employees to participate in the study.
Number of Study Participants
Questionnaire Data Collection: A maximum of 125 individuals may be included in the overall questionnaire study for all intervention treatment conditions. This includes 25-30 individuals per intervention and does not include replacement due to participant dropout. (See section B3.) A low non-response and dropout percentage is anticipated because of the plant’s participatory ergonomics program and employees’ active involvement in the ergonomics program.
Data Management, Security and Confidentiality
The study will collect data that may be considered sensitive (self-reported MSD symptoms and responses to work environment questionnaire). Personal identifiers (name, address, phone number, employee clock number) will be collected for the purpose of linking outcome data to individual employee characteristics. All data will be maintained such that it is identified with an assigned number, and stored in locked file cabinets and on secured computers, accessible only by password. The identification sheets and consent forms will be kept separate in locked file cabinets and will be available only to authorized NIOSH and contractor personnel.
Questionnaires will be administered by hard copy forms, and telephonic interviews, when necessary.
The confidentiality of all data collected will be protected to the extent legally possible, as covered by the Privacy Act of 1974, Title 5, United States Code, Section 522 (a). The method of handling the information complies with the Freedom of Information Act and the Privacy Act of 1974. Disclosure under the Privacy Act System is permitted: to private contractors assisting NIOSH; to collaborating researchers under certain limited circumstances to conduct further investigations; to the Department of Justice in the event of litigation; and to a congressional office assisting individuals in obtaining their records. Records management practices will adhere to all applicable federal, Health and Human Services (HHS), Centers for Disease Control (CDC), and NIOSH IT security policies and procedures [Security Requirements for Federal Information Technology Resources, January 2010; Health and Human Services Acquisition Regulation (HHSAR), Clause 352.239-72]. For example, data will be stored on encrypted CDs, flash drives, and/or ftp sites according to applicable Federal Information Processing Standards Publications (FIPS PUBS, see http://www.itl.nist.gov/fipspubs).
Use of Results
Results of the study (in de-identified and aggregated form) will be disseminated in the scientific literature and in educational materials by NIOSH and Toyota (website, publications).
Notification
Upon completion of the study, an overall summary report of the de-identified and aggregated results will be sent to participating employees who choose to receive this information. De-identified and aggregated results of the study will also be disseminated in the scientific literature and in educational materials directed at workers to make them more aware of the determined efficacy of the interventions.
If study participants leave their jobs during the study period, attempts will be made to contact them in order to determine whether those who leave the study are more or less likely to experience arm discomfort or MSD symptoms. Participants who leave the Chassis Assembly department, thus being no longer eligible to participate, but are still employed at the facility will be contacted in person; if they are no longer employed at the facility, they will be contacted by telephone. The telephone interview script (Attachment M) includes an explanation that the interview is voluntary and confidential.
Risks and Benefits
The study presents minimal physical risks to participants beyond those encountered during their regular daily work. In reference to vulnerable populations, pregnant women may be among the participants. Children (16 years or younger) are not employed at the Toyota facility and will not be allowed to be participants.
Interventions: The potential benefit of the tool support arm is expected to include reduced manual exertion to support the weight of the torque tool, resulting in reduced arm fatigue. The hypothesized benefit of the exercise program is improved functional capacity of the shoulder joint and a resulting reduction in fatigue of the joint from work activities. The risks associated with the shoulder specific exercise program are minimum. Individuals will be screened using the Physical Activity Readiness questionnaire (PAR-Q) in determining study eligibility. The nature of the particular study population is such that it is unlikely that any employee working in the relevant departments will be screened from participation. Minor muscle soreness may result after the first few exercise sessions. It will be explained to the participants that this is a normal response to exercise adaptation and should lessen after a few sessions.
Questionnaires: No individuals will be identified in published materials. No individuals will receive any benefits directly related to participation in the data collection other than their normal wage pay during work hours. An overall indirect benefit is that the information gained from the study may help to improve understanding of how to prevent upper extremity musculoskeletal disorders. The information may also be useful in the design of tools, equipment, and practices to improve manufacturing tasks.
Functional Capacity Evaluation (FCE): Participants in the E and TSE treatment conditions will be asked to participate in a shoulder FCE, which has been administered to both workers and also back disorder patients. Some workers who participate in the FCE may experience some mild discomfort, and delayed onset muscle soreness. These symptoms should resolve within a few days and should not prevent the individual from performing his/her normal daily work. Participants are instructed that they are in control of the test, and are advised to only to exert themselves in a comfortable level of exertion. Study participants will be advised that they may discontinue the test if they believe the exertion to be excessive.
No payments or other incentives will be made to participants by NIOSH because Toyota will permit all data collection to occur during normal work hours. The indirect benefits to the individual participants will include information on workplace controls for musculoskeletal disorders. What is learned from this study, when combined with the knowledge gained from other studies, may benefit workers by helping identify best practices and evidence-based controls that reduce the level of physical demands associated with overhead assembly work.
Informed Consent
Participation in this NIOSH study is completely voluntary and involves minimal risks. The informed consent forms (Attachment F) describe the potential benefits and risks of participation in the study. The grade level for the consent process has been estimated to be the 11th grade based on the Simple Measure of Gobbledygook (SMOG) formula (McLaughlin, 1969). This is consistent with the likely estimated grade level of the target respondents for this questionnaire study.
Emergency Procedures
In the event that an emergency develops during a study participant's involvement in the research, whether or not it is related to the research, emergency procedures for individual and facility wide incidents consistent with the Occupational Safety and Health Administration (OSHA) requirements as outlined by 1910.120(p)(8) and 1910.120(q)(1-8) will be followed.
B3. Methods to Maximize Response Rates and Deal with Nonresponse
Methods to Maximize Response Rate
This study is designed such that individual participants complete surveys every month for a 10-month period. Several methods (described below) will be utilized to maximize response rate.
Brief Survey: The questionnaires have been chosen to be as brief as possible. Baseline time burden per respondent is estimated to be 15 minutes at one month intervals and an additional 26 minutes will be required at baseline pre-intervention for the single administration of a secondary questionnaire. An additional 15 minutes will be required at one month intervals during the four-month intervention period. Then the procedure used at baseline will be repeated after the four month intervention period. This sums to 3.7 hours over the 10 month study period, or 22 minutes per month.
Participatory Ergonomics: The study sample will be drawn from a pool of employees in a corporate culture that fosters employee participation in health/safety programs. Toyota’s safety and ergonomics program stresses employee involvement and that culture permeates throughout manufacturing operations. NIOSH will work closely with TEMA and TMMK Environmental Health and Safety specialists to explain the purpose and importance of the study. NIOSH will begin recruiting individuals at each firm by personal contact and delivery of informational flyers (Attachment I) to employees at the work site. TMMK will be asked to provide a contact list for employees on the chassis assembly lines so that the NIOSH investigative team can assure that all eligible employees are invited to participate. It is anticipated that such a focused recruitment approach in combination with a committed participant pool will help maximize response rates once the study is underway.
Reminders to complete questionnaires: If the participant gives permission, he/she will be sent email reminders to complete questionnaires. The e-mail will be sent two working days prior to the scheduled completion day of the survey. A second e-mail will be sent on the scheduled completion day. The email and phone script for quarterly prompts will be as follows:
“You are participating in a CDC-NIOSH study. Your next scheduled data collection is now due. Please submit your completed surveys within 3 days. If you have any questions about your participation, contact NIOSH at XX.”
Methods to Deal With Non-Response
Any employee who withdraws from the study will be informally asked about the reason for drop out and to complete a final symptom questionnaire at that time. No coercion will be applied to do this. If the employee had completed at least two months of time using the intervention, no replacement employee will be sought. If two months of time with the intervention had not been completed, a replacement employee will be enrolled in the study after collecting a single baseline symptom report including the completion of all questionnaire instruments. This approach is intended to maximize the number of participants who use the intervention for at least two months and reduce any impact of participant drop-out. A two-month intervention period is considered the minimum period of time for valid interpretation of intervention effectiveness. Intention to treat analyses will be explored if intervention non-compliance becomes a threat. As mentioned previously, a low drop-out rate is anticipated, because TMMK has developed a successful participatory ergonomics program where employees and management work to implement MSD controls and work practices.
B4. Tests of Procedures or Methods to be Undertaken
Data Collection Forms
The primary questionnaire instruments: Shoulder Rating Questionnaire, Disabilities of the Arm Shoulder and Hand (DASH), and Standardized Nordic Questionnaire for Musculoskeletal Symptoms, are validated, published instruments that are clinically-accepted self-report methods for obtaining symptom prevalence of the shoulder, arm, and whole body. The instruments will be applied in their published format. Adopting commonly used instruments is advantageous because normative values exist for both diseased and non-diseased populations and knowledge of the time burden for completing these questionnaires were obtained from published studies with large sample sizes. The secondary work organization questionnaire has been used previously by NIOSH in a study of computer input devices. The time burden for the Work Organization Questionnaire is reported from data collected in that study.
Primary Questionnaires (administered to all 100-125 participants at baseline and every one month for 10 months; 15 minutes estimated time for all primary questionnaires combined per data collection):
Shoulder Rating Questionnaire (SRQ) (Attachment G2): This is a validated non-disease specific questionnaire pertaining to shoulder function and shoulder disability. (4.0 min average time to complete)
Disabilities of the Arm Shoulder and Hand (DASH) (Attachment G3): This is a validated non-disease specific questionnaire addressing upper extremity pain in the arm, shoulder, and hand. (6.2 min average time to complete)
Standardized Nordic Questionnaire for Musculoskeletal Symptoms (Attachment G4): This questionnaire is a widely used body map for the description of discomfort symptoms over the entire body. (4.0 min average time to complete)
Secondary Questionnaires
Work Organization and Non-Occupational Physical Activity Questionnaire (Attachment G5): This questionnaire contains 222 questions pertaining to information on the work environment, non-physical attributes of the work demands, and physical attributes of non-occupational activities. (approximately 26 min average time to complete, administered three times - at the beginning, middle and end of intervention period)
B5. Individuals Consulted on Statistical Aspects and/or Analyzing Data
NIOSH personnel will primarily design the data collection, will perform the data collection, and analyze the data. It is anticipated that contracted secondary support staff (to be determined) will also aid NIOSH in these data collection tasks. Below is a summary of individual NIOSH staff roles on this project.
Name |
Job Title |
Division |
Contact Information |
Roles on Project |
Brian Lowe, Ph.D. |
Research Industrial Engineer |
Division of Applied Research and Technology |
513.533.8161 |
Project Officer:
Designed data collection, will collect data, and analyze data |
Steve Wurzelbacher, Ph.D. |
Research Industrial Hygienist |
Division of Surveillance Hazard Evaluation and Field Studies (DSHEFS) |
513.841.4322 |
Intervention implementation, data collection |
Steve Hudock, Ph.D. |
Research Safety Engineer |
Division of Applied Research and Technology |
513.533.8183 |
Intervention implementation, data collection |
Peter Shaw, Ph.D. |
Statistician |
Division of Applied Research and Technology |
513.533.8579 |
Statistician; Statistical modeling and analysis |
Tapas Ray, Ph.D. |
Economist |
Division of Applied Research and Technology |
513.533.8627 |
Economist; Cost-benefit analysis |
Literature Cited
Adams J, Burridge J, Mullee M, Hammond A, Cooper C. Self-reported hand functional ability measured by the DASH in individuals with early rheumatoid arthritis. British Journal of Hand Therapy 2005; 10(1): 21-4.
Atroshi I, Gummesson C, Andersson B, Dahlgren E, Johansson A. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire. Reliability and validity of the Swedish version evaluated in 176 patients. Acta Orthopaedica Scandinavica 2000; 71(6): 613-618.
Beaton DE, Wright JG, Katz JN, Upper Extremity Collaborative Group. Development of the QuickDASH: Comparison of three item-reduction approaches. Journal of Bone & Joint Surgery - American Volume 2005; 87(5):1038-46.
Beaton DE, Davis AM, Hudak P, McConnell S. The DASH (Disabilities of the Arm, Shoulder and Hand) Outcome Measure: What Do We Know About It Now? British Journal of Hand Therapy 2001; 6(4):109-118
Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the Whole or the Parts? Validity, Reliability & Responsiveness of the Disabilities of the Arm, Shoulder, and Hand Outcome Measure in Different Regions of the Upper Extremity. Journal of Hand Therapy 2001; 14(2):128-146.
Beevis D. Ergonomics – Costs and Benefits revisited. Applied Ergonomics 34: 491-496 (2003).
Borg
G.: Psychophysical bases of perceived exertion. Medicine and Science
in Sports and Exercise. 14:377–381 (1982).
Bureau of
Labor Statistics. 2009. TABLE R8. Incidence rates1 for nonfatal
occupational injuries and illnesses involving days away from work2
per 10,000 full-time workers by industry and selected events or
exposures leading to injury or illness, 2006.
Bureau of
Labor Statistics. 2007. TABLE R8. Incidence rates1 for nonfatal
occupational injuries and illnesses involving days away from work2
per 10,000 full-time workers by industry and selected events or
exposures leading to injury or illness, 2006.
Bureau of Labor Statistics. 2009. TABLE 3. Hourly mean wage rates by industry and occupational group, May 2009.
Carande-Kulis V, Biddle E, Sotnikov S. (2009). Investing in Health and Safety: A Business Case Resource Guide. Centers for Disease Control. September 30, 2009.
Daltroy, L. H., Cats-Baril, W. L., Katz, J., Fossel, A. H., Liang, M. H. (1996). The North American Spine Society Lumbar Spine Outcome Assessment Instrument: Reliability and validity tests. Spine, 21, 741-749.
DHHS (2001). Guide to evaluating the effectiveness of strategies for preventing work injuries: How to show whether a safety intervention really works. Department of Health and Human Services, National Institute for Occupational Safety and Health Publication No. 2001-119.
Fitzmaurice, G. M. (2004). Applied longitudinal analysis. Wiley, Hoboken, New Jersey.
Frings-Dresen, M.H.W., and Sluiter, J.K. (2003). Development of a job-specific FCE protocol: The work demands of hospital nurses as an example. Journal of Occupational Rehabilitation, 13(4), 233-248.
Gay RE, Amadio PC, Johnson JC. Comparative responsiveness of the Disabilities of the Arm, Shoulder, and Hand, the Carpal Tunnel Questionnaire and the SF-36 to clinical change after carpal tunnel release. Journal of Hand Surgery (American) 2003; 28A(2): 250-254.
Hendrick
HW. Determining the cost-benefits of ergonomics projects and factors
that lead to their success. Applied Ergonomics 34: 419-427
(2003).
Hudak P, Amadio PC, Bombardier C, and the Upper
Extremity Collaborative Group. Development of an Upper Extremity
Outcome Measure: The DASH (Disabilities of the Arm, Shoulder, and
Hand). American Journal of Industrial Medicine 1996; 29:602-608.
Koepsell, T.D., Diehr, P.H., Cheadle, A., and Kristal, A. (1995). Invited commentary: symposium on community intervention trials. American Journal of Epidemiology, 142, 594-599.
Kuorinka, I., Jonsson, B., Kilbom, A., Vinterberg, H., Biering-Sorenson, F., Andersson, G., and Jorgensen, K. (1986). Standardised Nordic Questionnaire for the analysis of musculoskeletal symptoms. Applied Ergonomics, 18(3), 233-237.
L’Insalata, J.C.Warren, R.F., Cohen, S.B., Altcheck, D.W., and Peterson, M.G.E. (1997). A self-administered questionnaire for assessment of symptoms and function of the shoulder. Journal of Bone and Joint Surgery Am., 79, 738-748.
Lundblad, I., Elert, J., and Gerdle, B. (1999). Randomized controlled trial of physiotherapy and Feldenkrais interventions in female workers with neck-shoulder complaints. Journal of Occupational Rehabilitation, 9 (3), 179-194.
Oxenburgh
MS. Increasing Productivity and Profit through Health and Safety. CCH
International (1991).
Oxenburgh MS. Cost-Benefit analysis
of ergonomics programs. American Industrial Hygiene Association 58
(2): 150-156 (1997).
Sjögren, T., et al. (2005). Effects of a workplace physical exercise intervention on the intensity of headache and neck and shoulder symptoms and upper extremity muscular strength of office workers: A cluster randomized controlled cross-over trial. Pain, 116, 119-128.
Sjögren, T., et al. (2006). Effects of a workplace physical exercise intervention on the intensity of low back symptoms in office workers: A cluster randomized controlled cross-over design. Journal of Back and Musculoskeletal Rehabilitation, 19, 13-24.
Volinn
E. Do workplace interventions prevent low-back disorders? If so, why?
A
methodologic commentary. Ergonomics 1999;42:258–72.
| File Type | application/msword |
| File Title | NATIONAL SURVEY OF U |
| Author | Jan Birdsey |
| Last Modified By | Cenac, Carol E. (CDC/OD/OADS) |
| File Modified | 2015-11-04 |
| File Created | 2015-05-06 |
© 2026 OMB.report | Privacy Policy