DSC Example 1 testosterone
DSC Example 1_testosterone_Final.docx
Focus Groups About Drug Products As Used by The Food and Drug Administration
DSC Example 1 testosterone
OMB: 0910-0677
Testosterone Products Evaluation: FDA evaluating risk of stroke, heart attack and death with FDA-approved testosterone products
Safety Announcement
Drug |
FDA-Approved Testosterone Products |
Drug Safety Risk |
|
Patient Action |
|
Healthcare Professional Action |
|
Basis for Concern |
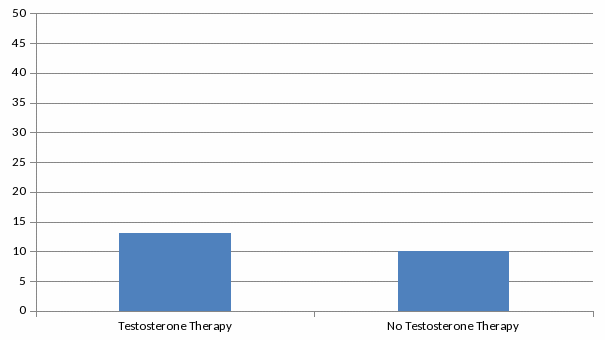
100 . 
Number of participants out of every 100 followed who experienced a cardiovascular event or death . .
|
FDA Response |
|
About Testosterone |
Testosterone is a hormone essential to the development of male growth and masculine characteristics.
|
About Testosterone Products |
|
Data Summary Information on This Risk
|
The first publication that prompted FDA to reassess the cardiovascular safety of testosterone therapy was an observational study of older men in the U.S. Veteran Affairs health system published in the Journal of the American Medical Association (JAMA) in November 2013. The men included in this study had low serum testosterone. They were undergoing imaging of the blood vessels of the heart, called coronary angiography, to assess for coronary artery disease. Some of the men received testosterone treatment while others did not. On average, the men who entered the study were about 60 years old, and many had underlying cardiovascular disease. This study suggested a 30 percent increased risk of stroke, heart attack, or death among men prescribed testosterone therapy. A second observational study reported an increased risk of heart attack in older men, as well as in younger men with pre-existing heart disease, who filled a prescription for testosterone therapy. The study reported that the risk of a heart attack doubled among men 65 years and older in the first 90 days following the first prescription. Among men younger than 65 years old with a pre-existing history of heart disease, the study reported a two to three times greater risk of heart attack in the first 90 days following a first prescription. However, younger men without a history of heart disease who filled a prescription for testosterone did not have an increased risk of heart attack. |
Additional Information |
Patients and health care professionals should report side effects to the FDA MedWatch program, using the information in the “Contact FDA” box at the bottom of the page |
What is FDA? |
FDA includes pharmacists, doctors, nurses, researchers and other health professionals. We also work with external experts. We protect public health by assuring drugs are safe and effective. |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Alison Ottenbreit |
| File Modified | 0000-00-00 |
| File Created | 2021-01-24 |
© 2026 OMB.report | Privacy Policy