Exposure Investigation Protocol
Att9 Anaconda EI II Protocol_final 0923-0048.docx
ATSDR Exposure Investigations (EIs)
Exposure Investigation Protocol
OMB: 0923-0048
Exposure Investigation
Protocol
Blood Lead and Urine Arsenic Levels in Anaconda, Montana
Anaconda Superfund Site,
Anaconda, Deer Lodge County,
Montana:
Anaconda EI II
Cost Recovery No: 8018
Prepared by:
Karen Scruton, MS
David Dorian, MS
Agency for Toxic Substances and Disease Registry (ATSDR)
Division of Community Health Investigations (DCHI)
Science Support Branch (SSB) and
ATSDR Western Branch (Region 8)
October 19, 2018
Table of Contents
Anaconda EI in September 2018 5
Objectives of the Anaconda II EI 5
The Investigators and Collaborators 6
Consent/Parental Permission/Assent Forms 8
Description of Risks and Benefits 11
Procedures for Consent/Parental Permission/Assent 12
Protection of Confidentiality 12
Feasibility and Limitations 12
Handling Unexpected or Adverse Events 13
Dissemination, Notification, and Reporting of Results 14
Appendix A: Anaconda EI Protocol 18
Appendix B: Privacy Act Statement and Consent Forms 94
Appendix D: Urine Collection Instructions 120
Appendix E: Health and Safety Plan 121
Appendix F: Data Management Plan 124
Appendix G: Template for Results Letters to Participants 130
ACRONYMS and ABBREVIATIONS
ADLC Anaconda Deer Lodge County
ARCO Atlantic Richfield Corporation
ATSDR Agency for Toxic Substances and Disease Registry
BLL blood lead level
CDC Centers for Disease Control and Prevention
DCHI Division of Community Health Investigations
DLS Division of Laboratory Sciences
DMA dimethylarsinic acid
DMP Data Management Plan
EDTA ethylenediamine tetra-acetic acid
EI exposure investigation
EPA (U.S.) Environmental Protection Agency
µg/dL microgram per deciliter
MDPHHS Montana Department of Public Health and Human Services
mg/kg milligram per kilogram
MMA monomethylarsonic acid
NCEH National Center for Environmental Health
NHANES National Health and Nutrition Examination Survey
NIOSH National Institute of Occupational Safety and Health
NPL National Priority List
OMB Office of Management and Budget
OU operable unit
Project Overview
An Exposure Investigation (EI) was conducted
at the Anaconda National Priority List (NPL) site in Anaconda,
Montana in September, 2018. The Anaconda area was contaminated with
heavy metals from past smelting activities. The EI included the
testing of 191 residents to determine the levels of lead in blood
and arsenic in urine in residents in the Anaconda community.
Community interest in the testing event was considerable and ATSDR
was not able to test all interested participants at that time. In
addition, very few young children and women of childbearing age were
tested. Therefore, EPA committed to fund additional testing in the
community. The second round of testing will be conducted by ATSDR
with the assistance of the ADLC health department and the MDPHHS.
This EI is a follow-up to the EI conducted in September 2018 and has
the same basic objectives and follow-up activities as the first EI:
Evaluate
blood lead levels (BLLs) and recommend case management for
participants with BLL ≥5 micrograms per deciliter (µg/dL)
(CDC reference level) Recommend
follow-up evaluation with a Primary Care Physician (PCP) for
retesting and developmental and behavioral screening, as needed Recommend
an early intervention program for children with developmental and
behavioral issues, as needed Evaluate
total and inorganic urine arsenic levels. Compare the
creatinine-corrected total urinary arsenic level to the most
up-to-date 95th
percentile value reported in NHANES. Currently, a value of 29.9
µg/g Cr is reported for children aged 6-11 years; 30.5 µg/g
Cr for children aged 12-19 years and 54.0 µg/g Cr for adults
aged 20 years and older (2013-2014 data) [CDC 2018].
For
participants whose creatinine-corrected total urinary arsenic
levels are above the appropriate 95th
percentile NHANES value, total inorganic urinary arsenic results
will be compared to the most up-to-date 95th
percentile values specific to age group that are reported in NHANES
[CDC 2018].
Recommend
ways to lower exposure to lead and arsenic in the home. Recommend
ways to lower exposure to lead- and arsenic-containing dust in
homes (e.g., attics) Assist the
community with the identification of available resources for home
assessments, including testing and remediation of yard soil and
attics
All
participants will have the option of discussing their lead and/or
arsenic findings with an ATSDR medical officer.
Provide
information on nutrition that may help to decrease the absorption
of lead into the body
Background
The previous protocol is provided as Attachment A. It provides information on site background including previous environmental sampling data and biological testing. The protocol also provides information on the potential health effects of environmental exposure to lead and arsenic. The justification for conducting an Exposure Investigation (EI) is provided.
Anaconda EI in September 2018
In September 2018, ATSDR conducted an EI in Anaconda. Blood lead levels and urine arsenic levels were tested in 191 participants in Anaconda. Results of the blood and urine testing are being processed and will be provided to participants when completed. A report will be written with aggregate summary data and presented to the community in a public meeting.
There was an overwhelming response from the Anaconda community to the testing event which exceeded the budget for the project. This resulted in the creation of a long waiting list that warranted an additional round of testing. EPA agreed to fund the laboratory costs associated with testing an additional 300 people in Anaconda.
As with the first round of testing, ATSDR will be assisted by the ADLC Health Department, and the MDPHHS in conducting the follow-up testing. For this EI, an effort will be made to recruit a higher percentage of young children and women who are pregnant or of childbearing age. A maximum of 300 residents of Anaconda will be tested in this EI, with 50 testing appointments reserved for young children.
Objectives of the Anaconda II EI
An EI was conducted at the Anaconda National Priority List (NPL) site in Anaconda, Montana in September 2018. The Anaconda area was contaminated with heavy metals from past smelting activities in the area. The EI included the testing of 191 residents to determine the levels of lead in blood and arsenic in urine in residents in the Anaconda community. Community interest in the testing event was considerable and ATSDR was not able to test all interested participants. Therefore, EPA committed to fund additional laboratory testing in the community; ATDSR, ADLC and MDPHHS will conduct the additional sample collection in the community.
The Anaconda II EI is an extension of the EI conducted in September 2018 and has the same basic objectives and follow-up activities as the first EI:
Evaluate blood lead levels (BLLs) and recommend case management for participants with BLL ≥5 micrograms per deciliter (µg/dL) (CDC reference level).
Recommend follow-up evaluation with a Primary Care Physician (PCP) for retesting and developmental and behavioral screening, as needed.
Recommend an early intervention program for children with developmental and behavioral issues, as needed.
Provide information on nutrition that may help to decrease the absorption of lead into the body.
Evaluate total and inorganic urine arsenic levels. Compare the creatinine-corrected total urinary arsenic level to the most up-to-date 95th percentile value reported in NHANES. Currently, a value of 29.9 µg/g Cr is reported for children aged 6-11 years; 30.5 µg/g Cr for children aged 12-19 years and 54.0 µg/g Cr for adults aged 20 years and older (2013-2014 data) [CDC 2018].
For participants whose creatinine-corrected total urinary arsenic levels are above the appropriate 95th percentile NHANES value, total inorganic urinary arsenic results will be compared to the most up-to-date 95th percentile values specific to age group that are reported in NHANES [CDC 2018].
Recommend ways to lower exposure to lead and arsenic in the home.
Recommend ways to lower exposure to lead- and arsenic-containing dust in homes (e.g., attics).
Assist the community with the identification of available resources for home assessments, including testing and remediation of yard soil and attics.
All participants will have the option of discussing their lead and/or arsenic findings with an ATSDR medical officer.
The Investigators and Collaborators
This EI is a collaborative project between ATSDR (headquarters and Region 8), Region 8 EPA, ADLC Health Department and the MDPHHS. The laboratory analysis will be completed by the National Center for Environmental Health (NCEH)/ Division of Laboratory Science (DLS). EPA is funding the laboratory analyses of samples collected for the Anaconda II EI.
EI Design
Given community interest in the testing in Anaconda, the goal of the EI is to collect blood and urine samples from additional participants. This effort will address the waitlist that was generated from the first testing event and will target participation by more young children and women of childbearing age who are most susceptible to exposure to metals, especially lead. Testing will occur in fall when soils are still likely to be exposed and not covered by snow.
In 2017, the population of Anaconda (consolidated city-county) was 9,139 persons with 93% of the population being white, 4% being American Indian or Alaskan Native, 3% being Hispanic or Latino and 2% being two or more races [Census Bureau 2010]. Of the 9,130 persons, 348 were children under 5 years old (4% of the population) and are considered to be the most vulnerable to health effects resulting from heavy metal contamination, especially lead [Census Bureau 2010] (Appendix A). Participants for the EI will be residents of the Anaconda and Opportunity communities.
Although all members of the community will be eligible for inclusion, young children and women who are pregnant or of childbearing age are most susceptible to the effects of elevated BLL. The strategy for recruitment and implementation of the second testing event in Anaconda will be:
A maximum of 300 participants will be tested for blood lead and urine arsenic levels, with a minimum of 50 appointments reserved for the testing of young children.
ATSDR will contact all people on the waiting list from the first testing event and offer them testing.
To recruit young children and women of childbearing age:
The ADLC health department will reach out to the local Head Start program and offer testing to young children and their families at the Head Start facility. The testing will be conducted at the Head Start facility on one of the testing days. Young children from the area daycares will be provided with the Anaconda EI fact sheet and will be offered testing at the Head Start facility.
The ADLC will provide the Lincoln Primary School with the Anaconda EI fact sheet to disseminate information on the testing event.
For those residents that have agreed to participate, a urine collection kit will be available at the ADLC health department or at the testing location (Anaconda Community Service Center or Head Start facility) for pickup prior to their blood testing appointment. The urine kit will include a collection cup (acid-washed) provided by the laboratory with instructions on how to collect and freeze a first-morning urine specimen that will be brought to the biological sample collection location. For young children, a collection unit that includes a urine collection bonnet will be provided. Consent/Parental Permission/Assent forms will be provided when the urine collection kits are delivered to participants; participants may sign the forms at home or may wait until they arrive for blood testing.
On the day of collection, participants will report to the blood collection location and
bring their frozen first morning urine sample to the collection site to provide to ATSDR personnel,
complete Consent/Parental Permission/Assent forms (if they did not complete them when the urine collection kits were delivered) and a questionnaire, and
provide a venous blood sample.
The EI will be performed according to a Health and Safety Plan.
Blood and urine samples will be submitted to the DLS laboratory for lead and urine analysis.
After the collection, participants will be
notified of their results if their BLL was ≥ 5 µg/dL. The State of Montana will also be notified of BLLs ≥ 5 µg/dL.
notified of their results if their urine arsenic levels are above NHANES values. Participants will be provided information on how they may reduce exposure to metals in the environment.
notified of the results of their blood lead and urine arsenic sampling in writing within 12 weeks, and
provided recommendations for follow-up for participants per EI levels defined above.
The results of the blood and urine testing will be evaluated to determine whether a public health hazard may exist in the community and will be presented in an EI report.
The findings and recommendations of the EI will be presented to the community in a community meeting after completion of the EI report.
A medical officer will be available throughout the EI process to address community concerns about environmental exposure to arsenic and lead and will be present at the community meeting to present the results of the EI.
An additional concern in the Anaconda community is occupational exposure for workers associated with exposure during remedial activities at the site, including work on the slag piles, yard remediation and remediation of attics. EPA has committed to implementing a worker testing program in the area for workers that are concerned about occupational exposure in Anaconda.
Consent/Parental Permission/Assent Forms
Participants of the testing will be provided with a Consent/Parental Permission/Assent form as appropriate prior to the sampling for all Anaconda residents. Participants will read and sign the Consent/Parental Permission/Assent form(s) while ATSDR personnel are present (Appendix B). The Consent/Parental Permission/Assent forms will be signed by participants when the urine sampling collection kits are delivered by ATSDR personnel. ATSDR EI personnel will answer any questions about the EI including questions about risks and benefits from participating in the EI which the community member may have. After the participants sign the forms, the ATSDR EI personnel will sign as a witness and collect one of the signed forms. The participants will be provided with a copy of the Consent/Parental Permission/Assent form.
The Consent/Parental Permission/Assent form and all other EI documents used to communicate with participants have been evaluated for reading level using Flesh-Kincaid Grade Level tool in Microsoft Word. The reading level is 8th grade or below. Appendix C includes copies of the Consent/Parental Permission/Assent forms.
Questionnaire
Participants will be asked questions regarding demographics, household attributes, soil exposure and daily activities (Appendix C). The responses will be used in the evaluation of the blood results.
Prior to use, the questionnaire will be approved through the Office of Management and Budget (OMB) using the existing OMB generic package for EIs (OMB number 0923-0048, expires 3/31/2019). The protocol, including the questionnaire, will be submitted to OMB as a GenIC package for the site.
Blood Sampling
Blood lead sampling is the most reliable method for measuring recent and ongoing exposure to lead. Blood will be collected by a certified phlebotomist using appropriate blood drawing protocols. Testing results will identify exposures from all sources. Specific exposure sources cannot be identified by a blood lead result.
A phlebotomist will collect 3 milliliters (ml) of blood from a vein from each participant who provides consent. The tubes for the collection have been selected and provided by DLS to ensure there is no contamination. Blood for lead analysis will be collected in 3 ml ethylenediamine tetra-acetic acid (EDTA) coated tubes provided by the laboratory.
The blood samples will be maintained at an appropriate refrigerator temperature (4º C) after collection. Samples will be shipped on ice packs by overnight delivery to DLS. The DLS will analyze blood samples for lead concentration using DLS method 30916.8-02 in whole blood by Inductively Coupled Plasma Dynamic Reaction Cell Mass Spectrometry (ICP DRC-MS). Reference: NHANES method 2009-2010:
https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2009
Urine Sampling
Urine arsenic is the most reliable method for measuring arsenic exposures occurring within a few days prior to the sample collection. A 24–hour urine collection is considered the optimal method to collect urine for arsenic sampling due to fluctuations in excretion rates. However, most studies use a first morning void or random spot sample because it is convenient for the participant and improves their compliance. These methods appear to correlate well with 24-hour collection results [Orloff et al. 2009, Hinwood et al. 2002]. Participants will be provided a urine collection kit that will include the urine collection cups (provided by DLS for quality control) and instructions for urine collection and freezing of the sample. DLS instructions for urine collection are included in Appendix D.
Frozen urine samples brought to the collection location will be placed on dry ice until all samples have been collected and then shipped frozen on dry ice by FedEx overnight to DLS in Atlanta for analysis. DLS will analyze the urine for arsenic by DLS method 30918.4-.02 Urine Multi-element by Inductively Coupled Plasma Dynamic Reaction Cell Mass Spectrometry (ICP DRC-MS).
All urine specimens will be analyzed for total arsenic, speciated arsenic, creatinine and specific gravity. To maintain privacy, the samples will be labeled with a coded identification number.
EI personnel will adhere to the Health and Safety Plan provided in Appendix E when handling and shipping blood and urine samples.
A Data Management Plan (Appendix F) outlines processes for ensuring confidentiality and preventing unauthorized release of data collected during the EI. To maintain privacy, the samples will be labeled with unique coded identification numbers. The Principal Investigator will maintain control of the coded identification numbers.
EI Time Line
Week 1 and 2:
Make appointments for testing for the people on the wait list.
Work with the ADLC to ensure that contact has been made with the appropriate groups associated with children (Head Start, elementary school).
Schedule phlebotomists and volunteers from the State of Montana.
Have urine kits available for pickup at the ACDL health department.
Week 3 in the Anaconda Community Service Center or the Head Start facility:
Call participants the day before their appointment as a reminder.
Register every participant upon arrival to the central testing location.
Collect first morning or spot urine samples provided by participants and handle appropriately for submission to the laboratory.
Provide the Consent/Parental Permission/Assent forms to participants to read and sign if they did not sign them prior to the blood testing, and explain the venous blood testing process. All potential participants have the right to refuse participation without penalty.
Administer a questionnaire to adults 18 years of age and above. The parent/guardian will answer the questions for their children.
Participation will depend on the individual’s capacity to give informed consent and respond to the questionnaire.
Collect blood samples after the Consent/Parental Permission/Assent and questionnaire are completed.
Send the blood and urine samples to DLS for analysis.
Week 5 to 7: Analyze the blood and urine samples in the CDC/NCEH/DLS:
Analyze blood and urine samples at NCEH/DLS.
Week 6 to 8: Review and interpret results:
Interpret the blood lead and urine arsenic results received from the laboratory. The questionnaire answers will help in the interpretation of the results.
Participants with BLL ≥ 5 µg/dL will be called and notified of their results and recommendations for appropriate follow-up.
Week 9 to 11: Notify the participants of their test results:
Notify the participants of individual tests results, interpretation, and recommendations via a letter. Sample letters are included in Appendix G.
Provide/Coordinate follow-up for participants with BLL above the CDC reference level and urine arsenic above the appropriate NHANES value.
Further evaluation for lead may include recommending follow-up with a PCP and providing information on lead health effects and ways to reduce exposure.
Further evaluation for arsenic may include providing information on arsenic health effects and ways to reduce exposure.
Report BLLs ≥5 ug/dL to the MDPHHS and enroll in case management.
Week 12 to 36: Prepare EI Report:
Draft the EI Report. Analyze BLL and urine arsenic data in relation to the environmental data and questionnaire information.
Submit the EI Report for review and release.
Make the report available in the repository at a central location in Anaconda.
A community meeting will be held when the document is released to provide the results of the EI to the Anaconda community.
Human Subjects
Residents living in and around the Anaconda NPL area will be eligible to participate. For the follow-up testing, young children and women who are pregnant or of childbearing age will be recruited. The EI will be conducted in accordance with an Anaconda EI Health and Safety Plan (Appendix E).
Sufficient resources are available to enroll up to 300 participants for the blood lead and urine arsenic testing. A minimum of 50 testing slots will be held for the testing of young children.
Description of Risks and Benefits
Risk: We anticipate the risks to be very low, but cannot entirely exclude them. There may be pain and/or bruising at the site where the blood was collected. There is the slight risk of feeling light headed during blood collection.
Benefits: Participants will be informed if they have recent and ongoing exposure to lead and recent exposure to arsenic. If we find elevated levels of lead in blood or arsenic in urine, we will provide recommendations to reduce exposure. There is no monetary incentive for participating in this EI, however, the EI participation, blood lead and urine arsenic testing and test interpretation are free of charge.
An additional benefit will be increased awareness of potential health effects associated with lead and arsenic exposure within the community and for local primary care physicians in Anaconda.
Procedures for Consent/Parental Permission/Assent
Participants will have time to read the Consent/Parental Permission/Assent form (Appendix B) while ATSDR EI personnel are present. ATSDR EI personnel will answer any questions about the EI, including risks and benefits. After the participant signs the Consent/Parental Permission/Assent form, ATSDR EI personnel will sign as witness and collect one of the two signed forms (the participant will keep one of the signed forms).
Parents/guardians will provide permission to participate for children <18 years of age. An assent form is also provided for participants aged 7 to 17 years. Consent/Parental Permission/Assent forms, as well as all other EI instruments of communication with participants, have been evaluated for readability using Flesh-Kincaid Grade Level tool in Microsoft Word.
Consent/Parental Permission/Assent forms will be provided when the urine collection kit is delivered to participants prior to the blood testing date.
Protection of Confidentiality
Participants’ confidentiality and personal information will be protected by ATSDR to the fullest extent possible by law. Individual tests results will not be made available to the public. Confidential information will be kept in locked cabinets and/or password protected computers. At the conclusion of the EI, ATSDR will prepare and publish an EI Report summarizing the findings, but will not reveal personal identifiers (e.g., name or address). Reports produced after the EI implementation will not identify specific individuals or residences.
All blood and urine samples that are sent to the laboratory for analysis will be labeled with a unique identifying number. De-identified results from the laboratory will be entered into an electronic database and sent to the ATSDR Principal Investigator (PI). Using a key, the PI will link laboratory and questionnaire data and maintain a complete de-identified dataset used for analysis.
Feasibility and Limitations
ATSDR has considerable experience conducting lead and arsenic exposure investigations. ATSDR is well positioned to address lead and arsenic issues and to respond to community concerns that may be voiced during this EI.
Potential limitations include:
We may not know the source of a participant’s exposure.
The blood lead and urine arsenic concentrations cannot be used to predict the future occurrence of disease nor be attributed as the cause of current health problems.
The number of participants recruited may not give ATSDR a complete understanding of the extent of exposure in the community.
The results of this EI will be applicable only to the individuals tested and cannot be generalized to the community or other populations.
Handling Unexpected or Adverse Events
There is a small chance of unexpected or adverse events occurring during the course of this EI.
The most likely adverse event is a participant feeling lightheaded or fainting during blood collection. The Health and Safety Plan (Appendix E) outlines the response to such an event. In addition, the on-site ATSDR medical officer and the phlebotomist collecting blood are trained in responding to such situations.
If any adverse event should occur, the Principal Investigator will notify local emergency services and ATSDR in Atlanta.
Data Handling
All data will be managed in accordance with the Anaconda Data Management Plan (DMP), included as Appendix F. All Consent/Parental Permission/Assent forms will be stored in a secure location while in Anaconda and then returned to ATSDR in Atlanta where they will be kept in a locked cabinet and/or password protected computer. Questionnaire results and blood lead and urine arsenic results (with unique identifying number), will be in electronic form and kept secure on password-protected CDC computers. All other confidential information will be kept in locked cabinets and/or password protected computers by the ATSDR Principal Investigator. ATSDR will share data with EPA and state partners, as appropriate; participants will have the option to agree to the data sharing in the consent forms.
Data Analysis
ATSDR staff will ship the venous blood and urine samples via FedEx overnight express mail to CDC/DLS in Atlanta for analysis. The laboratory will follow their standard procedures for analyzing blood for lead and arsenic in urine (see Biologic Sampling under Procedures/Methods). The analysis results from the CDC/DLS will be transmitted to the ATSDR Principal Investigator in an electronic spreadsheet format. No personal identifiers will be provided to the laboratory.
Appropriate Data Quality Assurance and Quality Control (QA/QC) will be performed by the CDC/DLS and will meet DLS accuracy and precision standards [Caudill et al., 2008]. After all the blood and urine samples are analyzed, the results will be provided to the ATSDR Principal Investigator for interpretation.
Results in the EI Report will be grouped to correspond to those found in the updated NHANES tables http://www.cdc.gov/exposurereport/pdf/fourthreport.pdf.
Dissemination, Notification, and Reporting of Results
Participants will be informed of their test results by letter which will be mailed via the US Postal Service. The texts of the draft letters will be addressed to participants or parents of children under 18 years of age. Sample letters that will be used to inform participants of their results are included in Appendix G.
An Exposure Investigation report will be written and made available by ATSDR to participants in a repository at a central location in Anaconda.
A public meeting and/or an availability session will take place in Anaconda after the EI Report is released. Speakers from ATSDR will be available to answer questions the community may have. A formal power point presentation about the EI will also be given to the community.
References:
[ARCO 2008] Atlantic Richfield Corporation. 2008. Draft Final Community Soils Interior and Attic dust Characterization Study Data Summary Report (DSR). Prepared by Pioneer Technical Services for ARCO. January 2008.
[ARCO 2014] Atlantic Richfield Corporation. 2013. Anaconda Smelter Community Soils Operable Unit: Lead and Arsenic Baseline Biomonitoring Study Report. Prepared for Atlantic Richfield Company by ENVIRON International Corporation. June 2014.
[ATSDR 2007a] Agency for Toxic Substances and Disease Registry. 2007. Health Consultation: Evaluation of Residential Soil Arsenic Action Level. Anaconda Co. Smelter Site, Anaconda, Deer Lodge County, MT. EPA Facility ID: MTD093291656
[ATSDR 2007b] Agency for Toxic Substances and Disease Registry. 2007. Toxicological profile for Arsenic. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
[ATSDR 2007c] Agency for Toxic Substances and Disease Registry. 2007. Toxicological profile for Lead. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
[ATSDR 2018] Agency for Toxic Substances and Disease Registry. 2018. Personal communication between David Dorian, ATSDR Region 8, and Charlie Partridge, Toxicologist, U.S. Environmental Protection Agency. July 10, 2018.
Baker EL, Hayes CG, Landrigan PJ, Handke JL, Leger RT, Housworth WJ, Harrington JM. 1977. A nationwide survey of heavy metal absorption in children living near primary copper, lead and zinc smelters. Am J Epidem 1977;106(4):261-273.
Binder S, Forney D, Kaye W, Pascal D. Arsenic exposure in children living near a former copper smelter. Bull Environ Contam Toxicool 1987;39:114-121.
Brink LL, Talbot EO, Sharma RK, March GM, Wu WC, Rager JR, Strosnider HM (2013). Do U.S. Ambient Air Lead Levels Have a Significant Impact on Childhood Blood Lead Levels: Result of a National Study. J. Environ and Public Health. Available at: http://www.hindawi.com/journals/jeph/2013/278042/.
Caudill SP, Schleicher RL, Pirkle JL, 2008. Multi-rule quality control for the age related eye disease study. Stat. Med. 27, 4094-4106.
[CDC 2005] Centers for Disease Control and Prevention. 2005. Preventing Lead Exposure in Young Children, 2005. http://www.cdc.gov/nceh/lead/publications/prevleadpoisoning.pdf
[CDC 2009] Centers for Disease Control and Prevention. 2009. The Fourth National Report on Human Exposure to Environmental Chemicals, 2009. Available at: http://www.cdc.gov/exposurereport/pdf/FourthReport.pdf
[CDC 2010] Centers for Disease Control and Prevention. 2010. CDC Guidelines for the Identification and Management of Lead Exposure in Pregnant and Lactating Women, November 2010. U.S. DHHS, Atlanta, GA. http://www.cdc.gov/nceh/lead/publications/leadandpregnancy2010.pdf
[CDC 2012a] Centers for Disease Control and Prevention. Lead (web page). 2012a. Update on Blood Lead Levels in Children. Last Updated October 30, 2012. Available online at: http://www.cdc.gov/nceh/lead/ACCLPP/blood_lead_levels.htm
[CDC 2012b] Centers for Disease Control and Prevention. 2012b. Low Level Lead Exposure Harms Children: A Renewed Call for Primary Prevention: Report of the Advisory Committee on Childhood Lead Poisoning Prevention, Centers for Disease Control and Prevention. U.S. Department of Health and Human Services, January. Available at: http://www.cdc.gov/nceh/lead/acclpp/final_document_010412.pdf
[CDC 2012c] Centers for Disease Control and Prevention. 2012c. CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in "Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention". Centers for Disease Control and Prevention, June 7, 2012. Available at: http://www.cdc.gov/nceh/lead/ACCLPP/CDC_Response_Lead_Exposure_Recs.pdf
[CDC 2018] Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, (March 2018). Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. https://www.cdc.gov/exposurereport/
[Census Bureau 2010] U.S. Census Bureau. 2010. Online. https://www.census.gov/
[DHHS 2010] U.S. Department of Health and Human Services. 2010. Atlanta, Georgia. Guidelines for the Identification and Management of Lead Exposure in Pregnant and Lactating Women. November 2010. U.S. Department of Health and Human Services.
[EPA 1996] U. S. Environmental Protection Agency. 1996. EPA Superfund Record of Decision: Anaconda Company Smelter. Denver: U.S. Environmental Protection Agency, Region 8. EPA/ROD/R08-96/127. September 1996. https://cumulis.epa.gov/supercpad/SiteProfiles/index.cfm?fuseaction=second.scs&id=0800403&doc=Y&colid=32604®ion=08&type=SC
[EPA 2008] U.S. Environmental Protection Agency. 2008. Residential Soils Interpretation and Analysis Report; Community Soils Operable Unit, Anaconda Smelter NPL Site. 2008.
[EPA 2013] U.S. Environmental Protection Agency. 2013. Record of Decision (ROD) Amendment, Community Soils Operable Unit, Anaconda Smelter National Priorities List Site. Anaconda, MT. EPA ID: MTD093291656. Community Soils Operable Unit. September 2013. https://cumulis.epa.gov/supercpad/SiteProfiles/index.cfm?fuseaction=second.scs&id=0800403&doc=Y&colid=32604®ion=08&type=SC
[EPA 2015] U.S. Environmental Protection Agency. 2015. Fifth Five-Year Review Report. Anaconda Smelter Superfund Site. Anaconda-Deer Lodge County, MT. EPA ID: MTD093291656. https://cumulis.epa.gov/supercpad/SiteProfiles/index.cfm?fuseaction=second.scs&id=0800403&doc=Y&colid=32604®ion=08&type=SC
Hartwell TD, Handy RW, Harris BS, Williams SR, Gehlbach SH. Heavy metal exposure in populations living around zinc and copper smelters. Arch Environ Health 1983;38(5):284-295.
Hinwood AL, Sim MR, de Klerk N, Drummer O, Gerostamoulos J, Bastone EB. Are 24-hour urine samples and creatinine adjustment required for analysis of inorganic arsenic in urine in population studies? Environ Res Section A. 88, 219–224.
Hwang Y-H, Bornschein RL, Grote J, Menrath W, Roda S. Environmental arsenic exposure of children around a former copper smelter site. Environ Res 1997;72:72-81.
Orloff K, Mistry K, Metcalf S. Biomonitoring for Environmental Exposures to Arsenic. Journal of Toxicology and Environmental Health, Part B: Critical Reviews. 2009. 12:7, 509-524. Available at http://dx.doi.org/10.1080/10937400903358934
Appendices
Appendix A: Anaconda EI Protocol
Appendix B: Privacy Act Statement and Consent Forms
B1: Privacy Act Statement
B2: Adult Consent Form
B3: Parental Permission form for children younger than 18 years
B4: Assent form for children between 7 and 17 years
Appendix C: Questionnaire
Appendix D: Urine Collection Instructions
Appendix E: Health and Safety Plan
Appendix F: Data Management Plan
Appendix G: Sample Results Letters to Participants
Appendix A: Anaconda EI Protocol
Exposure Investigation
Protocol
Blood Lead and Urine Arsenic Levels in Anaconda, Montana
Anaconda Superfund Site,
Anaconda, Deer Lodge County,
Montana
Cost Recovery No: 8018
Prepared by:
Karen Scruton, MS
David Dorian, MS
Agency for Toxic Substances and Disease Registry (ATSDR)
Division of Community Health Investigations (DCHI)
Science Support Branch (SSB) and
ATSDR Western Branch (Region 8)
August 17, 2018
Table of Contents
Previous Biological Testing 11
May 2018 Listening Session: Community Concerns 13
Health Impacts and Exposure to Lead in the Environment 14
Health Impacts and Exposure to Arsenic in the Environment 15
The Investigators and Collaborators 16
Consent/Parental Permission/Assent Forms 18
Description of Risks and Benefits 22
Procedures for Consent/Parental Permission/Assent 22
Protection of Confidentiality 22
Feasibility and Limitations 23
Handling Unexpected or Adverse Events 23
Dissemination, Notification, and Reporting of Results 24
Appendix A: Site and Demographic Map 29
Appendix B: Fact Sheet and Invitation to Participate 30
Appendix C: Privacy Act Statement and Consent Forms 36
Appendix E: Urine Collection Instructions 62
Appendix F: Health and Safety Plan 63
Appendix G: Data Management Plan 66
Appendix H: Template for Results Letters to Participants 71
List of Tables
Table 1. Soil Lead in Residential Yards: Arsenic below 250 mg/kg
Table 2: Soil Arsenic in Residential Yards
Table 3: 2015 and 2016 Residential Yards: Arsenic and Lead
Table 4: Summary of Arsenic and Lead Dust in Attic, Interior, and Exterior Surfaces
Table 5: Past Biomonitoring Event in Anaconda
Table 6: Blood Lead Results by Age (ARCO 2014)
Table 7: Urine Arsenic Results by Age (ARCO 2014)
ACRONYMS and ABBREVIATIONS
ADLC Anaconda Deer Lodge County
ARCO Atlantic Richfield Corporation
ATSDR Agency for Toxic Substances and Disease Registry
BLL blood lead level
CDC Centers for Disease Control and Prevention
DCHI Division of Community Health Investigations
DLS Division of Laboratory Sciences
DMA dimethylarsinic acid
DMP Data Management Plan
EDTA ethylenediamine tetra-acetic acid
EI exposure investigation
EPA (U.S.) Environmental Protection Agency
µg/dL microgram per deciliter
MDPHHS Montana Department of Public Health and Human Services
mg/kg milligram per kilogram
MMA monomethylarsonic acid
NCEH National Center for Environmental Health
NHANES National Health and Nutrition Examination Survey
NIOSH National Institute of Occupational Safety and Health
NPL National Priority List
OMB Office of Management and Budget
OU operable unit
Project Overview
An Exposure Investigation (EI) will be
conducted at the Anaconda National Priority List (NPL) site in
Anaconda, Montana. The Anaconda area was contaminated with heavy
metals from past smelting activities in the area. The EI will
include the testing of up to 200 residents to determine the levels
of lead in blood and arsenic in urine in residents in the Anaconda
community. The EI has the following objectives and follow-up
activities: Evaluate
blood lead levels (BLLs) for Anaconda residents that participate in
the investigation. Recommend
case management for participants with BLL ≥5 micrograms per
deciliter (µg/dL) (CDC reference level) Recommend
follow-up evaluation with a Primary Care Physician (PCP) for
retesting and developmental and behavioral screening, as needed Recommend
an early intervention program for children with developmental and
behavioral issues, as needed Provide
information on nutrition that may help to decrease the absorption
of lead into the body
Evaluate
total and inorganic urine arsenic levels for Anaconda residents
that participate in the investigation.
Each
participant’s creatinine-corrected, total urinary arsenic
level will be compared to the most up-to-date 95th
percentile value reported in the National Health and Nutrition
Examination Survey (NHANES). Currently, a value of 29.9 micrograms
per gram of creatinine (µg/g Cr) is reported for children
aged 6-11 years; 30.5 µg/g Cr for children aged 12-19 years
and 54.0 µg/g Cr for adults aged 20 years and older
(2013-2014 data) [CDC 2018].
For
participants whose creatinine-corrected, total urinary arsenic
level is above the appropriate 95th
percentile NHANES value, total inorganic urinary arsenic results
will be compared to the most up-to-date 95th
percentile values specific to age group that are reported in NHANES
[CDC 2018]. Recommend
ways to lower exposure to lead and arsenic in the home. Recommend
ways to lower exposure to lead- and arsenic-containing dust in
homes (e.g., attics)
Assist the
community with the identification of available resources for home
assessments All
participants will have the option of discussing their lead and/or
arsenic findings with an ATSDR medical officer.
Background
The Anaconda Company Smelter operated from 1884 through 1981. The Anaconda Copper Mining Company (ACM) mined copper ore in nearby Butte, Montana and operated a smelter on the north side of Warm Springs Creek, adjacent to the town of Anaconda from 1884 to 1901 (Appendix A). This area is now referred to as the “Old Works.” In 1902, ore processing commenced at the Washoe Reduction Works (the “New Works”) on Smelter Hill, east of town. Both smelters resulted in large volumes of waste that were disposed on the ground and in surface water, and used as fill. Airborne contaminants from stacks and waste (slag) piles resulted in widespread surface soil contamination with heavy metals (principally arsenic, cadmium, copper, lead and zinc) (Appendix A).
In 1983, the U.S. Environmental Protection Agency (EPA) placed the Anaconda Company Smelter site on the National Priorities List (NPL). Remediation of millions of cubic yards of mining wastes and roughly 20,000 acres of contaminated soil through the Superfund program continues to this day. Arsenic is the primary contaminant of concern and potential exposures to inorganic arsenic are the fundamental health risk addressed in site remediation [EPA 1996]. The copper mine in Butte is also under remediation through Superfund, and the combined Anaconda and Butte sites represent the largest Superfund complex in the United States.
Atlantic Richfield Corporation (ARCO) purchased the ACM in 1977 and conducts the site clean-up as the potentially responsible party. Smelter operations ceased in 1980, and the 585-foot landmark brick stack is the only remaining structure from the Washoe operations. Contamination from historic operations included 230 million cubic yards of concentrated mill tailings, 30 million cubic yards of furnace slag, and 500,000 cubic yards of flue dust. Leaching of these chemicals contaminated groundwater. Airborne emissions contaminated approximately 20,000 acres of soil above established action levels [EPA 2015]. Various remedial activities have been conducted to address the environmental contamination including permanent relocation of people living in the Mill Creek neighborhood; remediation of residential yards; and removal, consolidation, and on-site containment of waste material and contaminated soil.
While significant progress in site remediation and the concurrent reduction of potential exposures has been made to date, contamination, such as exposed slag and soils in residential areas, remains. Additionally, interior dust clean up, required by a 2013 Amendment to the Community Soils Operable Unit Record of Decision (ROD) to address arsenic and lead contamination, is not yet complete. The ROD was amended after sampling found “concentrations of lead in residential soils being significantly higher than those reported in the Remedial Investigation” [EPA 2013].
The site includes both the town of Anaconda and the town of Opportunity. For the purposes of the Superfund clean-up, the site is divided into five Operating Units (OUs): The Mill Creek OU, the Flue Dust OU, the Old Works/East Anaconda Development Area OU, the Community Soils OU, and the Anaconda Regional Water, Waste, and Soil OU.
In 1996, a Baseline Human Health Risk Assessment (BHHRA) was completed as part of the Remedial Investigation (RI). The document addressed several OUs at the site as well as community soils in Anaconda. The BHHRA evaluated residential surface soil and dust collected in 1992 and 1994. Risks from lead were determined to be within EPA’s acceptable range and risks from arsenic were considered to be unacceptable, resulting in arsenic being considered the risk driver at the site.
The Agency for Toxic Substances and Disease Registry (ATSDR) authored a Public Health Assessment for the site in 1987 and a Site Review and Update in 1992. In 2007, ATSDR evaluated the EPA’s Action Level for arsenic in residential soil, a level established in the 1998 ROD [ATSDR 2007a]. ATSDR concluded that exposures to soils at the 250 parts per million (ppm) action level would not likely result in adverse health effects, though that conclusion is not applicable to children who exhibit pica behavior [ATSDR 2007a]. In 2006, ATSDR evaluated potential health effects from dust blowing off the Opportunity Ponds (a community located 4 miles downwind of the smelter stack). ATSDR concluded that most people would not experience adverse health effects associated with the arsenic in the dust; however, sensitive populations might be at risk [ATSDR 2007a].
In May 2018 ATSDR, in coordination with the Montana Department of Public Health and Human Services (MDPHHS), which is funded, in part, by the ATSDR state cooperative agreement program, and the Anaconda Deer Lodge County (ADLC) Health Department, conducted a public listening session to assess community health concerns. The community identified exposure to arsenic and lead as the primary public health issues associated with environmental contamination.
Environmental Sampling Data
ARCO, under EPA oversight, has conducted extensive environmental sampling over the course of the site Superfund history. The information presented here provides only an overview of historic sampling followed by recent data that informs our understanding of present exposure pathways.
Residential Soils Overview
Through the Superfund process, ARCO and EPA together completed approximately 21 soil investigations between 1985 and 1995. Analytic results from the 1997-1998 Remedial Investigation/Feasibility Study (RI/FS) established the Community Soils Operating Unit (OU) as the focus of residential clean ups. This OU includes residential yards in Anaconda, Opportunity, and rural areas in the Anaconda vicinity. This focus area of remediation encompasses the population at greatest current risk for exposure to arsenic and lead. While ARCO has completed many remedial activities for residential soils, EPA’s Fifth Five Year Review Report in 2015 [EPA 2015] concludes that “the remedy for the Community Soils OU is not protective because exposure to lead contamination in residential soil and dust in not currently controlled.”
Soil studies have identified arsenic at levels of concern in both surface (0 to 2 inch depth) and subsurface soils, a pattern consistent with a near century of smelter emission deposition. Of the two, surface soil contamination presents a more immediate current health risk since children will contact surface soils when playing and adults will contact surface soils in routine yard maintenance.
ARCO commenced sampling of residential yards in the Community Soils OU in 2001. An evaluation of the data collected through 2007 presented the range of surface soil arsenic concentration from 5.6 to 1,690 milligrams per kilogram (mg/kg) from 4,449 samples with an average value of 204 mg/kg [CDM 2008].
In 2006, ARCO sampled over 1,400 residential yards in Anaconda and the surrounding rural area [EPA 2008]. Of these, 300 yards exceeded the 250 mg/kg action level for arsenic. Analysis of the sampling indicated that arsenic contamination was more widespread than indicated in previous decision documents. The highest arsenic levels occur in the Mill Creek/Aspen Hills section of Anaconda.
Also in 2006, ARCO evaluated residual lead concentrations in yards where arsenic concentrations measured below the 250 mg/kg clean up benchmark. The results indicated that lead concentrations in residential soils posed a significant exposure pathway for lead even where the arsenic level was below 250 mg/kg [EPA 2008]. The table below summarizes lead values in 554 subarea samples collected from 147 yards with arsenic below 250 mg/kg (Table 1).
Table 1: Soil Lead in Residential Yards: Arsenic below 250 mg/kg |
||
Lead Concentration (mg/kg) |
Number of Subarea Samples |
Percent of Samples |
0-400 |
207 |
37 |
400-800 |
238 |
43 |
800-1200 |
66 |
12 |
>1200 |
43 |
8 |
TOTAL |
554 |
100% |
For the Community Soils OU Remedial Action Phase 2 sampling (beginning 2007), ARCO collected both surface and subsurface soil samples for arsenic [EPA 2008] (Table 2).
Table 2: Soil Arsenic in Residential Yards* |
|||
Depth of Sample |
Number of Samples |
Range of Arsenic (mg/kg) |
Mean of Arsenic (mg/kg) |
0-2 inch (surface) |
595 |
53-1,690 |
388 |
2-6 in (subsurface) |
595 |
24-2,200 |
327 |
6-12 in (deeper subsurface) |
364 |
54-1,750 |
364 |
* EPA soil action level for arsenic at the Anaconda NPL site is 250 mg/kg.
Recent Residential Yard Sampling
Remediation actions continued from 2015 to 2017 for the Community Soils OU. Soil data collected for 2015 and 2016 contain both remediated and unremediated yards; hence, average and minimum values incorporate partial implementation of the remedy. The 2015 and 2016 data indicate continued risk for community exposure to lead and arsenic through residential soils (Table 3) [ATSDR 2018].
Table 3. 2015 and 2016 Residential Yards: Arsenic and Lead |
||||||
Year |
Number of Samples |
Number of residences |
Arsenic (mg/kg) |
Lead (mg/kg) |
||
Range |
Mean |
Range |
Mean |
|||
2015 |
188 |
12 |
12.7-853 |
191 |
12.6-2,380 |
479.1 |
2016 |
7,150 |
486 |
1.8-4,280 |
208 |
0.26-9,320 |
437 |
Dust in Attics and Home Interiors
Environmental sampling of attic dust has established that historic smelter emissions settled in home attics. The higher than anticipated soil arsenic concentrations (discussed above) prompted ARCO to sample attic dust in 52 homes [ARCO 2008]. ARCO collected dust from attics as well as other interior and exterior floor surfaces from homes in Anaconda, Opportunity, and Rural Areas. Arsenic and lead concentrations are summarized in Table 4.
Table 4. Summary of Arsenic and Lead Dust in Attic, Interior, and Exterior Surfaces
|
||||
Contaminant |
Dust Type |
Number of Samples |
Mean (mg/kg) |
Range (mg/kg) |
|
|
|
|
|
Arsenic |
Attic |
49 |
496 |
0.15-1,560 |
|
Interior |
52 |
64.9 |
11.1-322 |
|
Exterior |
52 |
63.4 |
17.2-213 |
|
|
|
|
|
Lead |
Attic |
49 |
721 |
2.61-2,620 |
|
Interior |
52 |
548 |
41.8-10,700 |
|
Exterior |
52 |
515 |
27.1-5,070 |
In its analysis of the correlation of contaminant levels found in residential interior, exterior, and attic dust EPA concluded that “attic dust may be a secondary source of interior dust arsenic and lead contamination” [EPA 2008]. The Community Soils Record of Decision [EPA 2013] established an attic remediation programs for residences located in the Community Soils OU. Of 99 attics sampled to date, 53 have been remediated, 21 are eligible for remediation, 12 were below the action level for remediation and 13 are still awaiting analysis by the laboratory. Attic dust is a current pathway of exposure to lead and arsenic; however, a full picture of how widespread the contamination is only now emerging.
Uncovered Waste in Place
EPA’s most recent Superfund Five-Year Review identified areas with uncovered wastes in place (some for historic preservation) [EPA 2015]. Some of these areas are accessible to trespassers resulting in a potential exposure pathway to arsenic and lead via particulates in air.
An area known as the Old Works Historic District, located in the Old Works/East Anaconda Development Area Operating Unit, contains ore processing waste from smelter operations dating from 1884-1902. In 2010, EPA estimated that 60,000 to 75,000 cubic yards of contaminated waste remained [EPA 2015]. Previous sampling has indicated that portions of this waste exceed the 1,000 mg/kg remedial action level established for recreational/open space/agricultural areas [EPA 2015].
Substantial progress in remediation has been made in the Anaconda Regional Water, Waste, and Soil Operating Unit; however, remediation is not complete for multiple remedial design units (RDUs) covering thousands of acres of contaminated land located within this OU. Three slag piles covering approximately 197 acres and consisting of approximately 25.5 million cubic yards of smelter slag are located within this OU. Wind and erosion control measures are in place; however, particulates are entrained in air during high wind events [EPA 2015].
Previous Biological Testing
In the past, several biomonitoring investigations have been conducted evaluating potential exposure to residents exposed to environmental contaminants from smelting activities. The ATSDR Health Consultation [ATSDR 2007a] provides information on the past biomonitoring events in the Anaconda area; they are summarized in Table 5.
Table 5. Past (1977-1997) Biomonitoring Evens in Anaconda* |
||
Reference |
Testing |
Conclusion |
Baker et al. 1977 |
Arsenic in hair and urine |
Nationwide survey of children living around copper, lead or zinc smelters, including Anaconda.
|
Hartwell et al. 1983 (testing completed from 1978-1979) |
Arsenic in hair, blood and urine (also evaluated air, soil, dust, and tap water) |
Nationwide survey of children living around copper, lead or zinc smelters, including Anaconda.
|
Anaconda Smelter closed in 1980 |
||
Binder et al. 1987 (testing completed in 1985) |
Arsenic urine (total) (also soil and house dust) |
Four locations were evaluated and compared to each other. In the Mill Creek neighborhood (downwind of smelter and adjacent to the stack): higher mean arsenic in soil and urinary arsenic in children vs Eastern Anaconda (upwind of stack), Opportunity (4 miles downwind of smelter) and the control town. Eight children relocated from Mill Creek based on this investigation.
|
Hwang et al. 1997 (testing completed in 1992-1993) |
Total and speciated arsenic in children in Anaconda |
Correlation between speciated urine arsenic concentration and soil arsenic level in bare yards.
|
* ATSDR (2007a)
The 2007 ATSDR Health Consultation concluded that “biomonitoring of children in the Anaconda area has been useful to indicate elevated past exposures while the smelter operated and, later, in the Mill Creek area of the site. More recent measurements showed average urine arsenic levels to be similar to control towns; however, some children still had elevated arsenic levels, indicating the need to continue to address arsenic exposures at the site” [ATSDR 2007a].
In 2013, ARCO contracted with ENVIRON International Corporation in consultation with ADLC to conduct a baseline blood lead and urinary arsenic biomonitoring study to evaluate potential exposure of residents in the Anaconda area. Blood lead and urine arsenic sampling was offered to community members and results are presented in Table 6 (lead) and Table 7 (arsenic) [ARCO 2014].
Table 6: Blood Lead Results by Age [ARCO 2014] |
||
|
< 7 years |
≥ 7 years |
Number detections (DL = 1.0 µg/dL) /number tested |
7/18 |
26/84 |
Range of detections (µg/dL) |
1-3.8 |
1-5.4 |
Table 7: Urine Arsenic Results [ARCO 2014] |
||||
|
<12 years |
≥12 years |
||
Arsenic Result |
Number of participants |
Range of Detections |
Number of Participants |
Range of Detections |
Total Arsenic (µg/L) |
32 |
4.24-39.5 |
74 |
1.36-363 |
Speciated Arsenic (µg/L)* |
32 |
4.61-25.4 |
74 |
1.15-90.6 |
Speciated Arsenic, Specific Gravity-Corrected (µg/L) |
29 |
4.56-22.5 |
61 |
1.87-77.5 |
Speciated Arsenic, Creatinine-Corrected (µg/g) |
30 |
4.69-33.4 |
66 |
1.83-63.7 |
*Combined inorganic species, arsenous (III) acid and arsenic (V) acid, and their methylated metabolites [monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA)] results.
The past biomonitoring studies in Anaconda indicate that environmental contamination has resulted in exposure of residents to lead and arsenic.
May 2018 Listening Session: Community Concerns
In May 2018, ATSDR, in partnership with the MDPHHS and the ADLC Health Department, hosted a listening session in Anaconda to address community concerns. Fifty-four people provided detailed accounts of their health concerns as they relate to contamination at the site.
Many concerns related to potential exposure to arsenic from soil, household dust, and particulate matter blowing off the slag piles on windy days. Community members also worried about children’s health and expressed concern that autoimmune and neurodegenerative diseases were elevated in Anaconda.
Justification for the EI
Exposure Investigations (EIs) are an approach developed by ATSDR that employ targeted biologic and environmental sampling to determine whether people are or have been exposed to unusual levels of pollutants at specific locations (e.g., where people live, spend leisure time, or anywhere they might come into contact with contaminants under investigation).
ATSDR uses EIs to fill a data gap that is essential for evaluating community exposure pathways and determining if a health hazard is present. The EI team conducts point of human-contact sampling (environmental and/or biological) focused on areas where exposures are expected to be high.
The EI team assesses potential EIs by answering the following four questions:
Can an exposed population be identified? Yes. Soil sampling data and biological testing data (blood lead and urine arsenic) indicate that sensitive populations could be at risk for exposure to harmful levels of lead and arsenic in this community.
Does a data gap exist that affects our ability to decide that a public health hazard exists? Yes. Additional blood lead and urine arsenic data will better identify whether residents may have been exposed to the lead and arsenic contamination in the community.
Can an Exposure Investigation address the data gap? Yes. Completing blood lead and urine arsenic testing will provide additional information on the impact of environmental contamination on the community.
How will the Exposure Investigation results impact public health decision making? The results of the blood lead and urine arsenic testing will assist the ADLC health department and the MCPHHS to evaluate and prioritize available resources from ARCO (e.g., funds for case management, lead paint screening in homes, dust sampling in attics in the community). Two Grand Round presentations will be made to primary care physicians in Anaconda and Butte prior to the EI testing to provide information on lead and arsenic exposure to assist them in patient management.
Objectives of the EI
The objectives of the EI in Anaconda include:
Evaluate blood lead levels (BLLs) for Anaconda residents that participate in the investigation.
Recommend case management for participants with BLL ≥5 micrograms per deciliter (µg/dL) (CDC reference level)
Recommend follow-up evaluation with a Primary Care Physician (PCP) for retesting and developmental and behavioral screening, as needed
Recommend an early intervention program for children with developmental and behavioral issues, as needed
Provide information on nutrition that may help to decrease the absorption of lead into the body
Evaluate total and inorganic urine arsenic levels for Anaconda residents that participate in the investigation.
Each participant’s creatinine-corrected, total urinary arsenic level will be compared to the most up-to-date 95th percentile value reported in the National Health and Nutrition Examination Survey (NHANES). Currently, a value of 29.9 micrograms per gram of creatinine (µg/g Cr) is reported for children aged 6-11 years; 30.5 µg/g Cr for children aged 12-19 years and 54.0 µg/g Cr for adults aged 20 years and older (2013-2014 data) [CDC 2018].
For participants whose creatinine-corrected, total urinary arsenic level is above the appropriate 95th percentile NHANES value, total inorganic urinary arsenic results will be compared to the most up-to-date 95th percentile values specific to age group that are reported in NHANES [CDC 2018].
Recommend ways to lower exposure to lead and arsenic in the home.
Recommend ways to lower exposure to lead- and arsenic-containing dust in homes (e.g., attics)
Assist the community with the identification of available resources for home assessments
All participants will have the option of discussing their lead and/or arsenic findings with an ATSDR medical officer.
Health Impacts and Exposure to Lead in the Environment
Lead is a naturally occurring element in the earth’s crust and is present in environmental media, including water and soil. Lead is found in many products such as old water pipes and their soldered connections, automobile radiators, ammunitions, some pottery produced in Mexico, some home remedies, and consumer products (e.g., contaminated food, make up, jewelry, and leaded crystal) [ATSDR 2007c]. Lead-based paint used in pre-1978 housing and lead contaminated dust are typically the most widespread and dangerous source of lead exposure for young children [CDC 2009].
No acceptable BLL has been identified that is free from deleterious health effects in children from 1 to 5 years of age [CDC 2012 a,b,c]. As a result, BLLs should be kept as low as possible and below the level of 5 µg/dL [CDC 2005, CDC 2010, CDC 2012a,b,c]. The CDC reference level of 5 µg/dL is based on the U.S. population of children ages 1 to 5 years who are in the highest 2.5% of blood levels in children [CDC 2012 a,b,c]. In 2015, the National Institute for Occupational Safety and Health (NIOSH) also recommended that BLLs in adults should be <5 µg/dL (http://www.cdc.gov/niosh/topics/ables/description.html). BLLs ≥5 µg/dL are reportable to the MDPHHS for all age groups, not just young children and pregnant women.
The emphasis on pregnant women and children younger than 6 years is because epidemiologic cohort studies suggest that prenatal lead exposure, even with maternal BLLs <10 µg/dL, is inversely related to fetal growth and neurodevelopment independent of the effects of postnatal lead exposure. The exact mechanism(s) by which low-level lead exposure, whether incurred prenatally or postnatally, may adversely affect child development remains uncertain [DHHS, 2010].
In general, people can be exposed to lead by:
Inhalation of dust contaminated with lead from contaminated soil or paint chips located in homes constructed prior to 1978,
drinking water containing lead,
accidentally ingesting contaminated dust or soil (hand-to-mouth activities in young children),
occupational exposure (e.g., mechanics, transportation workers, construction workers), and
intentionally ingesting non-food items (pica behavior).
Certain traits put people at higher risk for lead exposure [Brink et al. 2013]. Some characteristics contribute to susceptibilities (e.g., age, race, sex) and others to vulnerability (e.g., socioeconomic status). In addition to the risks posed by living in Anaconda with lead-contaminated soil, other major risk factors for higher lead levels are living in older housing and poverty (Appendix A). The questionnaire will ask questions related to these vulnerabilities and the answers will be used to assess the BLL and urine arsenic results.
Health Impacts and Exposure to Arsenic in the Environment
Arsenic exists naturally in organic and inorganic forms [ATSDR 2007b]. Arsenic is a widely distributed element on the earth’s surface, especially in its inorganic form.
In general, people can be exposed to arsenic by:
consuming foods containing arsenic (contaminated rice, chicken, fish/seafood and other food items),
drinking water containing arsenic,
accidentally ingesting contaminated dust or soil (hand-to-mouth activities in young children),
occupational exposure (e.g., sand blasting, wood preservation, arsenical pesticide production), and
intentionally ingesting non-food items (pica behavior).
Inorganic arsenic is well absorbed from the gastrointestinal tract and, to a lesser degree, from inhalation. Inorganic arsenic and its metabolites have elimination half-lives of around 2-4 days [ATSDR 2007b]. Inorganic arsenic is found in trivalent (arsenite) and pentavalent (arsenate) forms. Trivalent arsenic is substantially more toxic and carcinogenic than pentavalent arsenic [ATSDR 2007b]. Inorganic arsenic crosses the human placenta [ATSDR 2007b]. Inorganic arsenic has been used as an outdoor wood preservative, as semiconductor in dopant materials, in some pesticides and in certain medicines. Organic arsenic is found in primarily in seafood and is less toxic than its inorganic form [ATSDR 2007b].
All participants will be tested for total arsenic that will be speciated to differentiate organic from inorganic arsenic and for creatinine to correct for dilution variation. Total arsenic results will be creatinine-corrected, as age-appropriate, and be compared to the most up-to-date 95th percentile NHANES value available at the time of evaluation. Speciation will be completed on every urine sample. ATSDR will use a conservative approach to evaluate arsenic results by comparing the total of the participant’s inorganic arsenic species results [arsenic (V) acid and arsenous (III) acid and methylated metabolites: dimethylarsinic acid (DMA) and monomethylarsonic acid (MMA)] to the most up-to-date NHANES values [CDC 2018]. Participants who exceed the 95th percentile NHANES value for total arsenic and total inorganic arsenic will be contacted by the ATSDR Medical Officer to discuss whether further evaluation may be warranted. The results of the questionnaire will be used to assess organic arsenic, since most exposure to organic arsenic is dietary.
If further evaluation is recommended, participants will be referred to the PEHSU (Pediatric Environmental Health Specialty Units) or AOEC (Association of Occupational and Environmental Clinics) clinicians for further workup. The workup would include a complete history and physical to assess for any signs/symptoms of arsenic toxicity, provide counseling for reducing exposure, and potentially serial monitoring to establish that exposure has ceased.
The Investigators and Collaborators
This EI is a collaborative project between ATSDR (headquarters and Region 8), Region 8 EPA, ADLC health department and the MDPHHS. The laboratory analysis will be completed by the National Center for Environmental Health (NCEH)/ Division of Laboratory Science (DLS).
EI Design
The goal of the EI is to implement sample collection in the late summer/early fall of 2018 when contact with soil, and therefore, exposure to arsenic and lead, is expected to be high due to increased outdoor activity by residents. Exposure to soil is expected to be highest during months with good weather.
In 2017, the population of Anaconda (consolidated city-county) was 9,139 persons with 93% of the population being white, 4% being American Indian or Alaskan Native, 3% being Hispanic or Latino and 2% being two or more races [Census Bureau 2010]. Of the 9,130 persons, 348 were children under 5 years old (4% of the population) and are considered to be the most vulnerable to health effects resulting from heavy metal contamination, especially lead [Census Bureau 2010] (Appendix A). Participants for the EI will be recruited from the area surrounding the former smelter, including Anaconda and Opportunity.
All members of the community will be eligible for inclusion. Young children (younger than 6 years old) and women who are pregnant or of childbearing age are most susceptible to the effects of elevated BLL. However, given community concern about exposure across the lifespan, all members of the Anaconda community will be invited to participate in the lead and arsenic testing. ATSDR will test up to 200 persons for this investigation. The strategy for recruitment for the EI will be:
ATSDR will obtain a mailing list for the Anaconda area (approximately 4,500 residences) and will send a recruitment postcard to the homes inviting all residents to be tested (Appendix B). Contact information, including a toll free phone number, will be provided for participants to call to make appointments for the testing.
Fact sheets and posters providing information on the testing will be prepared by ATSDR and provided to the ADLC and MDPHHS to distribute to local residents (Appendix B).
For those residents that have agreed to participate, a urine collection kit will be hand-delivered by ATSDR personnel prior to the blood testing date. The urine kit will include a collection cup (acid-washed) provided by the laboratory with instructions on how to collect and freeze a first-morning urine specimen that will be brought to the biological sample collection location. For young children, a collection unit designed for young children will be provided. Consent/Parental Permission/Assent forms will be signed when the urine collection kits are delivered to participants.
On the day of collection, participants will report to the blood collection location and
bring their frozen first morning urine sample to the collection site to provide to ATSDR personnel,
complete Consent/Parental Permission/Assent forms (if they did not complete them when the urine collection kits were delivered) and a questionnaire, and
provide a venous blood sample.
The EI will be performed according to a Health and Safety Plan.
Blood and urine samples will be submitted to the DLS laboratory for lead and urine analysis.
After the collection, participants will be
notified of the results of their blood lead and urine arsenic sampling within 12 weeks, and
provided recommendations for follow-up for participants per EI levels defined above.
The results of the blood and urine testing will be evaluated to determine whether a public health hazard exists in the community and will be presented in an EI report.
The findings and recommendations of the EI will be presented to the community in a public availability session after completion of the EI report.
Recruitment
Media outlets, such as the Anaconda Leader and Montana Standard, will be contacted in advance with a request to publicize the upcoming recruitment and implementation for the EI in Anaconda.
Community members will be recruited for inclusion using the following methods:
Three weeks before the sampling, a postcard providing an invitation to participate will be mailed to all residential properties in the area around the former Anaconda smelter, including the communities of Anaconda and Opportunity (Appendix B). The invitation will provide information on when the EI will occur and how to sign up. The information will indicate that blood lead and urine arsenic sampling will be provided free of charge.
As outlined above, fact sheets and posters will be provided to publicly-accessible facilities within the community, such as preschools, grocery stores, community centers, public swimming pools, and libraries. The fact sheet will be provided to the directors of appropriate facilities with a request for the director to disseminate the information to families (Appendix B).
The ADLC health department and MDPHHS will use their local resources, such as social media and contacts within the community to publicize the EI testing.
Consent/Parental Permission/Assent Forms
Participants of the testing will be provided with a Consent/Parental Permission/Assent form as appropriate prior to the sampling for all Anaconda residents. Participants will read and sign the Consent/Parental Permission/Assent form(s) while ATSDR personnel are present (Appendix C). The Consent/Parental Permission/Assent forms will be signed by participants when the urine sampling collection kits are delivered by ATSDR personnel. ATSDR EI personnel will answer any questions about the EI including questions about risks and benefits from participating in the EI which the community member may have. After the participants sign the forms, the ATSDR EI personnel will sign as a witness and collect one of the signed forms. The participants will be provided with a copy of the Consent/Parental Permission/Assent form.
The Consent/Parental Permission/Assent form and all other EI documents used to communicate with participants have been evaluated for reading level using Flesh-Kincaid Grade Level tool in Microsoft Word. The reading level is 8th grade or below. Appendix C includes copies of the Consent/Parental Permission/Assent forms.
Questionnaire
Participants will be asked questions regarding demographics, household attributes, soil exposure and daily activities (Appendix D). The responses will be used in the evaluation of the blood results.
Prior to use, the questionnaire will be approved through the Office of Management and Budget (OMB) using the existing OMB generic package for EIs (OMB number 0923-0048, expires 3/31/2019). The protocol, including the questionnaire, will be submitted to OMB as a GenIC package for the site.
Blood Sampling
Blood lead sampling is the most reliable method for measuring recent and ongoing exposure to lead. Blood will be collected by a certified phlebotomist using appropriate blood drawing protocols. Testing results will identify exposures from all sources. Specific exposure sources cannot be identified by a blood lead result.
A phlebotomist will collect 3 milliliters (ml) of blood from a vein from each participant who provides consent. The tubes for the collection have been selected and provided by DLS to ensure there is not contamination. Blood for lead analysis will be collected in 3 ml ethylenediamine tetra-acetic acid (EDTA) coated tubes provided by the laboratory.
The blood samples will be maintained at an appropriate refrigerator temperature (4º C) after collection. Samples will be shipped on ice packs by overnight delivery to DLS. The DLS will analyze blood samples for lead concentration using DLS method 30916.8-02 in whole blood by Inductively Coupled Plasma Dynamic Reaction Cell Mass Spectrometry (ICP-DRC-MS). Reference: NHANES method 2009-2010:
https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2009
Urine Sampling
Urine arsenic is the most reliable method for measuring arsenic exposures occurring within a few days prior to the sample collection. A 24–hour urine collection is considered the optimal method to collect urine for arsenic sampling due to fluctuations in excretion rates. However, most studies use a first morning void or random spot sample because it is convenient for the participant and improves their compliance. These methods appear to correlate well with 24-hour collection results [Orloff et al. 2009, Hinwood et al. 2002]. Participants will be provided a urine collection kit that will include the urine collection cups (provided by DLS for quality control) and instructions for urine collection and freezing of the sample. DLS instructions for urine collection are included in Appendix E.
Frozen urine samples brought to the collection location will be placed on dry ice until all samples have been collected and then shipped frozen on dry ice by FedEx overnight to DLS in Atlanta for analysis. DLS will analyze the urine for arsenic by DLS method 30918.4-.02 Urine Multi-element by Inductively Coupled Plasma Dynamic Reaction Cell Mass Spectrometry (ICP DRC-MS).
All urine specimens will be analyzed for total arsenic, speciated arsenic, creatinine and specific gravity. To maintain privacy, the samples will be labeled with a coded identification number.
EI personnel will adhere to the Health and Safety Plan provided in Appendix F when handling and shipping blood and urine samples.
A Data Management Plan (Appendix G) outlines processes for ensuring confidentiality and preventing unauthorized release of data collected during the EI. To maintain privacy, the samples will be labeled with unique coded identification numbers. The Principal Investigator will maintain control of the coded identification numbers.
EI Time Line
Week 1 and 2:
Contact local media in advance requesting announcements of ATSDR’s recruitment process.
Mail recruitment postcards to resident’s homes and send recruitment materials to local government offices, public facilities, and businesses. The invitation will include an ATSDR-dedicated toll free 800 phone line in Atlanta to allow prospective participants to ask questions about the EI and make appointments for blood draw.
Week 2 to 3 :
Continue local recruitment efforts, including the use of social media and outreach by ADLC and MDPHHS.
Schedule testing appointments resulting from the postcards and recruitment efforts of local partners.
Schedule phlebotomists based on number and schedules of participants.
Week 4 in a Central Location in Anaconda:
Deliver urine collection kits to participants who made appointments and have the Consent/Parental Permission/Assent forms signed upon delivery.
Call participants the day before their appointment as a reminder.
Register every participant upon arrival to the central testing location.
Collect first morning or spot urine samples provided by participants and handle appropriately for submission to the laboratory.
Provide the Consent/Parental Permission/Assent forms to participants to read and sign if they did not sign them when the urine collection kits were delivered, and explain the venous blood testing process. All potential participants have the right to refuse participation without penalty.
Administer a questionnaire to adults 18 years of age and above. The parent/guardian will answer the questions for their children.
Participation will depend on the individual’s capacity to give informed consent and respond to the questionnaire.
Collect blood samples after the Consent/Parental Permission/Assent and questionnaire are completed.
Send the blood and urine samples to DLS for analysis.
Week 5 to 7: Analyze the blood and urine samples in the CDC/NCEH/DLS:
Analyze blood and urine samples at NCEH/DLS.
Week 6 to 8: Review and interpret results:
Interpret the blood lead and urine arsenic results received from the laboratory. The questionnaire answers will help in the interpretation of the results.
Week 9 to 11: Notify the participants of their test results:
Notify the participants of individual tests results, interpretation, and recommendations via a letter. Sample letters are included in Appendix H.
Provide/Coordinate follow-up for participants with BLL above the CDC reference level and urine arsenic above the appropriate NHANES value.
Further evaluation for lead may include recommending follow-up with a PCP and providing information on lead health effects and ways to reduce exposure.
Further evaluation for arsenic may include providing information on arsenic health effects and ways to reduce exposure.
Report BLLs ≥5 ug/dL to the MDPHHS and enroll in case management.
Week 12 to 36: Prepare EI Report:
Draft the EI Report. Analyze BLL and urine arsenic data in relation to the environmental data and questionnaire information.
Submit the EI Report for review and release.
Make the report available in the repository at a central location in Anaconda
Human Subjects
Residents living in and around the Anaconda NPL area will be eligible to participate. The EI will be conducted in accordance with an Anaconda EI Health and Safety Plan (Appendix F).
Sufficient resources are available to enroll up to 200 participants for the blood lead and urine arsenic testing.
Description of Risks and Benefits
Risk: We anticipate the risks to be very low, but cannot entirely exclude them. There may be pain and/or bruising at the site where the blood was collected. There is the slight risk of feeling light headed during blood collection.
Benefits: Participants will be informed if they have recent and ongoing exposure to lead and recent exposure to arsenic. If we find elevated levels of lead in blood or arsenic in urine, we will provide recommendations to reduce exposure. There is no monetary incentive for participating in this EI, however, the EI participation, blood lead and urine arsenic testing and test interpretation are free of charge.
An additional benefit will be increased awareness of potential health effects associated with lead and arsenic exposure within the community and for local primary care physicians in Anaconda.
Procedures for Consent/Parental Permission/Assent
Participants will have time to read the Consent/Parental Permission/Assent form (Appendix C) while ATSDR EI personnel are present. ATSDR EI personnel will answer any questions about the EI, including risks and benefits. After the participant signs the Consent/Parental Permission/Assent form, ATSDR EI personnel will sign as witness and collect one of the two signed forms (the participant will keep one of the signed forms).
Parents/guardians will provide permission to participate for children <18 years of age. An assent form is also provided for participants aged 7 to 17 years. Consent/Parental Permission/Assent forms, as well as all other EI instruments of communication with participants, have been evaluated for readability using Flesh-Kincaid Grade Level tool in Microsoft Word.
Consent/Parental Permission/Assent forms will be provided when the urine collection kit is delivered to participants prior to the blood testing date.
Protection of Confidentiality
Participants’ confidentiality and personal information will be protected by ATSDR to the fullest extent possible by law. Individual tests results will not be made available to the public. Confidential information will be kept in locked cabinets and/or password protected computers. At the conclusion of the EI, ATSDR will prepare and publish an EI Report summarizing the findings, but will not reveal personal identifiers (e.g., name or address). Reports produced after the EI implementation will not identify specific individuals or residences.
All blood and urine samples that are sent to the laboratory for analysis will be labeled with a unique identifying number. De-identified results from the laboratory will be entered into an electronic database and sent to the ATSDR Principal Investigator (PI). Using a key, the PI will link laboratory and questionnaire data and maintain a complete de-identified dataset used for analysis.
Feasibility and Limitations
ATSDR has considerable experience conducting lead and arsenic exposure investigations. ATSDR is well positioned to address lead and arsenic issues and to respond to community concerns that may be voiced during this EI.
Potential limitations include:
We may not know the source of a participant’s exposure.
The blood lead and urine arsenic concentrations cannot be used to predict the future occurrence of disease nor be attributed as the cause of current health problems.
The number of participants recruited may not give ATSDR a complete understanding of the extent of exposure in the community.
The results of this EI will be applicable only to the individuals tested and cannot be generalized to the community or other populations.
Handling Unexpected or Adverse Events
There is a small chance of unexpected or adverse events occurring during the course of this EI.
The most likely adverse event is a participant feeling lightheaded or fainting during blood collection. The Health and Safety Plan (Appendix F) outlines the response to such an event. In addition, the on-site ATSDR medical officer and the phlebotomist collecting blood are trained in responding to such situations.
If any adverse event should occur, the Principal Investigator will notify local emergency services and ATSDR in Atlanta.
Data Handling
All data will be managed in accordance with the Anaconda Data Management Plan (DMP), included as Appendix F. All Consent/Parental Permission/Assent forms will be stored in a secure location while in Anaconda and then returned to ATSDR in Atlanta where they will be kept in a locked cabinet and/or password protected computer. Questionnaire results and blood lead and urine arsenic results (with unique identifying number), will be in electronic form and kept secure on password-protected CDC computers. All other confidential information will be kept in locked cabinets and/or password protected computers by the ATSDR Principal Investigator. ATSDR will share data with EPA and state partners, as appropriate; participants will agree to the data sharing in the consent forms.
Data Analysis
ATSDR staff will ship the venous blood and urine samples via FedEx overnight express mail to CDC/DLS in Atlanta for analysis. The laboratory will follow their standard procedures for analyzing blood for lead and arsenic in urine (see Biologic Sampling under Procedures/Methods). The analysis results from the CDC/DLS will be transmitted to the ATSDR Principal Investigator in an electronic spreadsheet format. No personal identifiers will be provided to the laboratory.
Appropriate Data Quality Assurance and Quality Control (QA/QC) will be performed by the CDC/DLS and will meet DLS accuracy and precision standards [Caudill et al., 2008]. After all the blood samples are analyzed, the results will be provided to the ATSDR Principal Investigator for interpretation.
Results in the EI Report will be grouped to correspond to those found in the updated NHANES tables http://www.cdc.gov/exposurereport/pdf/fourthreport.pdf.
Dissemination, Notification, and Reporting of Results
Participants will be informed of their tests results by letter which will be mailed via the US Postal Service. The texts of the draft letters will be addressed to participants or parents of children under 18 years of age. Sample letters that will be used to inform participants of their results are included in Appendix H.
An Exposure Investigation report will be written and made available by ATSDR to participants in a repository at a central location in Anaconda.
A public meeting and/or an availability session will take place in Anaconda after the EI Report is released. Speakers from ATSDR will be available to answer questions the community may have. A formal power point presentation about the EI will also be given to the community.
References:
[ARCO 2008] Atlantic Richfield Corporation. 2008. Draft Final Community Soils Interior and Attic dust Characterization Study Data Summary Report (DSR). Prepared by Pioneer Technical Services for ARCO. January 2008.
[ARCO 2014] Atlantic Richfield Corporation. 2013. Anaconda Smelter Community Soils Operable Unit: Lead and Arsenic Baseline Biomonitoring Study Report. Prepared for Atlantic Richfield Company by ENVIRON International Corporation. June 2014.
[ATSDR 2007a] Agency for Toxic Substances and Disease Registry. 2007. Health Consultation: Evaluation of Residential Soil Arsenic Action Level. Anaconda Co. Smelter Site, Anaconda, Deer Lodge County, MT. EPA Facility ID: MTD093291656
[ATSDR 2007b] Agency for Toxic Substances and Disease Registry. 2007. Toxicological profile for Arsenic. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
[ATSDR 2007c] Agency for Toxic Substances and Disease Registry. 2007. Toxicological profile for Lead. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
[ATSDR 2018] Agency for Toxic Substances and Disease Registry. 2018. Personal communication between David Dorian, ATSDR Region 8, and Charlie Partridge, Toxicologist, U.S. Environmental Protection Agency. July 10, 2018.
Baker EL, Hayes CG, Landrigan PJ, Handke JL, Leger RT, Housworth WJ, Harrington JM. 1977. A nationwide survey of heavy metal absorption in children living near primary copper, lead and zinc smelters. Am J Epidem 1977;106(4):261-273.
Binder S, Forney D, Kaye W, Pascal D. Arsenic exposure in children living near a former copper smelter. Bull Environ Contam Toxicool 1987;39:114-121.
Brink LL, Talbot EO, Sharma RK, March GM, Wu WC, Rager JR, Strosnider HM (2013). Do U.S. Ambient Air Lead Levels Have a Significant Impact on Childhood Blood Lead Levels: Result of a National Study. J. Environ and Public Health. Available at: http://www.hindawi.com/journals/jeph/2013/278042/.
Caudill SP, Schleicher RL, Pirkle JL, 2008. Multi-rule quality control for the age related eye disease study. Stat. Med. 27, 4094-4106.
[CDC 2005] Centers for Disease Control and Prevention. 2005. Preventing Lead Exposure in Young Children, 2005. http://www.cdc.gov/nceh/lead/publications/prevleadpoisoning.pdf
[CDC 2009] Centers for Disease Control and Prevention. 2009. The Fourth National Report on Human Exposure to Environmental Chemicals, 2009. Available at: http://www.cdc.gov/exposurereport/pdf/FourthReport.pdf
[CDC 2010] Centers for Disease Control and Prevention. 2010. CDC Guidelines for the Identification and Management of Lead Exposure in Pregnant and Lactating Women, November 2010. U.S. DHHS, Atlanta, GA. http://www.cdc.gov/nceh/lead/publications/leadandpregnancy2010.pdf
[CDC 2012a] Centers for Disease Control and Prevention. Lead (web page). 2012a. Update on Blood Lead Levels in Children. Last Updated October 30, 2012. Available online at: http://www.cdc.gov/nceh/lead/ACCLPP/blood_lead_levels.htm
[CDC 2012b] Centers for Disease Control and Prevention. 2012b. Low Level Lead Exposure Harms Children: A Renewed Call for Primary Prevention: Report of the Advisory Committee on Childhood Lead Poisoning Prevention, Centers for Disease Control and Prevention. U.S. Department of Health and Human Services, January. Available at: http://www.cdc.gov/nceh/lead/acclpp/final_document_010412.pdf
[CDC 2012c] Centers for Disease Control and Prevention. 2012c. CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in "Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention". Centers for Disease Control and Prevention, June 7, 2012. Available at: http://www.cdc.gov/nceh/lead/ACCLPP/CDC_Response_Lead_Exposure_Recs.pdf
[CDC 2018] Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, (March 2018). Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. https://www.cdc.gov/exposurereport/
[Census Bureau 2010] U.S. Census Bureau. 2010. Online. https://www.census.gov/
[DHHS 2010] U.S. Department of Health and Human Services. 2010. Atlanta, Georgia. Guidelines for the Identification and Management of Lead Exposure in Pregnant and Lactating Women. November 2010. U.S. Department of Health and Human Services.
[EPA 1996] U. S. Environmental Protection Agency. 1996. EPA Superfund Record of Decision: Anaconda Company Smelter. Denver: U.S. Environmental Protection Agency, Region 8. EPA/ROD/R08-96/127. September 1996. https://cumulis.epa.gov/supercpad/SiteProfiles/index.cfm?fuseaction=second.scs&id=0800403&doc=Y&colid=32604®ion=08&type=SC
[EPA 2008] U.S. Environmental Protection Agency. 2008. Residential Soils Interpretation and Analysis Report; Community Soils Operable Unit, Anaconda Smelter NPL Site. 2008.
[EPA 2013] U.S. Environmental Protection Agency. 2013. Record of Decision (ROD) Amendment, Community Soils Operable Unit, Anaconda Smelter National Priorities List Site. Anaconda, MT. EPA ID: MTD093291656. Community Soils Operable Unit. September 2013. https://cumulis.epa.gov/supercpad/SiteProfiles/index.cfm?fuseaction=second.scs&id=0800403&doc=Y&colid=32604®ion=08&type=SC
[EPA 2015] U.S. Environmental Protection Agency. 2015. Fifth Five-Year Review Report. Anaconda Smelter Superfund Site. Anaconda-Deer Lodge County, MT. EPA ID: MTD093291656. https://cumulis.epa.gov/supercpad/SiteProfiles/index.cfm?fuseaction=second.scs&id=0800403&doc=Y&colid=32604®ion=08&type=SC
Hartwell TD, Handy RW, Harris BS, Williams SR, Gehlbach SH. Heavy metal exposure in populations living around zinc and copper smelters. Arch Environ Health 1983;38(5):284-295.
Hinwood AL, Sim MR, de Klerk N, Drummer O, Gerostamoulos J, Bastone EB. Are 24-hour urine samples and creatinine adjustment required for analysis of inorganic arsenic in urine in population studies? Environ Res Section A. 88, 219–224.
Hwang Y-H, Bornschein RL, Grote J, Menrath W, Roda S. Environmental arsenic exposure of children around a former copper smelter site. Environ Res 1997;72:72-81.
Orloff K, Mistry K, Metcalf S. Biomonitoring for Environmental Exposures to Arsenic. Journal of Toxicology and Environmental Health, Part B: Critical Reviews. 2009. 12:7, 509-524. Available at http://dx.doi.org/10.1080/10937400903358934
Appendices
Appendix A: Site Maps
A1: Figure 1. Anaconda Co. Smelter NPL Site (Montana): Site Location Map
A2: Figure 2. Anaconda Co. Smelter NPL Site (Montana): Site Features Map
Appendix B: Fact Sheet, Poster and Invitation to Participate
B1: Fact Sheet for EI
B2: Recruitment Poster
B3: Postcard Inviting Residents to Participate
Appendix C: Privacy Act Statement and Consent Forms
C1: Privacy Act Statement
C2: Adult Consent Form
C3: Parental Permission form for children younger than 18 years
C4: Assent form for children between 7 and 17 years
Appendix D: Questionnaire
Appendix E: Urine Collection Instructions
Appendix F: Health and Safety Plan
Appendix G: Data Management Plan
Appendix H: Sample Results Letters to Participants
Appendix A: Site and Demographic Map
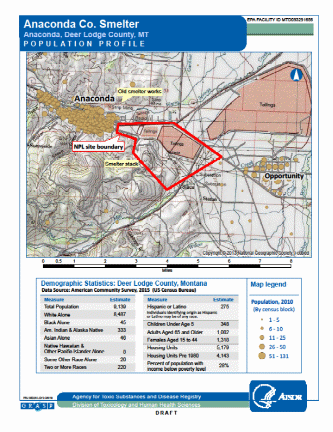
Appendix B: Fact Sheet and Invitation to Participate
B1: Fact Sheet for EI
B2: Recruitment Poster
B3: Recruitment Postcard
Appendix B1: Fact Sheet for EI


Panel to be included on the fact sheet on the second page:
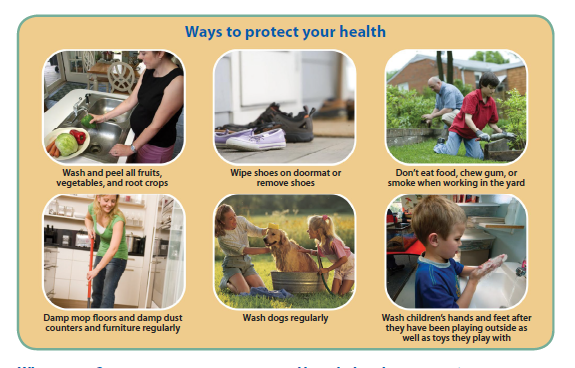
Appendix B2: Recruitment Poster

Appendix B3: Postcard Inviting Residents to Participate
Flesch-Kincaid Reading level –

Appendix C: Privacy Act Statement and Consent Forms
C1: Privacy Act Statement
C2: Adult Consent Form
C3: Parental Permission form for children younger than 18 years
C4: Assent form for children between 7 and 17 years
Appendix C1: Privacy Act Statement
PRIVACY ACT STATEMENT
FOR
Anaconda, Montana Exposure Investigation – Blood Lead and Urine Arsenic Testing
This statement provides the notice required by the Privacy Act of 1974 (5 USC § 552a(e)(3)).
Authority: The Agency for Toxic Substances and Disease Registry (ATSDR) has the authority to collect this information under the ‘‘Comprehensive Environmental Response, Compensation, and Liability Act of 1980’’ (CERCLA) as amended by ‘‘Superfund Amendments and Reauthorization Act of 1986’’ (SARA) (42 U.S.C. 9601, 9604).
Purpose: ATSDR is conducting this assessment to study your exposure to lead and arsenic in the Anaconda community as a result of past smelter activities. ATSDR is collecting this information on you or your child/ward for:
Adult consent, parental permission, and child assent to participate in questionnaires, and blood and urine collections.
Sending your or your child’s/ward’s testing results back to you.
Routine Uses:
ATSDR may disclose these records to the local health department to provide follow-up, as needed, for blood lead results.
Other routine uses as described in Statement of Records Notice (SORN) No. 09-19-0001 - ‘‘Records of Persons Exposed or Potentially Exposed to Toxic or Hazardous Substances.” See https://www.gpo.gov/fdsys/pkg/FR-2011-01-25/pdf/2010-33004.pdf.
Disclosure: Providing this information is voluntary. ATSDR needs this information for you or your child/ward to take part in the study. ATSDR may not include incomplete records in the data analysis. ATSDR needs up-to-date contact information to send your or your child’s/ward’s study results.
Appendix C2: Adult Consent Form
Flesch-Kincaid Reading level – 7.2
Adult Consent Form for Blood and Urine Testing
ATSDR Exposure Investigation (EI)
Anaconda, MT
Who are we?
We are from a federal public health agency, the Agency for Toxic Substances and Disease Registry (ATSDR)
Who are we working with?
Region 8 Environmental Protection Agency (EPA)
Anaconda Deer Lodge County (ADLC) Health Department
Montana Department of Public Health and Human Services (MDPHHS)
Why we are doing this Exposure Investigation (EI)?
We are doing this EI to respond to community concerns about lead and arsenic in the environment and to help people find out if they are exposed
We are testing lead in blood samples and arsenic in urine samples
What are we asking you to do?
You are invited to have your blood tested for lead, and urine tested for arsenic.
There is NO COST to you for the testing.
Collect a urine sample at home and bring it to the blood collection location.
Complete a brief questionnaire with that will ask questions regarding how you may be exposed to lead and arsenic.
Allow a licensed phlebotomist to take a sample of your blood.
What
is included in my participation?
There are three parts to
your participation.
Urine Collection and Testing for Arsenic
The first morning urine sample that you collected at home and froze was brought to the blood testing location.
We will send your urine to a lab to test it for arsenic.
The urine will not be tested for drugs, alcohol or HIV.
Answer a Short Questionnaire
We will ask you some questions about your home and how you might be exposed to lead and arsenic.
This should take about 20 minutes.
Blood Collection and Testing for Lead
We will collect less than 1 teaspoon (3 milliliters) from a vein in your arm.
This will take 10 minutes or less.
We will send your blood to a lab to test it for lead.
The blood will not be tested for drugs, alcohol or HIV.
What will happen to any leftover blood or urine after testing is finished?
The blood and urine will not be used for anything else.
The lab will throw out any leftover blood and urine.
When will you get the test results?
You will get your test results by mail about 12 weeks after testing.
What are the benefits of being in this EI?
You will know the levels of lead in your blood and arsenic in your urine.
If you are found to have high levels of lead or arsenic, ATSDR and ADLC will recommend you follow-up with your physician and will provide you with information that will help you reduce contact with lead and arsenic.
What are the risks of this EI?
The needle stick might hurt a little.
Some bruising may happen where the blood is taken.
You may feel a little lightheaded for a short time.
If you are pregnant there is no risk to the pregnancy from the blood collection.
How will we protect your privacy?
We will protect your privacy as much as the law allows.
Montana law requires that we report blood lead levels to the ADLC health department if the result is greater than 5 µg/dL.
Montana law requires that information given to the state may be made public if someone asks them for the information but your name and address will not be released.
We will share the results with other agencies only with your permission. We will require our government partners to treat your information as private.
We will give you an identification (ID) number.
Your ID number, not your name, will go on the tube of blood and urine sample.
We will keep a record, under lock-and-key, of your name, address, and ID number. The information will be used by ATSDR to link the results to each person and send your blood and urine test results to you.
We will not use your name in any report we write. Only group information that does not include individual names will be reported.
When can you ask questions about the testing?
If you have any questions about this testing, you can ask us now.
If you have questions later, you can call:
Dr. Luly Rosales-Guevara at 770-488-0744
Dr. Matt Karwowski at 404-718-5867
The Anaconda Exposure Investigation toll free number (888) 892-1320
Voluntary Consent
I agree to be tested.
I agree to answer questions.
I was given the chance to ask questions and I feel my questions were answered.
I know that having the test done is my choice.
I know that even though I have agreed to this testing, I may leave at any time without penalty.
Signature
I give my permission to be tested and agree to answer questions.
May we share the test results with other federal, state, and local health and environmental agencies? YES / NO (please circle one)
___________________________________ __________________ ______
Signature of Person Giving Consent Date Age
Address _____________________________ Telephone __________________
______________________________
______________________________
Lab ID Number____________________
Certification of Consent Form Administrator:
I read the consent form to the person named above. He/she had the opportunity to ask questions about the Exposure Investigation and had the questions answered.
_______________________________________
Signature of person administering the consent
Appendix C3: Parental Permission Form for Children younger than 18 Years of Age
Flesch-Kincaid Reading level – 7.4
Parental Permission Form for Blood and Urine Testing
Children younger than 18 years of age
ATSDR Exposure Investigation (EI)
Anaconda, Montana
Who are we?
We are from a federal public health agency, the Agency for Toxic Substances and Disease Registry (ATSDR)
Who are we working with?
Region 8 Environmental Protection Agency (EPA)
Anaconda Deer Lodge County (ADLC) Health Department
Montana Department of Public Health and Human Service (MDPHSS)
Why we are doing this Exposure Investigation (EI)?
We are doing this EI to respond to community concerns about lead and arsenic in the environment and to help people find out if they are exposed
We are testing lead in blood samples and arsenic in urine samples
What do we want you to do?
Your child/ward is invited to have his/her blood tested for lead and urine tested for arsenic.
There is NO COST to you for the testing of your child/ward.
Collect your child’s/ward’s urine sample at home and bring it to the blood collection location.
Complete a brief questionnaire with that will ask questions regarding how your child/ward may be exposed to lead and arsenic.
Allow a licensed phlebotomist to take a sample of your child’s/ward’s blood.
What is included in my child’s/ward’s participation?
There are three parts to your participation.
Urine Collection and Testing for Arsenic
The first morning urine sample from your child/ward that you collected at home and froze was brought to the blood testing location.
We will send your child’s/ward’s urine to a lab to test it for arsenic.
The urine will not be tested for drugs, alcohol or HIV.
Answer a Short Questionnaire
We will ask you some questions about your home and how your child/ward might be exposed to lead and arsenic.
This should take about 20 minutes.
Blood Collection and Testing for Lead
We will collect less than 1 teaspoon (3 milliliters) from a vein in your child’s/ward’s arm.
This will take 10 minutes or less.
We will send your child’s/ward’s blood to a lab to test it for lead.
The blood will not be tested for drugs, alcohol or HIV.
What will happen to any leftover blood after testing is finished?
The blood and urine will not be used or tested for anything else.
The lab will throw out any leftover blood and urine.
When will you get the test results?
You will get your child’s/ward’s test results by mail about 12 weeks after testing.
What are the benefits of being in this EI?
You will know the levels of lead in the blood and arsenic in the urine of your child/ward.
If your child/ward is found to have high levels of lead or arsenic, ATSDR and ADLC will recommend you follow-up with your child’s/ward’s physician and will provide you with information that will help you reduce contact with lead and arsenic.
What are the risks of this EI?
The needle stick might hurt a little.
Some bruising may happen where the blood is taken.
Your child/ward may feel a little lightheaded for a short time.
How will we protect your child’s/ward’s privacy?
We will protect your child’s/ward’s privacy as much as the law allows.
Montana law requires that we report blood lead levels to the ADLC if the result is greater than 5 µg/dL.
Montana law requires that information given to the state may be made public if someone asks them for the information but your name and address will not be released.
We will share the results with other agencies only with your permission. We will require our government partners to treat your information as private.
We will give your child/ward an identification (ID) number.
Your child’s/ward’s ID number, not their name, will go on the tube of blood and urine sample.
We will keep a record, under lock-and-key, of your child’s/ward’s name, address, and ID number. The information will be used by ATSDR to link the results to each person and send your blood and urine test results to you.
We will not use your child’s/ward’s name in any report we write. Only group information that does not include individual names will be reported.
When can you ask questions about the testing?
If you have any questions about this testing, you can ask us now.
If you have questions later, you can call:
Dr. Luly Rosales-Guevara at 770-488-0744
Dr. Matt Karwowski at 404-718-5867
The Anaconda Exposure Investigation toll free number (888) 892-1320
Parental/Guardian Voluntary Permission
I agree to have my child/ward tested.
I agree to answer questions about my child/ward.
I was given the chance to ask questions on behalf of my child/ward. I feel my questions have been answered.
I know that having these tests done is my choice for my child.
I know that even though we agreed to this testing, my child/ward may leave at any time without penalty.
Regardless of the results, may we share the test result with other federal, state, and local health and environmental agencies? YES / NO (please circle one)
If the results are 5 µg/dL or greater, can we provide your information to the Pediatric Environmental Health Specialty Unit (PEHSU), and may they contact you for follow-up? YES / NO (please circle one)
Signature
I give permission for my child/ward to be tested and agree to answer questions about my child/ward.
______________________________________
______ ___________
Printed name of child Age Sex of child
___________________________________ __________________
Signature of parent/guardian Date
___________________________________
Printed name of parent/guardian
Address of Child _____________________________ Telephone __________________
______________________________
______________________________
Lab ID Number____________________
Certification of Permission Form Administrator:
I read the permission form to the person named above. He/she had the opportunity to ask questions about the Exposure Investigation and had the questions answered.
_______________________________________
Signature of person administering permission
Appendix C4: Assent Form for Children younger than 18 Years of Age
Flesch-Kincaid Reading level – 6.6
Assent Form for Blood and Urine Testing
Children between 7 and 17 years of age
ATSDR Exposure Investigation (EI)
Anaconda, Montana
Who are we?
We are from a federal public health agency, the Agency for Toxic Substances and Disease Registry (ATSDR)
Who are we working with?
Region 8 Environmental Protection Agency (EPA)
Anaconda Deer Lodge County (ADLC) Health Department
Montana Department of Public Health and Human Service (MDPHHS)
Why we are doing this Exposure Investigation (EI)?
We are doing this EI to respond to community concerns about lead and arsenic in the environment and to help people find out if they are exposed
We are testing lead in blood samples and arsenic in urine samples
What are we asking you to do?
You are invited to have your blood tested for lead and urine tested for arsenic.
There is NO COST to you or your parents for the testing.
Collect a urine sample at home and bring it to the blood collection location.
Complete a brief questionnaire with that will ask questions regarding how you may be exposed to lead and arsenic.
Allow a licensed phlebotomist to take a sample of your blood.
What is included in my participation?
There are three parts to your participation.
Urine Collection and Testing for Arsenic
The first morning urine sample that you collected at home and froze was brought to the blood testing location.
We will send your urine to a lab to test it for arsenic.
The blood will not be tested for drugs, alcohol or HIV.
Answer a Short Questionnaire
We will ask you some questions about how you might be exposed to lead and arsenic. Your parents can help you answer the questions on the form.
This should take about 20 minutes
Blood Collection and Testing for Lead
We will collect less than 1 teaspoon (3 milliliters) from a vein in your arm.
This will take 10 minutes or less.
We will send your blood to a lab to test it for lead.
The urine will not be tested for drugs, alcohol or HIV.
What will happen to any leftover blood after testing is finished?
The blood and urine will not be used or tested for anything else.
The lab will throw out any leftover blood and urine.
When will you get the test results?
Your parents will get your test results by mail about 12 weeks after testing.
What are the benefits of being in this EI?
You and your parents will know the levels of lead in your blood and arsenic in your urine.
If you are found to have high levels of lead or arsenic, ATSDR and ADLC will recommend you follow-up with your physician and will provide you with information that will help you reduce contact with lead and arsenic.
What are the risks of this EI?
The needle stick might hurt a little.
Some bruising may happen where the blood is taken.
You may feel a little lightheaded for a short time.
How will we protect your privacy?
We will protect your privacy as much as the law allows.
Montana law requires that we report blood lead levels to the ADLC if the result is greater than 5 µg/dL.
Montana law requires that information given to the state may be made public if someone asks them for the information but your name and address will not be released.
We will share the results with other agencies only with your permission. We will require our government partners to treat your information as private.
We will give you an identification (ID) number.
Your ID number, not your name, will go on the tube of blood and urine sample.
We will keep a record, under lock-and-key, of your name, address, and ID number. The information will be used by ATSDR to link the results to each person and send your blood and urine test results to your parents.
We will not use your name in any report we write. Only group information that does not include individual names will be reported.
When can you ask questions about the testing?
If you have any questions about this testing, you can ask us now.
If you have questions later, you can call:
Dr. Luly Rosales-Guevara at 770-488-0744
Dr. Matt Karwowski at 404-718-5867
The Anaconda Exposure Investigation toll free number (888) 892-1320
Child Assent
Your parent/guardian said it is all right for you to have the blood and urine tests.
Your parent/guardian said it is all right for you to answer some questions.
You don’t have to have these tests to answer questions if you don’t want to.
Voluntary Assent
I agree to be tested.
I agree to answer questions.
I was given the chance to ask questions and feel my questions were answered.
I know that having these tests done is my choice.
I know that even though I have agreed to this testing, I may leave at any time without penalty.
Signature
I agree to be tested and to answer questions.
_________________________________________ ___________ ______________
Printed name of child Age of child Sex of child
______________________________________________ __________________
Signature or written name of child in child’s handwriting Date
__________________________
Printed name of parent/guardian
Address of child ______________________________ Telephone __________________
______________________________
______________________________
Lab ID Number____________________
Certification of Assent Form Administrator:
I read the assent form to the person named above. He/she had the opportunity to ask questions about the Exposure Investigation and had the questions answered.
_______________________________________
Signature of person administering the assent
Appendix D: Questionnaire
OMB # 0923-0048 Expires 3/31/2019
Flesch-Kincaid Level – 5.5
Introduction - Hello my name is {SAY NAME}.
We are doing an Exposure Investigation for the Agency for Toxic Substances and Disease Registry, or ATSDR. ATSDR is a sister agency to the Centers for Disease Control and Prevention (CDC). As part of the investigation, we will be asking you some common questions like your name and address. We will also ask questions on your contact with lead and arsenic. We are asking these questions to better understand all the data we collect.
The questions should take about 20 minutes. After that, we will be offering free blood and urine testing for participants in this exposure investigation. Your total time in the investigation will be about 30 minutes. Once we are done with this investigation, you will be given a copy and details of the testing results for you and your children (if you have them). Generally, we are able to get results to you within 12 weeks.
Cost Recovery Number: 8018
Person Administering Questionnaire _______________________________________
Date Questionnaire Administered _________________________________________
Participant last name ___________________________________________________
Participants first name __________________________________________________
Address: _____________________________________________________________
______________________________________________________________
______________________________________________________________
Mailing address if different from home address: ______________________________
______________________________________________________________
______________________________________________________________
Laboratory ID ________________________________________________________
Public reporting burden of this collection of information is estimated to average 20 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (0923-0048).
Now I want to ask you questions about how I can contact you. I will also be asking how long you have lived at or visited certain places. This is needed to find out how long you may have had contact with lead and arsenic and how long it may have lasted. We will also ask your age, address, race, and about how you spend your time (e.g, child at daycare, how often they play outside, your jobs and hobbies). This is useful to help us better understand your test results.
Is the person being interviewed a minor?
Yes
No (skip to question 17)
Name of person answering questions for minor child:
Relationship to child:
Mother
Father
Grandparent
Guardian
Has your child ever had their blood tested for lead?
Yes
No (skip to question 13)
If yes, when, where and what was the result?
Does the child put their hands or toys in their mouth?
Yes
No (skip to question 21)
If yes, what and how often?
Have you noticed the child eating dirt while playing outside?
Yes
No (skip to question 23)
If yes, how often?
Demographic Questions. Script: The next questions are about qualities of the person who is being tested (you or your child/ward). This information and will help us better understand your test results.
What is your or your child/ward’s sex?
Male
Female
What is your or your child/ward’s age and date of birth?
Age
Date of Birth
Are you or your child/ward Hispanic, Latino/a, or Spanish Origin? (one or more categories may be selected)
No, not of Hispanic Latino/a, or Spanish origin
Yes, Mexican, Mexican American, Chicano/a
Yes, Puerto Rican
Yes, Cuban
Yes, Other Hispanic, Latino, or Spanish Origin
What is your or your child/ward’s race? (one or more categories may be selected)
American Indian or Alaska Native
Native Hawaiian or Other Pacific Islander
Asian
White
Black or African American
Don’t Know
Refused to Answer
If female between 15-44 yrs, are you pregnant? If yes in what month of pregnancy?
Don't know
No
Yes, 0 to 3 months
Yes, 4 to 6 months
Yes, 7 to 9 months
Do you or your child/ward spend time outside the home (e.g., work or daycare/school)?
Yes
No (skip to question 25)
If yes, how long are your or your child/ward out of the house during the day?
1 to 4 hours
5 to 8 hours
Over 8 hours
Don’t know
If you or your child/ward are out of the house during the day, how many times per week?
1-3 days per week
4 or more days per week
Don’t know
How many hours per day do you or your child/ward typically spend outdoors?
Do not spend time outdoors
Less than 2 hours per day
2 to 4 hours per day
4 to 6 hours per day
Over 6 hours per day
Don’t know
How many hours per day do you or your child/ward typically spend in your attic?
Do not spend time in the attic
Less than 2 hours per day
2 to 4 hours per day
4 to 6 hours per day
Over 6 hours per day
Don’t know
Does you or your child/ward wash hands before eating?
Always
Sometimes
Never
How long have you lived at this address?
Less than 6 months
6 months to less than 2 years
2 to 5 years
6 to 10 years
More than 10 years
How long have you lived in Anaconda, MT?
Less than 6 months
6 months to less than 2 years
2 to 5 years
6 to 10 years
More than 10 years
Do you speak a language other than English at home? (5 years or older)
Yes
No (skip to question 32)
If you speak another language in the household, do you prefer receiving followup information in another language? What is this language? (5 years old and older)
Yes, Spanish
Yes, Other __________________________
Attributes of the Structure or Home. The following questions are about the qualities and characteristics of your home.
Do you live in a(n):
Apartment
Single Family Home
Townhouse or Condominium
Mobile Home
Other
Approximately when was the building built?
2000—present
1990—1999
1980—1989
1970—1979
1960—1969
1950—1959
1940—1949
1939 or earlier
Don’t know
What is the condition of your home or building?
Good
Fair
Poor
Do the windows (e.g., sills) have peeling paint?
Yes No
Is there peeling paint in other places such as cabinets, interior walls and/or exterior walls?
Yes
No
Don’t know
How often do you clean your home using a wet mop?
Daily
Several times a week
Weekly
Monthly
Other
How often do you clean your home using a vacuum cleaner?
Daily
Several times a week
Weekly
Monthly
Other
Do you have an attic in your home?
Yes
No (skip to question 42)
If you have an attic in your home, how often do you enter the attic?
Daily
Weekly
Monthly
Yearly
Never
Has your attic been cleaned by a professional?
Yes
No
If yes, when was it cleaned?
Soil Information (Tracking inside home)
Does your home have a yard with bare dirt?
Yes
No
Has soil in your yard been removed and replaced with clean soil?
Yes
No (skip to question 46)
If yes, when was it done?
How often do you or your child/ward remove shoes before entering your home?
Never do this
Seldom do this
Sometimes do this
Always do this
Does anyone in the home work primarily outdoors in a job with frequent soil or slag contact? (slag reprocessor, construction worker, landscaping, etc.) (if NO, skip to question 49)
Yes
No
Don’t know
How often do they change clothing when entering the home after work outdoors?
Never do this
Seldom do this
Sometimes do this
Always do this
Do you have a job that may bring you into contact with lead?
Mechanic
Transportation worker
Construction worker
Other
Do you have a job that may bring you into contact with arsenic?
Wood preservation
Arsenate pesticide production
Sand blaster
Other
Other Sources of Lead Exposure
Have you or your child/ward used any Mexican pottery in the past month?
Yes
No
Don’t know
Have you or your child/ward used any home (folk) remedies (used in Indian, Asian and Hispanic cultures) in the past month for any illnesses?
Yes
No
Don’t know
Have you or your child/ward eaten any Mexican candy (containing chili powder or tamarind) in the past month?
Yes
No
Don’t know
Do you or your child/ward own any imported toy or costume jewelry that are over 10 years old?
Yes
No
Don’t know
Do you or your child/ward have any hobbies that may involve exposure to lead?
No
Don’t know
Stained Glass
Firing Range
Leaded fishing lures
Other (list out)
Frequency of Eating Food That May Contain Arsenic
How many portions of fish and other seafood (including shrimp) did you or your child/ward eat in the past week?
None
1-2
3-4
5 or more
Don’t know
How many portions of rice (white or brown) did you or your child/ward eat in the past week?
None
1-2
3-4
5 or more
Don’t know
How many portions of chicken did you or your child/ward eat in the past week?
None
1-2
3-4
5 or more
Don’t know
Is there anything you want us to know about you or your child that we did not ask about?
Appendix E: Urine Collection Instructions
URINE COLLECTION INSTRUCTIONS
ANACONDA EXPOSURE INVESTIGATION
Please READ CAREFULLY:
Enclosed is a plastic cup and a plastic bag with an absorbent pad inside.
DO NOT take the cap off the cup until you are about to collect your urine.
We would like you to collect your first morning urine, if possible
Make sure you fill out all the items on the plastic cup label before you put your urine in the freezer.
Instructions for collecting your urine:


Wash your hands with soap and water.
Rinse and dry your hands with a clean towel.
Keep the cup closed until you are ready to collect your urine.
DO NOT TOUCH the inside of the cup or cap.
Open the cup and leave the cap turned up.
Collect your urine inside the cup.
Put the cap back on the filled container and tighten it.
Wash your hands with soap and water again.
Fill out the label on the plastic cup as follows:
Name
Date
Time of urine collection
First Morning Urine? Yes or No
Time urine put into freezer
Put the closed cup filled with urine on the absorbent pad in the plastic bag we gave you,
SEAL the BAG,
Put the sealed plastic bag with the filled urine cup in the FREEZER.
Bring the CUP of FROZEN URINE in the sealed plastic bag to your scheduled blood draw appointment.
Thanks!
Appendix F: Health and Safety Plan
Anaconda Site Health and Safety Plan
Blood Lead Levels and Urine Arsenic Levels in Anaconda, MT
Anaconda, MT
Principle Investigator
Karen Scruton, MS
Division of Community Health Investigations (DCHI)
Agency for Toxic Substances and Disease Registry (ATSDR)
Atlanta, GA
Introduction
This Site Health and Safety Plan (SHSP) defines applicability and responsibility regarding compliance with the Agency for Toxic Substances and Disease Registry (ATSDR) Health and Safety Program for Hazardous Substance Field Activities.
This SHSP defines site requirements and protocol applicable during all activities. It extends to all ATSDR employees, ATSDR contractors, and site visitors invited by ATSDR.
Site emergency response procedures and any potential fire, explosion, health, or safety hazards of the operation must be communicated to all personnel. Noncompliance with site safety procedures will not be tolerated. Personnel not observing safety procedures could be suspended from participation in site activities.
Development of this plan included consideration of current safety standards and recommendations as defined by the Environmental Protection Agency (EPA), the Occupational Safety and Health Administration (OSHA), the National Institute for Occupational Safety and Health (NIOSH), the American Conference of Governmental Industrial Hygienists (ACGIH), health effects and standards for known contaminants, and procedures designed to account for potential exposure to unknown substances.
Personnel Training Requirements
All site personnel will be trained in accordance with the requirements contained in the CDC/ATSDR Mandatory Training Requirements. At a minimum, all personnel will be trained to recognize on-site hazards, the provisions of this SHSP, and identification of responsible personnel.
All personnel are required to complete the following training courses:
Blood Borne Pathogen Training
Safety Survival Skills Part 1 - General Responsibilities
HAZWOPER (current 8-hour refresher)
Human Research Protections Training
First aid/CPR/Automated External Defibrillator (AED) Training
If you need one or more of those trainings, please go to: http://intranet.cdc.gov/od/hcrmo/CDCU/mandatorytraining.shtml
Personal Protective Equipment
Anyone working in the area must be fully aware of and protected against potential hazards. The purpose of personal protective equipment (PPE) is to shield or isolate individuals from chemical, physical, and biological hazards that could be encountered at the site.
Personnel working with blood are required to wear Level D PPE to include closed toed shoes, long pants, and gloves. Eye protection and respirators are not required. Gloves should be changed in between handling each participant’s sample. Blood collection materials should be placed in appropriate biohazard containers.
Emergency Procedures
On-site personnel will use the following standard emergency procedures. Notify the principle investigator of any on-site emergencies. The principle investigator is responsible for ensuring that appropriate emergency procedures are followed.
In the event that participants feel lightheaded or faint during the blood draw, ATSDR’s onsite Medical Officer (Dr. Lourdes Rosales-Guevara) and the phlebotomist are trained to provide assistance and will ensure the participant has recovered prior to leaving the blood collection location.
Personal Injury
When an injury occurs the principle investigator will assess its nature. A qualified first aid provider should initiate appropriate first aid and continue appropriate emergency medical services. If necessary, injured personnel will be transported to the hospital listed below.
Community Hospital of Anaconda
401 W Pennsylvania Ave
Anaconda, MT 59711
Hospital Phone: (406) 563-8500
Fire or Explosion
If a fire or explosion occurs on site, the emergency will be announced and all personnel will leave the area through emergency exits (unless directed otherwise). The fire department shall be contacted (911), and all personnel shall be moved a safe distance from the involved area. If it is safe to do so, site personnel can take the following actions:
Use on site fire-fighting equipment to control or extinguish the fire; and
Remove or isolate flammable or other hazardous materials that could contribute to the fire.
Natural Hazards
The principle investigator has responsibility for safety of ATSDR personnel if natural hazards (e.g., thunderstorms, tornadoes, hurricanes, etc.) occur. The principle investigator will inform personnel of current and impending weather conditions.
Equipment Failure
If any site worker experiences a protective equipment failure or alteration that affects the protection factor, that person shall immediately wash hands as needed and replace the failed equipment.
If any other on-site equipment fails to operate properly, the principle investigator shall be notified and will then determine the effect of this failure on continuing operations at the site.
Appendix G: Data Management Plan
NCEH/ATSDR Data Management Plan Form
This plan describes the anticipated use and release by CDC of the dataset named below. All CDC DMPs are required to be in compliance with the CDC/ATSDR Policy on Releasing and Sharing data, available at http://isp-v-maso-apps.cdc.gov/Policy/Doc/policy385.pdf. This plan is modifiable and does not represent a legal contract between CDC and any other entity. The elements included do not necessarily constitute an exhaustive list of all possible elements for a DMP, so users should add elements as needed.
The DMP is submitted through eClearance for review and approval. Use “TBD” if you cannot determine some of this information at the time of submission. Elements with an asterisk (*) are required data fields for metadata.
Table 1 – Core DMP Elements (should be filled out when project approval is sought)
Label (Definition) P1810-0015 |
*Title: Blood Lead Levels and Urine Arsenic Levels in Anaconda, MT, Anaconda Smelter NPL site, Anaconda Deer Lodge County, Montana: Exposure Investigation II.
|
*Description (Human-readable description with sufficient detail to enable a user to quickly understand whether the project or data set is of interest. Short clear description is ideal.) This exposure investigation (EI) will sample up to 300 persons to evaluate lead and arsenic exposure in the Anaconda area. In September 2018, 191 participants were tested for blood lead and urine arsenic in Anaconda. Given community concern, an additional round of testing will be completed in mid-fall of 2018 for lead in blood and arsenic in urine. This EI will be a collaboration between ATSDR headquarters, ATSDR Region 8, EPA Region 8, the Anaconda Deer Lodge County (ADLC) health department and the Montana Department of Public Health and Human Services (MDPHHS). |
*Last DMP Update: September 4, 2018
|
Contact Name and Email |
CDC PI or POC Name (last, first): Scruton, Karen |
CDC PI or POC e-mail address: [email protected] |
CDC PI or POC phone number: 770-488-1325 |
Organization
ATSDR/DCHI/SSB
|
*Unique Identifier (A unique identifier for the project as maintained within an Agency catalog or database. For intramural submissions, protocol/S3P number can be used. For extramural submissions, grant/Co Ag/contract number can be used to map to related documents.)
8018 |
Public Access Level (The degree to which the data collected as part of this project could be made publicly available, regardless of whether it has been made available.) |
□ Public release (Data set can be made available without restrictions; data steward no longer controls data.) |
□ Release by request (Data set is available to members of the public by request only; data steward no longer controls data.) |
X Restricted use data sharing (Data set is available to particular parties under certain use restrictions; data not always under CDC custody.) |
□ Restricted access data sharing (Data set is only available in an RDC; data need to remain under CDC custody.) |
□ Summary data or data tables only (Underlying data set cannot be released or shared, but summary data or data tables can.) |
□ No release or data sharing |
Access Rights/Restrictions (Include information regarding access or restrictions based on privacy, security, or other policies of the owner of the data. Include an explanation for the selected “Public Access Level” above.)
Venous blood lead and urine arsenic data will be available as aggregated data without identifiers in the ATSDR Exposure Investigation Report.
|
License/Other Agreements (The license or non-license [i.e. Public Domain] status with which the data set will be published. See Open Licenses for more information. May include DTA, MTA, IAA, MOU or other agreements concerning data use and access.)
None |
*Publisher/Owner (The publishing entity and optionally their parent organization(s). This could be the “owner” of the data.)
ATSDR/DCHI/SSB
|
Access URL, If Known (URL providing indirect access to the DMP, data set, data dictionary [variable names and valid values], data collection instrument and other relevant information, including the research protocol if possible.)
None
|
Download URL, If Known (URL providing direct access to a downloadable file of the data set, summary data, or data tables.)
Not applicable.
|
Spatial (The range of spatial applicability of a data set. Could include a geographic region or a named place [city, county, state, region, country].)
Anaconda, Montana
|
Temporal (The range of temporal applicability of project [i.e., a start and end year of applicability for the data]. Include the years for the project or data set.)
September 2018 to September 2019
|
Table 2 – Additional DMP Elements (should be filled out where possible when project approval is sought; however, many fields can only be filled out later when publication/report is cleared)
Label (Definition) |
*Tags (Keywords to help users discover the data set; include terms that would be used by technical and non-technical users.)
Exposure Investigation Blood Lead Levels Urine Arsenic Levels Anaconda Smelter NPL site Anaconda, MT
|
Project Type (Multiple selections may apply.) |
X Intramural |
□ Extramural (grant, cooperative agreement, contract, IAA, CDC Foundation, other) Specify mechanism: |
□ Surveillance |
□ Research |
□ Ongoing collection |
□ Other |
Project Status Estimated start date (Fall 2018): Estimated date of release (Fall 2019): |
Data Category (For explanation of D1 to D10 codes, see Table on page 1) |
X D1 □ D2 □ D3 |
□ D4 □ D5 □ D6 □ D7 |
□ D8 □ D9 □ D10 |
Population Represented (e.g., “residents of x,” “inpatients at x,” “users of product x”)
Residents from the City of Anaconda, MT are invited to participate. |
Data Collection Protocol (Brief description with reference to document or website that provides detailed information.)
Data collection is described in the Blood Lead and Urine Arsenic Levels in Anaconda, Montana EI Protocol.
Given community concern, all residents of Anaconda are invited to participate. Participants will be recruited and tested in Fall 2018. This is a follow-up testing event given the amount of community concern.
|
Data Management Protocol (Brief description with reference to system/sources where data will be housed (internal SQL database, external SQL database, etc.) and to data formats (proprietary vs. open source data formats.)
Data will be housed within the ATSDR/DCHI Exposure Investigation Database that contains metadata about the EI process, contaminants, locations of the protocol and the raw data and no PII. The consent forms and questionnaires will be housed in a secured ATSDR server and hard copies will be kept in a locked cabinet at ATSDR.
|
Process for Omitting Identifying Information (Description of what identifiers are in the database, how they will be removed, and by whom.)
Identifiers (names and addresses) will be flagged and housed in special subdirectory by the Database manager and will not be released unless directed by the CDC Office of General Counsel.
|
Data Quality Protocol (to address issues of confidentiality protection and statistical stability) (Brief description with reference to document or website that provides detailed information. Describe methods for data validation and error resolution, removal or shielding of any proprietary information, removal or shielding of sensitive information [i.e. data with dual use applicability], removal or shielding of any individually identifying information including indirect identification.)
The Blood Lead and Urine Arsenic Levels in Anaconda, MT EI protocol describes the Data Quality Objectives.
ATSDR will share blood lead and urine arsenic testing results with state and Federal partners provided that consent is obtained and the government partner signs a CDC/ATSDR data sharing agreement.
|
Data Retention/Disposal Plan (State when and how the dataset will be archived or destroyed [in accordance with CDC/ATSDR Records Control Schedule: http://isp-v-maso-apps/RecSched/images/RCS.pdf ].)
EI data are considered as a component of the ATSDR “Site Files” (Section 3.6, page 64). These files are maintained in the Records and Information Management Branch. Transfer to an FRC 5 years after publication of final health assessment, consultation, or advisory report. Destroy when 30 years old.
|
Data Analysis Plan (Brief description of planned use of the data. Can include reference to document [e.g. Information Collection Request, Research Protocol, or other] that provides more detailed information.)
The data will be used to determine whether the concentrations of lead and arsenic in soil and in attics in Anaconda may result in elevated blood lead and urine arsenic levels in the Anaconda community. The blood lead and urine arsenic results will be used to determine if a public health risk exists in Anaconda.
|
Publication Plan (Brief description of planned CDC-authored and CDC-coauthored publications, including topic, type of publication, and estimated timeline.)
ATSDR will use the data to publish an ATSDR Exposure Investigation Report |
Access URL (URL providing indirect access to the DMP, data set, data dictionary [variable names and valid values], data collection instrument and other relevant information, including the research protocol if possible.)
Not applicable
|
Download URL (URL providing direct access to a downloadable file of the data set, summary data, or data tables.)
Not applicable
|
Data Set Name
To be determined
|
Data Release Documentation (List documents provided to users, e.g. variable definitions, codebook, metadata file, guidance on data use.)
To be determined
|
Data Release Format (Specify dataset formats, e.g., Excel, SAS, ASCII, etc.; interactive data query website; mixed mode. Also specify data dictionary file format, e.g., JSON, RDF, SAS.)
Excel will be used to store the blood lead and urine arsenic level data for the EI
|
Data Release Notification (State how potential users will be informed of dataset availability.)
ATSDR will include a notice in the final Blood Lead and Urine Arsenic Levels in Anaconda, MT Exposure Investigation Report.
|
Date Form Completed: 07/19/2018 By: Karen Scruton, MS
Name, Title
Date Form Last Revised: 10/10/2018 By: Karen Scruton, MS
Appendix H: Template for Results Letters to Participants
Appendix H: Example Results Letter to the Participant (Adult or Parent of Child)
Note: The format and content of this example letter could change depending on the results of the EI. Final letters will also go through CDC/ATSDR clearance.
(Flesch-Kincaid Reading Level: grade XX)
(ATSDR Letterhead)
DATE ________________
Name XXXXX
Address XXXX
Dear ____________
Thank you for participating in (or allowing your child/ward, _______________, to take part in) the Agency for Toxic Substances and Disease Registry’s (ATSDR) Exposure Investigation in Anaconda, Montana. The goal of the Exposure Investigation is to determine whether people living in Anaconda, MT are being exposed to lead and arsenic.
This letter contains the results of your (your child’s/ward’s) blood lead and urine arsenic tests. ATSDR collected a blood and urine sample from you/your child/ward for testing on [Month Day, Year].
Test results of your (your child’s/ward’s) samples are shown in the enclosed Table 1.
Lead in Blood:
if BLL is <5 µg/dL:
Your (your child’s/ward’s) blood lead level was below the reference level for blood lead. Although there is no safe level of blood lead, the results indicate that your (your child’s/ward’s) blood lead level is similar to most other people’s results in the United States. Therefore, no additional action with regard to lead is needed based on these results.
if BLL is ≥5 µg/dL
Your (your child’s/ward’s) blood lead level was equal to or above the reference level for blood lead. As a result, the Anaconda Deer Lodge County (ADLC) health department will contact you to help identify the possible source of exposure. ADLC is also available to speak with you about ways to reduce your exposure to lead. You can contact ADLC at XXX-XXX-XXXX. The exposure could be coming from various sources including hobbies, contaminated soil and dust, or lead-based paint.
The U.S. Centers for Disease Control and Prevention (CDC) sets a reference level for blood lead for children under the age of 6 years. The current CDC reference level is 5 microgram of lead per deciliter of blood (µg/dL). Because lead can pass from a mother to her unborn baby, ATSDR uses this child blood lead reference level for pregnant women. For this Exposure Investigation, the level of 5 µg/dL is used for all age groups and pregnancy status.
Although there is no known safe exposure level for lead, blood lead levels above the reference level do not mean that you or your child will develop health effects. Health effects depend on the blood lead level, length of exposure, age and present health status. Potential health effects for pregnant women include increased risk of miscarriage, low birth weight or premature birth. Potential health effects for baby from lead exposure can include: learning problems such as speech and language delay; problems with attention; decreased intelligence quotient (IQ); anemia (fewer red blood cells than normal).
Your health care provider should retest your blood lead level as soon as possible.
Arsenic in Urine:
Total arsenic is measured by adding two different types of arsenic together: organic and inorganic.
Organic arsenic, often found in fish and seafood, is not typically associated with health concerns. Organic arsenic can be increased in urine when a person eats fish or seafood a few days before the urine is collected for testing
Inorganic arsenic, found in many places in the environment including soil and water, is known to be associated with health concerns when elevated.
ATSDR uses total arsenic levels as a first step in measuring a person’s exposure. If total arsenic is above the investigation follow-up level, we look to see what form of arsenic is in the sample: organic and inorganic forms. If a person’s organic arsenic measurement is above the follow-up level, it is usually the result of a recent meal that included food that may contain arsenic. If a person’s inorganic arsenic measurement is above the follow-up level, it means that person may consider ways to lower their exposure to arsenic or may see a healthcare provider if they are concerned about how arsenic may cause health problems.
If total arsenic was below the NHANES:
Your (your child’s/ward’s) levels were below the reference level for total arsenic. This indicates that your (your child’s/ward’s) results are similar to most other people’s results in the United States. Therefore, no additional action is needed.
If total arsenic was above the NHANES:
Your (your child’s/ward’s) total arsenic level(s) was above the exposure investigation follow-up level. Because the level of total urine arsenic was above the follow-up level we looked at the additional test to determine the levels of organic and inorganic arsenic present in your (your child’s/ward’s) urine sample.
Inorganic arsenic:
The inorganic arsenic detected in your (your child’s/ward’s) urine samples was below the level that is representative of inorganic arsenic in the U.S. population, indicating that the elevated total arsenic level is not the result of inorganic arsenic and is probably the result of organic arsenic in your (your child’s/ward’s) diet.
OR
The inorganic arsenic detected in your (your child’s/ward’s) urine samples was above the level that is representative of inorganic arsenic in the U.S. population. This means that the increased total arsenic level in your (your child’s/ward’s) urine is the result of inorganic arsenic, which may cause health effects. It is recommended that people with higher inorganic arsenic levels may evaluate ways to reduce arsenic exposure and may consider seeing a physician if you are concerned about potential effects of arsenic on your (your child’s/ward’s) health.
Organic arsenic:
Organic arsenic in your (your child’s/ward’s) was below the level that is representative of organic arsenic in the U.S. population, indicating the elevated total arsenic level is not the result of organic arsenic and is likely the result of inorganic arsenic.
OR
Organic arsenic in your (your child’s/ward’s) was above the level that is representative of organic arsenic in the U.S. population. This means the increased total arsenic level in your (your child’s/ward’s) urine may contain organic arsenic, and suggests that your (your child’s/ward’s) diet likely contributed to the elevated level.
If you have questions concerning this Exposure Investigation or your (your child’s/ward’s) test results, please contact me at 888-320-5291or by email at [email protected].
Sincerely,
Lourdes Rosales-Guevara, MD
Medical Officer – Anaconda MT Exposure Investigation
ATSDR Division of Community Health Investigations, Exposure Investigation Team
Table 1: Test Results for Firstname Lastname |
||
Test |
Test Result |
Follow-Up Level |
Blood Lead |
XXX µg/mL |
5 µg/dL1 |
Total Urine Arsenic |
XXX µg/g of creatinine |
XX2 |
Inorganic Arsenic |
XX |
XX |
Organic Arsenic |
XX |
XX |
1 The reference value for Blood Lead is taken from the CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention [CDC 2012].
2 The total urine arsenic reference value is the lowest 95th percentile total urine arsenic value reported for the appropriate age group in the 2013-2014 CDC National Health and Nutrition Examination Survey. Results are reported in micrograms per gram of creatinine (µg/g) [CDC 2018]. Creatinine testing is a standard method used to measure the amount of arsenic present in urine samples. |
||
References:
[CDC 2012] Centers for Disease Control and Prevention. 2012. CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in "Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention". Centers for Disease Control and Prevention, June 7, 2012. Available at: http://www.cdc.gov/nceh/lead/ACCLPP/CDC_Response_Lead_Exposure_Recs.pdf
[CDC 2018] Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, (March 2018). Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. https://www.cdc.gov/exposurereport/
Appendix B: Privacy Act Statement and Consent Forms
B1: Privacy Act Statement
B2: Adult Consent Form
B3: Parental Permission form for children younger than 18 years
B4: Assent form for children between 7 and 17 years
Appendix B1: Privacy Act Statement
PRIVACY ACT STATEMENT
FOR
Anaconda, Montana Exposure Investigation II – Blood Lead and Urine Arsenic Testing
This statement provides the notice required by the Privacy Act of 1974 (5 USC § 552a(e)(3)).
Authority: The Agency for Toxic Substances and Disease Registry (ATSDR) has the authority to collect this information under the ‘‘Comprehensive Environmental Response, Compensation, and Liability Act of 1980’’ (CERCLA) as amended by ‘‘Superfund Amendments and Reauthorization Act of 1986’’ (SARA) (42 U.S.C. 9601, 9604).
Purpose: ATSDR is conducting this investigation to assess your exposure to lead and arsenic in the Anaconda community as a result of past smelter activities. ATSDR is collecting this information on you or your child/ward for:
Adult consent or parental permission and child assent to participate in questionnaires, and blood and urine collections.
Sending your or your child’s/ward’s testing results back to you.
Routine Uses:
ATSDR may disclose these records to the local health department to provide follow-up, as needed, for blood lead results.
Other routine uses as described in Statement of Records Notice (SORN) No. 09-19-0001 - ‘‘Records of Persons Exposed or Potentially Exposed to Toxic or Hazardous Substances.” See https://www.gpo.gov/fdsys/pkg/FR-2011-01-25/pdf/2010-33004.pdf.
Disclosure: Providing this information is voluntary. ATSDR needs this information for you or your child/ward to take part in the investigation. ATSDR may not include incomplete records in the data analysis. ATSDR needs up-to-date contact information to send your or your child’s/ward’s study results.
Appendix B2: Adult Consent Form
Flesch-Kincaid Reading level – 7.2
Adult Consent Form for Blood and Urine Testing
ATSDR Exposure Investigation (EI)
Anaconda, Montana II
Who are we?
We are from a federal public health agency, the Agency for Toxic Substances and Disease Registry (ATSDR).
Who are we working with?
Region 8 Environmental Protection Agency (EPA)
Anaconda Deer Lodge County (ADLC) Health Department
Montana Department of Public Health and Human Services (MDPHHS)
Why we are doing this Exposure Investigation (EI)?
We are doing this EI to respond to community concerns about lead and arsenic in the environment and to help people find out if they are exposed.
We are testing lead in blood samples and arsenic in urine samples.
What are we asking you to do?
You are invited to have your blood tested for lead, and urine tested for arsenic.
There is NO COST to you for the testing.
Collect a urine sample at home and bring it to the blood collection location.
Complete a brief questionnaire with that will ask questions regarding how you may be exposed to lead and arsenic.
Allow a licensed phlebotomist to take a sample of your blood.
What is included in my participation?
There are three parts to your participation.
Urine Collection and Testing for Arsenic
You will bring the first morning urine sample that you collected at home and froze to the blood testing location.
We will send your urine to a lab to test it for arsenic.
The urine will not be tested for drugs, alcohol or HIV.
Answer a Short Questionnaire
We will ask you some questions about your home and how you might be exposed to lead and arsenic.
This should take about 20 minutes.
Blood Collection and Testing for Lead
We will collect less than 1 teaspoon (3 milliliters) from a vein in your arm.
This will take 10 minutes or less.
We will send your blood to a lab to test it for lead.
The blood will not be tested for drugs, alcohol or HIV.
What will happen to any leftover blood or urine after testing is finished?
The blood and urine will not be used for anything else.
The lab will throw out any leftover blood and urine.
When will you get the test results?
You will get your test results by mail about 12 weeks after testing.
What are the benefits of being in this EI?
You will know the levels of lead in your blood and arsenic in your urine.
If you are found to have high levels of lead or arsenic, ATSDR and ADLC will recommend that you follow-up with your physician and will provide you with information that will help you reduce contact with lead and arsenic.
What are the risks of this EI?
The needle stick might hurt a little.
Some bruising may happen where the blood is taken.
You may feel a little lightheaded for a short time.
If you are pregnant there is no risk to the pregnancy from the blood collection.
How will we protect your privacy?
We will protect your privacy as much as the law allows.
Montana law requires that we report blood lead levels to the ADLC health department if the result is greater than 5 µg/dL.
Montana law requires that information given to the state may be made public if someone asks them for the information but your name and address will not be released.
We will share the results with other agencies only with your permission. We will require our government partners to treat your information as private.
We will give you an identification (ID) number.
Your ID number, not your name, will go on the tube of blood and urine sample.
We will keep a record, under lock-and-key, of your name, address, and ID number. The information will be used by ATSDR to link the results to each person and send your blood and urine test results to you.
We will not use your name in any report we write. Only group information that does not include individual names will be reported.
When can you ask questions about the testing?
If you have any questions about this testing, you can ask us now.
If you have questions later, you can call:
Dr. Arthur Wendel at 206-553-0454
Dr. Matt Karwowski at 404-718-5867
The Anaconda Exposure Investigation toll free number 888-892-1320
Voluntary Consent
I agree to be tested.
I agree to answer questions.
I was given the chance to ask questions and I feel my questions were answered.
I know that having the test done is my choice.
I know that even though I have agreed to this testing, I may leave at any time without penalty.
Signature
I give my permission to be tested and agree to answer questions.
May we share the test results with other federal, state, and local health and environmental agencies? YES / NO (please circle one)
___________________________________ __________________ ______
Signature of Person Giving Consent Date Age
Address _____________________________ Telephone __________________
______________________________
______________________________
Lab ID Number____________________
Certification of Consent Form Administrator:
I read the consent form to the person named above. He/she had the opportunity to ask questions about the Exposure Investigation and had the questions answered.
_______________________________________
Signature of person administering the consent
Appendix B3: Parental Permission Form for Children younger than 18 Years of Age
Flesch-Kincaid Reading level – 7.4
Parental Permission Form for Blood and Urine Testing
Children younger than 18 years of age
ATSDR Exposure Investigation (EI)
Anaconda, Montana II
Who are we?
We are from a federal public health agency, the Agency for Toxic Substances and Disease Registry (ATSDR)
Who are we working with?
Region 8 Environmental Protection Agency (EPA)
Anaconda Deer Lodge County (ADLC) Health Department
Montana Department of Public Health and Human Service (MDPHSS)
Why we are doing this Exposure Investigation (EI)?
We are doing this EI to respond to community concerns about lead and arsenic in the environment and to help people find out if they are exposed
We are testing lead in blood samples and arsenic in urine samples
What do we want you to do?
Your child/ward is invited to have his/her blood tested for lead and urine tested for arsenic.
There is NO COST to you for the testing of your child/ward.
Collect your child’s/ward’s urine sample at home and bring it to the blood collection location.
Complete a brief questionnaire with that will ask questions regarding how your child/ward may be exposed to lead and arsenic.
Allow a licensed phlebotomist to take a sample of your child’s/ward’s blood.
What is included in my child’s/ward’s participation?
There are three parts to your participation.
Urine Collection and Testing for Arsenic
You will bring the first morning urine sample from your child/ward that you collected at home and froze to the blood testing location.
We will send your child’s/ward’s urine to a lab to test it for arsenic.
The urine will not be tested for drugs, alcohol or HIV.
Answer a Short Questionnaire
We will ask you some questions about your home and how your child/ward might be exposed to lead and arsenic.
This should take about 20 minutes.
Blood Collection and Testing for Lead
We will collect less than 1 teaspoon (3 milliliters) from a vein in your child’s/ward’s arm.
This will take 10 minutes or less.
We will send your child’s/ward’s blood to a lab to test it for lead.
The blood will not be tested for drugs, alcohol or HIV.
What will happen to any leftover blood after testing is finished?
The blood and urine will not be used or tested for anything else.
The lab will throw out any leftover blood and urine.
When will you get the test results?
You will get your child’s/ward’s test results by mail about 12 weeks after testing.
What are the benefits of being in this EI?
You will know the levels of lead in the blood and arsenic in the urine of your child/ward.
If your child/ward is found to have high levels of lead or arsenic, ATSDR and ADLC will recommend that you follow-up with your child’s/ward’s physician and will provide you with information that will help you reduce contact with lead and arsenic.
What are the risks of this EI?
The needle stick might hurt a little.
Some bruising may happen where the blood is taken.
Your child/ward may feel a little lightheaded for a short time.
How will we protect your child’s/ward’s privacy?
We will protect your child’s/ward’s privacy as much as the law allows.
Montana law requires that we report blood lead levels to the ADLC if the result is greater than 5 µg/dL.
Montana law requires that information given to the state may be made public if someone asks them for the information but your name and address will not be released.
We will share the results with other agencies only with your permission. We will require our government partners to treat your information as private.
We will give your child/ward an identification (ID) number.
Your child’s/ward’s ID number, not their name, will go on the tube of blood and urine sample.
We will keep a record, under lock-and-key, of your child’s/ward’s name, address, and ID number. The information will be used by ATSDR to link the results to each person and send your blood and urine test results to you.
We will not use your child’s/ward’s name in any report we write. Only group information that does not include individual names will be reported.
When can you ask questions about the testing?
If you have any questions about this testing, you can ask us now.
If you have questions later, you can call:
Dr. Arthur Wendel at 206-553-0454
Dr. Matt Karwowski at 404-718-5867
The Anaconda Exposure Investigation toll free number 888-892-1320
Parental/Guardian Voluntary Permission
I agree to have my child/ward tested.
I agree to answer questions about my child/ward.
I was given the chance to ask questions on behalf of my child/ward. I feel my questions have been answered.
I know that having these tests done is my choice for my child.
I know that even though we agreed to this testing, my child/ward may leave at any time without penalty.
Regardless of the results, may we share the test result with other federal, state, and local health and environmental agencies? YES / NO (please circle one)
If the results are 5 µg/dL or greater, can we provide your information to the Pediatric Environmental Health Specialty Unit (PEHSU), and may they contact you for follow-up? YES / NO (please circle one)
Signature
I give permission for my child/ward to be tested and agree to answer questions about my child/ward.
______________________________________
______ ___________
Printed name of child Age Sex of child
___________________________________ __________________
Signature of parent/guardian Date
___________________________________
Printed name of parent/guardian
Address of Child _____________________________ Telephone __________________
______________________________
______________________________
Lab ID Number____________________
Certification of Permission Form Administrator:
I read the permission form to the person named above. He/she had the opportunity to ask questions about the Exposure Investigation and had the questions answered.
_______________________________________
Signature of person administering permission
Appendix B4: Assent Form for Children younger than 18 Years of Age
Flesch-Kincaid Reading level – 6.6
Assent Form for Blood and Urine Testing
Children between 7 and 17 years of age
ATSDR Exposure Investigation (EI)
Anaconda, Montana II
Who are we?
We are from a federal public health agency, the Agency for Toxic Substances and Disease Registry (ATSDR)
Who are we working with?
Region 8 Environmental Protection Agency (EPA)
Anaconda Deer Lodge County (ADLC) Health Department
Montana Department of Public Health and Human Service (MDPHHS)
Why we are doing this Exposure Investigation (EI)?
We are doing this EI to respond to community concerns about lead and arsenic in the environment and to help people find out if they are exposed
We are testing lead in blood samples and arsenic in urine samples
What are we asking you to do?
You are invited to have your blood tested for lead and urine tested for arsenic.
There is NO COST to you or your parents for the testing.
Collect a urine sample at home and bring it to the blood collection location.
Complete a brief questionnaire with that will ask questions regarding how you may be exposed to lead and arsenic.
Allow a licensed phlebotomist to take a sample of your blood.
What is included in my participation?
There are three parts to your participation.
Urine Collection and Testing for Arsenic
You will bring the first morning urine sample that you collected at home and froze was brought to the blood testing location.
We will send your urine to a lab to test it for arsenic.
The blood will not be tested for drugs, alcohol or HIV.
Answer a Short Questionnaire
We will ask you some questions about how you might be exposed to lead and arsenic. Your parents can help you answer the questions on the form.
This should take about 20 minutes
Blood Collection and Testing for Lead
We will collect less than 1 teaspoon (3 milliliters) from a vein in your arm.
This will take 10 minutes or less.
We will send your blood to a lab to test it for lead.
The urine will not be tested for drugs, alcohol or HIV.
What will happen to any leftover blood after testing is finished?
The blood and urine will not be used or tested for anything else.
The lab will throw out any leftover blood and urine.
When will you get the test results?
Your parents will get your test results by mail about 12 weeks after testing.
What are the benefits of being in this EI?
You and your parents will know the levels of lead in your blood and arsenic in your urine.
If you are found to have high levels of lead or arsenic, ATSDR and ADLC will recommend you follow-up with your physician and will provide you with information that will help you reduce contact with lead and arsenic.
What are the risks of this EI?
The needle stick might hurt a little.
Some bruising may happen where the blood is taken.
You may feel a little lightheaded for a short time.
How will we protect your privacy?
We will protect your privacy as much as the law allows.
Montana law requires that we report blood lead levels to the ADLC if the result is greater than 5 µg/dL.
Montana law requires that information given to the state may be made public if someone asks them for the information but your name and address will not be released.
We will share the results with other agencies only with your permission. We will require our government partners to treat your information as private.
We will give you an identification (ID) number.
Your ID number, not your name, will go on the tube of blood and urine sample.
We will keep a record, under lock-and-key, of your name, address, and ID number. The information will be used by ATSDR to link the results to each person and send your blood and urine test results to your parents.
We will not use your name in any report we write. Only group information that does not include individual names will be reported.
When can you ask questions about the testing?
If you have any questions about this testing, you can ask us now.
If you have questions later, you can call:
Dr. Arthur Wendel at 206-553-0454
Dr. Matt Karwowski at 404-718-5867
The Anaconda Exposure Investigation toll free number 888-892-1320
Child Assent
Your parent/guardian said it is all right for you to have the blood and urine tests.
Your parent/guardian said it is all right for you to answer some questions.
You don’t have to have these tests to answer questions if you don’t want to.
Voluntary Assent
I agree to be tested.
I agree to answer questions.
I was given the chance to ask questions and feel my questions were answered.
I know that having these tests done is my choice.
I know that even though I have agreed to this testing, I may leave at any time without penalty.
Signature
I agree to be tested and to answer questions.
_________________________________________ ___________ ______________
Printed name of child Age of child Sex of child
______________________________________________ __________________
Signature or written name of child in child’s handwriting Date
__________________________
Printed name of parent/guardian
Address of child ______________________________ Telephone __________________
______________________________
______________________________
Lab ID Number____________________
Certification of Assent Form Administrator:
I read the assent form to the person named above. He/she had the opportunity to ask questions about the Exposure Investigation and had the questions answered.
_______________________________________
Signature of person administering the assent
Appendix C: Questionnaire
OMB # 0923-0048 Expires 3/31/2019
Flesch-Kincaid Level – 6.6
[The interviewer will not state “don’t know” and “refused” as response options. The interviewer will mark such responses only if provided by the participant. This holds true to all questions, including ethnicity and race.]
Introduction - Hello my name is {SAY NAME}.
We are doing an Exposure Investigation for the Agency for Toxic Substances and Disease Registry, or ATSDR. ATSDR is a sister agency to the Centers for Disease Control and Prevention (CDC). As part of the investigation, we will be asking you some common questions like your name and address. We will also ask questions on your contact with lead and arsenic. We are asking these questions to better understand all the data we collect.
The questions should take about 20 minutes. After that, we will be offering free blood and urine testing for participants in this exposure investigation. Your total time in the investigation will be about 30 minutes. Once we are done with this investigation, you will be given a copy and details of the testing results for you and your children (if you have them). Generally, we are able to get results to you within 12 weeks.
Cost Recovery Number: 8018
Person Administering Questionnaire _______________________________________
Date Questionnaire Administered _________________________________________
Participant last name ___________________________________________________
Participants first name __________________________________________________
Address: _____________________________________________________________
______________________________________________________________
______________________________________________________________
Mailing address if different from home address: ______________________________
______________________________________________________________
______________________________________________________________
Laboratory ID ________________________________________________________
Public reporting burden of this collection of information is estimated to average 20 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (0923-0048).
Now I want to ask you questions about how I can contact you. I will also be asking how long you have lived at or visited certain places. This is needed to find out how long you may have had contact with lead and arsenic and how long it may have lasted. We will also ask your age, address, race, and about how you spend your time (e.g, child at daycare, how often they play outside, your jobs and hobbies). This is useful to help us better understand your test results.
Is the person being interviewed a minor?
Yes
No (skip to question 10)
Name of person answering questions for minor child:
Relationship to child:
Mother
Father
Grandparent
Guardian
Has your child ever had their blood tested for lead?
Yes
No (skip to question 6)
If yes, when, where and what was the result?
Does the child put their hands or toys in their mouth?
Yes
No (skip to question 10)
If yes, what and how often?
Have you noticed the child eating dirt while playing outside?
Yes
No (skip to question 10)
If yes, how often?
Demographic Questions. Script: The next questions are about qualities of the person who is being tested (you or your child/ward). This information and will help us better understand your test results.
What is your or your child/ward’s sex?
Male
Female
What is your or your child/ward’s age and date of birth?
Age
Date of Birth
Are you or your child/ward Hispanic or Latino?
No
Yes
What is your or your child/ward’s race? (One or more categories may be selected)
American Indian or Alaska Native
Native Hawaiian or Other Pacific Islander
Asian
White
Black or African American
If female between 15-44 yrs, are you pregnant? If yes in what month of pregnancy?
Don't know
No
Yes, 0 to 3 months
Yes, 4 to 6 months
Yes, 7 to 9 months
Do you or your child/ward spend time outside the home (e.g., work or daycare/school)?
Yes
No (skip to question 18)
If yes, how long are your or your child/ward out of the house during the day?
1 to 4 hours
5 to 8 hours
Over 8 hours
Don’t know
If you or your child/ward are out of the house during the day, how many times per week?
1-3 days per week
4 or more days per week
Don’t know
How many hours per day do you or your child/ward typically spend outdoors?
Do not spend time outdoors
Less than 2 hours per day
2 to 4 hours per day
4 to 6 hours per day
Over 6 hours per day
Don’t know
How many hours per day do you or your child/ward typically spend in your attic?
Do not spend time in the attic
Less than 2 hours per day
2 to 4 hours per day
4 to 6 hours per day
Over 6 hours per day
Don’t know
Does you or your child/ward wash hands before eating?
Always
Sometimes
Never
How long have you lived at this address?
Less than 6 months
6 months to less than 2 years
2 to 5 years
6 to 10 years
More than 10 years
How long have you lived in Anaconda, MT?
Less than 6 months
6 months to less than 2 years
2 to 5 years
6 to 10 years
More than 10 years
Do you speak a language other than English at home? (5 years or older)
Yes
No (skip to question 25)
If you speak another language in the household, do you prefer receiving followup information in another language? What is this language? (5 years old and older)
Yes, Spanish
Yes, Other __________________________
Attributes of the Structure or Home. The following questions are about the qualities and characteristics of your home.
Do you live in a(n):
Apartment
Single Family Home
Townhouse or Condominium
Mobile Home
Other
Approximately when was the building built?
2000—present
1990—1999
1980—1989
1970—1979
1960—1969
1950—1959
1940—1949
1939 or earlier
Don’t know
What is the condition of your home or building?
Good
Fair
Poor
Do the windows (e.g., sills) have peeling paint?
Yes No
Is there peeling paint in other places such as cabinets, interior walls and/or exterior walls?
Yes
No
Don’t know
How often do you clean your home using a wet mop?
Daily
Several times a week
Weekly
Monthly
Other
How often do you clean your home using a vacuum cleaner?
Daily
Several times a week
Weekly
Monthly
Other
Do you have an attic in your home?
Yes
No (skip to question 36)
If you have an attic in your home, how often do you enter the attic?
Daily
Weekly
Monthly
Yearly
Never
Has your attic been cleaned by a professional?
Yes
No
If yes, when was it cleaned?
Soil Information (Tracking inside home)
Does your home have a yard with bare dirt?
Yes
No
Has soil in your yard been removed and replaced with clean soil?
Yes
No (skip to question 46)
If yes, when was it done?
How often do you or your child/ward remove shoes before entering your home?
Never do this
Seldom do this
Sometimes do this
Always do this
Does anyone in the home work primarily outdoors in a job with frequent soil or slag contact? (slag reprocessor, construction worker, landscaping, etc.) (if NO, skip to question 42)
Yes
No
Don’t know
How often do they change clothing when entering the home after work outdoors?
Never do this
Seldom do this
Sometimes do this
Always do this
Do you have a job that may bring you into contact with lead?
Mechanic
Transportation worker
Construction worker
Other
Do you have a job that may bring you into contact with arsenic?
Wood preservation
Arsenate pesticide production
Sand blaster
Other
Other Sources of Lead Exposure
Have you or your child/ward used any Mexican pottery in the past month?
Yes
No
Don’t know
Have you or your child/ward used any home (folk) remedies (used in Indian, Asian and Hispanic cultures) in the past month for any illnesses?
Yes
No
Don’t know
Have you or your child/ward eaten any Mexican candy (containing chili powder or tamarind) in the past month?
Yes
No
Don’t know
Do you or your child/ward own any imported toy or costume jewelry that are over 10 years old?
Yes
No
Don’t know
Do you or your child/ward have any hobbies that may involve exposure to lead?
No
Don’t know
Stained Glass
Firing Range
Leaded fishing lures
Other (list out)
Frequency of Eating Food That May Contain Arsenic
How many portions of fish and other seafood (including shrimp) did you or your child/ward eat in the past week?
None
1-2
3-4
5 or more
Don’t know
How many portions of rice (white or brown) did you or your child/ward eat in the past week?
None
1-2
3-4
5 or more
Don’t know
How many portions of chicken did you or your child/ward eat in the past week?
None
1-2
3-4
5 or more
Don’t know
Is there anything you want us to know about?
Appendix D: Urine Collection Instructions
URINE COLLECTION INSTRUCTIONS
ANACONDA EXPOSURE INVESTIGATION
Please READ CAREFULLY:
Enclosed is a plastic cup and a plastic bag with an absorbent pad inside.
DO NOT take the cap off the cup until you are about to collect your urine.
We would like you to collect your first morning urine, if possible.
Make sure you fill out all the items on the plastic cup label before you put your urine in the freezer.
Instructions for collecting your urine:


Wash your hands with soap and water.
Rinse and dry your hands with a clean towel.
Keep the cup closed until you are ready to collect your urine.
DO NOT TOUCH the inside of the cup or cap.
Open the cup and leave the cap turned up.
Collect your urine inside the cup.
Put the cap back on the filled container and tighten it.
Wash your hands with soap and water again.
Fill out the label on the plastic cup as follows:
Name
Date
Time of urine collection
First Morning Urine? Yes or No
Time urine put into freezer
Put the closed cup filled with urine on the absorbent pad in the plastic bag we gave you,
SEAL the BAG,
Put the sealed plastic bag with the filled urine cup in the FREEZER.
Bring the CUP of FROZEN URINE in the sealed plastic bag to your scheduled blood draw appointment.
Thanks!
Appendix E: Health and Safety Plan
Anaconda Site Health and Safety Plan
Blood Lead Levels and Urine Arsenic Levels in Anaconda, MT
Anaconda, MT
Principle Investigator
Karen Scruton, MS
Division of Community Health Investigations (DCHI)
Agency for Toxic Substances and Disease Registry (ATSDR)
Atlanta, GA
This Site Health and Safety Plan (SHSP) defines applicability and responsibility regarding compliance with the Agency for Toxic Substances and Disease Registry (ATSDR) Health and Safety Program for Hazardous Substance Field Activities.
This SHSP defines site requirements and protocol applicable during all activities. It extends to all ATSDR employees, ATSDR contractors, and site visitors invited by ATSDR.
Site emergency response procedures and any potential fire, explosion, health, or safety hazards of the operation must be communicated to all personnel. Noncompliance with site safety procedures will not be tolerated. Personnel not observing safety procedures could be suspended from participation in site activities.
Development of this plan included consideration of current safety standards and recommendations as defined by the Environmental Protection Agency (EPA), the Occupational Safety and Health Administration (OSHA), the National Institute for Occupational Safety and Health (NIOSH), the American Conference of Governmental Industrial Hygienists (ACGIH), health effects and standards for known contaminants, and procedures designed to account for potential exposure to unknown substances.
Personnel Training Requirements
All site personnel will be trained in accordance with the requirements contained in the CDC/ATSDR Mandatory Training Requirements. At a minimum, all personnel will be trained to recognize on-site hazards, the provisions of this SHSP, and identification of responsible personnel.
All personnel are required to complete the following training courses:
Blood Borne Pathogen Training
Safety Survival Skills Part 1 - General Responsibilities
HAZWOPER (current 8-hour refresher)
Human Research Protections Training
First aid/CPR/Automated External Defibrillator (AED) Training
If you need one or more of those trainings, please go to: http://intranet.cdc.gov/od/hcrmo/CDCU/mandatorytraining.shtml
Anyone working in the area must be fully aware of and protected against potential hazards. The purpose of personal protective equipment (PPE) is to shield or isolate individuals from chemical, physical, and biological hazards that could be encountered at the site.
Personnel working with blood are required to wear Level D PPE to include closed toed shoes, long pants, and gloves. Eye protection and respirators are not required. Gloves should be changed in between handling each participant’s sample. Blood collection materials should be placed in appropriate biohazard containers.
On-site personnel will use the following standard emergency procedures. Notify the principle investigator of any on-site emergencies. The principle investigator is responsible for ensuring that appropriate emergency procedures are followed.
In the event that participants feel lightheaded or faint during the blood draw, ATSDR’s onsite Medical Officer (Dr. Arthur Wendel) and the phlebotomist are trained to provide assistance and will ensure the participant has recovered prior to leaving the blood collection location.
When an injury occurs the principle investigator will assess its nature. A qualified first aid provider should initiate appropriate first aid and continue appropriate emergency medical services. If necessary, injured personnel will be transported to the hospital listed below.
Community Hospital of Anaconda
401 W Pennsylvania Ave
Anaconda, MT 59711
Hospital Phone: (406) 563-8500
If a fire or explosion occurs on site, the emergency will be announced and all personnel will leave the area through emergency exits (unless directed otherwise). The fire department shall be contacted (911), and all personnel shall be moved a safe distance from the involved area. If it is safe to do so, site personnel can take the following actions:
Use on site fire-fighting equipment to control or extinguish the fire; and
Remove or isolate flammable or other hazardous materials that could contribute to the fire.
The principle investigator has responsibility for safety of ATSDR personnel if natural hazards (e.g., thunderstorms, tornadoes, hurricanes, etc.) occur. The principle investigator will inform personnel of current and impending weather conditions.
If any site worker experiences a protective equipment failure or alteration that affects the protection factor, that person shall immediately wash hands as needed and replace the failed equipment.
If any other on-site equipment fails to operate properly, the principle investigator shall be notified and will then determine the effect of this failure on continuing operations at the site.
Appendix F: Data Management Plan
NCEH/ATSDR Data Management Plan Form
This plan describes the anticipated use and release by CDC of the dataset named below. All CDC DMPs are required to be in compliance with the CDC/ATSDR Policy on Releasing and Sharing data, available at http://isp-v-maso-apps.cdc.gov/Policy/Doc/policy385.pdf. This plan is modifiable and does not represent a legal contract between CDC and any other entity. The elements included do not necessarily constitute an exhaustive list of all possible elements for a DMP, so users should add elements as needed.
The DMP is submitted through eClearance for review and approval. Use “TBD” if you cannot determine some of this information at the time of submission. Elements with an asterisk (*) are required data fields for metadata.
Table 1 – Core DMP Elements (should be filled out when project approval is sought)
Label (Definition) |
*Title: Blood Lead Levels and Urine Arsenic Levels in Anaconda, MT, Anaconda Smelter NPL site, Anaconda Deer Lodge County, Montana: Exposure Investigation.
|
*Description (Human-readable description with sufficient detail to enable a user to quickly understand whether the project or data set is of interest. Short clear description is ideal.) This exposure investigation (EI) will sample up to 300 persons to evaluate lead and arsenic exposure in the Anaconda area. In September 2018, 191 participants were tested for blood lead and urine arsenic in Anaconda. Given community concern, an additional round of testing will be completed in mid-fall of 2018 for lead in blood and arsenic in urine. This EI will be a collaboration between ATSDR headquarters, ATSDR Region 8, EPA Region 8, the Anaconda Deer Lodge County (ADLC) health department and the Montana Department of Public Health and Human Services (MDPHHS). |
*Last DMP Update: September 4, 2018
|
Contact Name and Email |
CDC PI or POC Name (last, first): Scruton, Karen |
CDC PI or POC e-mail address: [email protected] |
CDC PI or POC phone number: 770-488-1325 |
Organization
ATSDR/DCHI/SSB
|
*Unique Identifier (A unique identifier for the project as maintained within an Agency catalog or database. For intramural submissions, protocol/S3P number can be used. For extramural submissions, grant/Co Ag/contract number can be used to map to related documents.)
8018 |
Public Access Level (The degree to which the data collected as part of this project could be made publicly available, regardless of whether it has been made available.) |
□ Public release (Data set can be made available without restrictions; data steward no longer controls data.) |
□ Release by request (Data set is available to members of the public by request only; data steward no longer controls data.) |
X Restricted use data sharing (Data set is available to particular parties under certain use restrictions; data not always under CDC custody.) |
□ Restricted access data sharing (Data set is only available in an RDC; data need to remain under CDC custody.) |
□ Summary data or data tables only (Underlying data set cannot be released or shared, but summary data or data tables can.) |
□ No release or data sharing |
Access Rights/Restrictions (Include information regarding access or restrictions based on privacy, security, or other policies of the owner of the data. Include an explanation for the selected “Public Access Level” above.)
Venous blood lead and urine arsenic data will be available as aggregated data without identifiers in the ATSDR Exposure Investigation Report.
|
License/Other Agreements (The license or non-license [i.e. Public Domain] status with which the data set will be published. See Open Licenses for more information. May include DTA, MTA, IAA, MOU or other agreements concerning data use and access.)
None |
*Publisher/Owner (The publishing entity and optionally their parent organization(s). This could be the “owner” of the data.)
ATSDR/DCHI/SSB
|
Access URL, If Known (URL providing indirect access to the DMP, data set, data dictionary [variable names and valid values], data collection instrument and other relevant information, including the research protocol if possible.)
None
|
Download URL, If Known (URL providing direct access to a downloadable file of the data set, summary data, or data tables.)
Not applicable.
|
Spatial (The range of spatial applicability of a data set. Could include a geographic region or a named place [city, county, state, region, country].)
Anaconda, Montana
|
Temporal (The range of temporal applicability of project [i.e., a start and end year of applicability for the data]. Include the years for the project or data set.)
September 2018 to September 2019
|
Table 2 – Additional DMP Elements (should be filled out where possible when project approval is sought; however, many fields can only be filled out later when publication/report is cleared)
Label (Definition) |
*Tags (Keywords to help users discover the data set; include terms that would be used by technical and non-technical users.)
Exposure Investigation Blood Lead Levels Urine Arsenic Levels Anaconda Smelter NPL site Anaconda, MT
|
Project Type (Multiple selections may apply.) |
X Intramural |
□ Extramural (grant, cooperative agreement, contract, IAA, CDC Foundation, other) Specify mechanism: |
□ Surveillance |
□ Research |
□ Ongoing collection |
□ Other |
Project Status Estimated start date (Fall 2018): Estimated date of release (Fall 2019): |
Data Category (For explanation of D1 to D10 codes, see Table on page 1) |
X D1 □ D2 □ D3 |
□ D4 □ D5 □ D6 □ D7 |
□ D8 □ D9 □ D10 |
Population Represented (e.g., “residents of x,” “inpatients at x,” “users of product x”)
Residents from the City of Anaconda, MT are invited to participate. |
Data Collection Protocol (Brief description with reference to document or website that provides detailed information.)
Data collection is described in the Blood Lead and Urine Arsenic Levels in Anaconda, Montana EI Protocol.
Given community concern, all residents of Anaconda are invited to participate. Participants will be recruited and tested in mid-Fall 2018. This is a followup testing event given the amount of community concern.
|
Data Management Protocol (Brief description with reference to system/sources where data will be housed (internal SQL database, external SQL database, etc.) and to data formats (proprietary vs. open source data formats.)
Data will be housed within the ATSDR/DCHI Exposure Investigation Database that contains metadata about the EI process, contaminants, locations of the protocol and the raw data and no PII. The consent forms and questionnaires will be housed in a secured ATSDR server and hard copies will be kept in a locked cabinet at ATSDR.
|
Process for Omitting Identifying Information (Description of what identifiers are in the database, how they will be removed, and by whom.)
Identifiers (names and addresses) will be flagged and housed in special subdirectory by the Database manager and will not be released unless directed by the CDC Office of General Counsel.
|
Data Quality Protocol (to address issues of confidentiality protection and statistical stability) (Brief description with reference to document or website that provides detailed information. Describe methods for data validation and error resolution, removal or shielding of any proprietary information, removal or shielding of sensitive information [i.e. data with dual use applicability], removal or shielding of any individually identifying information including indirect identification.)
The Blood Lead and Urine Arsenic Levels in Anaconda, MT EI protocol describes the Data Quality Objectives.
ATSDR will share blood lead and urine arsenic testing results with state and Federal partners provided that consent is obtained and the government partner signs a CDC/ATSDR data sharing agreement.
|
Data Retention/Disposal Plan (State when and how the dataset will be archived or destroyed [in accordance with CDC/ATSDR Records Control Schedule: http://isp-v-maso-apps/RecSched/images/RCS.pdf ].)
EI data are considered as a component of the ATSDR “Site Files” (Section 3.6, page 64). These files are maintained in the Records and Information Management Branch. Transfer to an FRC 5 years after publication of final health assessment, consultation, or advisory report. Destroy when 30 years old.
|
Data Analysis Plan (Brief description of planned use of the data. Can include reference to document [e.g. Information Collection Request, Research Protocol, or other] that provides more detailed information.)
The data will be used to determine whether the concentrations of lead and arsenic in soil and in attics in Anaconda may result in elevated blood lead and urine arsenic levels in the Anaconda community. The blood lead and urine arsenic results will be used to determine if a public health risk exists in Anaconda.
|
Publication Plan (Brief description of planned CDC-authored and CDC-coauthored publications, including topic, type of publication, and estimated timeline.)
ATSDR will use the data to publish an ATSDR Exposure Investigation Report |
Access URL (URL providing indirect access to the DMP, data set, data dictionary [variable names and valid values], data collection instrument and other relevant information, including the research protocol if possible.)
Not applicable
|
Download URL (URL providing direct access to a downloadable file of the data set, summary data, or data tables.)
Not applicable
|
Data Set Name
To be determined
|
Data Release Documentation (List documents provided to users, e.g. variable definitions, codebook, metadata file, guidance on data use.)
To be determined
|
Data Release Format (Specify dataset formats, e.g., Excel, SAS, ASCII, etc.; interactive data query website; mixed mode. Also specify data dictionary file format, e.g., JSON, RDF, SAS.)
Excel will be used to store the blood lead and urine arsenic level data for the EI
|
Data Release Notification (State how potential users will be informed of dataset availability.)
ATSDR will include a notice in the final Blood Lead and Urine Arsenic Levels in Anaconda, MT Exposure Investigation Report.
|
Date Form Completed: 07/19/2018 By: Karen Scruton, MS
Name, Title
Date Form Last Revised: 10/10/2018 By: Karen Scruton, MS
Appendix G: Template for Results Letters to Participants
Appendix H: Example Results Letter to the Participant (Adult or Parent of Child)
Note: The format and content of this example letter could change depending on the results of the EI. Final letters will also go through CDC/ATSDR clearance.
(Flesch-Kincaid Reading Level: grade XX)
(ATSDR Letterhead)
DATE ________________
Name XXXXX
Address XXXX
Dear ____________
Thank you for participating in (or allowing your child/ward, _______________, to take part in) the Agency for Toxic Substances and Disease Registry’s (ATSDR) Exposure Investigation in Anaconda, Montana. The goal of the Exposure Investigation is to determine whether people living in Anaconda, MT are being exposed to lead and arsenic.
This letter contains the results of your (your child’s/ward’s) blood lead and urine arsenic tests. ATSDR collected a blood and urine sample from you/your child/ward for testing on [Month Day, Year].
Test results of your (your child’s/ward’s) samples are shown in the enclosed Table 1.
Lead in Blood:
if BLL is <5 µg/dL:
Your (your child’s/ward’s) blood lead level was below the reference level for blood lead. Although there is no safe level of blood lead, the results indicate that your (your child’s/ward’s) blood lead level is similar to most other people’s results in the United States. Therefore, no additional action with regard to lead is needed based on these results.
if BLL is ≥5 µg/dL
Your (your child’s/ward’s) blood lead level was equal to or above the reference level for blood lead. As a result, the Anaconda Deer Lodge County (ADLC) health department will contact you to help identify the possible source of exposure. ADLC is also available to speak with you about ways to reduce your exposure to lead. You can contact ADLC at XXX-XXX-XXXX. The exposure could be coming from various sources including hobbies, contaminated soil and dust, or lead-based paint.
The U.S. Centers for Disease Control and Prevention (CDC) sets a reference level for blood lead for children under the age of 6 years. The current CDC reference level is 5 microgram of lead per deciliter of blood (µg/dL). Because lead can pass from a mother to her unborn baby, ATSDR uses this child blood lead reference level for pregnant women. For this Exposure Investigation, the level of 5 µg/dL is used for all age groups and pregnancy status.
Although there is no known safe exposure level for lead, blood lead levels above the reference level do not mean that you or your child will develop health effects. Health effects depend on the blood lead level, length of exposure, age and present health status. Potential health effects for pregnant women include increased risk of miscarriage, low birth weight or premature birth. Potential health effects for baby from lead exposure can include: learning problems such as speech and language delay; problems with attention; decreased intelligence quotient (IQ); anemia (fewer red blood cells than normal).
Your health care provider should retest your blood lead level as soon as possible.
Arsenic in Urine:
Total arsenic is measured by adding two different types of arsenic together: organic and inorganic.
Organic arsenic, often found in fish and seafood, is not typically associated with health concerns. Organic arsenic can be increased in urine when a person eats fish or seafood a few days before the urine is collected for testing
Inorganic arsenic, found in many places in the environment including soil and water, is known to be associated with health concerns when elevated.
ATSDR uses total arsenic levels as a first step in measuring a person’s exposure. If total arsenic is above the investigation follow-up level, we look to see what form of arsenic is in the sample: organic and inorganic forms. If a person’s organic arsenic measurement is above the follow-up level, it is usually the result of a recent meal that included food that may contain arsenic. If a person’s inorganic arsenic measurement is above the follow-up level, it means that person may consider ways to lower their exposure to arsenic or may see a healthcare provider if they are concerned about how arsenic may cause health problems.
If total arsenic was below the NHANES:
Your (your child’s/ward’s) levels were below the reference level for total arsenic. This indicates that your (your child’s/ward’s) results are similar to most other people’s results in the United States. Therefore, no additional action is needed.
If total arsenic was above the NHANES:
Your (your child’s/ward’s) total arsenic level(s) was above the exposure investigation follow-up level. Because the level of total urine arsenic was above the follow-up level we looked at the additional test to determine the levels of organic and inorganic arsenic present in your (your child’s/ward’s) urine sample.
Inorganic arsenic:
The inorganic arsenic detected in your (your child’s/ward’s) urine samples was below the level that is representative of inorganic arsenic in the U.S. population, indicating that the elevated total arsenic level is not the result of inorganic arsenic and is probably the result of organic arsenic in your (your child’s/ward’s) diet.
OR
The inorganic arsenic detected in your (your child’s/ward’s) urine samples was above the level that is representative of inorganic arsenic in the U.S. population. This means that the increased total arsenic level in your (your child’s/ward’s) urine is the result of inorganic arsenic, which may cause health effects. It is recommended that people with higher inorganic arsenic levels may evaluate ways to reduce arsenic exposure and may consider seeing a physician if you are concerned about potential effects of arsenic on your (your child’s/ward’s) health.
Organic arsenic:
Organic arsenic in your (your child’s/ward’s) was below the level that is representative of organic arsenic in the U.S. population, indicating the elevated total arsenic level is not the result of organic arsenic and is likely the result of inorganic arsenic.
OR
Organic arsenic in your (your child’s/ward’s) was above the level that is representative of organic arsenic in the U.S. population. This means the increased total arsenic level in your (your child’s/ward’s) urine may contain organic arsenic, and suggests that your (your child’s/ward’s) diet likely contributed to the elevated level.
If you have questions concerning this Exposure Investigation or your (your child’s/ward’s) test results, please contact me at 206-553-0454 or by email at [email protected].
Sincerely,
Arthur Wendel, MD
Medical Officer – Anaconda MT Exposure Investigation
ATSDR Division of Community Health Investigations, Exposure Investigation Team
Table 1: Test Results for Firstname Lastname |
||
Test |
Test Result |
Follow-Up Level |
Blood Lead |
XXX µg/mL |
5 µg/dL1 |
Total Urine Arsenic |
XXX µg/g of creatinine |
XX2 |
Inorganic Arsenic |
XX |
XX |
Organic Arsenic |
XX |
XX |
1 The reference value for Blood Lead is taken from the CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention [CDC 2012].
2 The total urine arsenic reference value is the lowest 95th percentile total urine arsenic value reported for the appropriate age group in the 2013-2014 CDC National Health and Nutrition Examination Survey. Results are reported in micrograms per gram of creatinine (µg/g) [CDC 2018]. Creatinine testing is a standard method used to measure the amount of arsenic present in urine samples. |
||
References:
[CDC 2012] Centers for Disease Control and Prevention. 2012. CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in "Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention". Centers for Disease Control and Prevention, June 7, 2012. Available at: http://www.cdc.gov/nceh/lead/ACCLPP/CDC_Response_Lead_Exposure_Recs.pdf
[CDC 2018] Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, (March 2018). Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. https://www.cdc.gov/exposurereport/
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | NCEH/ATSDR OFFICEofSCIENCE |
| File Modified | 0000-00-00 |
| File Created | 2021-01-20 |
© 2026 OMB.report | Privacy Policy