Biennial Update of Infomation by Firms Already on the List of U.S. Dairy Producers For Export
Establishing a List of U.S. Dairy Producers for Export
Screenshots 0509 Dairy Listing Module 6-8-2016
Biennial Update of Infomation by Firms Already on the List of U.S. Dairy Producers For Export
OMB: 0910-0509
Dairy Listing Module (DLM) Screens for OMB approval
Version: 2.0
June 3, 2016
Submitting a Manufacturer or Processor’s Application Electronically to the dairy list with intent to export
Table of Contents
Dairy Listing Module (DLM) Common Page Components
After the external user has logged in on the FDA’s Unified Registration Listing System (FURLS) Online Account Administration (OAA) page and given their account access to the “Dairy Listing Module”, choose the link "Dairy Listing Module" from the list of systems available on the FURLS home page. This will take you to the webpage “DLM Main Menu” with the banner “DAIRY LISTING MODULE”. Each screen in the DLM electronic submission process has this banner.
At the top right of each page are the links “FURLS Home”, which will take you to the FURLS home page, and "DLM Home," which will take you to the DLM Main Menu. Choose the link “FURLS Home” to log out.
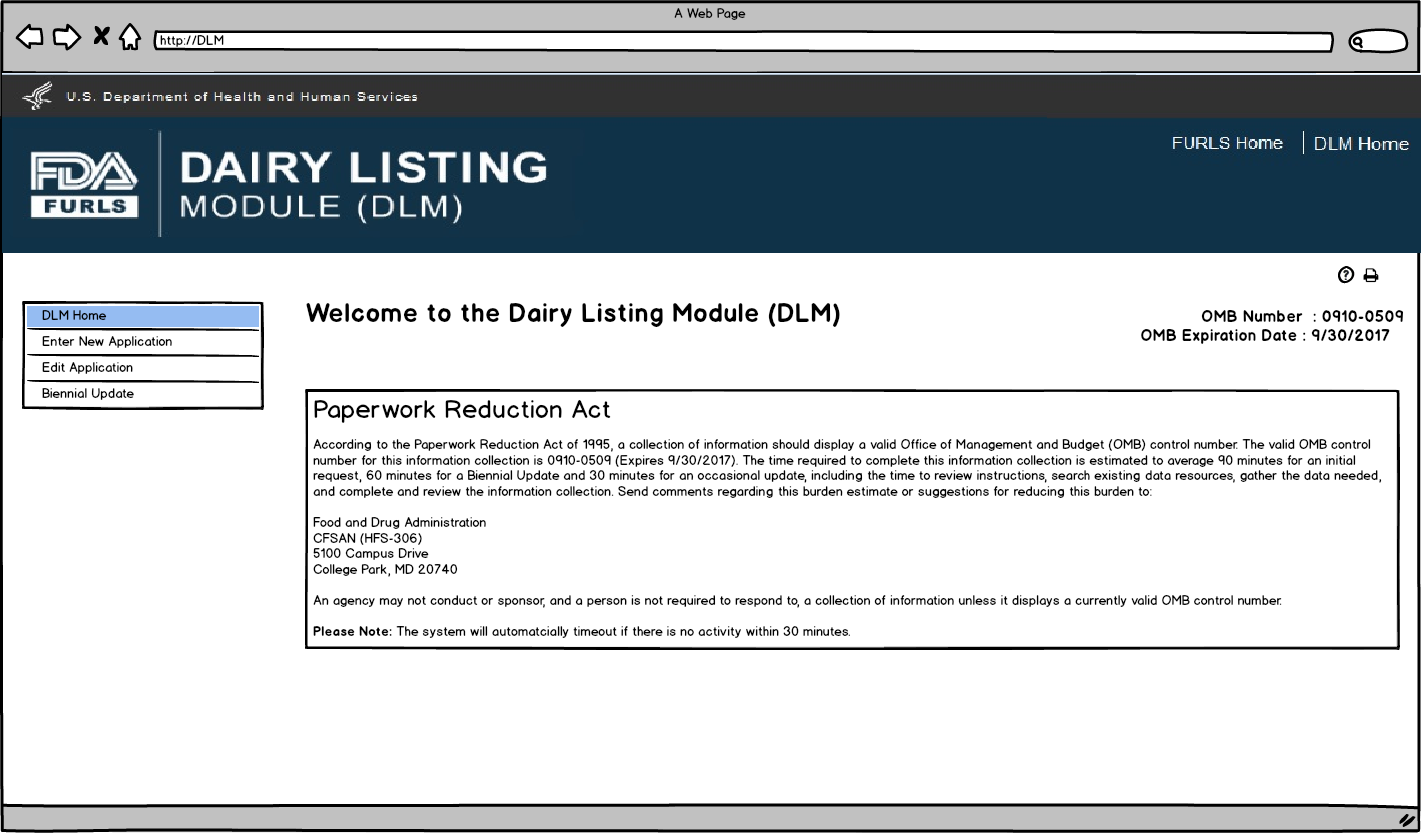
To submit an application for a new facility to be added to the dairy list, select the link “Enter New Application” (see “Enter New Application: Figure 1”)
The screens throughout the DLM online application process will have the following features:
At the top of every screen is a status bar that will track your progress through each step of the process.
A "?" Get-Help icon above the menu bar on the right side of the page will provide help. For an overview of all the help files available, see the FDA Industry Systems Index of Help Pages available separately through the “?”Get-Help icon.
A “Print” icon which prints the screen displayed on the browser.
At the bottom of each screen you will see 1, 2 or 3 navigation buttons; depending on the step in the online application process.
“Previous” will take you back one step in the process to the previous section of the application. Please note that unsaved information entered on the form is lost if you press the “Previous” button.
“Next” takes you to the next screen for entering application data.
”Cancel” takes the user back to the DLM Main Menu. (Will be added to all applicable screens).
In the last step, the button labeled “Submit” on the bottom far right submits the application to the FDA.
Flow 1: Enter New Application: External User
Enter New Application: Figure 1
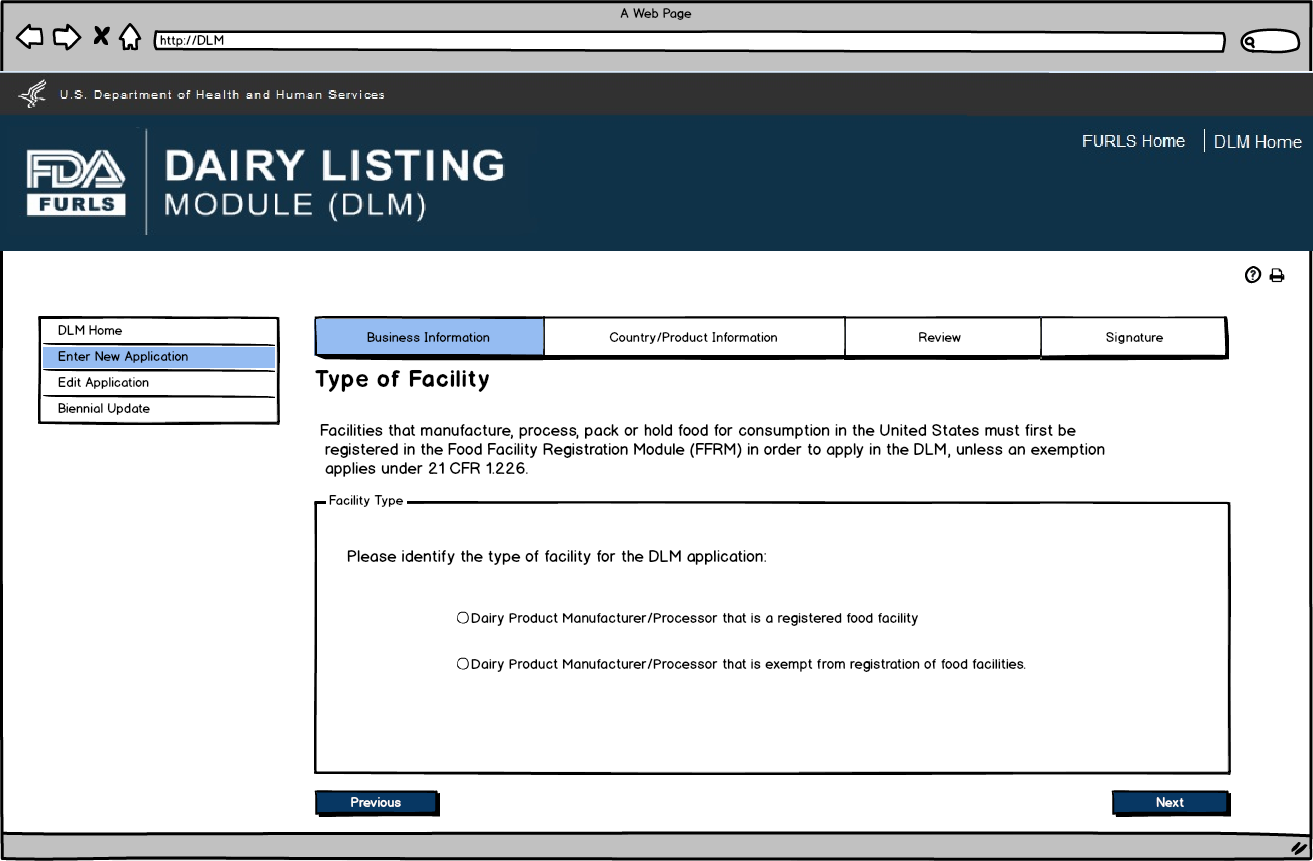
 Enter
New Application: Figure 2
Enter
New Application: Figure 2
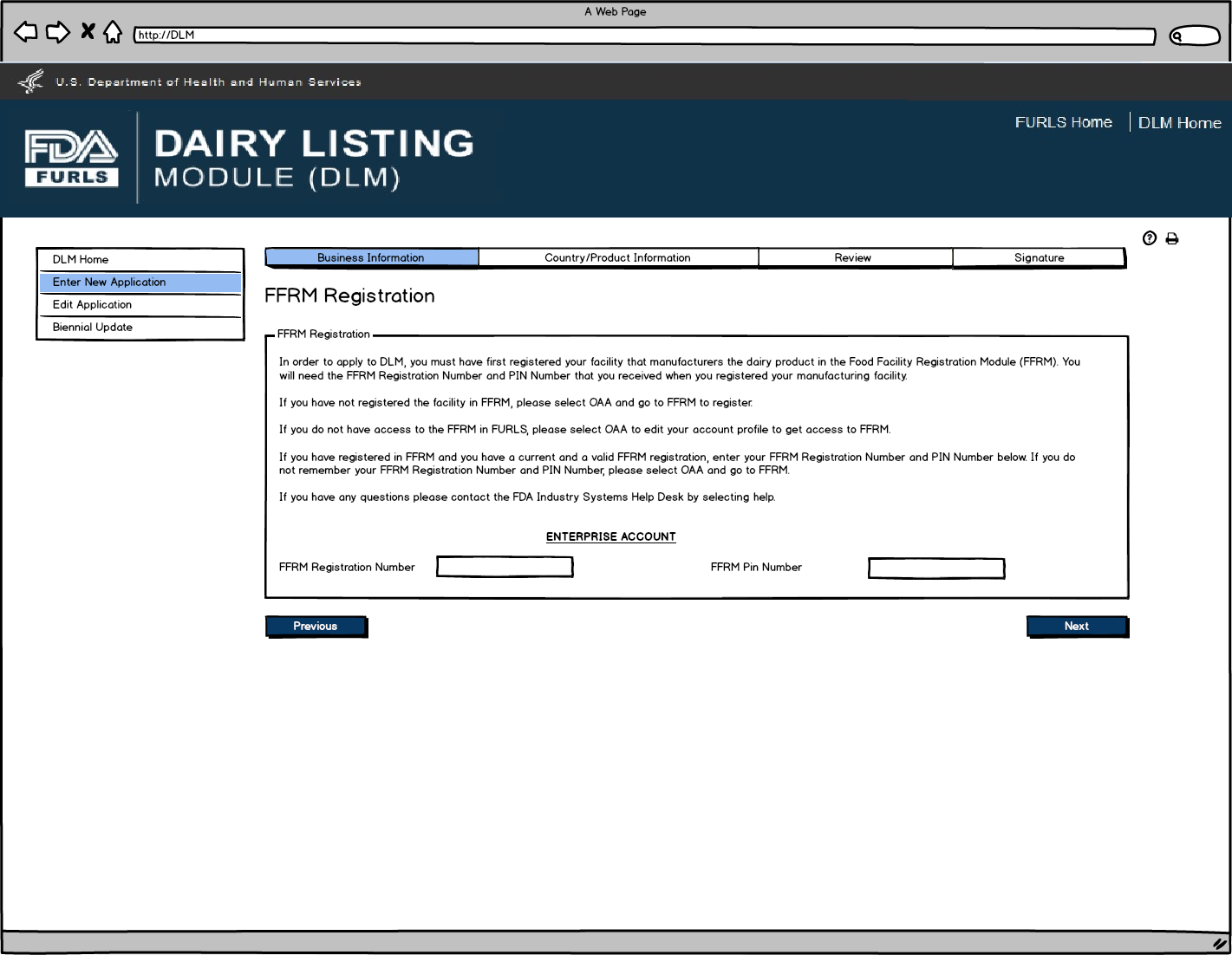
Enter New Application: Figure 3
Food Facility Manufacturers: Food Facility Registration Module (FFRM) Enterprise Account Login. Each firm will have a main enterprise account holder with access to the Food Facility Registration (FFR) Number and FFR Pin Number.
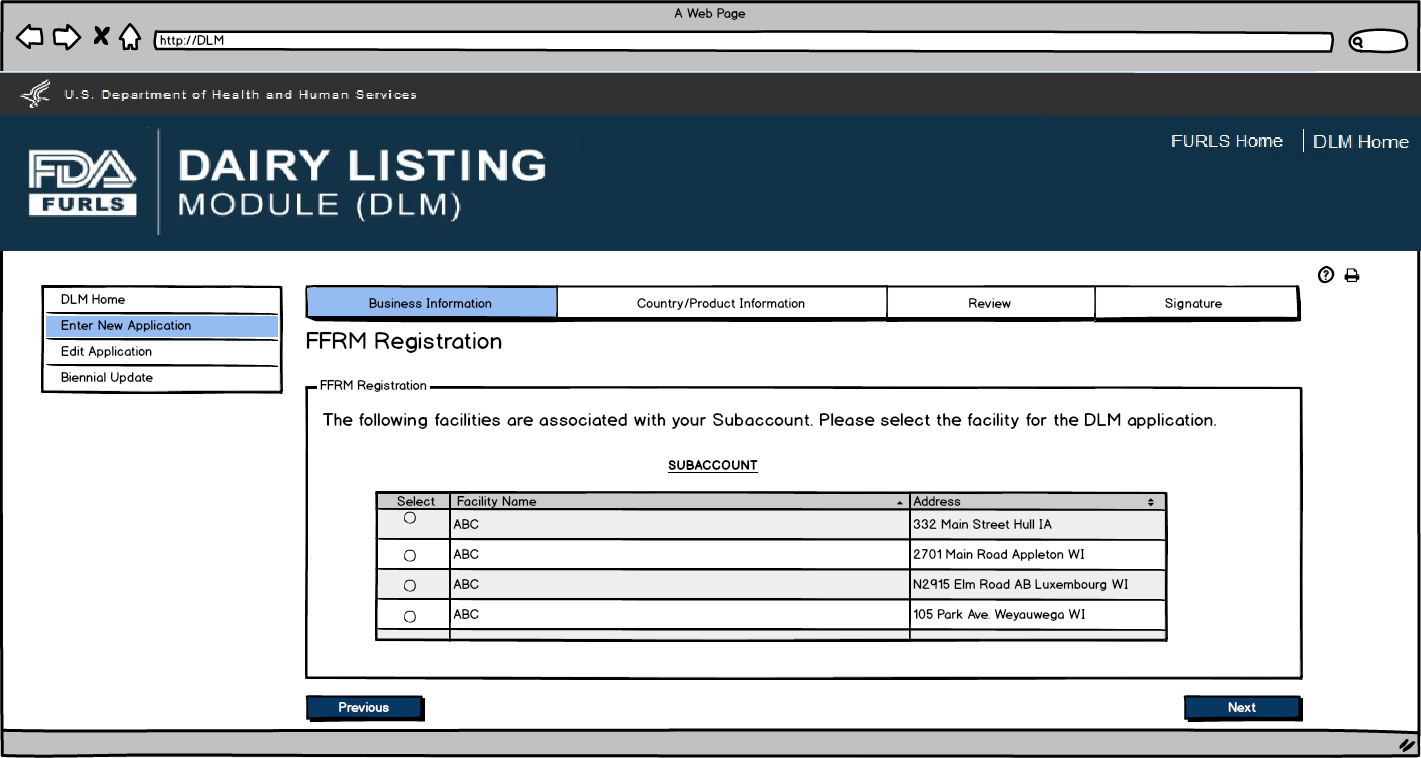
Enter New Application: Figure 4
Dairy Product Manufacturer/Processor that is a registered food facility: FFRM Subaccount Login. The FFRM Enterprise Account holder is able to assign subaccounts when more than one person at a firm needs to access DLM. When the subaccount user logs in with their OAA account, the system will recognize the facilities that they have access to.
Enter New Application: Figure 5
Dairy Product Manufacturer/Processor that is a registered food facility: Business Information, Manufacturer Address and Main Contact Information will be prepopulated for Food Facility Manufacturers since they will be registered in FFRM. All the fields except Doing Business As, Manufacturer Type, and the Inspection details will be read only and cannot be edited.
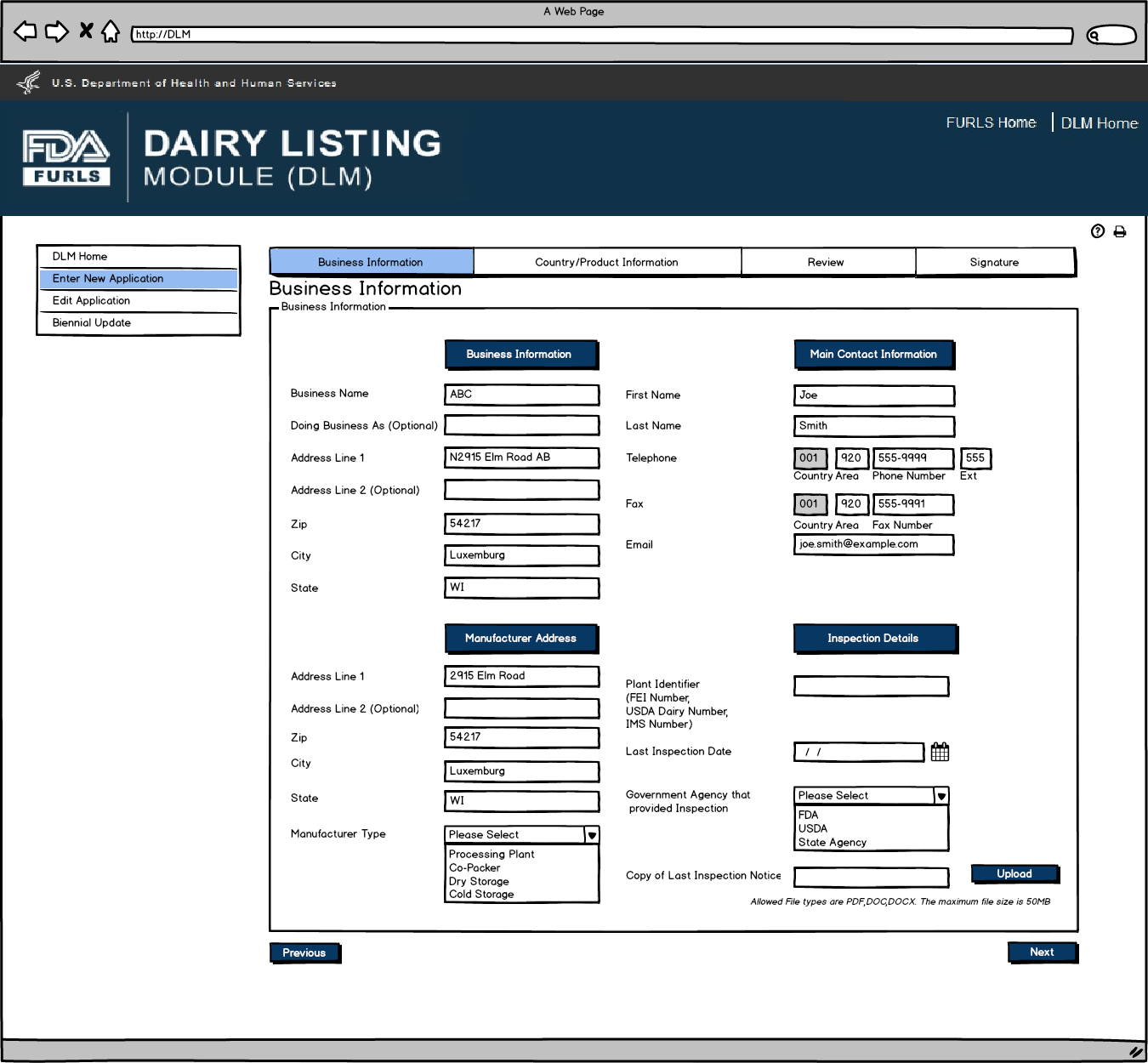
Enter New Application: Figure 6
Dairy Product Manufacturer/Processor that is exempt from registration of food facilities: Business Information Screen. This screen will appear if the user selects Dairy Product Manufacturer/Processor that is exempt from registration of food facilities in Figure 2. Inspection Details will be required except for Plant Identifier. External user will have to enter information.
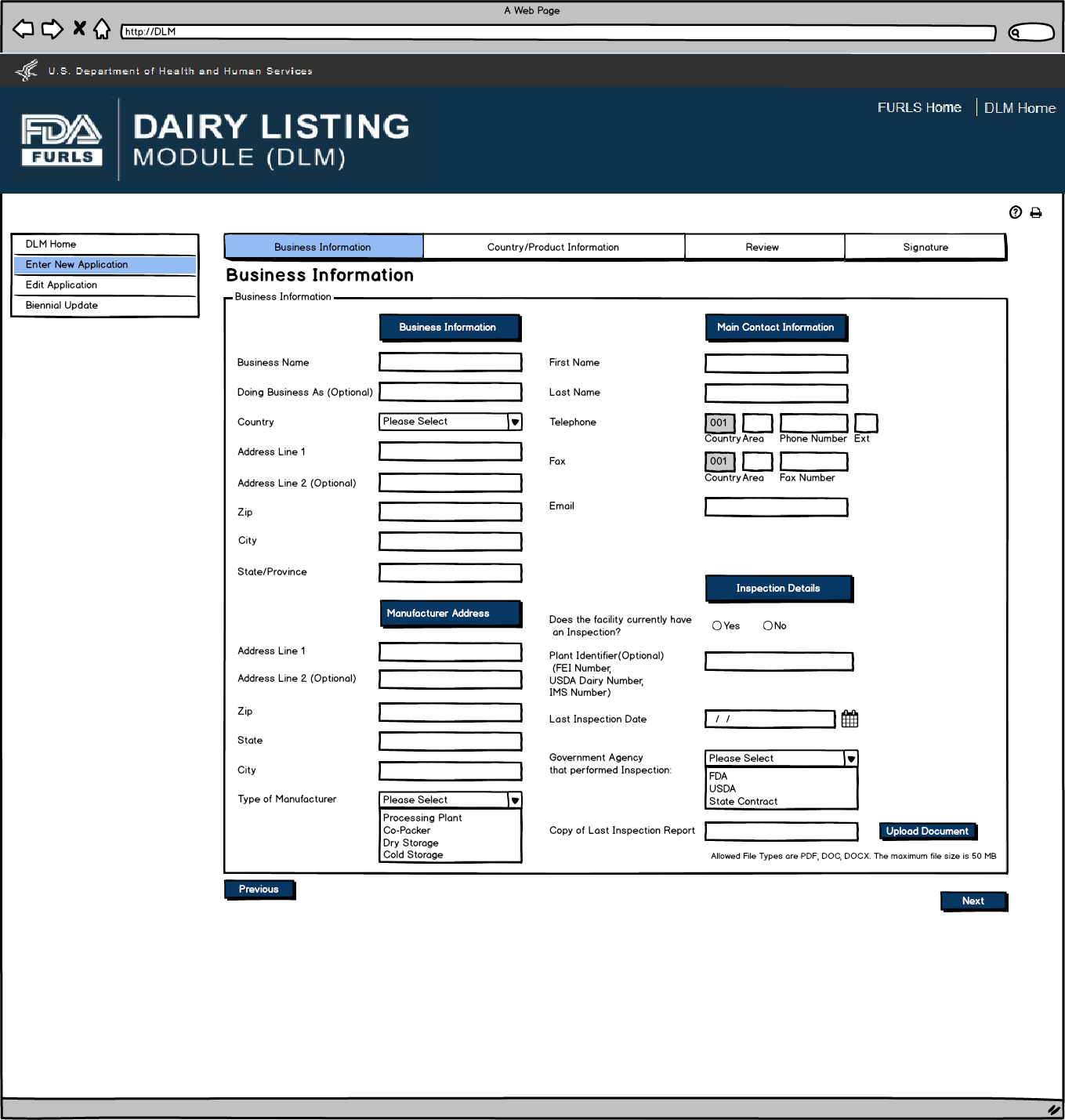
Enter New Application: Figure 7
User will select the exporting country for which the products are being entered.
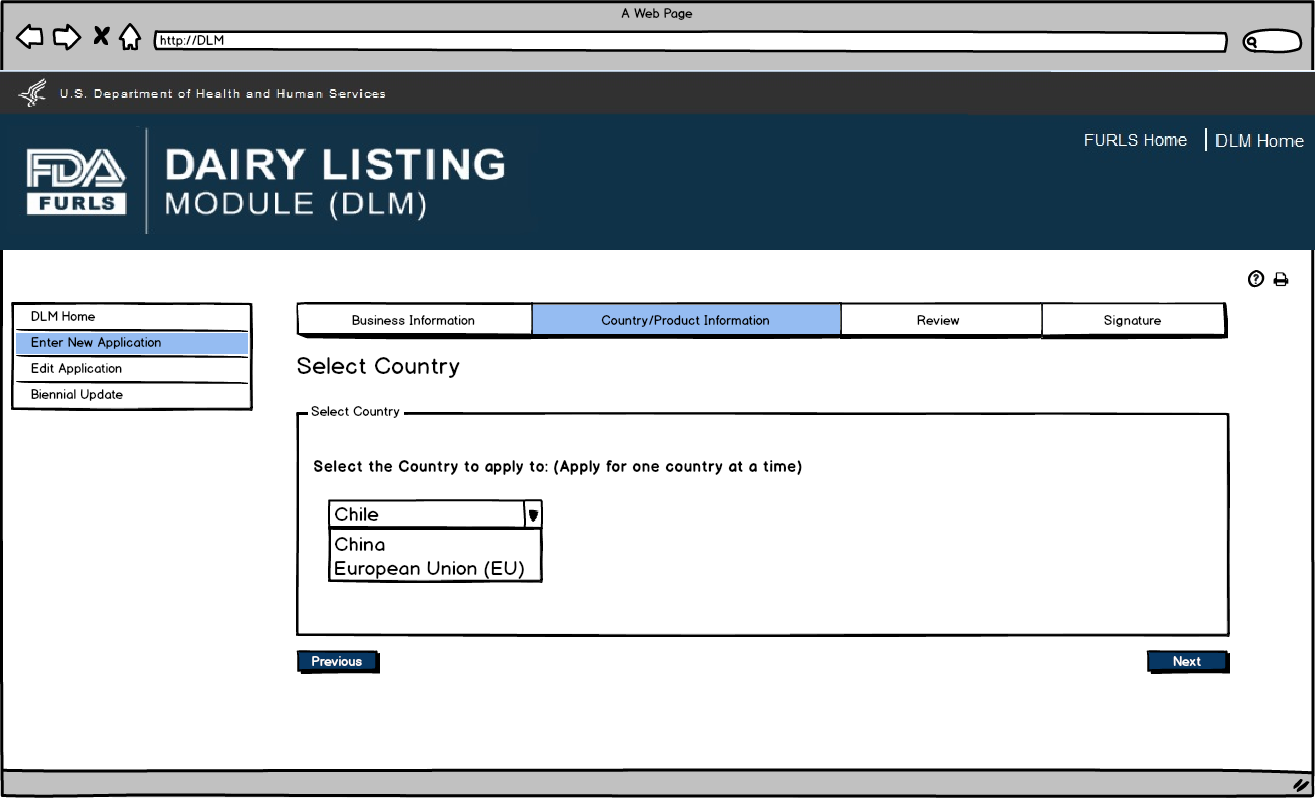
Enter New Application: Figure 8
Contact Information for Country Selected. Users can autofill the Contact Information from the Main Contact Information on the Business Information Screen (Figure 5 or 6) or enter data.
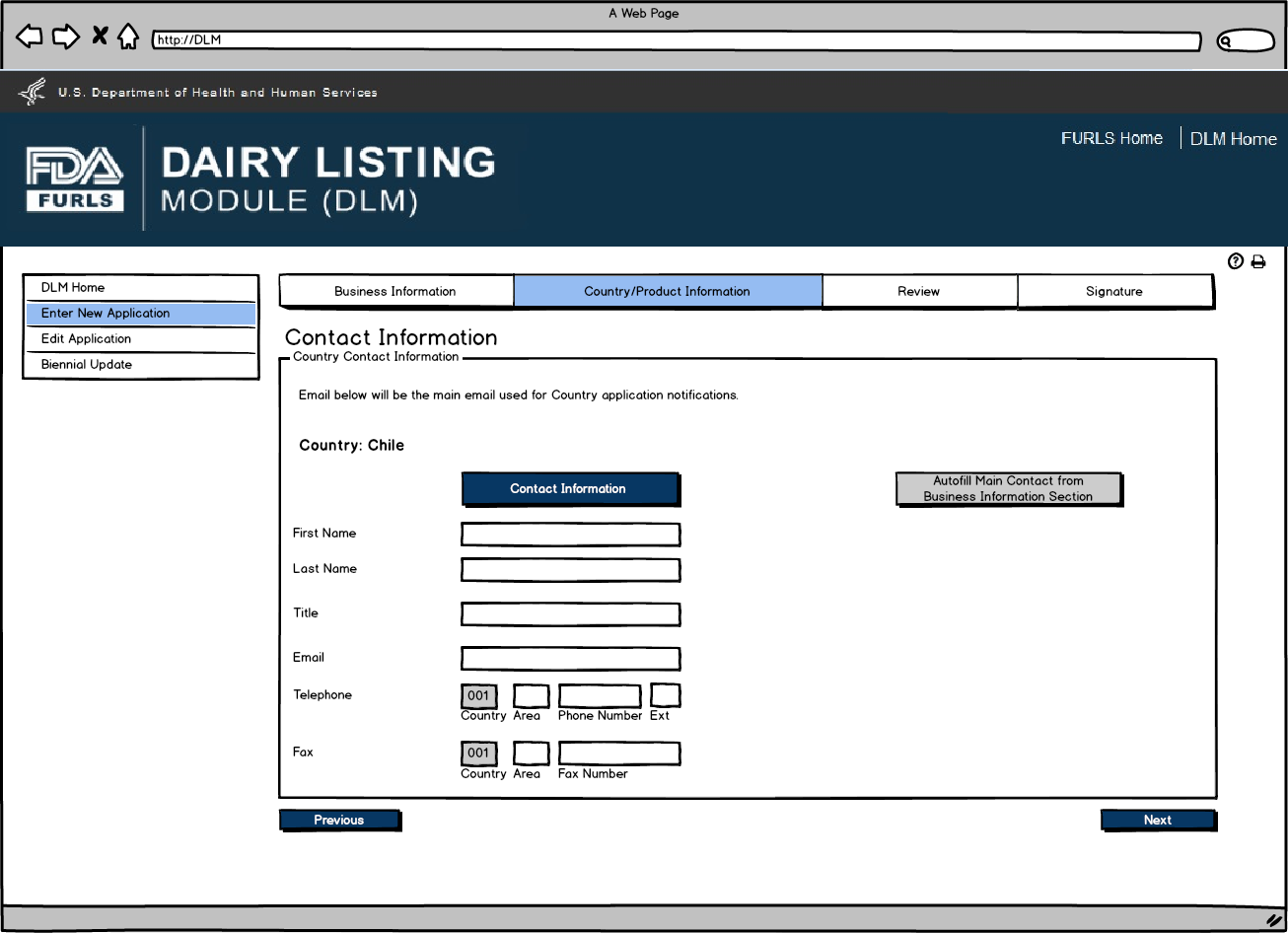
Enter New Application: Figure 9
Product Information Screen is based on Country selected in Figure 7. Animal Origin will be a required field for all countries. Labels for China will be “Product Category” and “Product Name”. Label for Chile and EU will be “Product”.
Questions will be based on country requirements.
When applying for the China dairy list, users will be prompted “Is this product currently being manufactured and ready to ship?”
When applying for the Chile or EU dairy list, users will be prompted “Is this product shipping within the next two years?”
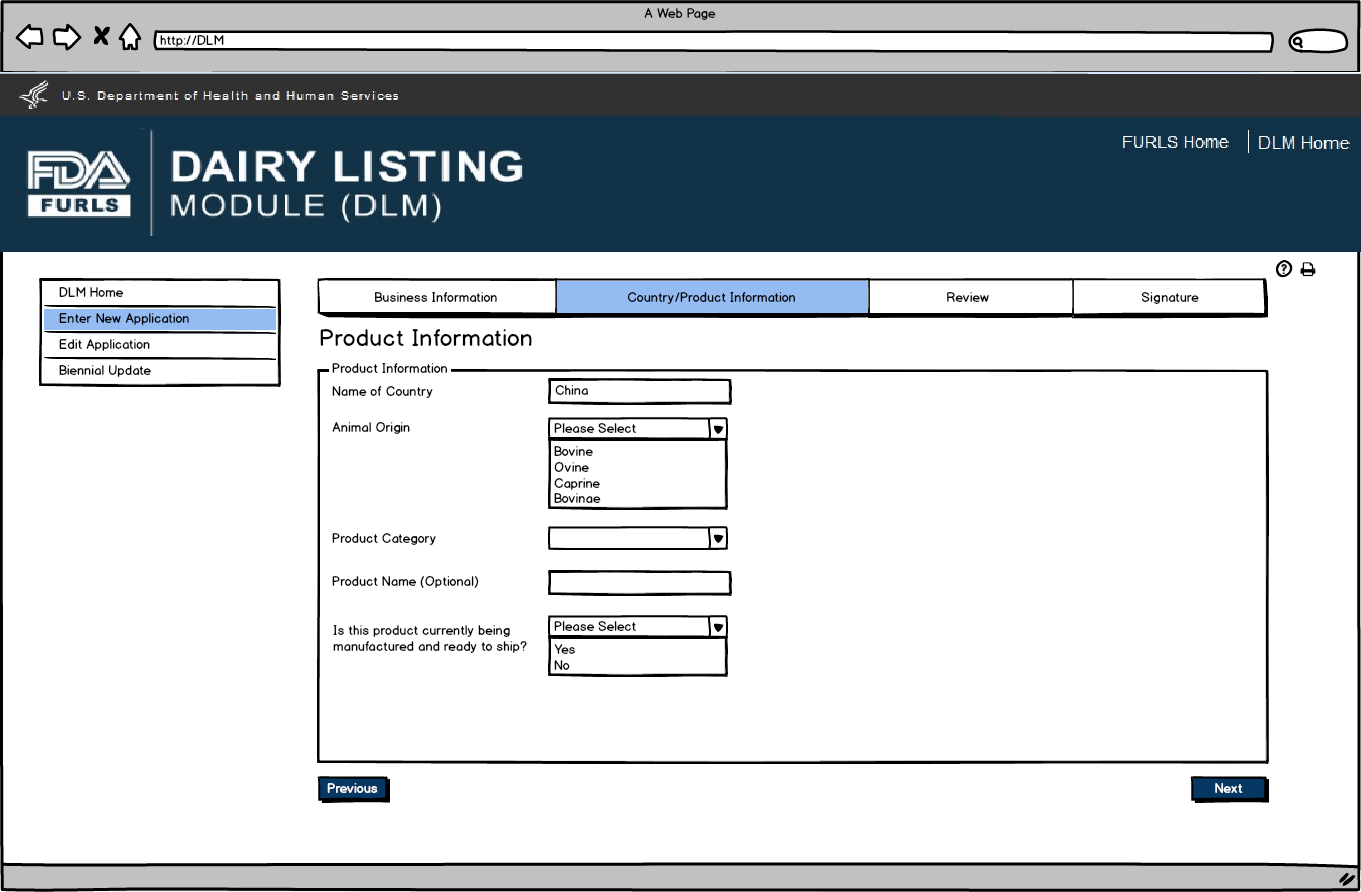
Enter New Application: Figure 10
After user has completed entering Products in Figure 9, they can now view the Product List to confirm the list of products that have been entered for their firm for the current DLM session.
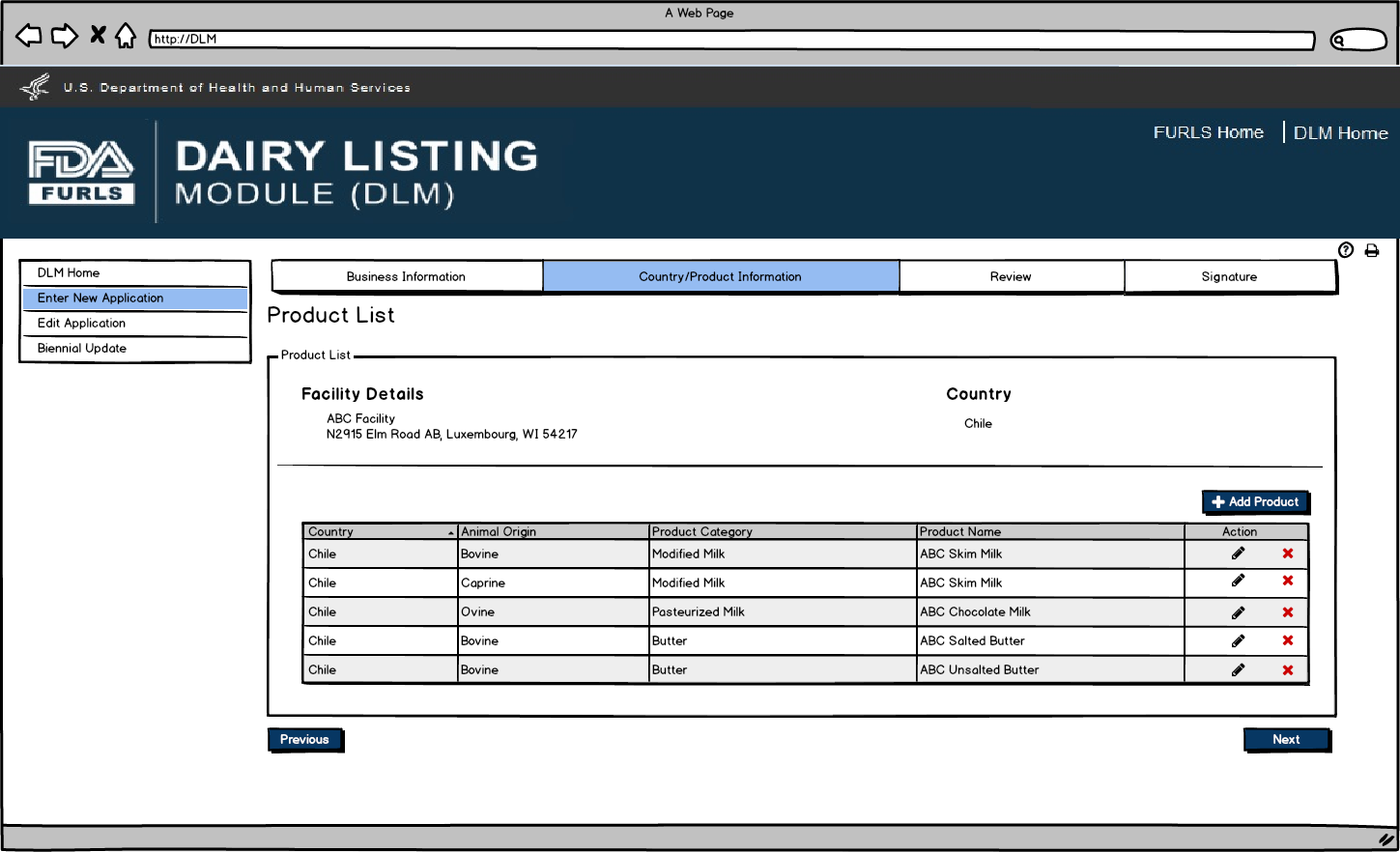
Enter New Application: Figure 11
Review Screen allows users to review
information submitted for the country applied to and edit information
as needed prior to submitting.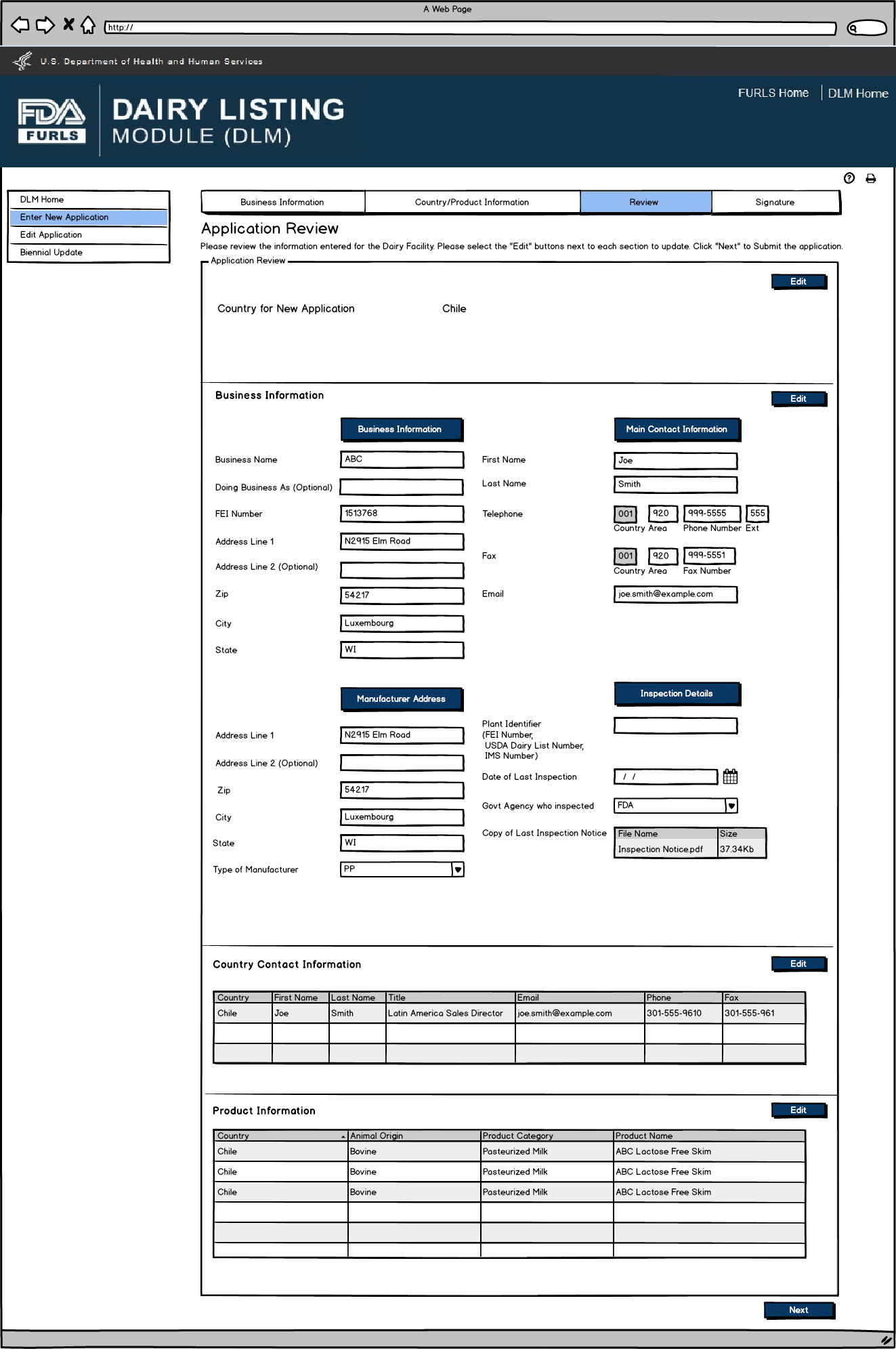
Enter New Application: Figure 12
Signature Page allows users to sign off on the application submission.
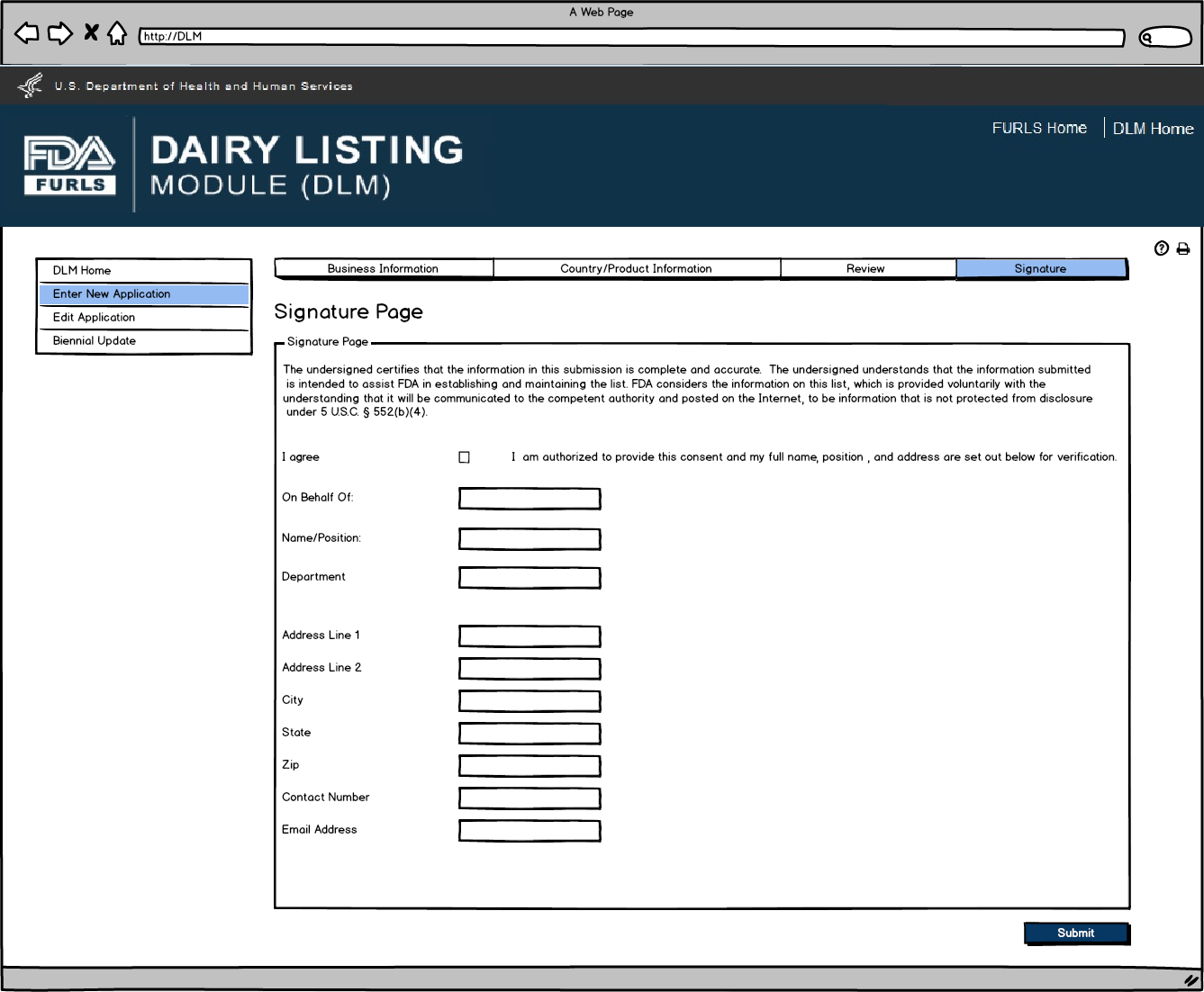
Enter New Application: Figure 13
This screen confirms that the application has been successfully submitted along with the Application Number and Country applied to.
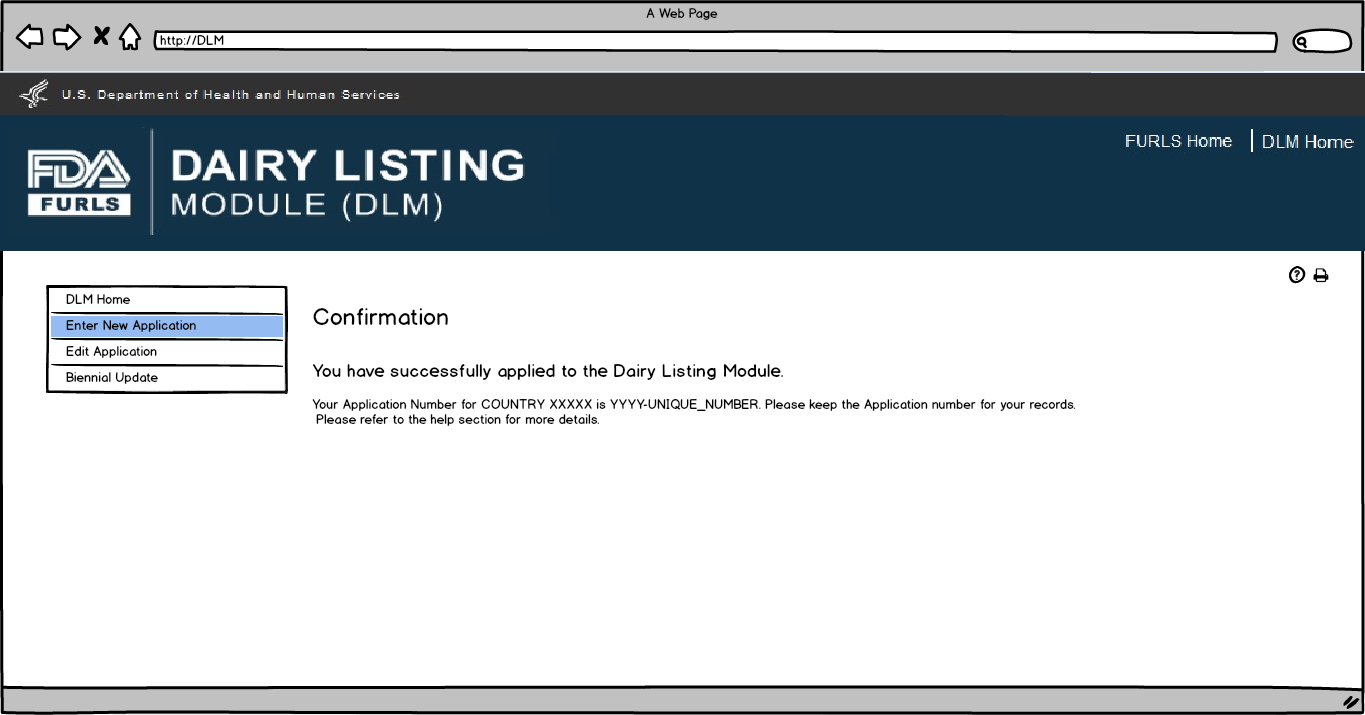
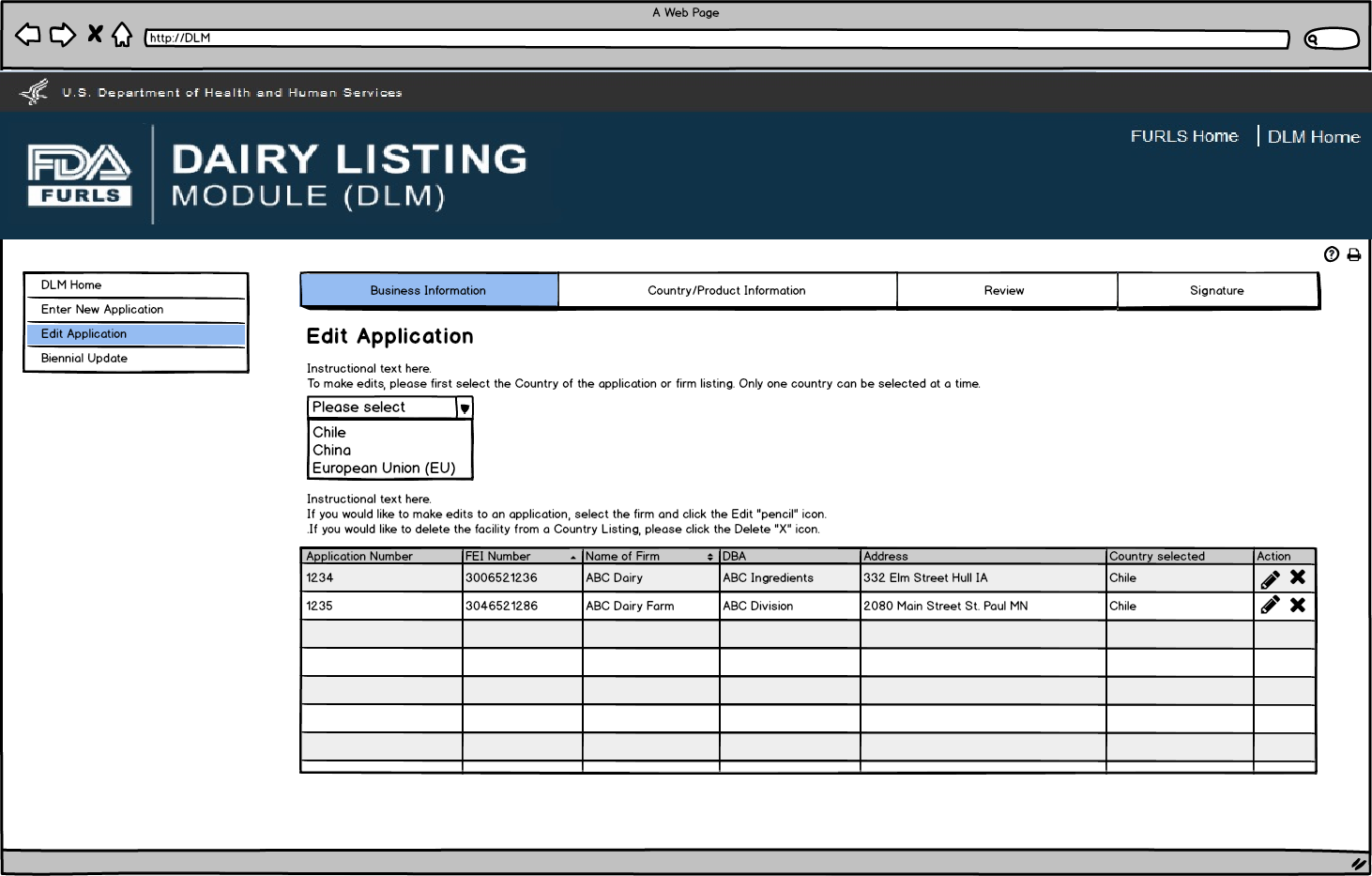
Flow 2: Edit Application
Edit Application: Figure 1
User first selects the country to edit the application. The table will then appear with the facilities for the selected country under the user’s OAA account. User can select the Edit “pencil” icon to edit the application for the selected facility. User can select the Delete “X” icon to request removal of selected facility and the associated products from the selected country list.
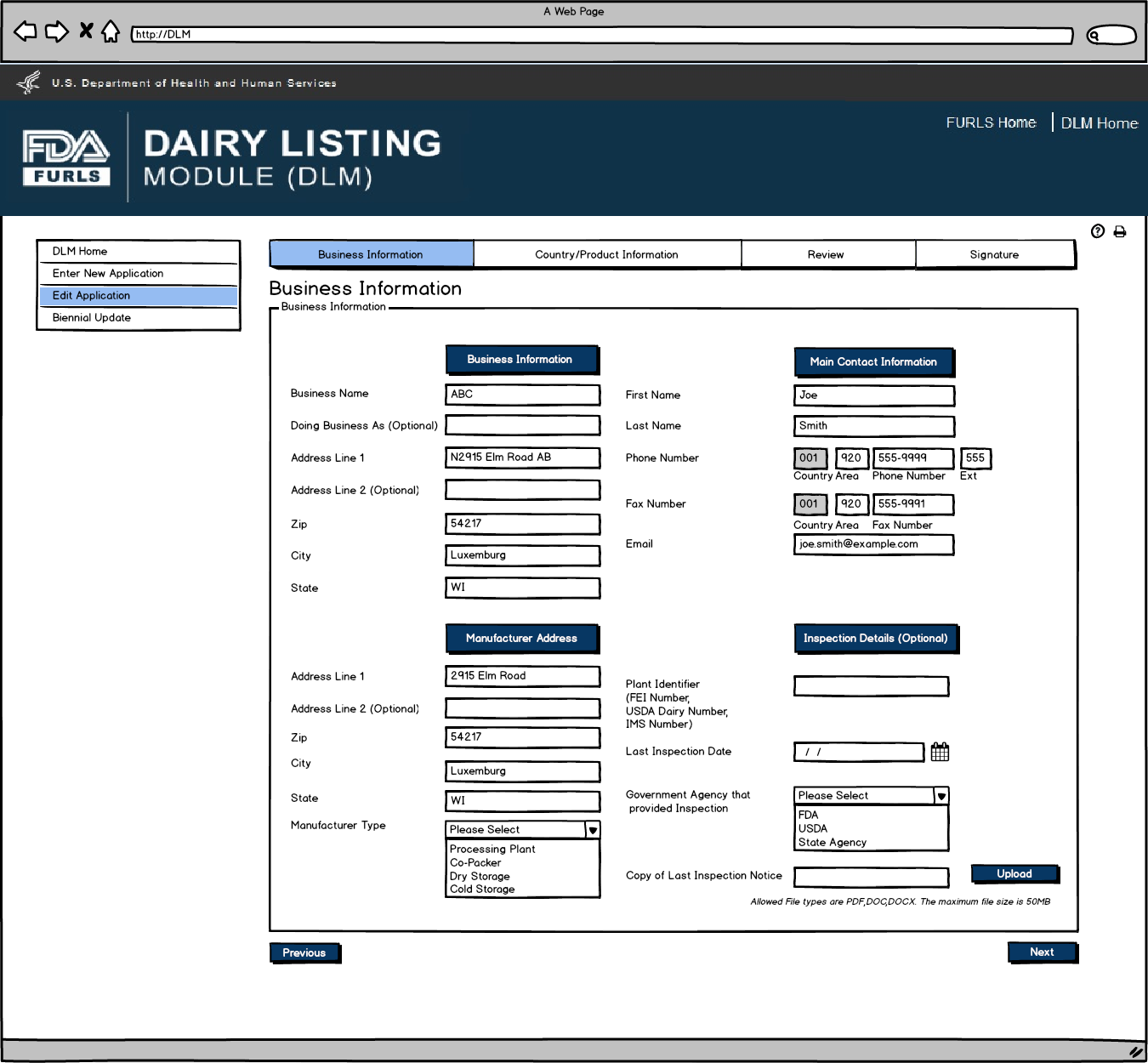
Edit Application: Figure 2
If the firm is a Dairy Product Manufacturer/Processor that is a registered food facility, all the fields except Doing Business As, Manufacturer Type, and the Inspection details will be read only and cannot be edited. User will need to change any Business Information in FFRM. The information that will be editable for Dairy Product Manufacturer/Processor that is a registered food facility will be Doing Business As, Manufacturer Type, and every field in Inspection Details section. For Dairy Product Manufacturer/Processor that is exempt from registration of food facilities, all fields will be editable.
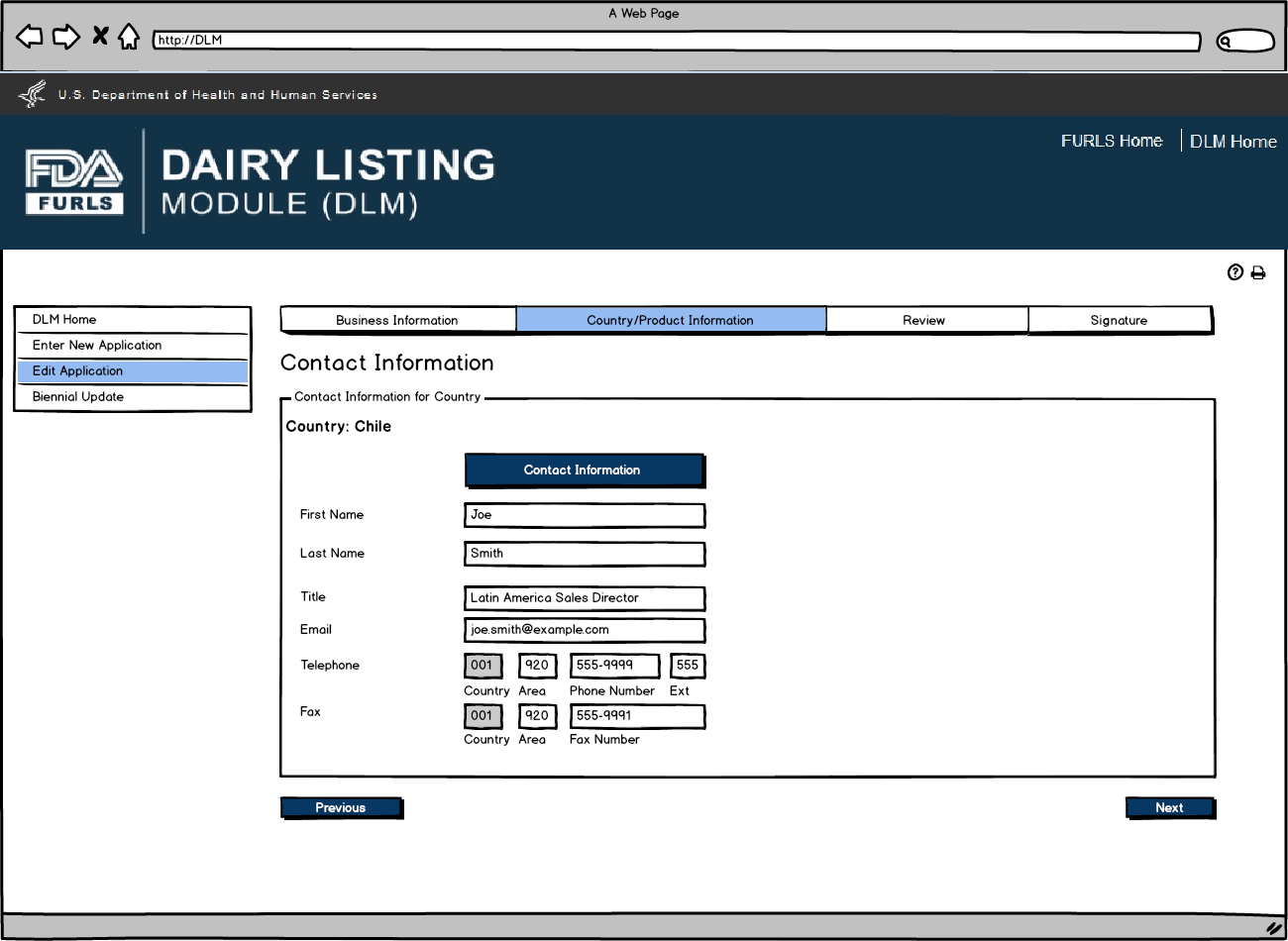
Edit Application: Figure 3
User will review Country Contact Information and edit information if needed.
Edit Application: Figure 4
If there are no changes to the product listing, then the user confirms their choice by selecting “There are no changes to this Product List”. If user would like to edit product information, they can click on the pencil icon to edit the product.
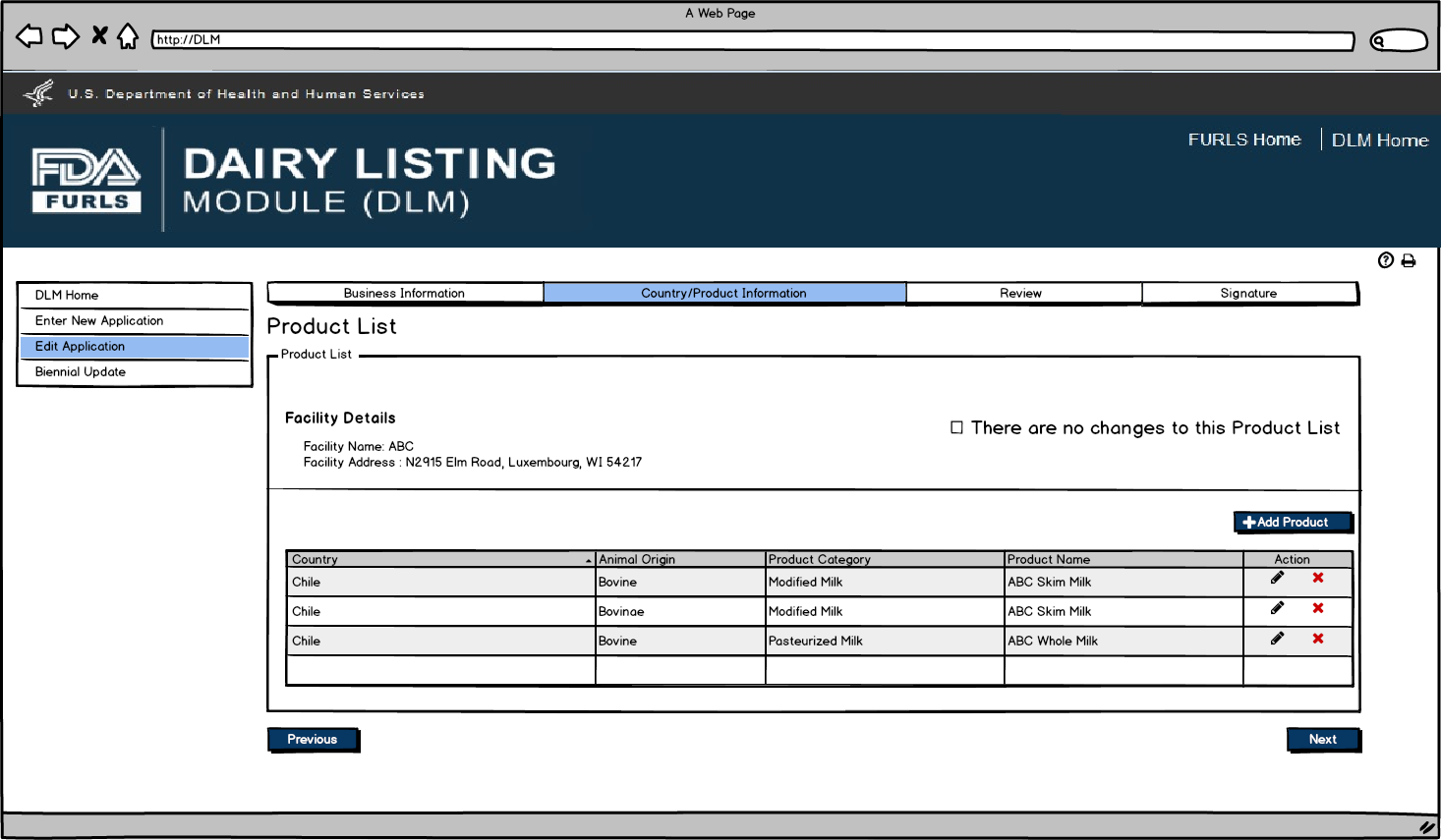
Edit Application: Figure 5
After user clicks Pencil Icon in Figure 5, they can now edit information for the Product selected if needed.
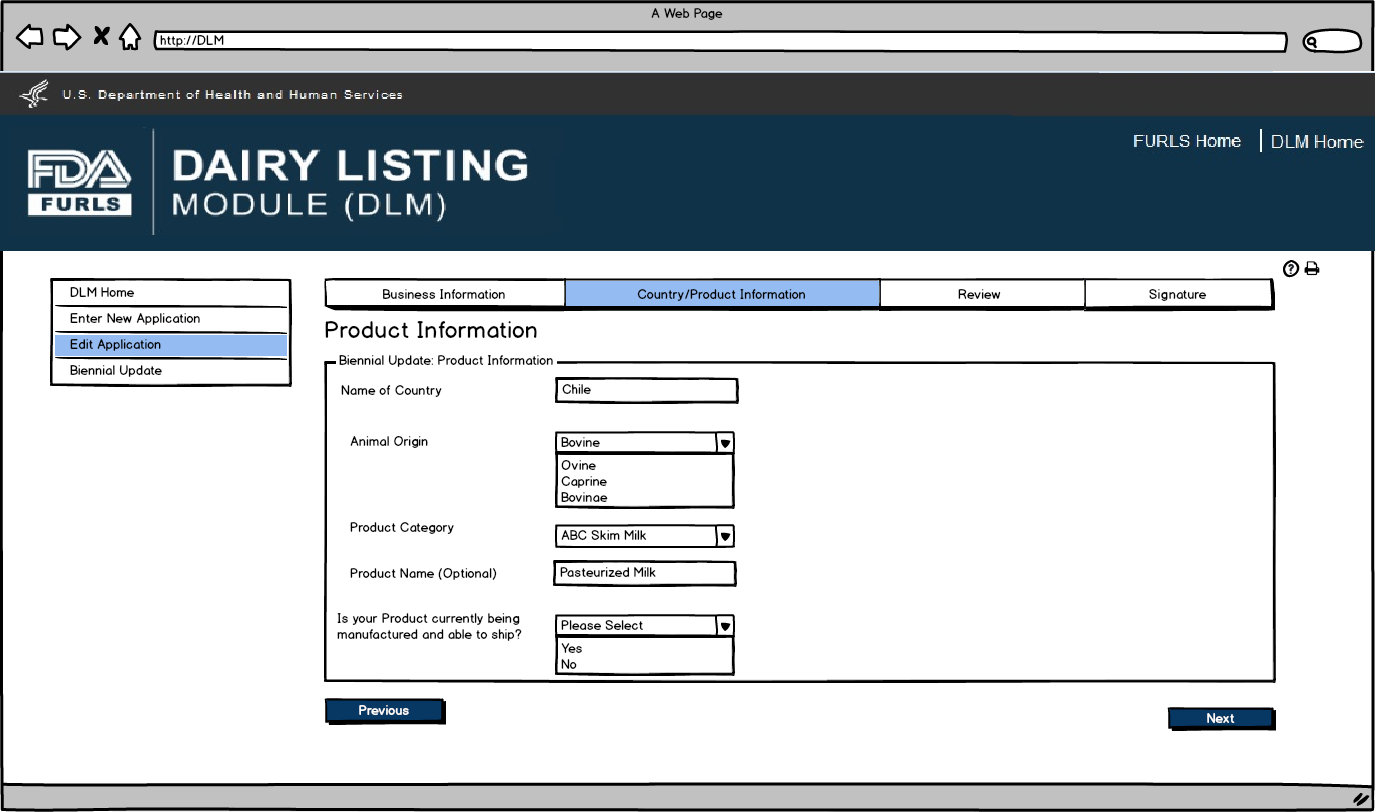
Edit Application: Figure 6
User will see the Product List to confirm the Products entered with their firm.

Edit Application: Figure 7
Application Review screen for users.
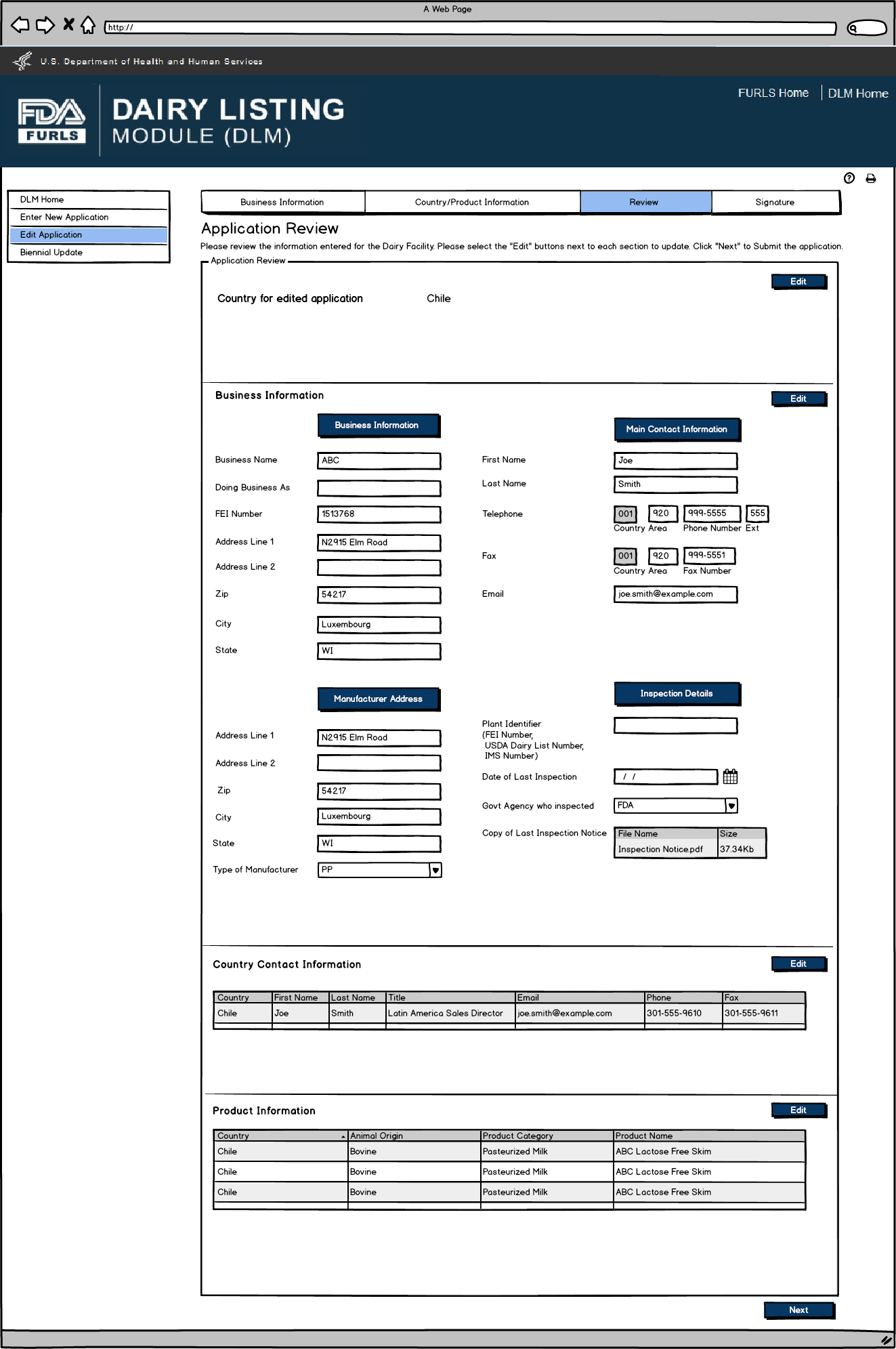
Edit Application: Figure 8
Signature Page allows users to sign off on the edited application submission.
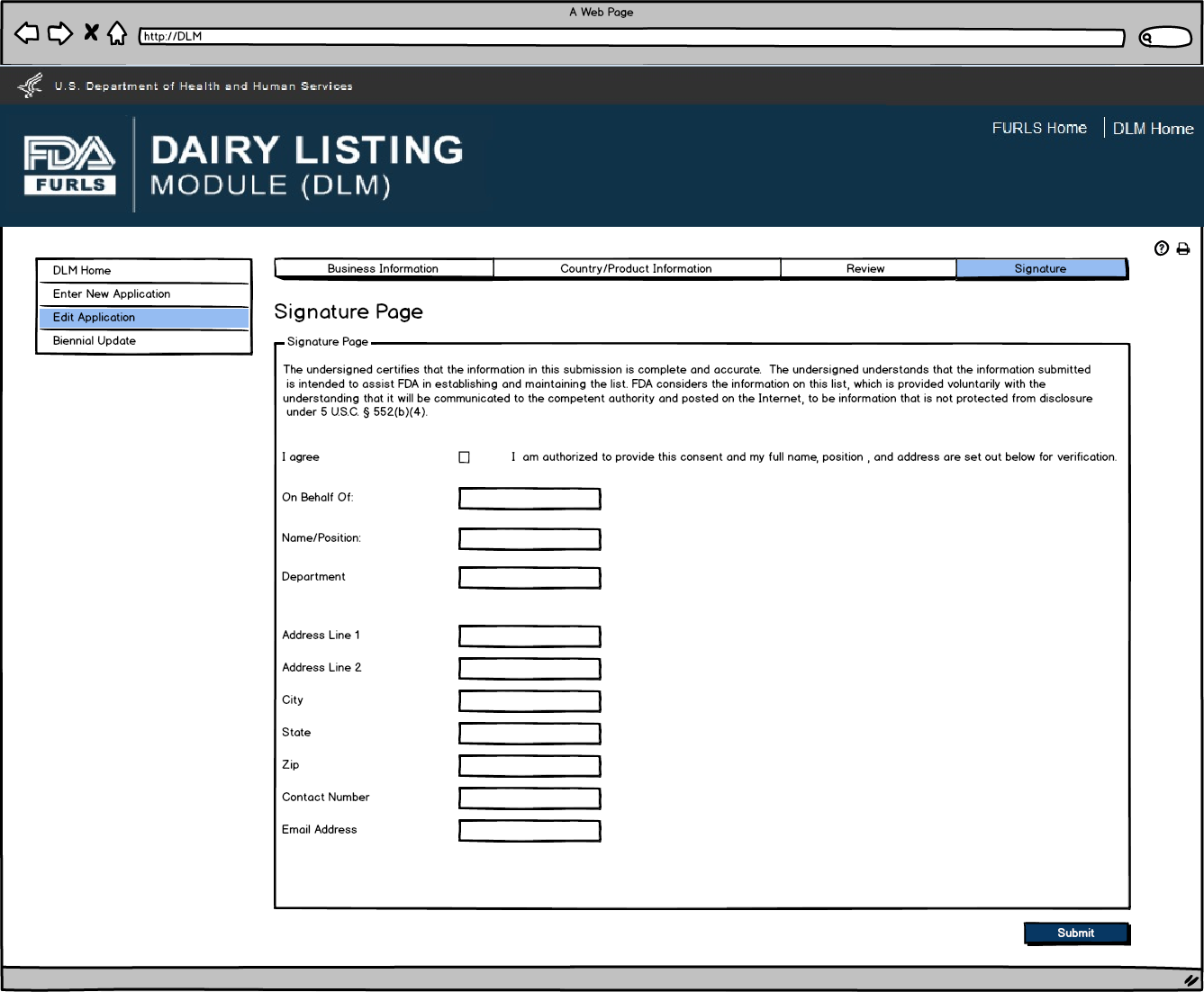
Edit Application: Figure 9
This screen confirms that the application changes have been successfully submitted along with the Application Number and Country applied to.
Flow 3: Biennial Update for External User
Biennial Update: Figure 1
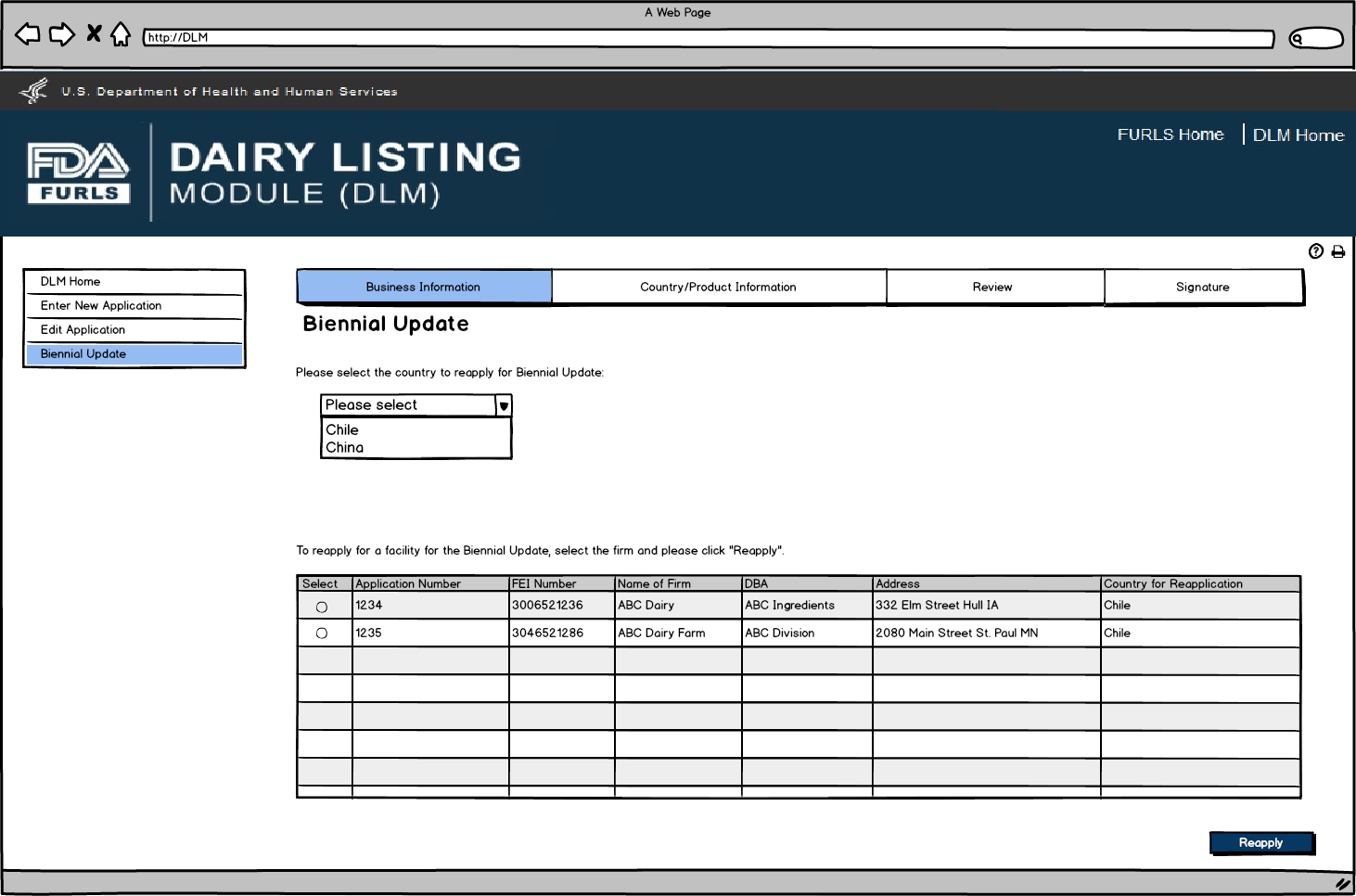
User first selects the country for Biennial Update. The table will then appear with the facilities under the user’s account. The user will select the facility for reapplication and then select the Reapply button.
 Biennial
Update: Figure 2
Biennial
Update: Figure 2
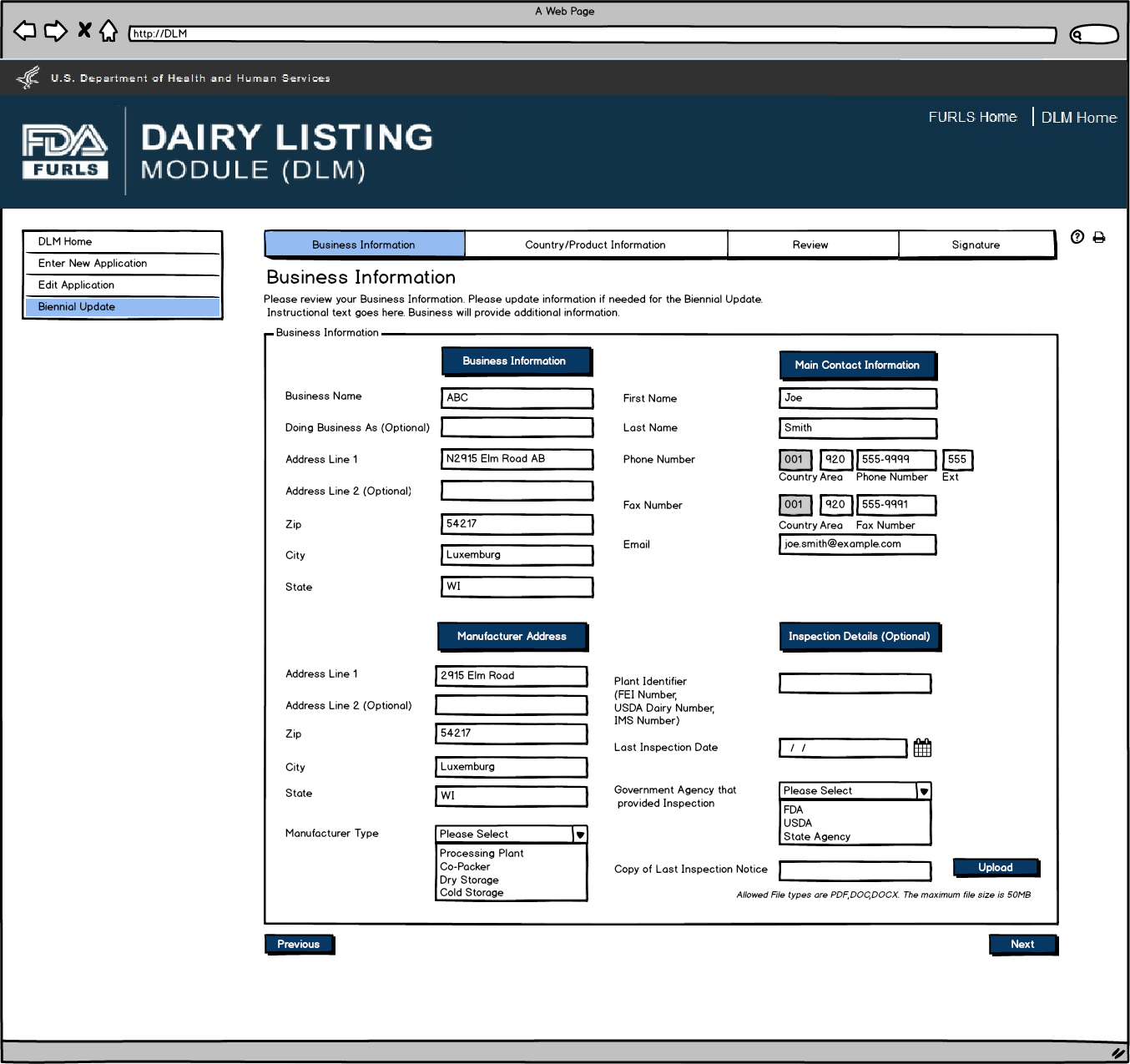
If the firm is a Dairy Product Manufacturer/Processor that is a registered food facility, all the fields except Doing Business As, Manufacturer Type, and the Inspection details will be read only and cannot be edited. Dairy Product Manufacturer/Processor that is a registered food facility user will need to change any Business Information in FFRM. The information that will be editable for Dairy Product Manufacturer/Processor that is a registered food facility will be Doing Business As, Manufacturer Type, and every field in Inspection Details section. For Dairy Product Manufacturer/Processor that is exempt from registration of food facilities, all fields will be editable.
Biennial Update: Figure 3
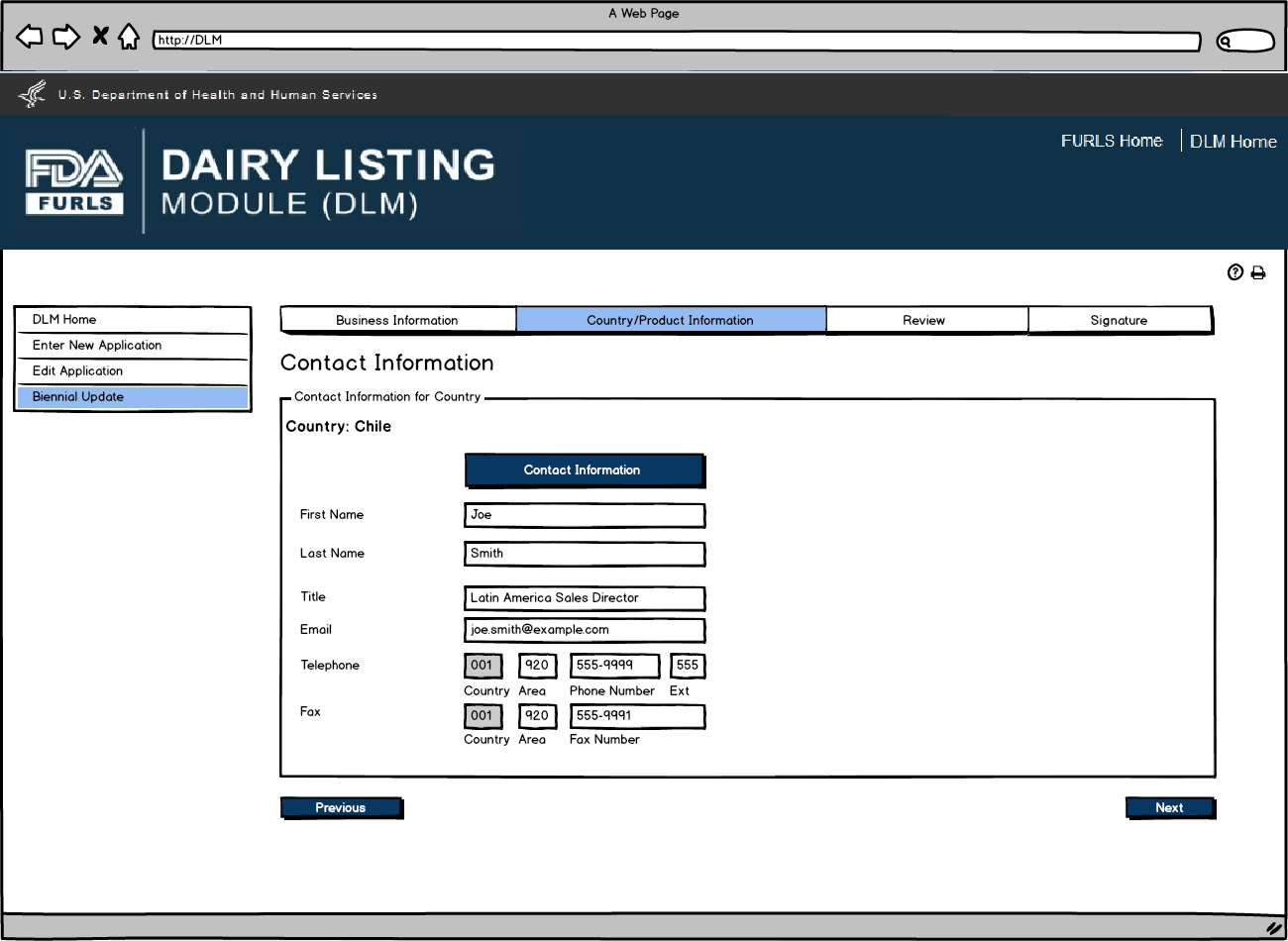
User will review Country Contact Information and edit information if needed.
Biennial Update: Figure 4
If there are no changes to the product listing, then the user confirms their choice by selecting the “There are no changes to this Product List”. If user would like to edit product information, they can click on the pencil icon to edit the product.
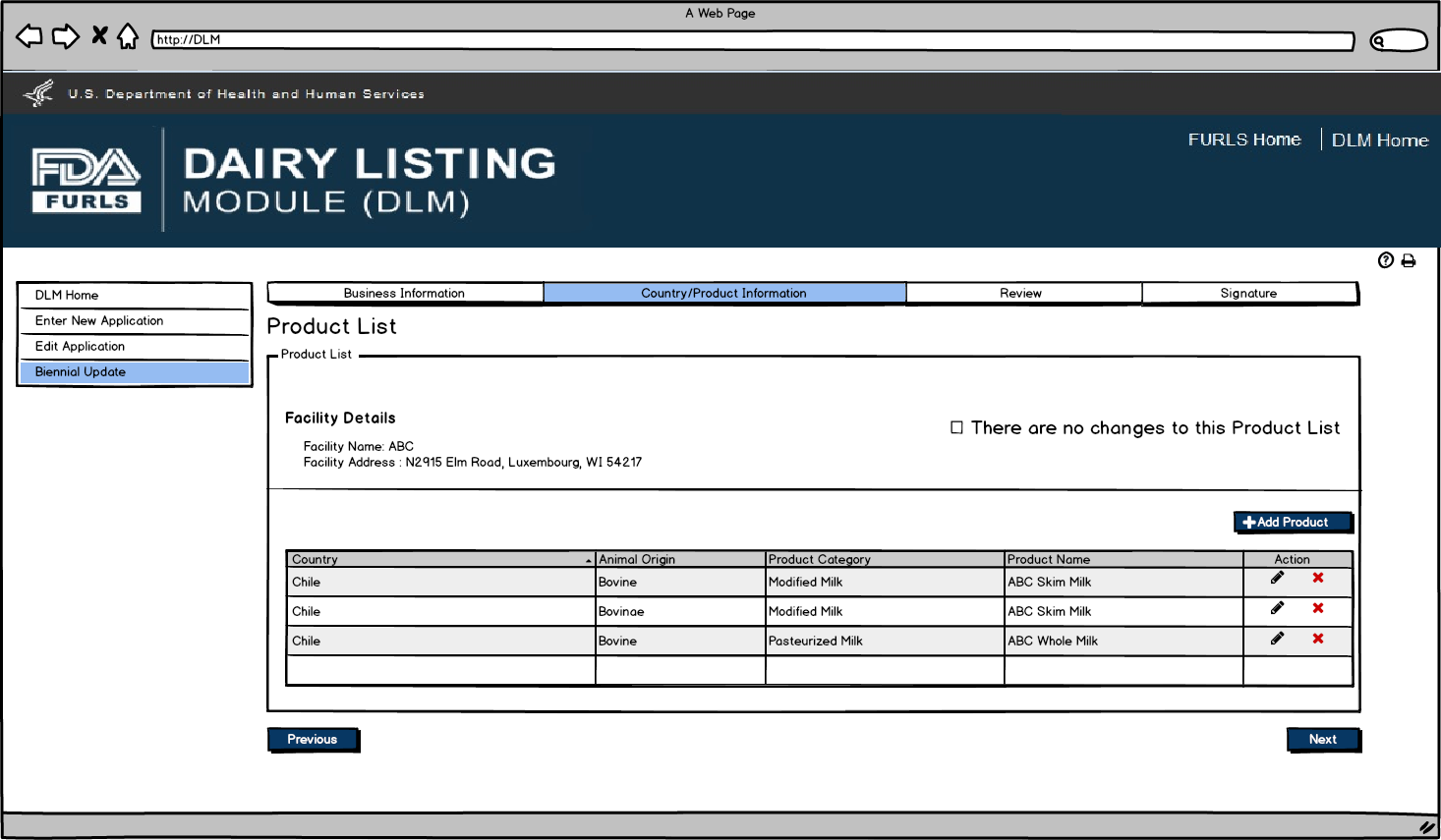
Biennial Update: Figure 5
After user clicks Pencil Icon in Figure 5, they can now edit information for the Product selected if needed.
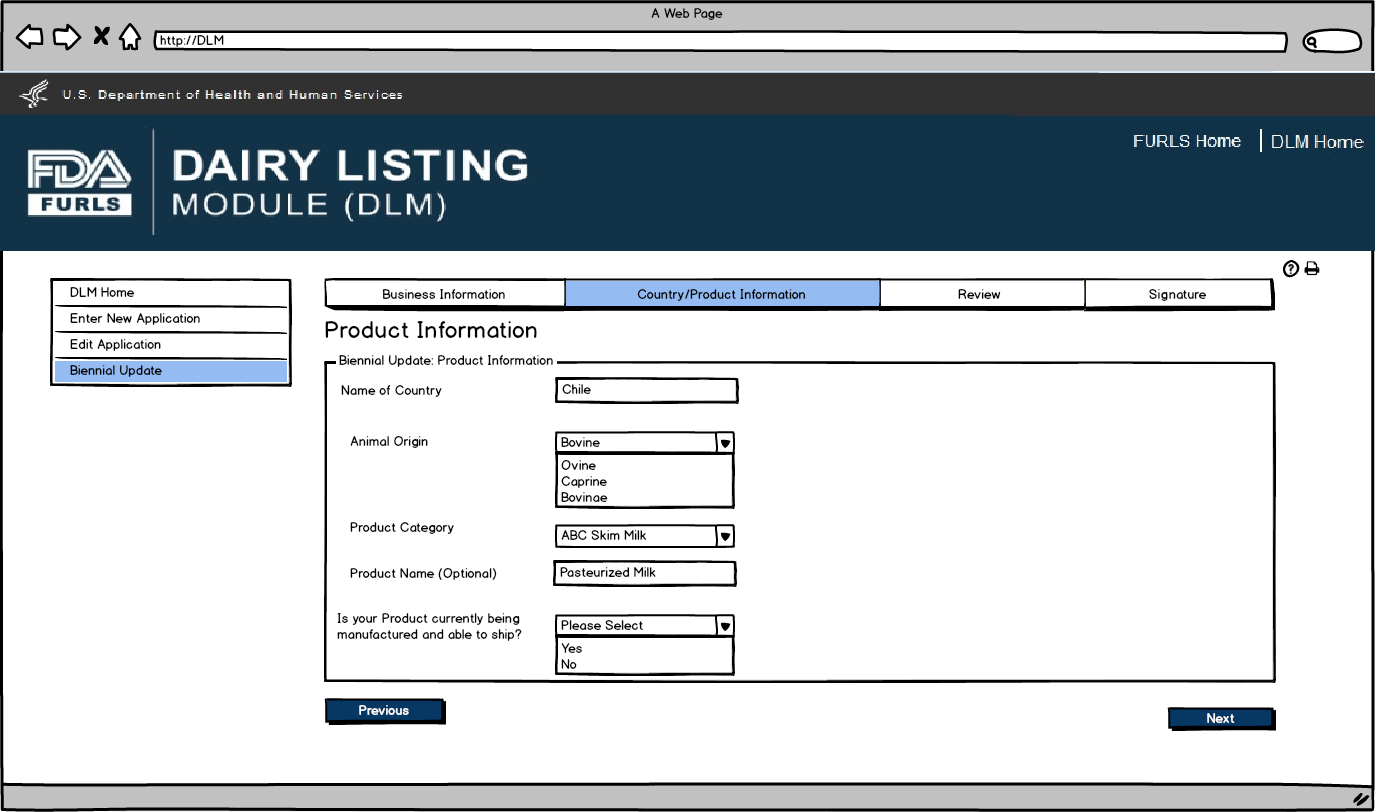
Biennial Update: Figure 6
User will see the Product List to confirm the Products for their firm.

Biennial Update: Figure 7
Biennial Update Application Review screen for users.
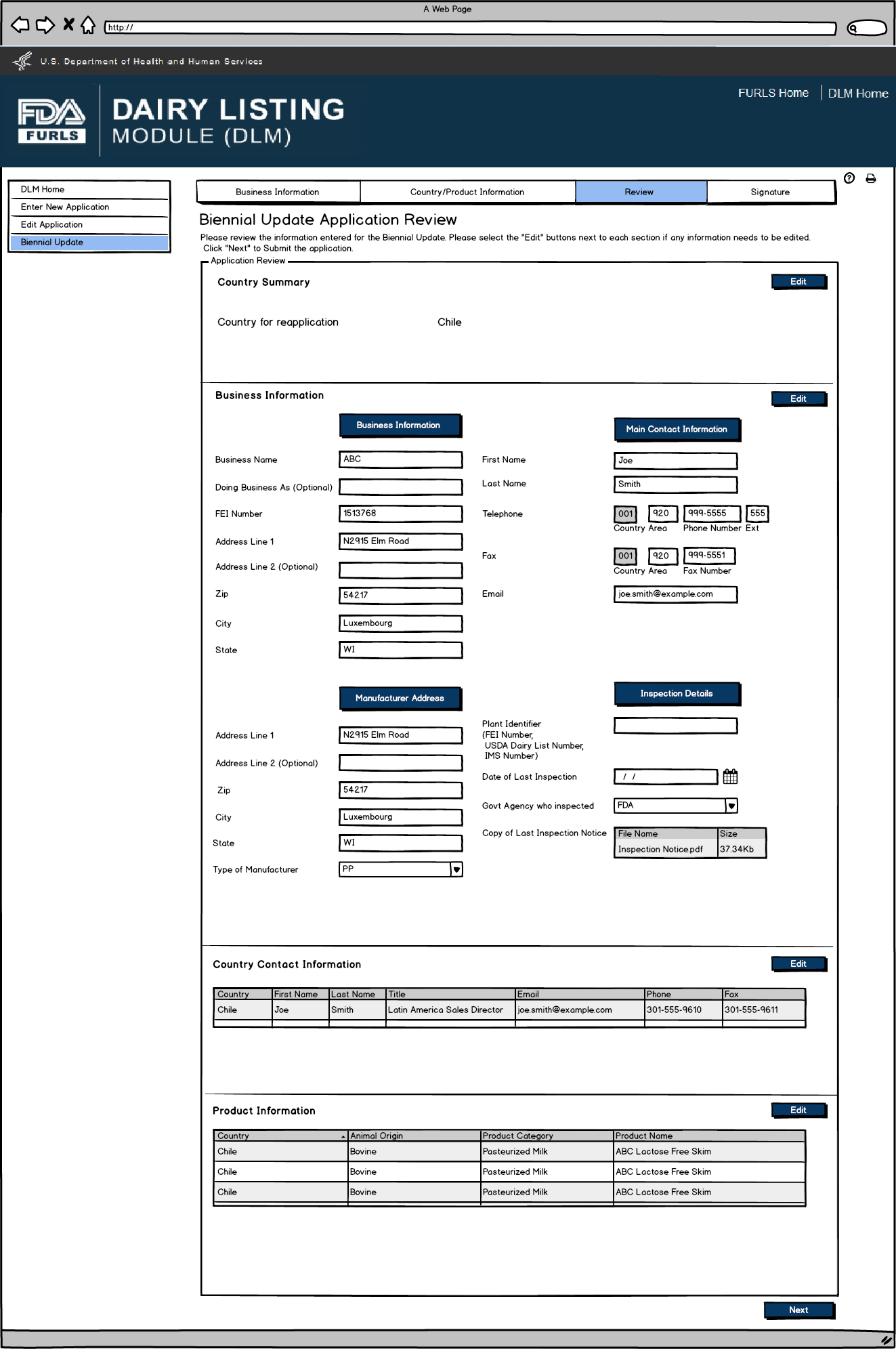
Biennial Update: Figure 8
Signature Page allows users to sign off on the Biennial Update application submission.
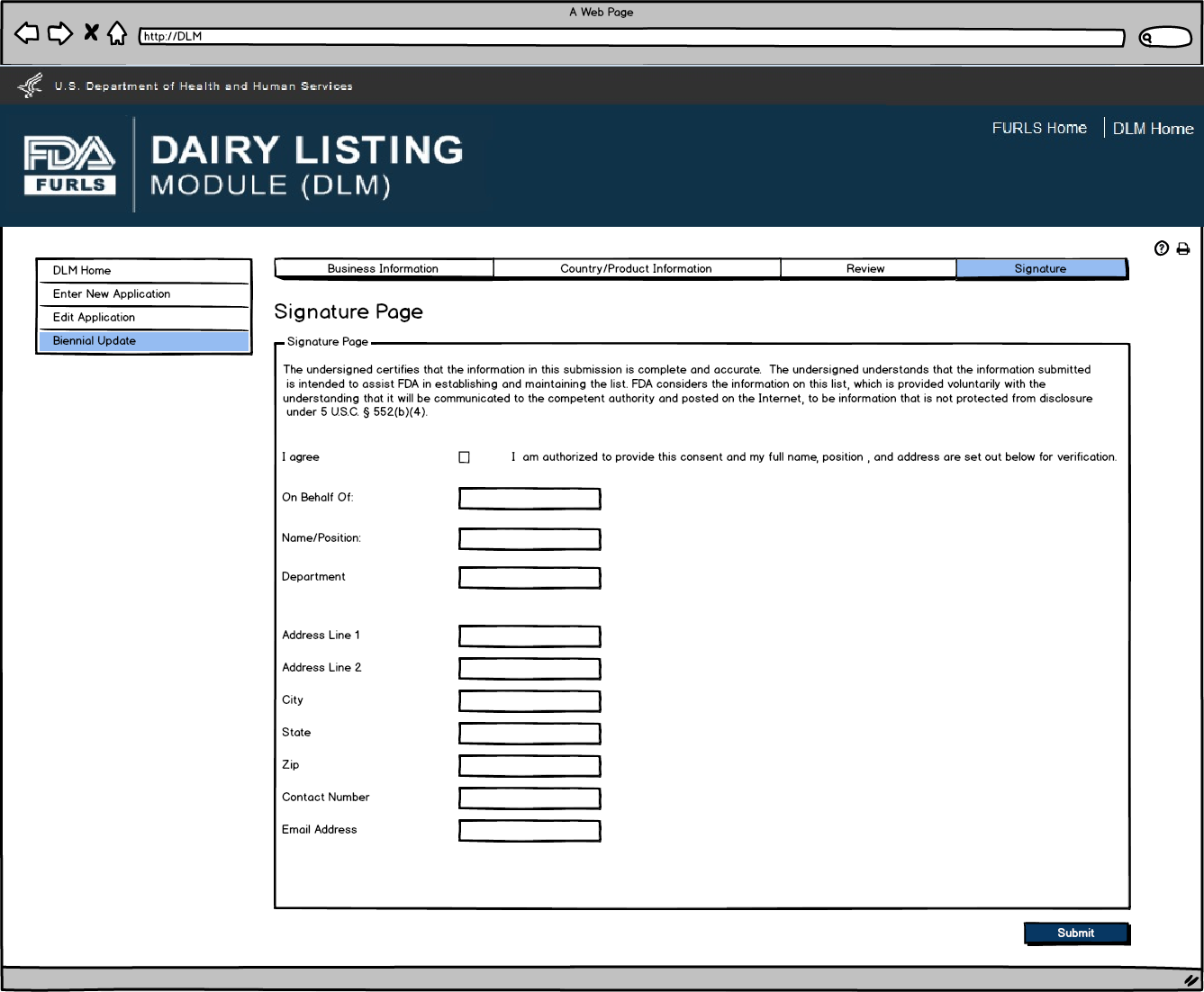
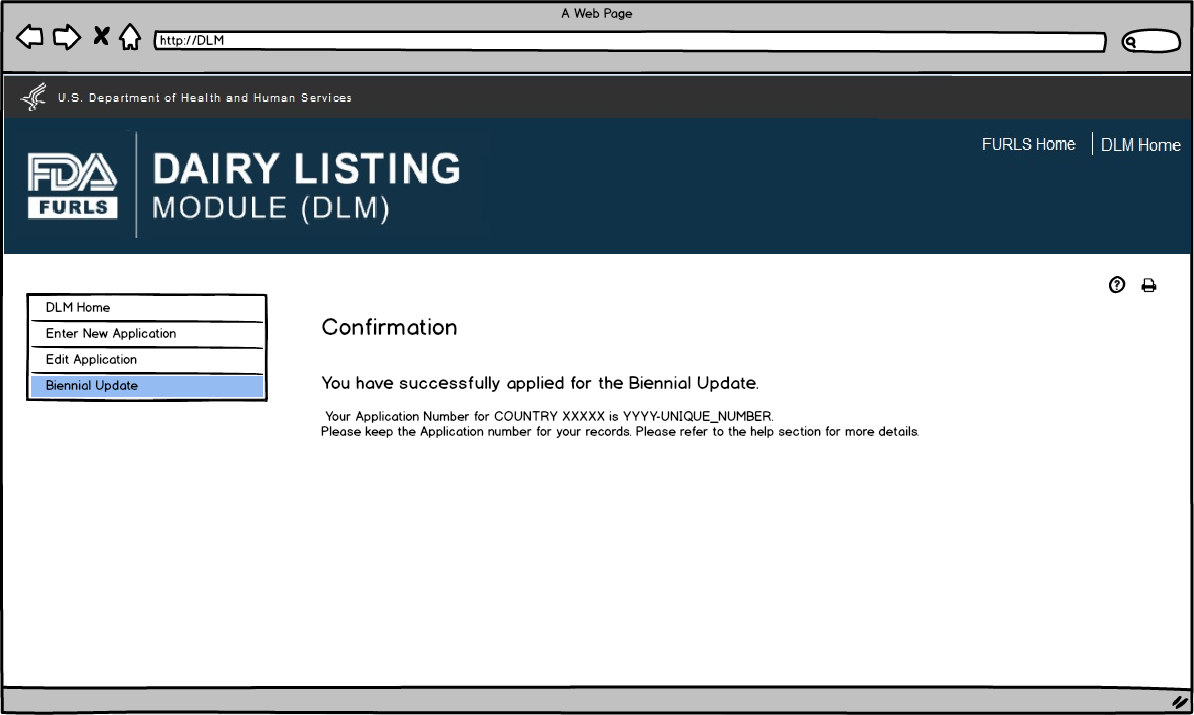
Biennial Update: Figure 9
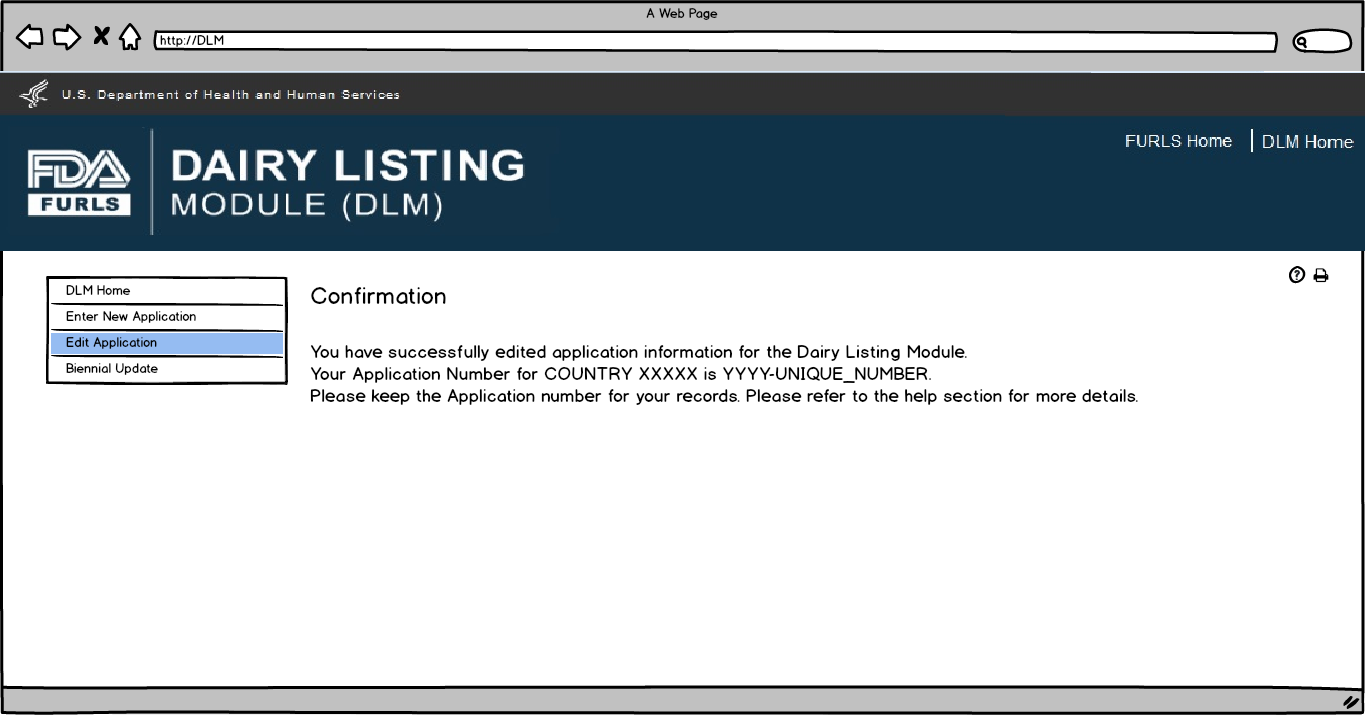
This screen confirms that the Biennial update application has been successfully submitted along with the Application Number and Country applied to.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Hardy, Mary * |
| File Modified | 0000-00-00 |
| File Created | 2021-01-23 |
© 2026 OMB.report | Privacy Policy