OMB Justification for Generic Clearance for Educating Groups Influencing Generic Drug Use
OMB Justification Memo for Generic Clearance 0677 for Educating Groups Influending GDUse.doc
Focus Groups About Drug Products As Used by The Food and Drug Administration
OMB Justification for Generic Clearance for Educating Groups Influencing Generic Drug Use
OMB: 0910-0677
FDA DOCUMENTATION FOR THE GENERIC CLEARANCE,
“FOCUS GROUPS ABOUT DRUG PRODUCTS”
(0910-0677)

Focus groups do not yield meaningful quantitative findings. They can provide public input, but they do not yield data about public opinion that can be generalized. As such, they cannot be used to drive the development of policies, programs, and services. Policy makers and educators can use focus groups findings to test and refine their ideas, but should then conduct further research before making important decisions such as adopting new policies and allocating or redirecting significant resources to support these policies.
TITLE OF INFORMATION COLLECTION: Educating Groups Influencing Generic Drug Use
DESCRIPTION OF THIS SPECIFIC COLLECTION
Statement of need:
The Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Office of Generic Drugs (OGD) is seeking OMB approval under the generic clearance 0910-0677 to conduct focus groups for the project “Educating Groups Influencing Generic Drug Use.”
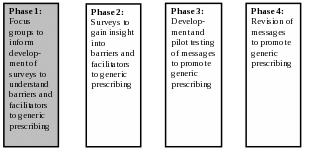
Based on the supporting statement for generic clearance 0910-0677,1 focus groups may be used “to help develop communication messages.” The specific collection described in this memo aims to use focus groups to inform the development of surveys, with the ultimate goal of developing communication messages to promote the prescribing of generic drugs. Exhibit 1 illustrates the full set of research phases for this project; please note that this information collection request concerns only Phase 1.
Exhibit 1. Overview of Research Phases
Congress passed the Generic Drug User Fee Amendments (GDUFA, Title III of the Food and Drug Administration Safety and Innovation Act (Public Law 112-144)) in July 2012. Under GDUFA, FDA obtains industry and public input to create regulatory science initiatives regarding research on generic drugs that advance public health.2 Once marketed, certain generic drugs are often not preferred over brand drugs3 even though generic drugs generally cost less than brand drugs.4 Research to characterize the key influencers of generic drug use (particularly for drug classes with low generic drug use), including their knowledge base and perceptions toward generic drugs, is needed to effectively design and deliver communications about generic drugs to the key groups influencing consumer acceptance and use of generic drugs. To address this regulatory science need regarding generic drugs, the FDA entered into an agreement with the University of Chicago (UChicago).
As prescribers of brand or generic drugs, healthcare providers can influence consumer use of generic drugs. Many healthcare providers have been slow to adopt generic drugs5 despite data suggesting clinical equivalence between generic and brand drugs.6
In primary care practice, both clinicians and patients express concerns regarding use of certain generic drug classes such as antidepressants, oral contraceptives, and cholesterol lowering drugs.7 Through an agreement with UChicago, the barriers and facilitators to consumer use of these drug classes will be explored by conducting focus groups comprised of primary care physicians and nurse practitioner prescribers.
Intended use of information:
Information collected in these focus groups will ultimately be used to help develop communication messages to promote the prescribing of generic drugs. The information collected will be used to inform the development of a survey, to be submitted as a separate information collection request for OMB approval at a later date. The survey will be used to obtain further insight into barriers and facilitators of generic drug use, with a particular interest in knowledge gaps regarding generic drugs.
Information collected in the Phase 1 focus groups (and Phase 2 surveys) will be used to inform development of educational messages regarding generic drugs (Phase 3). By understanding why healthcare providers may sometimes not prescribe generic drugs, effective educational messages may be developed.
Description of respondents:
A total of four (4) focus groups are planned. Focus groups will be conducted at the 2016 American College of Physicians (ACP) Internal Medicine meeting (two (2) focus groups) and the 2016 American Academy of Nurse Practitioners (AANP) national conference (two (2) focus groups). Focus group participants will be general internist primary care physicians attending the ACP meeting and nurse practitioners attending the AANP meeting.
ACP is the largest medical-specialty organization and the second largest physician group in the United States. General internist primary care physicians typically provide comprehensive longitudinal care and coordinate complex treatment for adults.
AANP is a union of the American Academy of Nurse Practitioners and the American College of Nurse Practitioners. It is currently the largest full-service national professional membership organization for NPs of all specialties. AANP maintains the only national NP database of all practicing NPs. NPs are authorized to prescribe medications in all fifty states and Washington, D.C.
The well-established networks and infrastructures of the ACP and AANP guarantee the success of this study by ensuring feasibility and a representative recruitment pool that can be surveyed at professional society conferences with minimal logistical burden.
Date(s) to be conducted and location(s):
The ACP focus groups will be held on May 6, 2016 in Washington, D.C.
The AANP focus groups will be held on June 24 and/or 25, 2016 in San Antonio, TX.
How the Information is being collected:
Recruitment
ACP conducts a periodic census of its members and keeps a database of demographic information which they use to screen for eligible participants. ACP will select eligible members based on the screening criteria (attached) using this database. ACP will send emails to recruit eligible focus group participants who will be asked to RSVP. Participants who meet these qualifications and express interest will be randomly selected to participate in the focus groups.
Focus group participants at AANP will be recruited through AANP’s Conference Registrant Newsletters, which are distributed to all conference registrants (~5500 NPs). The first Conference Registrant Newsletter is generally sent out six weeks prior to the conference. Newsletter messaging will call specifically for NPs that meet criteria specified in the participant screener (attached). Participants who meet these qualifications and express interest will be randomly selected to participate in the focus groups.
A minimum of 12 participants will be included in each session. Participants will be provided basic information about the topic and length of time for the focus group as well as instructions to ensure arrival at the proper locations on the agreed upon dates and times. Participants are intentionally over-recruited to ensure the minimum number of participants needed for each scheduled time slot. Confirmation and reminder correspondences containing participant consent forms are emailed to recruited participants to help ensure attendance one week and one day prior to the focus groups. Participation in the ACP and AANP focus groups is voluntary.
Focus Group Discussions
UChicago investigators who are trained facilitators will moderate the focus groups to ensure that all topics of interest are addressed and all participants have opportunities to speak. FDA staff will observe some of the focus group sessions. The moderators will use the attached moderator’s guides. Time is allowed to address ideas and questions spontaneously generated from the discussion. Reliability and validity are assessed iteratively by revisiting respondents’ verbalizations and asking for clarifications. This is done both within the course of individual discussions and between separate group discussions.
Each focus group will consist of 12 participants and last 60 minutes, as is standard practice for ACP and AANP focus groups and a condition of partnership (any deviation may jeopardize recruitment by deterring participants). The focus group discussions will be recorded and transcripts will be made from these recordings. Transcripts and notes taken by the project staff will be the bases for data analysis. Before the group discussion begins, the facilitators will obtain written consent from the participants to audiotape the sessions. The consent forms also mention the audiotaping. The consent forms will be reviewed with each participant and signed prior to them joining the discussion group. For all focus groups, there will be general discussion about generic drugs and generic prescribing.
Confidentiality of Respondents
UChicago and FDA will comply with safeguards for ensuring participant information is kept private to the extent permitted by law. Participants are to use first names only during group discussions, and the last names of the participants will not appear on any focus group materials (typed lists of participants, name placards, transcripts, draft or final reports). Participants will also have the option of using a pseudonym.
The focus group participants will be informed about how the audio files are used in the analyses, and assured that the recorded data are kept on a secure web-based database that will be maintained at University of Chicago. Any paper data will be stored in locked file cabinets in locked offices of the investigators. For any recordings that are transcribed, the transcription service will be instructed to include no names of any sort; instead they will indicate that a name was mentioned with the annotation “xxx.” If names are needed for the identification of issues related to the study, the Principal Investigator will replace the specified “xxx” with a study code number.
For the participants, the information they provide will be linked to them via a code and will be available only to the research team. The code key and data files will be stored separately on a password-protected computer that only investigators can access. Audio recordings will be listened to only by the study team in closed, private rooms.
Data will be kept for six years after the close of the study. The recordings will be destroyed using commercial software applications designed to remove all data from the storage device. If any transcripts are made, they will be cross-shredded at the conclusion of the study.
Verbatim quotes included in the final report will not be attributed to any individual. Data will be used in the aggregate. Data from this study may be used in medical publications or presentations. Participant names and other identifying information will be removed before the data is used or shared with stakeholders such as the FDA.
Number of focus groups:
Four focus groups are proposed. As seen in Exhibit 2, one ACP focus group and one AANP focus group will include discussion about antidepressants and (time permitting) lipid-lowering drugs. One ACP focus group and one AANP focus group will include discussion about oral contraceptives and (time permitting) lipid-lowering drugs.
Exhibit 2. Focus Group Design
-
Focus Groups*
TOTAL
Antidepressants and lipid-lowering drugs
Oral contraceptives and lipid-lowering drugs
ACP
1 groups
1 group
2 groups
AANP
1 groups
1 group
2 groups
TOTAL
2 groups
2 groups
4 groups
(12 per group,
N = 48)
* all focus groups include general discussion about generic drugs and generic prescribing
Amount and justification for any proposed incentive:
Compensation to subjects participating in focus groups is proposed in accordance with the ACP and the AANP standard practices regarding focus groups. To maintain equipoise with other focus groups at these conferences and prevent jeopardizing recruitment, physician and nurse practitioner focus group participants are proposed to receive ACP/AANP credit that participants can use towards their continuing education. ACP standards call for a $100 ACP credit while AANP standards call for AANP credit commensurate with average hourly NP compensation (approximately $60), which members may use towards continuing education.
The incentive is not a reward or salary. Rather, it is a stimulus to attend the session and arrive on time. Offering an incentive below the accepted rate may result in increased costs exceeding the amount saved with a lower incentive. Consequences of insufficient incentives include increased time and cost of recruitment; increased “no-show” rate; and increased probability of cancelled or postponed focus groups due to insufficient participants on the scheduled date.
Questions of a Sensitive Nature:
There will be no questions of a sensitive nature asked of participants.
Description of Statistical Methods (I.E. Sample Size & Method of Selection):
This is a qualitative study using a convenience sample. As such, the analyses do not entail the use of statistics though the analytical methods are described below.
Transcription of the audio recordings will be used to analyze participant responses. Transcripts provided by a professional transcription service will be loaded into a qualitative software package, ATLAS.ti, which facilitates the storage, coding, and retrieval of qualitative data. All transcripts will be analyzed using the constant comparative method with no a priori hypotheses. First, two researchers will code a small subset of the transcripts to identify preliminary codes, allowing for grouping or reclassification of codes. All discrepancies will be resolved by discussion to consensus. In adherence to standards for qualitative research, the remainder of the transcripts will be coded independently until inter-rater reliability (kappa > 0.6) is achieved.
The raw data will be the words, phrases, sentences, and non-verbal responses of the participants. A report based on the discursive data gathered from each group will detail the characteristics of each group and highlight significant variations and commonalities between groups.
BURDEN HOUR COMPUTATION (Number of responses (X) estimated response or participation time in minutes (/60) = annual burden hours):
Approximately sixty (60) hours in total based on approximately twelve (12) participants for each of four (4) focus group sessions and time (75 minutes) spent reviewing consent and recruitment materials prior.
Type/Category of Respondent |
No. of Respondents |
Participation Time (minutes) |
Burden (hours) |
General internist primary care physicians |
24 |
75 |
30 |
Nurse practitioners |
24 |
75 |
30 |
Total |
48 |
150 |
60 |
REQUESTED APPROVAL DATE: March 1, 2016. While approval by March 1, 2016 is ideal for study subject recruitment purposes, we have some limited flexibility. Approval after March 14, 2016, however, would be detrimental to this study moving forward.
NAME OF PRA ANALYST & PROGRAM CONTACT:
Ila S. Mizrachi
Paperwork Reduction Act Staff
301.796.7726
Lucie Yang, MD, PhD, MBA
Director (Acting), Division of Quality Management Systems
Office of Generic Drugs
301.796.5112
FDA CENTER: Center for Drug Research and Evaluation
3 Scher, S. (2013) The Branded Advantage. Ophthamol Mgmt. July: p18 http://www.ophthalmologymanagement.com/printarticle.aspx?articleID=108618
Alloway, RR, Isaacs R, Lake K, Hoyer P, First R, Helderman H, Bunnapradist S, Leichtman A, Bennett MW, Tejani A, Takemoto SK. (2003) Report of the American Society of Transplantation Conference on Immunosuppressive Drugs and the Use of Generic Immunosuppressants. A J Transpl 3: 1211.
Liow K, Barkley GL, Pollard JR, Harden CL, Bazil CW. (2007) Position statement on the coverage of anticonvulsant drugs for the treatment of epilepsy. Neurology 68: 1249.
American Thyroid Association, The Endocrine Society, and American Association of Clinical Endocrinologists. (2004) Joint Statement on the U.S. Food and Drug Administration’s Decision Regarding Bioequivalence of Levothyroxine Sodium. Thyroid 14: 486.
4 Avoidable Costs in U.S. Healthcare: The $200 Billion Opportunity from Using Medicines More Responsibly. Report by the IMS Institute for Healthcare Informatics. http://www.imshealth.com/deployedfiles/imshealth/Global/Content/Corporate/IMS Institute/RUOM- 2013/IHII_Responsible_Use_Medicines_2013.pdf
5 Shrank WH, Liberman JN, Fischer MA, Girdish C, Brennan TA, Choudhry NK. Physician Perceptions about Generic Drugs. Ann Pharmacother. 2011;45(1):31-8.
6 Kesselheim AS, Misono AS, Lee JL, et al. Clinical Equivalence of Generic and Brand-Name Drugs Used in Cardiovascular Disease: A Systematic Review and Meta-Analysis. JAMA: the journal of the American Medical Association. 2008;300(21):2514-2526.
7 Bolton JM, Dahl M, Sareen J, Enns MW, Leslie WD, Collins DM, Alessi-Severini S. A population-based study of the use of selective serotonin reuptake inhibitors before and after introduction of generic equivalents. Can J Psychiatry. 2012;57(4):223-9.
Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 375: Brand versus generic oral contraceptives. Obstet Gynecol. 2007;110 (2 Pt 1):447-8.
Green JB, Ross JS, Jackevicius CA, Shah ND, Krumholz HM. When choosing statin therapy: the case for generics. JAMA Intern Med. 2013;173(3):229-32.
Drug Benefit News. As Competitors Encroach, Pfizer Seizes A Few More Glory Days With Lipitor Promo. http://www.silverlink.com/assets/pdfs/silverlinknews/dbn020411.pdf
| File Type | application/msword |
| File Title | OMBMemoMERCPtP |
| Subject | MERC OMB MEP |
| Author | Hillabrant |
| Last Modified By | Mizrachi, Ila |
| File Modified | 2016-02-12 |
| File Created | 2016-02-12 |
© 2026 OMB.report | Privacy Policy