CMS-367a, CMS-367b Medicaid Drug Program Monthly and Quarterly Drug Reporti
Medicaid Drug Rebate Program - Manufacturers and Supporting Regulation at 42 CFR 447.534 (CMS-367)
DDR Screenshots for CMS-367a_b_c_3.18.16
Medicaid Drug Rebate Program - Manufacturers and Supporting Regulation at 42 CFR 447.534 (CMS-367)
OMB: 0938-0578
Online Quarterly Pricing Data Entry Screen (CMS-367a)
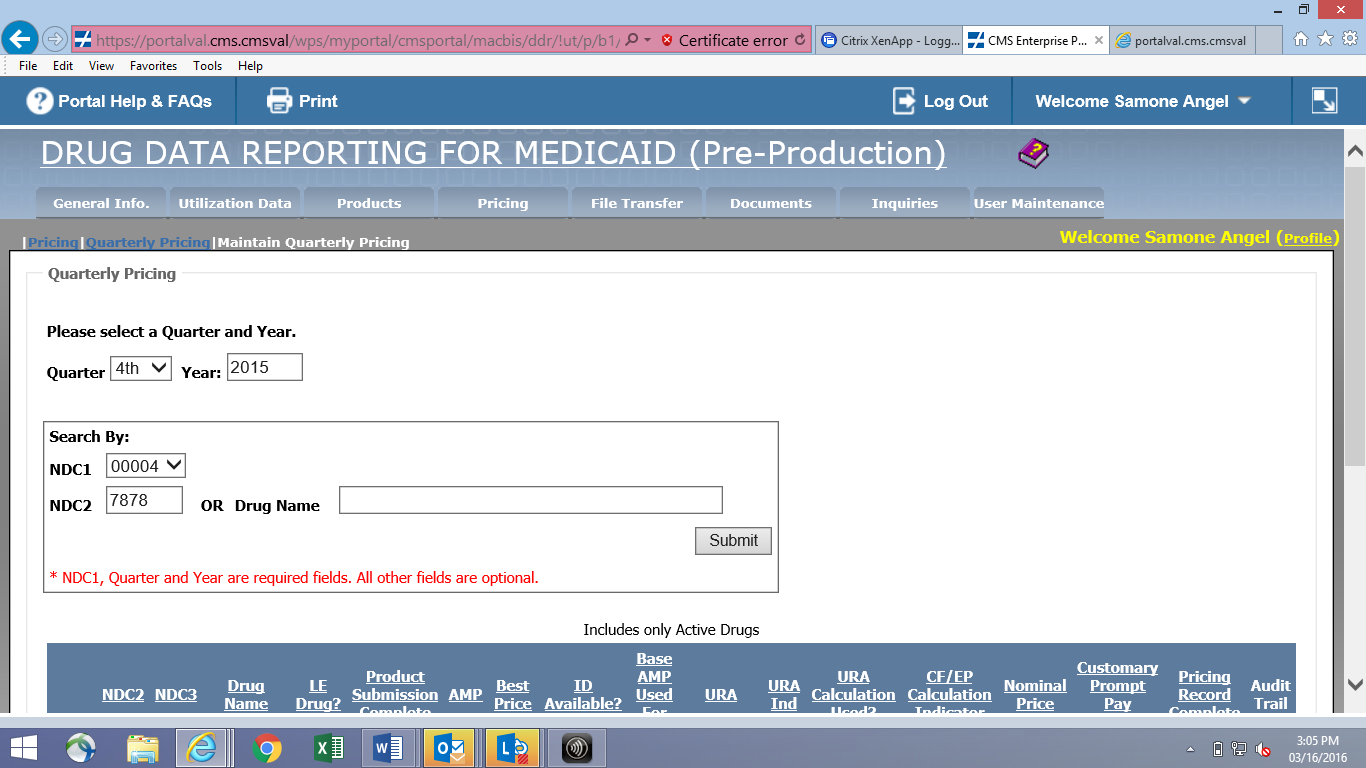
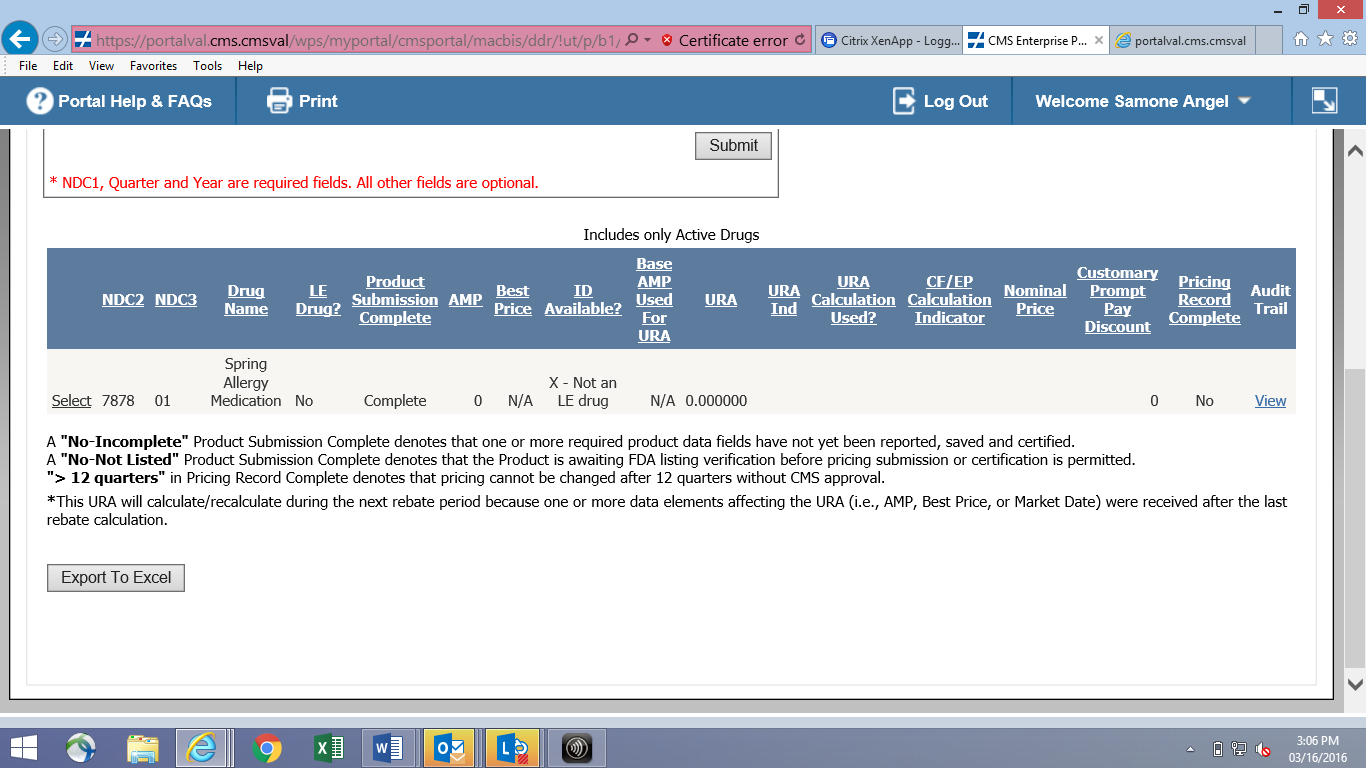
File Transfer Quarterly Pricing Layout (CMS-367a)
Field |
Size |
Position |
Remarks |
Record ID |
1 |
1 - 1 |
Constant of “Q” |
Labeler Code |
5 |
2 - 6 |
NDC #1 |
Product Code |
4 |
7 - 10 |
NDC #2 |
Package Size |
2 |
11 – 12 |
NDC #3 |
Period Covered |
5 |
13 – 17 |
QYYYY (Qtr/Yr) |
Average Mfr Price |
12 |
18 – 29 |
99999.999999 |
Best Price |
12 |
30 – 41 |
99999.999999 |
Nominal Price |
9 |
42 – 50 |
999999999 |
Customary Prompt Pay Disc. |
9 |
51 – 59 |
999999999 |
Initial Drug Available for LE |
1 |
60-60 |
Y, N, X or Z |
Initial Drug |
9 |
61-69 |
9 digits alpha-numeric |
Online Monthly Pricing Data Entry Screen (CMS-367b)
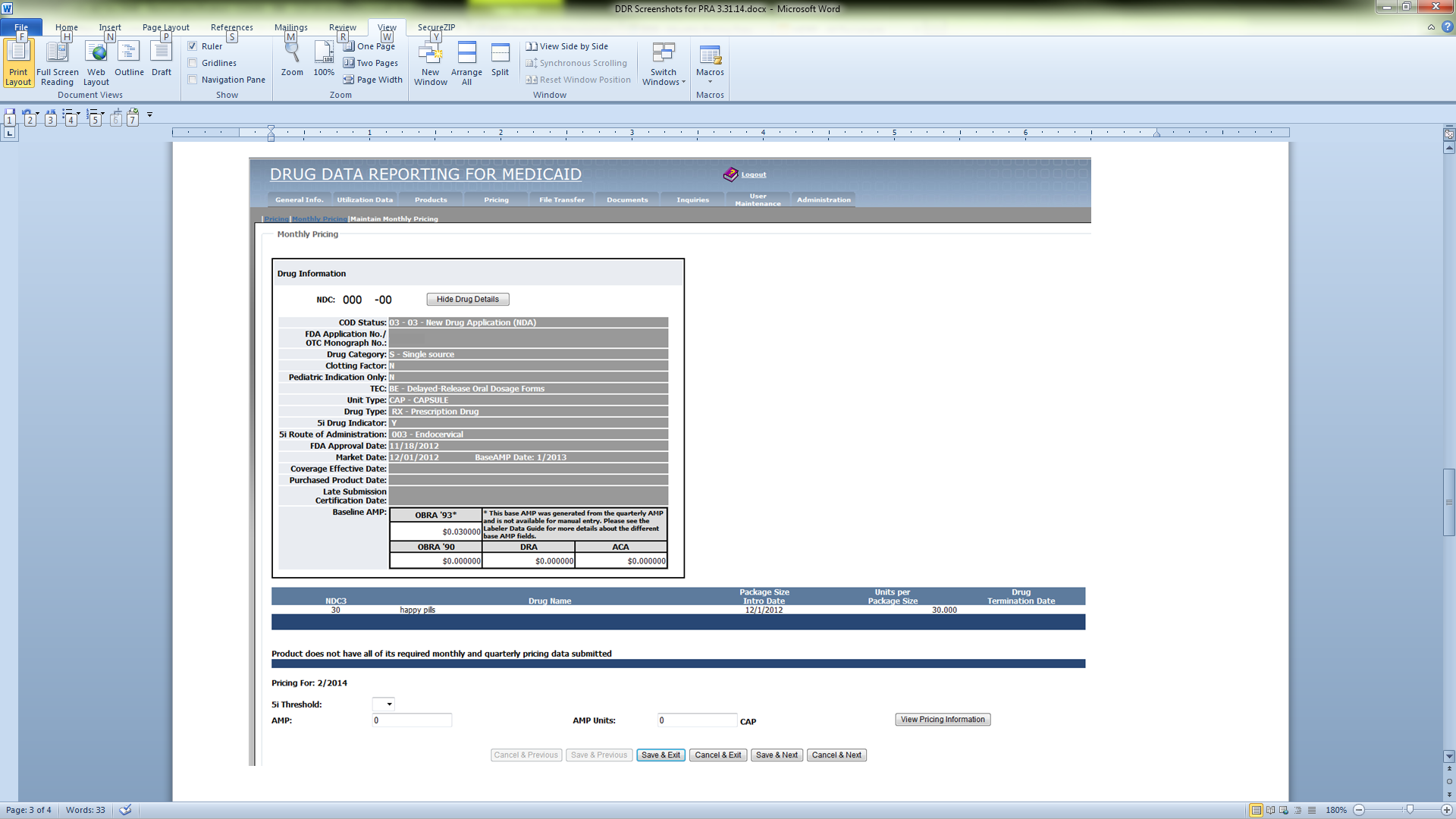
File Transfer Monthly Pricing Layout (CMS-367b)
Field |
Size |
Position |
Remarks |
Record ID |
1 |
1 – 1 |
Constant of “M” |
Labeler Code |
5 |
2 – 6 |
NDC #1 |
Product Code |
4 |
7 – 10 |
NDC #2 |
Package Size |
2 |
11 – 12 |
NDC #3 |
Month |
2 |
13 – 14 |
MM |
Year |
4 |
15 – 18 |
YYYY |
Average Mfr Price |
12 |
19 – 30 |
99999.999999 |
AMP Units |
14 |
31 – 44 |
99999999999.99 |
5i Threshold |
1 |
45 - 45 |
Y, N, X, or Z |
Online Product Data Entry Screen (CMS-367c)
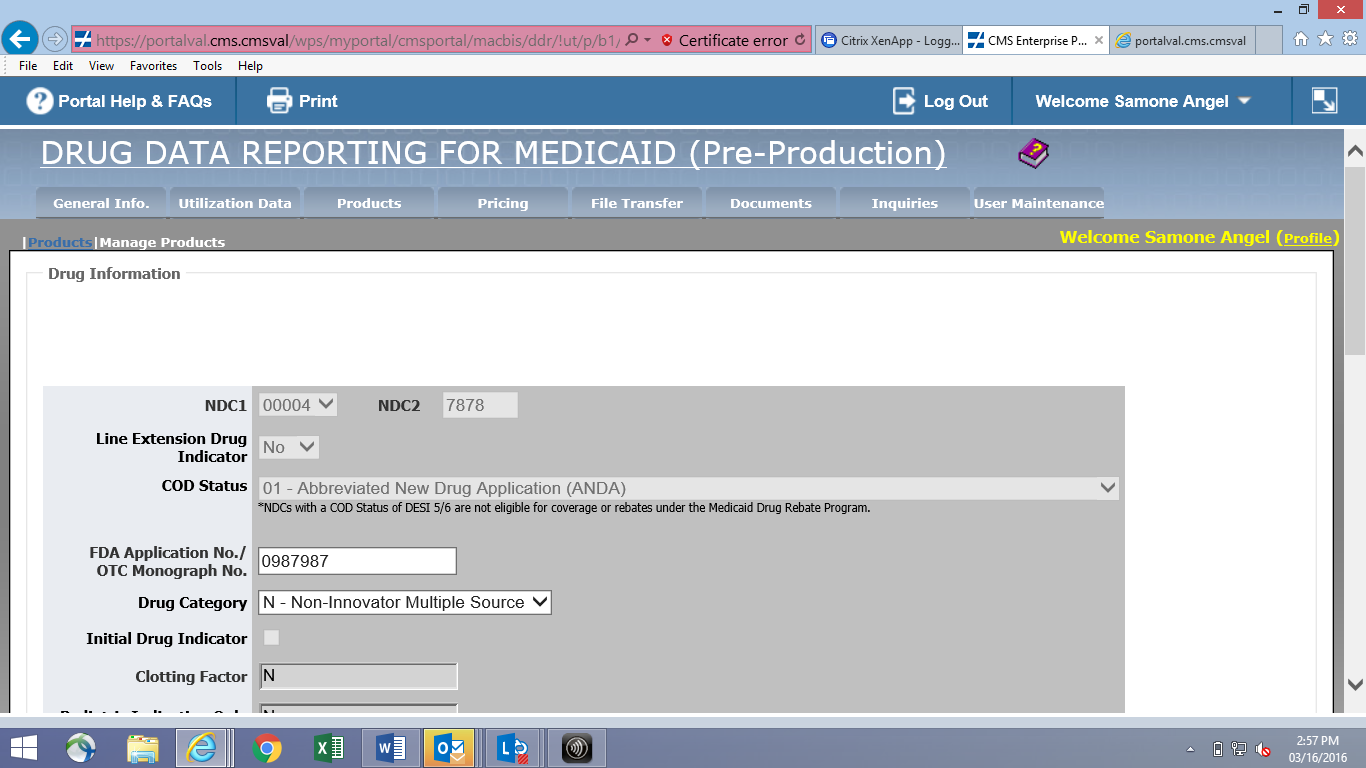
![]()
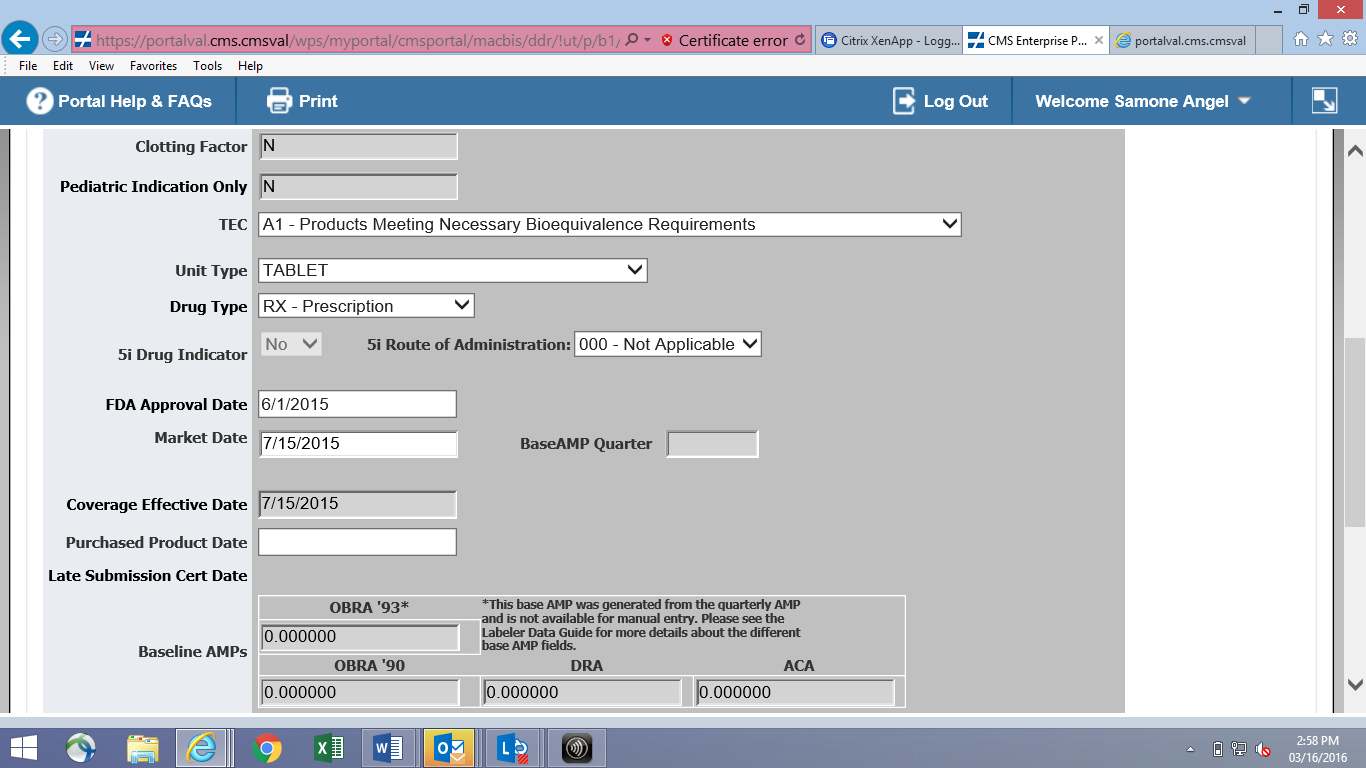
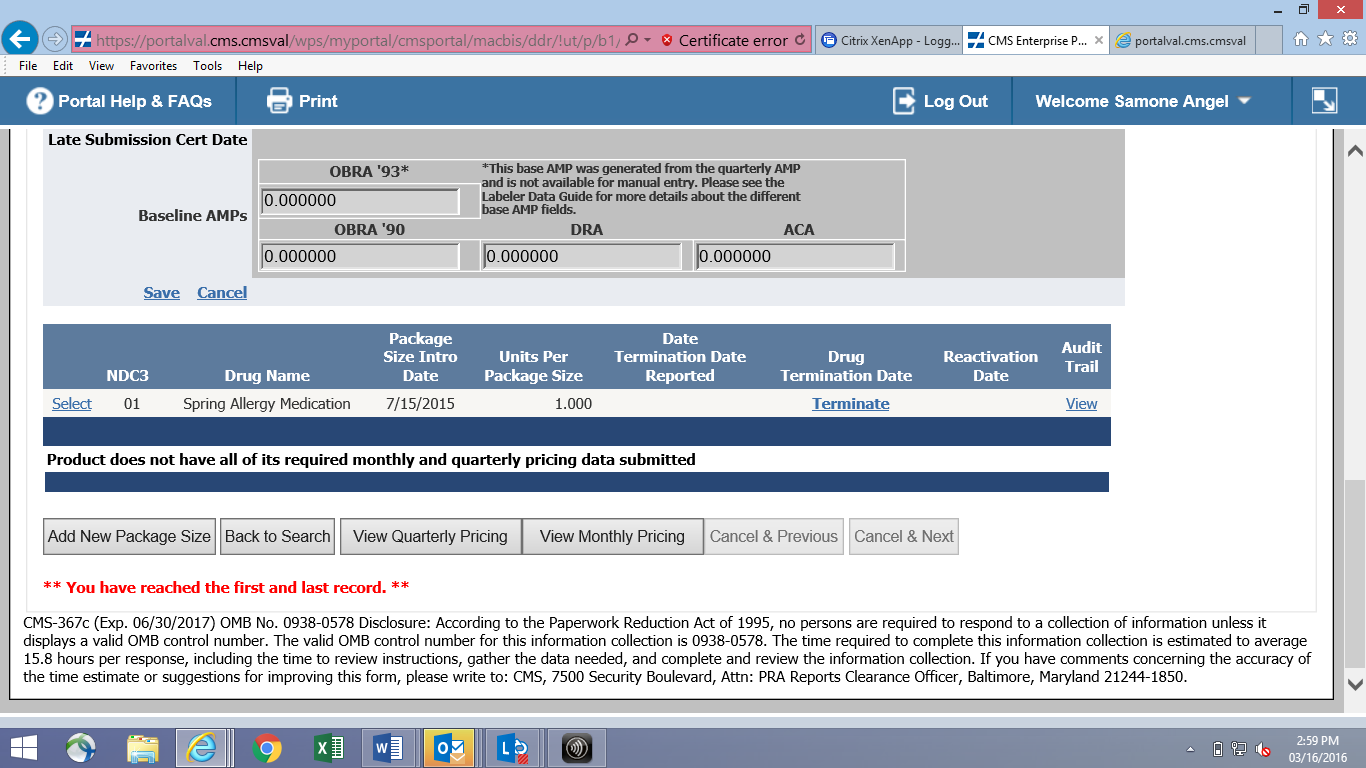
File Transfer Product Layout (CMS-367c)
Field |
Size |
Position |
Remarks |
Record ID |
1 |
1 – 1 |
Constant of “P” |
Labeler Code |
5 |
2 – 6 |
NDC #1 |
Product Code |
4 |
7 – 10 |
NDC #2 |
Package Size Code |
2 |
11 - 12 |
NDC #3 |
Drug Category |
1 |
13 - 13 |
See Data Element Definitions |
Unit Type |
3 |
14 - 16 |
See Data Element Definitions |
FDA Approval Date |
8 |
17 - 24 |
MMDDYYYY |
FDA Thera. Eq. Code |
2 |
25 - 26 |
See Data Element Definitions |
Market Date |
8 |
27 - 34 |
MMDDYYYY |
Termination Date |
8 |
35 - 42 |
MMDDYYYY |
Drug Type Indicator |
1 |
43 – 43 |
See Data Element Definitions |
OBRA’90 Baseline AMP |
12 |
44 – 55 |
99999.999999 |
Units Per Pkg Size |
11 |
56 – 66 |
9999999.999 |
FDA Product Name |
63 |
67 – 129 |
FDA Product Name |
DRA Baseline AMP |
12 |
130 – 141 |
99999.999999 |
Package Size Intro Date |
8 |
142 – 149 |
MMDDYYYY |
Purchased Product Date |
8 |
150 – 157 |
MMDDYYYY |
5i Drug Indicator |
1 |
158 – 158 |
See Data Element Definitions |
5i Route of Administration |
3 |
159 – 161 |
See Data Element Definitions |
ACA Baseline AMP |
12 |
162 - 173 |
99999.999999 |
COD Status |
2 |
174 – 175 |
See Data Element Definitions |
FDA Appl. No./OTC Mono. No. |
7 |
176 – 182 |
See Data Element Definitions |
Line Extension Drug Indicator |
1 |
183 – 183 |
See Data Element Definitions |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Modified | 0000-00-00 |
| File Created | 2021-01-24 |
© 2026 OMB.report | Privacy Policy