Ebola Sierra Leone_SSA-15BFV_11APR2016_30NOVrev_CLEAN
Ebola Sierra Leone_SSA-15BFV_11APR2016_30NOVrev_CLEAN.docx
Persistence of Ebola Virus in Body Fluids of Ebola Virus Disease Survivors in Sierra Leone
OMB: 0920-1149
Persistence of Ebola Virus in Body Fluids of Ebola Virus Disease Survivors in Sierra Leone
Request for OMB Approval for an
Existing Information Collection in Use Without an OMB Control Number
Supporting Statement A
Based on Protocol Version March 4, 2016
Submitted: March 15, 2016
Program Official/Project Officer
Barbara Knust, DVM, MPH, DACVPM
CDR, US Public Health Service
Viral Special Pathogens Branch
US Centers for Disease Control
404-639-1104 phone
404-639-1118 fax
404-218-9626 mobile
Table of Contents
1. Circumstances Making the Collection of Information Necessary 5
2. Purpose and Use of the Information Collection 7
3. Use of Improved Information Technology and Burden Reduction 8
4. Efforts to Identify Duplication and Use of Similar Information 8
5. Impact on Small Businesses or Other Small Entities 8
6. Consequences of Collecting the Information Less Frequently 8
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 9
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency 9
9. Explanation of Any Payment or Gift to Respondents 10
10. Protection of the Privacy and Confidentiality of Information Provided by Respondents 10
11. Justification for Sensitive Questions 16
12. Estimates of Annualized Burden Hours and Costs 16
13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers 19
14. Annualized Cost to the Government 19
15. Explanation for Program Changes or Adjustments 20
16. Plans for Tabulation and Publication and Project Time Schedule 20
17. Reason(s) Display of OMB Expiration Date is Inappropriate 24
18. Exceptions to Certification for Paperwork Reduction Act Submissions 24
Persistence of Ebola Virus in Body Fluids of Ebola Virus Disease Survivors in Sierra Leone:
Emergency Request for OMB Approval
Goal of the research study:
Assess the presence and duration of infectious Ebola virus (EBOV)
in semen, vaginal fluids, and other body fluids of Ebola Virus
Disease (EVD) and its relation to humoral immune response in
survivors in Sierra Leone, which will help inform EBOV transmission
prevention strategies during EVD outbreaks.
Within this study, there is a
pilot cohort of 100 male EVD survivors, and a main study cohort of
120 male and 120 female EVD survivors.
Intended use of the resulting
data: Provide health care and public health practitioners working
on the Ebola response with insight into how long EBOV persists in
body fluids other than blood. This information is needed in order
to understand transmission risk of EBOV to others in the community
from survivors for whom the virus is no longer active in blood. Methods to be used to collect:
This is an observational cohort study that will collect
socio-demographic and health data from all enrolled study
participants using information collection instruments and body
fluid specimens tested using standard laboratory techniques.
Information will be collected until a participant’s specimens
from all relevant body fluids are EBOV negative, defined as having
two consecutive negative reverse
transcription polymerase chain reaction
(RT-PCR) results.
Population to be studied: EVD
survivors ages 18 and older in Sierra Leone. How data will be analyzed:
Descriptive data will be followed by cross tabulations to
understand transmission patterns and potentially associated
factors. of viral transmission are identified, multivariate
techniques that include logistic regression will also be used.
Part A — Justification
Background
The epidemic of EVD that began in 2014 in West Africa was unprecedented in scale and duration. As of November 29, 2016, there have been over 28,000 reported cases and over 11,000 deaths, in the three most affected countries: Sierra Leone, Guinea, and Liberia. No new cases of EVD have been reported in any of these countries since March-April 2016. The case fatality rate is estimated to be between 53% and 64% in these three countries. The precise number of survivors is unknown, but is in the thousands. The Ebola outbreak was a public health emergency with devastating and persistent socioeconomic and humanitarian consequences. To avoid new cases, all possible routes of transmission must be investigated and understood in order to prevent another chain of transmission.
Survivors are released from Ebola treatment units (ETUs) with a discharge certificate after two tests for Ebola virus (EBOV) in the blood have been negative by reverse transcription polymerase chain reaction (RT-PCR). Anecdotal reports suggest that some new cases might be occurring from sexual transmission of the virus between EVD survivors and their sexual partners. In addition to concerns about semen and vaginal fluid, concerns exist regarding the unknown persistence of EBOV in other body fluids of survivors (e.g., saliva, sweat, tears, urine, rectal swabs, menstrual blood, and breast milk), and their potential as a source of ongoing transmission; the literature does not rule out these possibilities.
After clinical improvement and elimination of viremia, limited data exist concerning EBOV clearance, persistence, and shedding during convalescence. It has been previously reported that live EBOV can be cultured in seminal fluids of a convalescent man at 82 days after onset of symptoms and results from initial samples collected from participants in the pilot study have shown that RNA can be detected at 284 days (~9months) post symptom onset. Limited evidence suggests that live EBOV can persist in urine for 26 days following symptom onset. Evidence regarding EBOV in vaginal secretions is also limited. EBOV RNA has been detected in vaginal secretions at 33 days after symptom onset; due to the type of diagnostic test used (RT-PCR), it was not clear whether these traces represented live virus. Similarly, traces of EBOV RNA were detected in sweat at 40 days after symptom onset, but no live virus was isolated. In addition, rectal swabs and conjunctival fluid have been found by RT-PCR to contain EBOV RNA at 29 and 22 days after illness onset, respectively. A limited number of saliva specimens have been tested by RT-PCR, all yielding negative results. There have been a small number of reports of suspected sexual transmission during the current outbreak.
The risk of sexual transmission of EBOV is unknown. Current guidance from the World Health Organization (WHO) recommends that male Ebola survivors should have their semen tested starting at 3 months post EVD symptom onset and every month thereafter until two consecutive negative tests. If testing is not available, male survivors should continue to abstain from sexual intercourse for 12 months (365 days) after the onset of symptoms, or use condoms if abstinence is not possible. CDC recommends abstinence or condom use until more information becomes available through scientific research. A recent publication outlining the preliminary results of this study found that Ebola virus RNA was detected in the semen of all 9 men who had a specimen obtained 2 to 3 months after the onset of EVD, in the semen of 26 of 40 (65%) who had a specimen obtained 4 to 6 months after onset, and in the semen of 11 of 43 (26%) who had a specimen obtained 7 to 9 months after onset.1 Another recent publication has been released, providing molecular evidence of sexual transmission of EVD that was unrelated to the virus persistence study2. The last cluster of reported cases occurred in March-April 2016, and the original patient in this cluster was a female who had unprotected sex with a male survivor nearly 500 days after his onset of EVD3. Semen tested from the male survivor found a high quantity of virus RNA that matched the cluster sequence closely. The cluster involved 13 patients in both Guinea and Liberia, emphasizing that even a single sexual transmission of EBOV can lead to another significant outbreak.
Additionally, there is limited data on EBOV in breast milk. In one published case series, two mothers each provided a single breast milk specimen after EBOV was no longer detectable in their blood, which were tested by virus isolation. One mother’s specimen had detectable virus at 7 days after disease onset and the second mother’s specimen had detectable virus at 15 days after disease onset. Another study reported that three breastfed infants of mothers with EVD and one neonate with an unknown method of feeding died shortly after their mothers. In general, infants breastfeeding from women who recently had laboratory-confirmed EVD are considered to be at high risk of developing EVD themselves.
Given limited knowledge related to the presence and potential risk of transmission of EBOV via breast milk, the current WHO and CDC recommendation is that women with and recovering from EVD, who are caring for an infant who does not have EVD, should discontinue breastfeeding when safe replacements for breastfeeding and infant care exist. Another organization, UNICEF, recommends that breastfeeding be discontinued for at least eight weeks following recovery from EVD.
The potential public health impact of EBOV transmission by contact with body fluids other than blood from convalescent EVD survivors is substantial. Given large numbers of survivors from the outbreak in West Africa, even a small percentage of survivors with EBOV persisting in body fluids who transmit EBOV to their sex partners, children, or other contacts could prolong clusters of infection or spread EBOV to new communities.
CDC is requesting a review of the following information collection pursuant to the Office of Management and Budget (OMB) procedures established at 4 CFR 1320, Controlling Paperwork Burdens on the Public. Even a single case of Ebola Virus Disease (EVD) caused by an unknown transmission source, if not rapidly identified and controlled, could set off another chain of transmission in Sierra Leone. Thus, maintaining zero new cases is essential for CDC to fulfill its public health mission to assist the government of Sierra Leone end the Ebola outbreak as completely as possible.
This new information collection is authorized by Section 301 of the Public Health Service Act (42 U.S.C. 241) (Appendix A). This is an existing information collection without an OMB control number intended to replace a previously approved emergency information collection (OMB Approval no.: 0920-1064: Persistence of Ebola Virus in Body Fluids of Ebola Virus Disease Survivors in Sierra Leone, Exp. Date- 11/30/2015). CDC requests OMB approval for an additional 12 months to complete collection of data. This information collection is necessary in order to complete the mission of this study: based on preliminary findings, Ebola virus RNA may persist in semen in 26% of men 7 to 9 months after the onset of EVD. Thus, the initial emergency approval period of 180 days was not sufficient to ascertain the duration of Ebola virus persistence in the body fluids of EVD survivors. CDC anticipates that this information collection will continue for 12 months.
2. Purpose and Use of the Information Collection
The purpose of this study is to assess the presence and duration of infectious EBOV in semen, vaginal fluids, and other body fluids of EVD survivors in Sierra Leone, which is critically important in understanding potential risk of EBOV transmission from survivors. Given the large number of survivors and the lack of evidence for virus persistence in various body fluids, the urgency to collect this needed information is of upmost importance in controlling future cases and potential clusters, and refining recommendations and public health practices. Describing persistence of EBOV in semen, vaginal secretions, and other body fluids (e.g., saliva, sweat, tears, urine, rectal swabs, menstrual blood, and breast milk) among male and female EVD survivors in Sierra Leone is needed in order to eliminate transmission in ways currently not being addressed.
Information collected will be used to address two objectives:
To analyze EBOV persistence in body fluids (semen or vaginal secretions, menstrual blood, breast milk, rectal swabs, saliva, sweat, urine, tears) using RT-PCR for all collected specimens, and virus isolation for RT-PCR positive specimens
To assess concordance between RT-PCR and virus isolation test results
The specific objectives of the study components are:
Pilot Study (Persistence of Ebola virus in semen among EVD survivors)
Inform the process and implementation of the study
Provide rapid results on the prevalence of EBOV by RT-PCR in semen of survivors
Assess the prevalence of live Ebola RNA detected by culture/viral isolation from RT-PCR semen
Main study (Persistence of Ebola virus in semen, vaginal secretions, and other body fluids among EVD survivors)
Assess the prevalence and duration after EVD recovery of any Ebola ribonucleic acid (RNA) (live or dead) detected by RT-PCR in: i) semen, ii) vaginal swab, iii) rectal swab, iv) sweat, v) urine, vi) saliva, vii) tears, viii) breast milk, and ix) menstrual blood.
Assess the prevalence after EVD recovery of live Ebola RNA detected by culture/viral isolation from i) semen, ii) vaginal swab, iii) rectal swab, iv) sweat, v) urine, vi) saliva, vii) tears, viii) breast milk, and ix) menstrual blood.
Evaluate the time to EBOV clearance using RT-PCR and viral isolation assays among persons with specimens that are positive by RT-PCR at the baseline visit.
Describe concordance between RT-PCR and viral culture results for EBOV in these body fluids.
For the pilot study and Main study, the study site(s) will be the setting for all initial and follow-up visits of survivor participants, including interviews, specimen collection, and counselling regarding test results. Study participants can be referred to HIV and survivor services within the hospitals where the study sites are located.
For the study:
Eligible study participants must demonstrate an EVD discharge certificate and a national identification card in order to be recruited; to ensure survivor status and eligibility for the study, collaborators may cross-check laboratory information as available; this information will be recorded in the Intake Form (Attachment 1) and a unique ID will be assigned.
Efforts will be made to recruit as many “recent survivors” as possible, recognizing that due to the successful efforts in containing the epidemic and delays in implementing the study it is possible that some participants will have > 1 year elapsed since their initial acute disease.
Survivors with experiences of experimental treatment during their EVD as well as any pregnant women will be included in the study
Entry will then begin with the informed Consent Form (Attachment 2a,c/b,d; for pilot study/Main study, respectively).
A Participant Study ID Card (Attachment 3) will be issued to the participant for verification of identity at subsequent study visits.
The participant will then complete a Survivor Questionnaire (Attachment 4a and 4b) and initial specimen collection. At baseline, all applicable body fluid specimens are collected depending on gender, lactation status, and which arm of the study the participant is enrolled in (Pilot vs. Main study).
Pilot samples collected prior to Oct 19, 2015 will be tested for EBOV RNA by RT-PCR in Sierra Leone at the CDC laboratory facility in Bo. Pilot sample collected Oct. 19, 2015 and onwards and Main study samples will be tested by China CDC laboratory in Jui;
Any specimen positive for EBOV by RT-PCR except semen collected from participants in the Main Study will be frozen and shipped to CDC for culture/virus isolation in a BSL-4 laboratory facility in Atlanta, GA.
Participants with a RT-PCR positive result in any of the initial specimens will be invited to complete a Survivor Follow-up Questionnaire (Attachment 5a and 5b) at every subsequent study visit and to provide prospective specimens (i.e., repeat specimen collection) of each RT-PCR positive body fluid according to the following schedule:
Semen collection every 2 weeks until the RT-PCR is negative in two consecutive samples (pilot).
Specimen (i.e., semen or vaginal secretions, rectal swabs, sweat, urine, saliva, tears, menstrual blood, breast milk) collection will be scheduled every 2 weeks until the RT-PCR is negative twice in every fluid collected (Main study).
At 3 and 6 months after completion of the body fluid testing, the participant will return for a follow-up visit where a questionnaire (Attachment 8a and 8b) will be administered that will ascertain the participant’s health since last visit, and a blood specimen and body fluid specimens will be collected as described above.
All laboratory results will be entered into a Laboratory Results Form (Attachment 6) marked with the participant’s study ID and kept in confidential storage for entering of follow-up results.
At follow up visits, laboratory results will be shared with the participant, and transmission prevention and potentially other counselling messages will be provided via a Counselling Script (Attachment 7a and 7b). Additional specimens are collected at follow-up visits for all body fluids that have not yet had two consecutive RT-PCR negative results.
Confirmatory virus culture results will be reported back to participants when available.
Once a participant has had two consecutive negative test results for all body fluid specimens collected in this study, there will be no further follow-up.
Study instruments will be translated into Krio and other regional languages as necessary by qualified translators. Data collectors will be trained extensively in conducting interviews and the ethical protection of human subjects. Clinic staff will enter participant questionnaire responses into laptop computers which will have the ability to connect to the internet and upload data into a secure questionnaire database. They will also be skilled in specimen collection and ways to communicate instructions for specimen collection to participants. A trained data manager will complete the laboratory results form (Attachment 6) each time a participant submits a specimen for testing. Data for laboratory specimen results will be stored in a secure specimen database after being recorded in computers at the laboratories conducting testing.
Data Flow Diagram
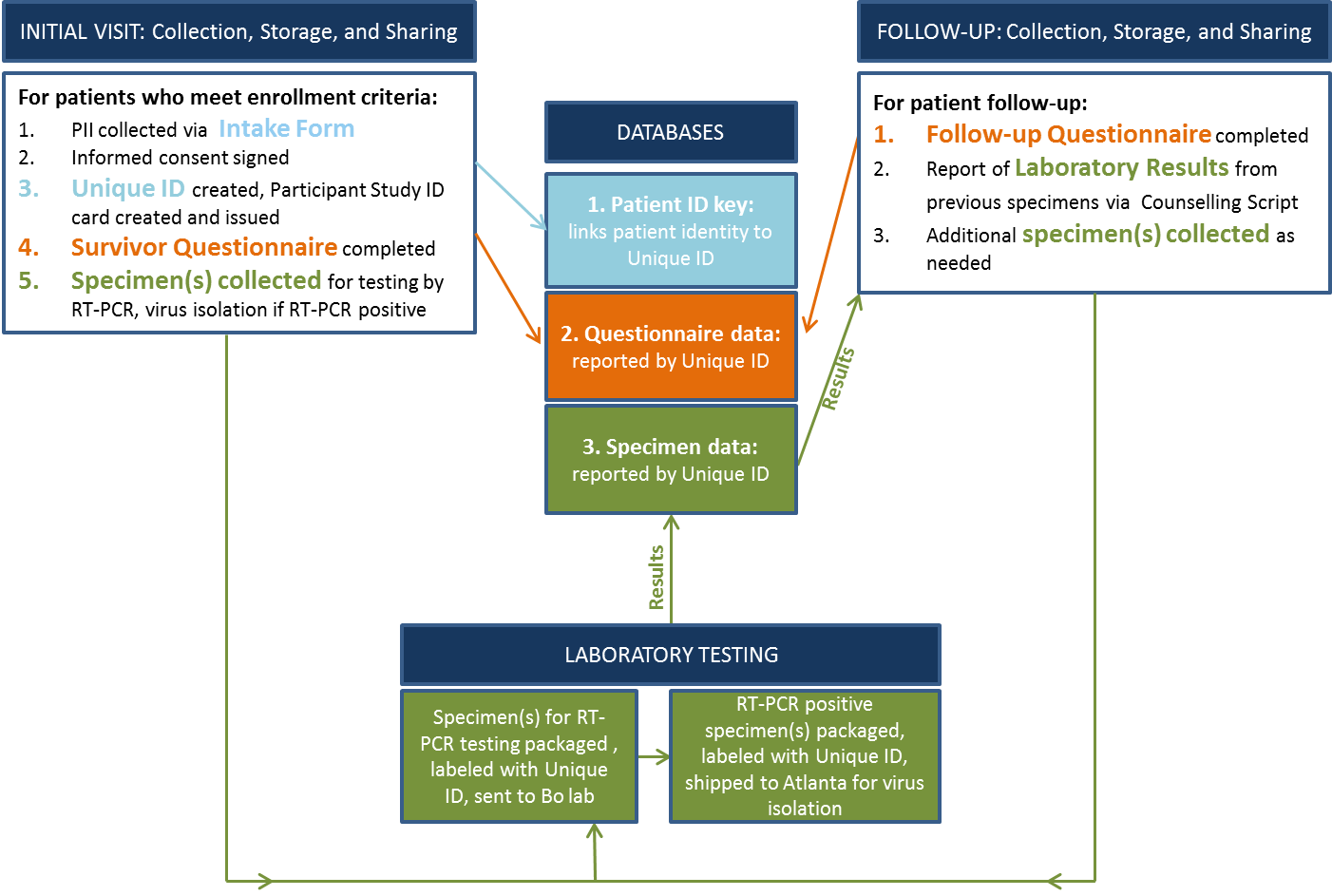
Components of the different study visits
|
Baseline visit |
Follow-up visitsa |
|
Additional specimen collection |
Dischargeb |
||
Informed consentc and enrolment |
X |
|
|
Assignment of a unique study identification number |
X |
|
|
Standardized questionnairec |
|
|
|
Socio-demographicsd, Ebola virus disease |
X |
|
|
(EVD) health status during and after EVD and sexual history (current/since last study visit) |
X |
X |
X |
Pre- EBOV-test counsellingc |
X |
X |
|
Post EBOV-test counsellingc according to test results |
|
X |
X |
Collection of body fluids |
X |
X |
|
Risk-reduction behavioral counsellingc |
X |
X |
|
Optional HIV counsellingc and testing |
X |
X |
X |
Optional pregnancy testing (when applicable) |
X |
X |
X |
Medical referral (when applicable) |
X |
X |
X |
a During initial set of visits, and three and six months after initial discharge.
b Participants were discharged when semen/all body fluids tested qRT-PCR negative for EBOV twice consecutively during the initial set of visits, and once during the three- and six-month follow-up after initial discharge.
c Performed in a language of the participants choice,
dbaseline visit only.
The EVD survivors with at least one of the body fluid positive at RT-PCR will be asked to return for follow-up study visits, first time after two weeks then every two to four weeks until there have been two consecutive negative RT-PCR test results for each body fluid. Participants will be asked to continue follow-up until all body fluid specimens are negative for two consecutive visits.
Lactating women will receive their results within 3 days after the collection of the specimen. Women with positive results in breast-milk will be offered testing every 3 days till 2 consecutive tests are negative; women with a first negative test will come back after 3 days to do a confirmatory test.
Menstruating women will be asked to provide a specimen as close to the first day of their period as possible, and a second specimen before the end of the period. Menstrual blood sampling may be done at the same time as other body fluid testing, or may occur after testing of other body fluids has been completed.
Results and analysis will be used to update public health interventions and messaging, relevant counselling messages, and other recommendations from CDC, WHO, and the Sierra Leone Ministry of Health and Sanitation (MoHS).
3. Use of Improved Information Technology and Burden Reduction
A CDC data manager will oversee data management and data integration activities in close partnership with WHO and MoHS collaborators. All questionnaire data will be collected via secure laptop computers using in-person interviews. Collected data will be directly recorded on computers or tablets to minimize data recording and entry errors and minimize delays in data availability. Interview and laboratory data will be organized in databases stored on secure local servers within the Sierra Leone MoHS and WHO and will be backed-up regularly. Given that the study will be conducted in Sierra Leone under resource-constrained conditions, the ability to utilize more advanced technology in data collection approaches will be limited. Information collection instruments were each designed to collect the minimum information necessary for the purposes of this project.
4. Efforts to Identify Duplication and Use of Similar Information
As described in the background section, the baseline results from the pilot phase of the study were critical discoveries in terms of the length of time and percentages of male survivors who had EBOV detected in their semen. Previous to this study, only a small number of male survivors had their semen tested and recommendations were based on these limited tests. It was critical that the Virus Persistence Study test semen from a large population of male survivors in order to demonstrate how prevalent EBOV RNA persistence in semen was, and to continue collecting specimens and testing these individuals in order to fully describe the duration of this persistence in the population. In the second phase of the study, additional males and females have had non-invasive body fluids collected in order to further improve scientific understanding of EBOV RNA persistence in semen but also in these other body fluids (sweat, tears, saliva, breast milk, rectal fluid, vaginal fluid, menstural blood). This information has not been collected from a comprehensive population of EVD survivors, and serves an important role in our understanding of whether EBOV may remain after recovery from EVD, and in which body compartments and which fluids.
5. Impact on Small Businesses or Other Small Entities
No small businesses will be involved in this information collection.
6. Consequences of Collecting the Information Less Frequently
This request is for an existing information collection without an OMB control number for a one-time data collection. There are no legal obstacles to reduce the burden.
The EVD survivors with at least one of the body fluid positive at RT-PCR will be asked to return for follow-up study visits, first time after two weeks then every two to four weeks until there have been two consecutive negative RT-PCR test results for each body fluid. Participants will be asked to continue follow-up until all body fluid specimens are negative for two consecutive visits.
Lactating women will receive their results within 3 days after the collection of the specimen. Women with positive results in breast-milk will be offered testing every 3 days till 2 consecutive tests are negative; women with a first negative test will come back after 3 days to do a confirmatory test.
Menstruating women will be asked to provide a specimen as close to the first day of their period as possible, and a second specimen before the end of the period. Menstrual blood sampling may be done at the same time as other body fluid testing, or may occur after testing of other body fluids has been completed.
If no data are collected, CDC, WHO, and MoHS will be unable to:
Understand the potential for EBOV transmission though body fluids other than blood (e.g., semen or vaginal secretions, menstrual blood, breast milk, rectal swabs, saliva, sweat, urine, tears)
Completely control EBOV transmission in Sierra Leone
Develop recommendations for public health and clinical partners responding to the Ebola outbreak
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
There are no special circumstances with this information collection package. This request fully complies with the regulation 5 CFR 1320.5 and will be voluntary.
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A. A 60-day Federal Register Notice was published on September 21, 2015, Vol. 80, No. 182; pp.56993–55. One comment was received but was not substantive in nature (Appendix C.)
B. Efforts to consult with persons outside the agency: For this project, the Sierra Leone MoHS and WHO are integrally involved in the concept, design, planning, staff hiring, data collection, analysis, reporting, and data storage together with CDC. A member of Sierra Leone MoHS is serving as the principal investigator. The Chinese Center for Disease Control (C-CDC) is leading laboratory testing of RT-PCR in Sierra Leone and is the lead for specimen archiving and maintenance. US-CDC study roles, to date and future, are described below:
Initiated the idea for the project. The information obtained will assist in creating and targeting public health interventions to decrease spread of EVD in Sierra Leone.
Designed and had significant input or control into the design of the data collection instruments.
Will analyze laboratory specimens shipped to Atlanta for repeat RT-PCR and virus isolation testing and will report jointly with MoHS, China-CDC, and WHO staff.
Will assist in the training of locally hired staff to conduct interviews and maintain the ethical protection of human subjects.
Joint data analysis will be collaboratively performed by the MoHS, WHO, China-CDC and CDC staff.
The CDC will be co-authors on manuscripts from this research study.
CDC staff have worked with the following research partners on all aspects of the research protocol and project management:
Sierra Leone MoHS; Ministry of Social Welfare, Gender, Children’s Affairs (MSWGCA) |
PI: Dr. Gibrilla Fadlu Deen ([email protected]) |
Co-PIs: Dr. Amara Jambai ([email protected]), Dr. Alie Wurie ([email protected]) |
Additional staff: Abdul Kamara, Tina Davies ([email protected]) |
WHO |
Coordination HQ: sexually transmitted infection, epidemiology subject matter expert: Dr. Nathalie Broutet ([email protected]) |
Epidemiologist infectious diseases subject matter experts: Dr. Anna Thorson ([email protected]) |
Research coordinator, SL: Suzanna MacDonald ([email protected]), Jaclyn Marrinan ([email protected]), Phillippe Gallard ([email protected] |
WHO representative – Ebola response: Dr. Anshu Banerjee |
Laboratory Virology: Dr. Pierre Formenty |
Reproductive health experts: Dr. Tim O’Dempsey, Dr. Lisa Thomas |
China-CDC |
Team Lead of China P3 Lab: Dr. William J. Liu Previous Team leads: Dr. Wenbo Xu, Dr. Hongtu Liu, Dr. Wenjiao Yin |
9. Explanation of Any Payment or Gift to Respondents
At each study visit, participants will receive a token of appreciation of 120,000 Leones (approximately $28 US dollars), as well as a 5-week supply of condoms, counselling, and linkages to health services as needed. This token of appreciation is in recognition of the effort required for participants to remain in the study potentially long-term, and for travel to study sites. Participant referral will be provided to EVD survivor services for post-recovery complications, as needed, and human immunodeficiency virus (HIV) testing will be offered to all participants. HIV-positive study participants will be referred to national HIV services. Counselling on potential breastfeeding transmission among lactating female participants will be executed by trained counsellors and alternative feeding will be provided. Breast-feeding women who test positive for EVD in breastmilk will be offered free formula feeding in line with current WHO guidelines.
Participants will receive these tokens of appreciation for attending a study visit even if they do not complete the interview or specimen collection. Participants who do not complete multiple study visits may be deemed ineligible from participating further. For more details regarding specimen collection during study visits, please see Section 2.
10. Protection of the Privacy and Confidentiality of Information Provided by Respondents
This ICR has been reviewed by the OMB PRA Advisor for the CDC emergency response who determined that the Privacy Act does not apply.
The Privacy Act does not apply and no system of records is being created:
The Privacy Act covers only records in the possession and control of Federal agencies.
A system of records consists of any item, collection, or grouping of information about an individual, where those records can be retrieved by the name of the individual or by some other type of identifier unique to the individual.
To qualify as a Privacy Act record, the information must identify an individual.
The Sierra Leone MoHS is the owner of the data and will not deliver data for storage on CDC servers except in coded or aggregate formats. These records will not be retrievable by any identifiers.
The Privacy Act applies to records maintained on a living individual who is “a citizen of the United States or an alien lawfully admitted for permanent residence.” All participants will be from Sierra Leone; therefore, their records will not be afforded privacy protections under the Act.
Regardless of the Privacy Act requirements, CDC will take all efforts to protect the privacy of study participants’ records.
The Sierra Leone MoHS will ensure coordination of project activities with routine MoHS response activities. Data from routine MoHS response activities (case investigation and contact tracing) will be shared for project purposes as needed. The Sierra Leone MoHS will advise in hiring local clinical and data entry staff. Clinical staff will interact with participants; clinical and data entry staff will have access to individually identifiable data. CDC and WHO will provide technical assistance in all project aspects and will supervise clinical staff and lead data analysis. As supervisory staff, CDC and WHO field staff will interact with participants and have access to individually identifiable data.
The MoHS identified two study sites in two high EVD burden districts (Western, and Port Loko) for the study:
M34 hospital, Freetown (pilot site location)
Lungi General Hospital, Port Loko
Several items containing personally identifiable patient information will be collected:
Name
Date of Birth
Photographic Identifiers
Mailing Address
Phone Numbers
Email Address
Medical Information and Notes
Medical Records Numbers
Biological Specimens
Certificates
Legal Documents
Employment Status
Other
The personal identifiers collected through the Intake Form (Attachment 1) and the assigned unique ID will be stored in a secure database. A Participant Study ID Card (Attachment 3) will be given to the patient with his/her unique ID number.
A letter of agreement between CDC and the Sierra Leone MoHS regarding sharing of data and laboratory specimens has been in place since September 2014, but a new Materials Transfer Agreement (MTA) and a Memorandum of Understanding (MOU) was created between CDC, WHO, and Sierra Leone; these agreements covers shipment of specimens from Sierra Leone to the United States. Sierra Leone MoHS will determine how and with whom their research data will be shared. For this study, it has been agreed that data will belong to the Sierra Leone MoHS, and joint analysis will be collaboratively performed by MoHS, WHO, China-CDC, and CDC staff. The data will be stored in a Sierra Leone MoHS-owned database. While in the field, CDC will have access to identifiable information. Data delivered to the CDC for statistical analysis will be identified by unique study ID only.
Data are treated in a private manner, unless otherwise compelled by law. Highly sensitive information is being collected and would affect a respondent’s privacy if there were a breach. MoHS, CDC, China-CDC, and WHO research partners will make every effort to secure the information as described in Section A.10.1.7.Respondents are informed about the voluntary nature of their participation during the consent process (Attachments 2a, b, c, and d).Consent scripts are included on the consent form attachment (Attachments 2a, b, c, and d ). Project assistants will obtain written consent from respondents prior to conducting all questionnaires.
How the information will be secured
All MoHS-owned data related to a study participant will be assigned the participant’s unique study ID. No patient-identifying information (i.e., names) will leave Sierra Leone.
Interview data will be organized in databases stored on secure local servers within the MoHS in Sierra Leone and WHO and will be backed-up regularly. Collected data will be directly recorded on computers or tablets to minimize data recording and entry errors and minimize delays in data availability. If paper forms must be used, interview responses will be entered into the database either daily or as a group at the close of data collection; and 10% of entered forms will be re-checked to identify any problems with data entry accuracy that must be addressed.
Electronic equipment and files will be kept password-protected.
Paper forms and electronic devices will be kept locked when not in use.
All individual data identifying direct patient identifiers will be removed from the dataset before analysis and replaced with a unique participant code that can be linked back to individuals via a master key at a centralized secure server and database.
Individual records and the key linking the participant code number will be kept secure, accessible only to the local study team under the supervision of the study PI and MoHS in collaboration with WHO and CDC.
Paper interview forms, if used, will be destroyed within one year after all data are entered and verified.
Laboratory results will be batch processed and complete PCR results for all specimen types will be reported back within 1 week of specimen receipt to each site coordinator. A positive RT-PCR result on any specimen should be entered in the secured data base and reported within 8 hours to the study PI and WHO and CDC local coordinators as well as the site coordinator where the specimen was collected. Laboratory staff will identify specimens only by the labelled study ID, and will not have access to any personally identifying information.
Site coordinators will provide laboratory results only in person and only to participants who (1) return for a follow-up study visit; and (2) present their study ID card or confirm their identity and study ID number; and (3) request to receive their own results. Individual results will not be shared with anyone other than the study participant. Results will be presented according to the counselling script.
Viral isolation results from CDC Atlanta will be reported promptly to the study PI and entered in the secured data base as they arrive. These confirmatory results will not be available within a clinically relevant time frame (6 months) although positive results will be reported to participants.
.
11. Institutional Review Board (IRB) and Justification for Sensitive Questions
The Human Subjects Regulatory Advisor for the CDC emergency response has reviewed the proposed information collection, which is determined to be research. The CDC has entered into a reliance agreement with the Sierra Leone MoHS Institutional Review Board, which has reviewed and approved the research protocol (Appendix D). The project is dependent on willingness from participants to share intimate information on sexual behavior, and to provide biological specimens. For the individual, there is a risk of the intimate interview and sampling procedure creating anxiety for the interviewee and for his/her family members. Questions will be asked about EVD symptoms experienced (vomiting, diarrhea, etc.), current health (health status, co-morbidities), and sex-related items (using condoms, sore in genital area, type of sexual practice, etc.) that might be embarrassing.
It is also anticipated that study participants receiving their specimen test results may experience psychological and social stress, especially when results are of unknown clinical utility and may be subject to misinterpretation. At the community level, there is a risk of stigma or discrimination enacted towards study participants. However, this information is essential to understanding EBOV persistence in different body fluids and efficiently detecting a public health threat from contact with EVD survivors. This information will allow public health officials, including CDC, to rapidly implement appropriate public health control measures to prevent the introduction and spread of further EVD in communities.
Several survivors’ support groups are actively working to provide counselling and support of various qualities to EVD survivors in Sierra Leone, in collaboration with UNICEF and the national survivors’ association, which study collaborators anticipate will mitigate community concerns.
Benefits of the study also include possibilities for participants to gain information about EBOV persistence in body fluids, and for the community to receive accurate recommendations on how to stop transmission chains.
12. Estimates of Annualized Burden Hours and Costs
The total estimated annualized time burden to Sierra Leone respondents is 1,850 hours at a total cost burden in respondent wages of $6,996.50 in US Dollars (USD).
Estimated Burden Hours
Using WHO funds only, the study team previously held a focus group with 15 male EVD survivors at the Military 34 hospital grounds; CDC personnel observed, but did not conduct, this focus group. The study team provided an overview of the study and explained the rationale, followed by a review of the informed consent and questionnaire with the group. The group provided feedback on their comprehension of the informed consent, the acceptability of the subject matter, and questionnaire content. Revisions were made to the informed consent and questionnaire based on this feedback. The group did not find the informed consent or questionnaire process burdensome. From this process, it is estimated that the average time to complete the:
Survivor questionnaire (Attachment 4a and 4b) (including time for reviewing instructions, gathering needed information and completing the instrument) is 30 minutes;
Follow-up questionnaire (Attachment 5a and 5b) is 15 minutes; and
Patient laboratory report (Attachment 6) is 10 minutes.
For the primary outcome of live virus isolated in tissue culture, even one positive (live EBOV virus) outcome is of interest; as such a finding would be expected to have important implications for guidance to future caregivers, patients, and their families. Similarly, we would not want to conclude that there is no probability of persistent live EBOV virus in the fluids we test if, in reality (within the larger population), there is. Therefore, the primary power calculation of interest is the degree of confidence we can have in a null (no positives) result from our tissue culture testing. In other words, we need to know the upper confidence limit for a calculated proportion of 0% in our study.
We arrived on a sample size of 100 men for the Pilot phase of the study not based on sample size calculations in relation to power, but on feasibility for assessing study process and generating enough knowledge for main study. For the main study, we arrived at our sample size based on the assumption of a 1st sample period prevalence in body fluids other than semen approaching zero. We estimated that a target sample of 100 would provide a 95% confidence interval in gender specific sub-analysis of 0-3.6% and if all specimens are negative for both sexes a collapsed group analysis with a 95% CI of 0-1.8%. We then added an additional 20% of participants to account for potential drop-out, giving a total sample size of 120 men and 120 women.
The estimated number of responses per respondent is based on several criteria:
The intake form (Attachment 1) will be completed by the trained data manager once for all participants from the pilot and Main study.
The initial survivor questionnaire (Attachment 4a and 4b) will be administered once for all participants from the pilot and Main study.
The frequency of the follow-up questionnaire (Attachment 5a and 5b) in the pilot and Main study is dependent on positive test results, which will vary among study participants. Follow-up visits will continue at specified intervals for the pilot and Main study until RT-PCR for pertinent specimens is negative.
Estimated number of follow-up visits, or the expected number of responses per respondent, is based on previously published literature. Male participants are expected to have the highest number of follow-up visits given the persistence of detectable EBOV (PCR and/or virus isolation) in semen several months post symptoms onset. Study collaborators do not expect participants to drop out of the study; however, if this occurs, the provided estimated burden hours would actually be overestimated.
Because laboratory tests are planned at each clinical visit, the estimated number of responses for the data manager entering laboratory results (Attachment 6) was calculated by multiplying the number of respondents by their estimated number of responses.
Table 12.A. Estimated Burden Hours
Type of Respondent |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Average Burden per Response (hours) |
Total Burden Hours |
Data manager |
Intake Form |
1 |
550 |
20/60 |
183 |
Pilot participants |
Survivor Questionnaire |
100 |
1 |
30/60 |
50 |
Pilot participants |
Survivor Follow-up Questionnaire |
100 |
5 |
15/60 |
125 |
Pilot participants |
3 & 6 Month Follow up Questionnaire |
100 |
2 |
15/60 |
50 |
Main study male participants |
Survivor Questionnaire |
120 |
1 |
30/60 |
60 |
Main study male participants |
Survivor Follow-up Questionnaire |
120 |
12 |
15/60 |
360 |
Main study male participants |
3 & 6 Month Follow up Questionnaire |
120 |
2 |
15/60 |
60 |
Main study female participants |
Survivor Questionnaire |
120 |
1 |
30/60 |
60 |
Main study female participants |
Survivor Follow-up Questionnaire |
120 |
4 |
15/60 |
120 |
Main study female participants |
3 & 6 Month Follow up Questionnaire |
120 |
2 |
15/60 |
60 |
Data manager |
Laboratory Results Form |
1 |
4,250 |
10/60 |
708 |
Total |
|
1,836 |
|||
Estimated Annualized Burden Costs
For Sierra Leone, we based our wage estimate on internet news pages where increases in 2015 minimum wages are published (Appendix E).
To convert from monthly to hourly wages, we also assumed the following relationships: 8 hours/day; 40 hours/week; 4 weeks/month; 160 hours/month. We used the following equation:
Hourly
minimum wage rate = USD per hour =
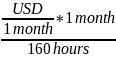 or
or
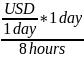
Table 12.B.1. Minimum Wage in Sierra Leone
West Africa |
Official Language |
2013 Population Estimate |
Minimum Wage Estimates |
Source |
||
Country Wage |
US Dollar2 |
|||||
Monthly |
Hourly |
|||||
Sierra Leone |
English |
6.09m |
500,000 SL leone/month |
112.65 |
0.70 |
Internet News 2015 |
Due to lack of documented information from existing sources, we assume that all survivor participants will be unskilled workers to which minimum wage applies, and that trained data managers will be skilled workers and have wages that are 10 times the minimum wage ($7.00/hour).
Table 12.B.2. Estimated Annualized Burden Costs
Type of Respondent |
Form Name |
Total Burden Hours |
Hourly Wage Rate |
Total Respondent Costs |
Data manager |
Intake Form |
183 |
$7.00 |
$1,281.00 |
Pilot participants |
Survivor Questionnaire |
50 |
$0.70 |
$35.00 |
Pilot participants |
Survivor Follow-up Questionnaire |
125 |
$0.70 |
$87.50 |
Pilot participants |
3 & 6 Month Follow up Questionnaire |
50 |
$0.70 |
$35.00 |
Main study male participants |
Survivor Questionnaire |
60 |
$0.70 |
$42.00 |
Main study male participants |
Survivor Follow-up Questionnaire |
360 |
$0.70 |
$252.00 |
Main study male participants |
3 & 6 Month Follow up Questionnaire |
60 |
$0.70 |
$42.00 |
Main study female participants |
Survivor Questionnaire |
60 |
$0.70 |
$42.00 |
Main study female participants |
Survivor Follow-up Questionnaire |
120 |
$0.70 |
$84.00 |
Main study female participants |
3 & 6 Month Follow up Questionnaire |
60 |
$0.70 |
$42.00 |
Data manager |
Laboratory Results Form |
708 |
$7.00 |
$4,956.00 |
Total |
|
$6,898.50 |
||
13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
There will be no direct costs to the respondents other than their time to participate in each information collection.
14. Annualized Cost to the Government
The total estimated annualized cost of this research study is $1,450,505.10.
The CDC is funding this study under cooperative agreement for the total estimated amount of $176,933.50. The annualized wage and travel cost to the federal government is estimated at $1,273,571.60. This is based Table 14, which represents the amount of time for CDC staff to advise and design the data collection methodology, provide data management and statistical support, perform laboratory testing, and aggregate and summarize the study data both in the field and in Atlanta during the emergency approval period. Travel costs are included, although there are no equipment or overhead costs.
Table 14. Estimated 180-day Federal Government Wages and Travel Costs
Staff (FTE) |
Est. Hours |
Average Hourly Rate |
Average Cost |
Atlanta-based Support Hourly Wage |
|||
Design of methods |
1 FTE 240 hours |
GS14 $49.38 |
$11,851.20 |
2 FTE 800 hours |
GS13 $41.79 |
$66,864.00 |
|
Laboratory testing: virus isolation |
3 FTE 800 hours |
GS12 $35.14 |
$42,168.00 |
Field-based Support Hourly Wage |
|||
Field operations support |
2 FTE 7680 hours |
GS13 $41.79 |
$641,894.40 |
Data management: aggregation and summary |
1 FTE 200 hours |
GS14 $49.38 |
$9,876.00 |
1 FTE 200 hours |
GS13 $41.79 |
$8,358.00 |
|
Laboratory testing: RT-PCR |
4 FTE 2000 hours |
GS12 $35.14 |
$140,560.00 |
Travel expenses |
|||
Flights (2 FTEs, 16 flights each, estimated $2,000 per flight) |
$64,000.00 |
||
Per diem (estimated $300/day, 960 days) |
$288,000.00 |
||
Total Cost |
$1,273,571.60 |
||
http://www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/salary-tables/pdf/2015/ATL_h.pdf |
|||
15. Explanation for Program Changes or Adjustments
N/A
16. Plans for Tabulation and Publication and Project Time Schedule
Descriptive analysis of the study participants will be performed by the study PI and staff with the support of WHO and CDC. WHO and CDC technical assistance may be provided as needed for these and any additional analyses of the study data.
Pilot study data collection and analysis results will inform the Main study. Results and analysis (both interim and final) will be used to update and refine relevant counselling messages and recommendations from WHO and CDC. Potential products include scientific abstracts and manuscripts, presentations, guidance documents, and others. The MTA, MOU, and a data sharing/data use agreement will be used between MoHS, WHO and CDC in order to ensure collaboration and appropriate recognition of all contributing coauthors. All parties (MoHS, WHO, and CDC) will share ownership of all data in the study; although, CDC will retain only de-identified data on federal servers. Publication policy will follow international guidelines for authorship applying Vancouver criteria, and all publications will be shared and reviewed by the three parties before submission for publication. Local partnerships are involved with the UNAIDS country office, with MSWGCA and local survivors’ organizations. These local partners will support the study process in different respects, during the data collection process and/or in relation to specific needs of study participants. Local partners will be offered co-authorship in relation to fulfilling the Vancouver criteria for authorship.
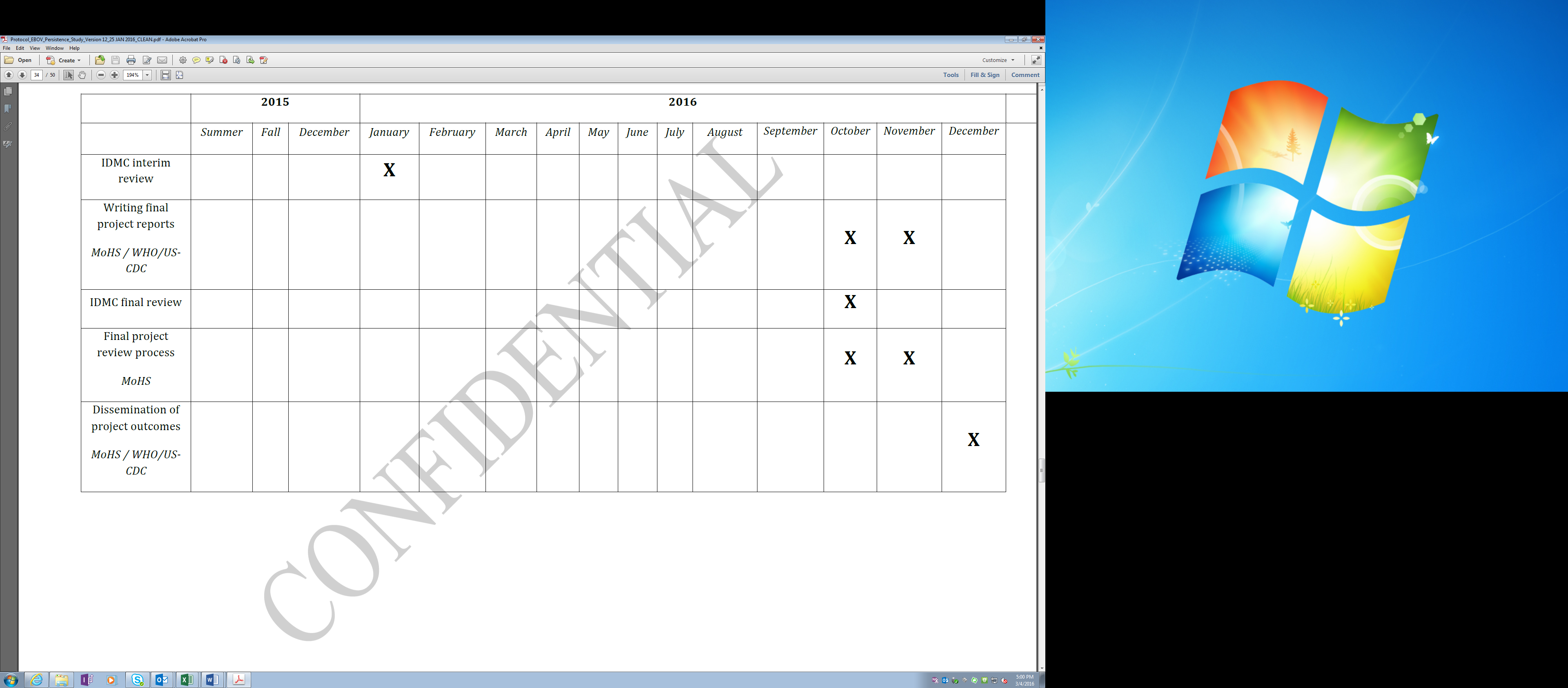
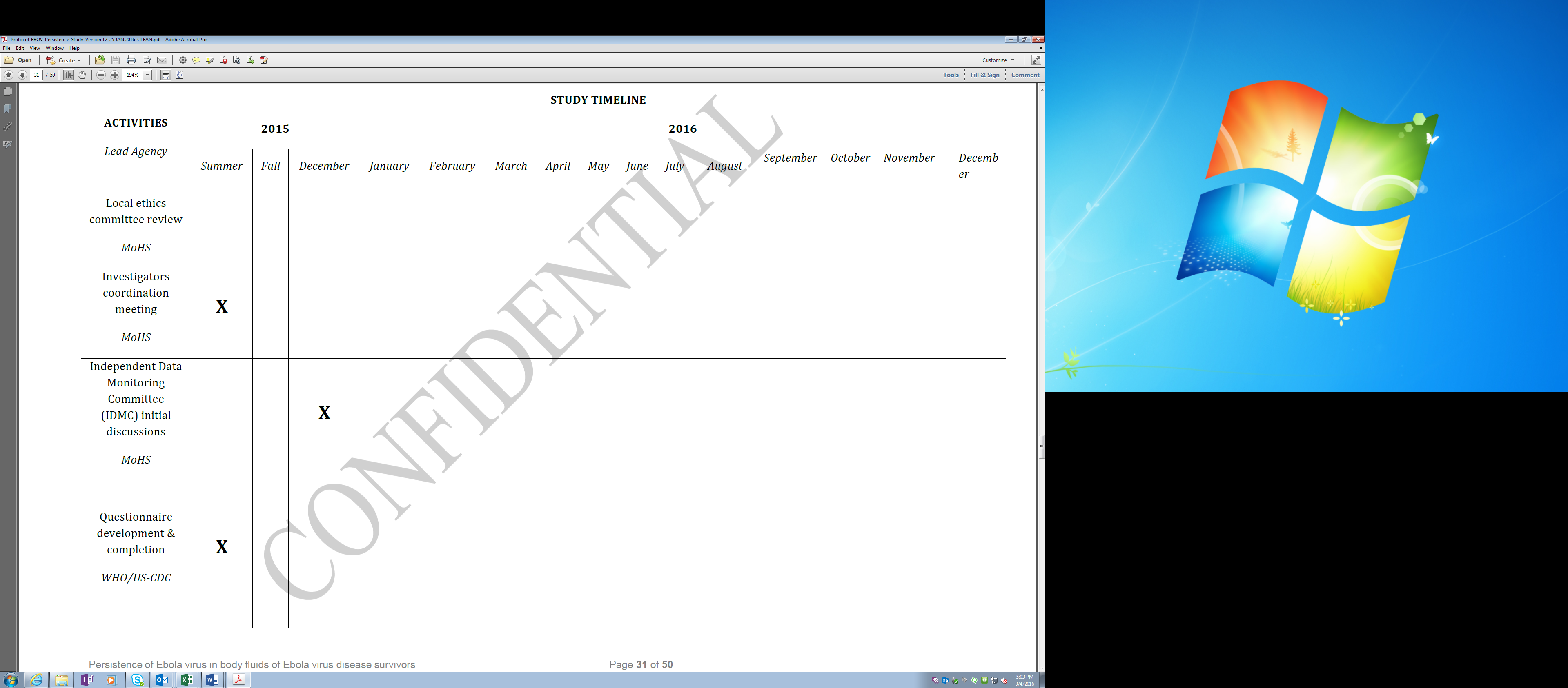
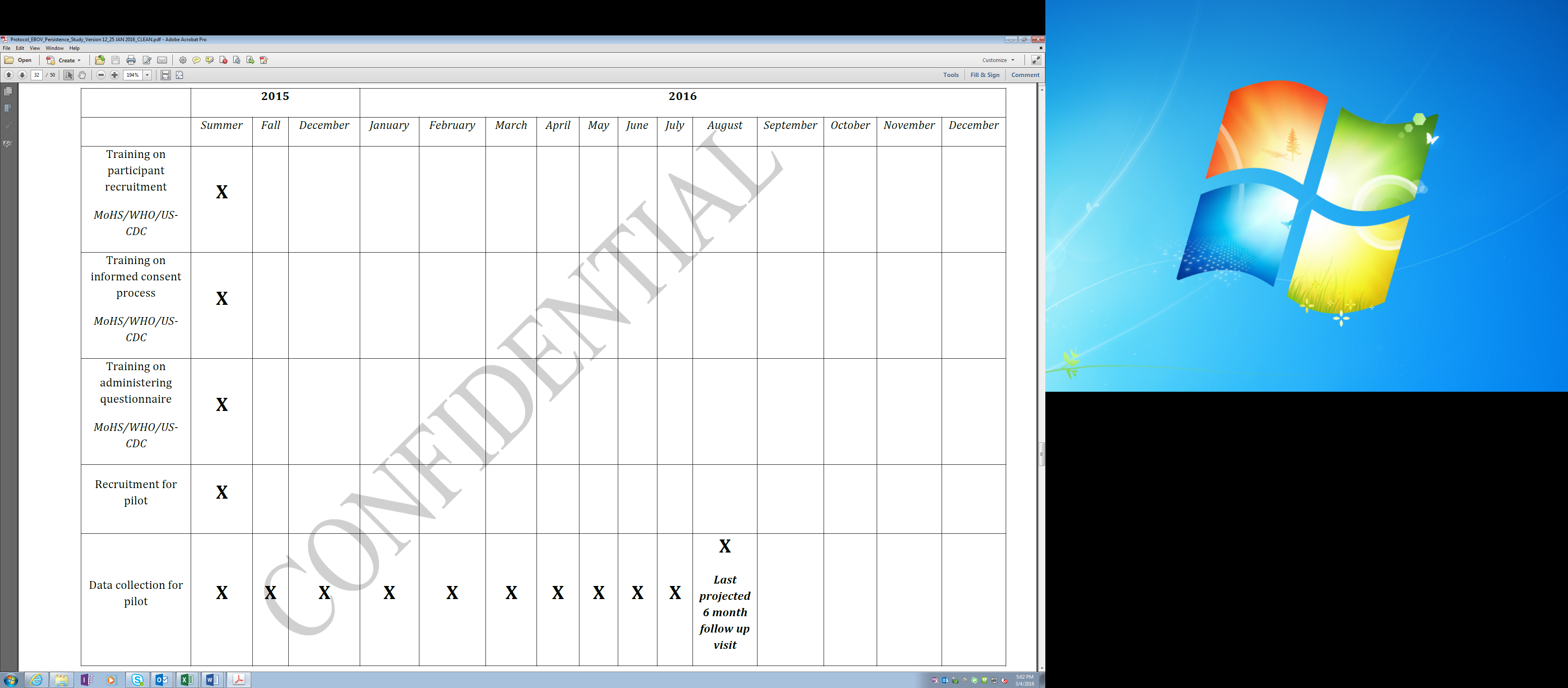
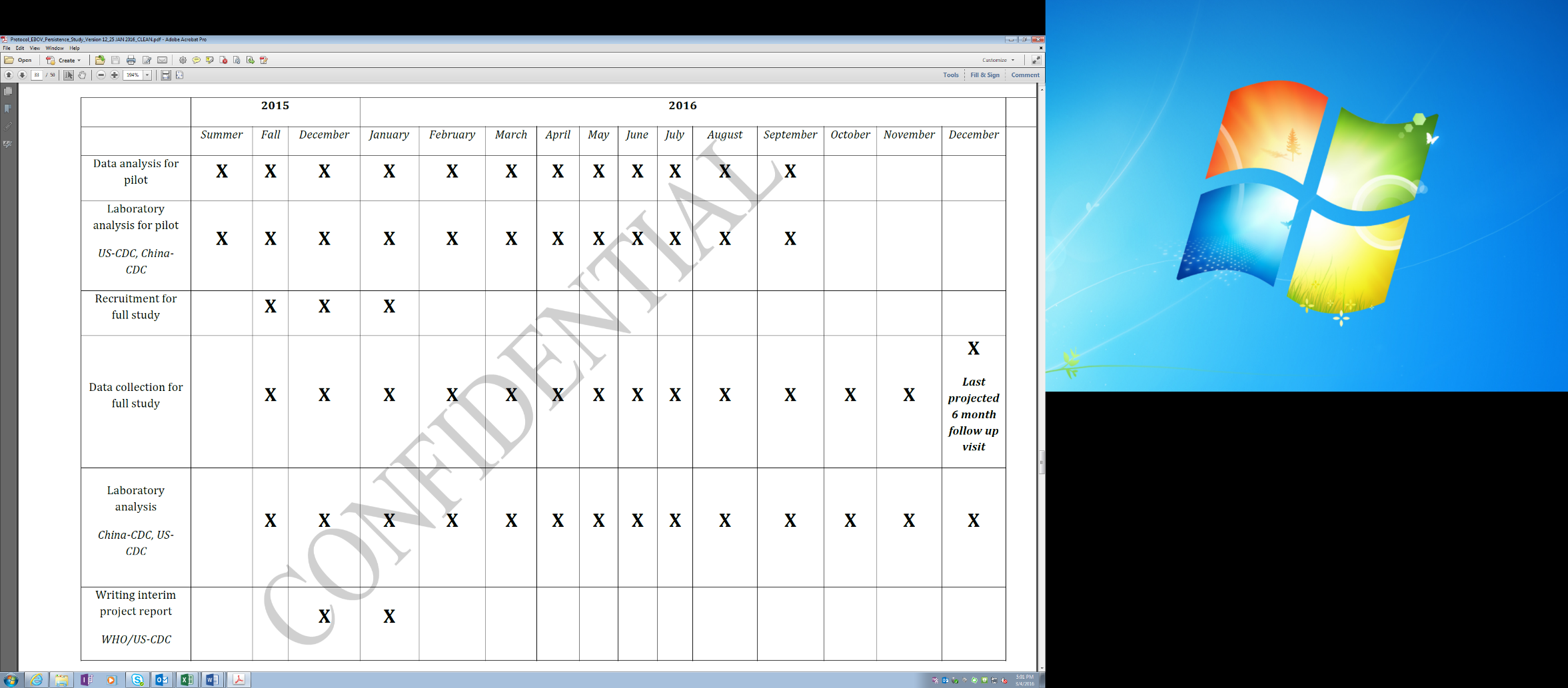
A more detailed 2015 study timeline is as follows:
Work Plan |
||||||||||||
ACTIVITIES - Lead agency (other agency involvement TBD) |
Study Timeline (2015-2016) |
|||||||||||
Month 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|
Local ethics committee review - MoHS |
1 |
|
|
|
|
|
|
|
|
|
|
|
Investigators coordination meeting - MoHS |
|
2 |
|
|
|
|
|
|
|
|
|
|
Data and Safety Monitoring Board (DSMB) initial discussions - MoHS |
1 |
|
|
|
|
|
|
|
|
|
|
|
Questionnaire development & completion - WHO/CDC |
1 |
|
|
|
|
|
|
|
|
|
|
|
Training on participant recruitment – MoHS/WHO/CDC |
|
2 |
|
|
|
|
|
|
|
|
|
|
Training on informed consent process - MoHS/WHO/CDC |
|
2 |
|
|
|
|
|
|
|
|
|
|
Training on administering questionnaire - MoHS/WHO/CDC |
|
2 |
|
|
|
|
|
|
|
|
|
|
Months after OMB Approval |
||||||||||||
Recruitment for pilot - MoHS |
|
2 |
|
|
|
|
|
|
|
|
|
|
Data collection for pilot - MoHS/WHO/CDC |
|
|
3 |
4 |
5 |
|
|
|
|
|
|
|
Data analysis for pilot - WHO/CDC |
|
|
3 |
4 |
|
|
|
|
|
|
|
|
Laboratory analysis for pilot - CDC |
|
|
3 |
4 |
|
|
|
|
|
|
|
|
Recruitment for Main study - MoHS |
|
|
|
4 |
5 |
|
|
|
|
|
|
|
Data collection for Main study - MoHS / WHO/CDC |
|
|
|
4 |
5 |
6 |
7 |
8 |
9 |
|
|
|
Data Management (for pilot, Main Study) - MoHS / WHO/CDC |
|
|
|
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
|
Data analysis - WHO/CDC |
|
|
|
|
|
|
|
|
|
10 |
11 |
12 |
Laboratory analysis (for pilot, Main study) - CDC |
|
|
|
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
Writing interim project report - WHO/CDC |
|
|
|
|
|
6 |
7 |
|
|
|
|
|
DSMB interim review - MoHS |
|
|
|
|
|
6 |
|
|
|
|
|
|
Writing final project reports - WHO/CDC |
|
|
|
|
|
|
|
|
|
10 |
11 |
12 |
DSMB final review - MoHS |
|
|
|
|
|
|
|
|
|
|
|
12 |
Final project review process - MoHS |
|
|
|
|
|
|
|
|
|
|
|
12 |
Dissemination of project outcomes - MoHS / WHO/CDC |
|
|
|
|
|
|
|
|
|
|
|
12 |
Updated Timeline: Pilot Study
Updated Timeline: Main Study
17. Reason(s) Display of OMB Expiration Date is Inappropriate
The display of the OMB expiration date is appropriate.
18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification. These activities comply with the requirements in 5 CFR 1320.9.
LIST OF APPENDICES
APPENDIX A. Authorizing Legislation
APPENDIX B. 60-Day Federal Register Notice
APPENDIX C. Public Comments
APPENDIX D. Human Subjects Review and Approvals
APPENDIX E. Sierra Leone News 2015 Minimum Wage
LIST OF ATTACHMENTS
ATTACHMENT 1. Intake Form
ATTACHMENT 2a. Consent Form – Pilot Study
ATTACHMENT 2b. Consent Form – Main Study
ATTACHMENT 2c. Re-Consent Form - Pilot Study
ATTACHMENT 2d. Re-Consent Form - Main Study
ATTACHMENT 3. Participant Study ID Card
ATTACHMENT 4a. Survivor Questionnaire – Male
ATTACHMENT 4b. Survivor Questionnaire – Female
ATTACHMENT 5. Survivor Questionnaire/Survivor Follow-up Questionnaire - Pilot
ATTACHMENT 5a. Survivor Follow-up Questionnaire - Male
ATTACHMENT 5b. Survivor Follow-up Questionnaire - Female
ATTACHMENT 6. Laboratory Results Form
ATTACHMENT 7a. Counselling Script – Male
ATTACHMENT 7b. Counselling Script - Female
ATTACHMENT 8a. 3-6 Month Follow-up Questionnaire – Male
ATTACHMENT 8b. 3-6 Month Follow-up Questionnaire – Female
1 Deen GF, Knust B, Broutet N, et al. Ebola RNA Persistence in Semen of Ebola Virus Disease Survivors – Preliminary Report. N Engl J Med. DOI: 10.1056/NEJMoa1511410.
2 Mate, SE, Kugelman, JR, Nyenswah, TG, et al. (2015). Molecular Evidence of Sexual Transmission of Ebola Virus. N Engl J Med. Epub PMID: 26465384.
3 Diallo B, Sissoko D, Loman NJ, et al. Resurgence of Ebola virus disease in Guinea linked to a survivor with virus persistence in seminal fluid for more than 500 Days. Clin Inf Dis, 2016.
2 USD conversion performed 02/12/2015 at eXchangeRate.com at http://www.exchangerate.com/currency-converter/XOF/USD/60,000%20/?XR-200Plus_Converter=convert&calc_short_from_iso=59&calc_short_to_iso=239
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | O2C2-Supporting-Statement-A-Template-CDCID-NICKNAME-SSA |
| Author | CDC User |
| File Modified | 0000-00-00 |
| File Created | 2021-01-24 |
© 2026 OMB.report | Privacy Policy