SSA Biobank Final 5.2016
SSA Biobank Final 5.2016.docx
Survey to Assess the Feasibility of Establishing a Gynecologic Specimen Bank (NCI)
OMB: 0925-0745
Supporting Statement A for
Survey to assess the feasibility of establishing a gynecologic specimen bank (NCI)
Federal Government Employee Address:
Name: Goli Samimi
Address: Breast and Gynecologic Cancer Research Group, Division of Cancer Prevention
National Cancer Institute, National Institutes of Health
9609 Medical Center Drive
Room 5E-336, MSC 9783
Bethesda, MD 20892
Telephone: (240) 276-6582
Fax: (240) 276-7828
Email: [email protected]
Check off which applies:
X New
Revision
Reinstatement with Change
Reinstatement without Change
Extension
Emergency
Existing
Table of Contents
A. JUSTIFICATION
A.1 Circumstances Making the Collection of Information Necessary
A.2. Purpose and Use of the Information COLLECTION
A.3 Use of Information Technology and Burden Reduction
A.4 Efforts to Identify Duplication and Use of Similar Information
A.5 Impact on Small Businesses or Other Small Entities
A.6 Consequences of Collecting the Information Less Frequently
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
A.8 Comments in Response to the Federal Register Notice and Efforts to Consult Outside Agency
A.9 Explanation of Any Payment of Gift to Respondents
A.10 Assurance of Confidentiality Provided to Respondents
A.11 Justification for Sensitive Questions
A.12 Estimates of Hour Burden Including Annualized Hourly Costs
A.13 Estimate of Other Total Annual Cost Burden to Respondents or Record keepers
A.14 Annualized Cost to the Federal Government
A.15 Explanation for Program Changes or Adjustments
A.16 Plans for Tabulation and Publication and Project Time Schedule
A.17 Reason(s) Display of OMB Expiration Date is Inappropriate
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions
List of Attachments
Attachment #1: Invitation
Attachment #2: Advertisement
Attachment #3: Paper Survey
Attachment #4: Survey Screenshots
Attachment #5: Privacy Act Memo
A. Justification
This is a request for a New OMB number for a period of 1 year. The National Cancer Institute is assessing the feasibility of developing a tissue bank that would include tube and ovary tissues from women undergoing surgery for benign conditions, risk reduction and early stage cancer. Collecting tissues from tubes and ovaries containing clinically unsuspected precursors or early stage cancer is challenging, especially among women that are not at increased genetic risk. However, given that many pathology laboratories have enhanced their processing protocols for gynecologic surgical specimens removed for benign indications, it may be possible to develop a tissue resource. Accordingly, we are requesting information via a survey about the volume of samples that are accessioned at different pathology laboratories, and the methods used to process these samples. These data would provide information necessary to assess the feasibility of establishing a tissue bank for research and provide insights into the best design of a pilot study.
A.1 Circumstances Making the Collection of Information Necessary
The National Cancer Institute (NCI), established under the National Cancer Act of 1937, is the Federal Government's principal agency for research on cancer cause, prevention, detection, diagnosis, treatment, and rehabilitation, and for the dissemination of information for the control of cancer. Current authorization for NCI's education and information collection and dissemination activities is contained in Section 410 of the Public Health Service Act (42 USC § 285).
Increasing evidence suggests that a substantial fraction of high-grade serous carcinomas (HGSC) arise from the fallopian tube among carriers of germline BRCA1/2 mutations. However, the percentage of HGSC that arise from the tube among BRCA1/2 mutation carriers is unknown, and the origin of HGSC among women who are not carriers of BRCA1/2 mutations is poorly studied. Defining the sites of origin(s) of HGSC among women who are not BRCA1/2 mutation carriers is a priority because these women account for over 80% of HGSC.
The National Cancer Institute is assessing the feasibility of developing a tissue bank that would include tube and ovary tissues from women undergoing surgery for benign conditions, risk reduction and early stage cancer. Collecting tissues from tubes and ovaries containing clinically unsuspected precursors or early stage cancer is challenging, especially among women that are not at increased genetic risk. However, given that many pathology laboratories have enhanced their processing protocols for gynecologic surgical specimens removed for benign indications, it may be possible to develop a tissue resource. Accordingly, we are requesting information via a survey about the volume of samples that are accessioned at different pathology laboratories, and the methods used to process these samples. These data would provide information necessary to assess the feasibility of establishing a tissue bank for research and provide insights into the best design of a pilot study.
A.2 Purpose and Use of the Information Collection
The information will inform how the NCI develops a new resource that is relevant, useful and appropriate. We will send 500 invitations (Attachment 1) to Pathology Lab Managers throughout the United States. Managers will be identified through Pathology lab databases, Society Membership lists and personal recommendations. We will also advertise the survey in relevant pathology journals and society meetings (Attachment 2). Individuals will receive an invitation (Attachment 1) by email or postal mail to participate in the survey. They will have the option to complete the hard copy of the survey (Attachment 3) to be returned in a self-addressed stamped envelope, or a web-based survey (Attachment 4) via an online link included on the invitation. We expect to send out approximately 500 invitations, with 250 respondents representing 250 Pathology Laboratories. After 4-6 weeks the original email invitation (Attachment 1) will be sent to non-responders.
Specifically, the survey (Attachments 3 and 4) will collect information about:
Approximate annual surgical pathology specimen volume at each laboratory
The processing and methodology of samples obtained from risk-reduction surgery vs. early stage disease
The feasibility of collecting tissues and pathology reports for a national specimen bank/resource organized by the National Cancer Institute
A.3 Use of Information Technology and Burden Reduction
Respondents will have the choice of answering the survey online (Attachment 4), or as a hard paper copy (Attachment 3). This option reduces the time burden for respondents, as they can access and respond to the survey in a manner and at a time and place most convenient for them.
A.4 Efforts to Identify Duplication and Use of Similar Information
The information regarding the processing of samples from non-high-risk woman, and feasibility of tissue collection is not currently available from the literature or national pathology societies. To our knowledge, there is no nationally-sponsored bio-bank containing gynecologic tissues from risk reduction or early stage cancer surgeries.
A.5 Impact on Small Businesses or Other Small Entities
No small entities are impacted.
A.6 Consequences of Collecting the Information Less Frequently
This is a one-time collection of information.
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This survey will be implemented in a manner that fully complies with 5 C.F.R. 1320.5.
A.8.1 Comments in Response to the Federal Register Notice
The 60-Day Federal Register Notice was published on March 8, 2016, Vol. 81, page 12111. No comments were received.
A.8.2 Efforts to Consult Outside Agency
There was no consultation with any entities outside of NCI.
A.9 Explanation of Any Payment of Gift to Respondents
No payments or gifts will be given to respondents.
A.10 Assurance of Confidentiality Provided to Respondents
All information will be kept private to the extent allowable under law. No personally identifiable information is being collected. Per the Privacy Officer, the Privacy Act does not apply (Attachment 5). A PIA is not needed as PII is not being collected.
A.11 Justification for Sensitive Questions
There are no sensitive questions being asked.
A.12.1 Estimated Annualized Burden Hours
The annualized burden hours are 42 from a total 250 respondents. We expect to send out approximately 500 invitations and expecting 250 respondents representing 250 Pathology Laboratories. The survey is expected to take 10 mins to complete.
Table A12-1. Estimates of Hour Burden
Category of Respondent |
Form Name |
Number of Respondents |
Frequency of Response per Respondent |
Time Per Response (in hours) |
Burden Hours |
Individuals |
Survey |
250 |
1 |
10/60 |
42 |
Totals |
|
250 |
|
|
42 |
A.12-2 Annualized Cost to respondents
The annualized cost to respondents is $1,892.51. This amount was calculated using the mean hourly wage rate of $45.06 for occupation title “Medical Scientists Except Epidemiologists” occupation code “19-1042” from the May 2015 National Occupational Employment and Wage Estimates in the United States (http://www.bls.gov/oes/current/oes191042.htm). Table A12.2 provides the cost to respondents.
Table A12-2. Annualized Cost to the Respondents
Type of Respondent |
Average Burden Per Response (in hours) |
Hourly Wage Rate |
Total Respondent Cost |
Individuals (Pathology Lab Head) |
42 |
$45.06 |
$1,892.51 |
Total |
42 |
|
$1,892.51 |
A.13 Estimate of Other Total Annual Cost Burden to Respondents or Record Keepers
There are no capital costs, operating costs, or maintenance costs to report.
A.14 Annualized Cost to the Federal Government
The annualized total cost to the Federal Government is solely associated with FTE costs and is estimated to be $5,989 (Table A14-1). Federal personnel will be designing and distributing the survey and invitation, as well as collecting the data.
Table A14. Annualized Cost to the Federal Government
Staff |
Grade/Step |
Salary |
% of Effort |
Fringe (if applicable) |
Total Cost to Gov’t |
Federal Oversight |
|
|
|
|
|
Program Director |
13/10 |
$119,794 |
2.5% |
|
$2,995 |
Investigator |
14/4 |
$119,776 |
2.5% |
|
$2,994 |
|
|
|
|
|
|
Contractor Cost |
|
|
|
|
$0 |
|
|
|
|
|
|
Travel |
|
|
|
|
$0 |
Other Cost |
|
|
|
|
$0 |
|
|
|
|
|
|
Total |
|
|
|
|
$5,989 |
A.15 Explanation for Program Changes or Adjustments
This is a new collection of information.
A.16 Plans for Tabulation and Publication and Project Time Schedule
Simple descriptive statistics will be employed for the survey questions, with some degree of qualitative techniques used to analyze and identify common themes.
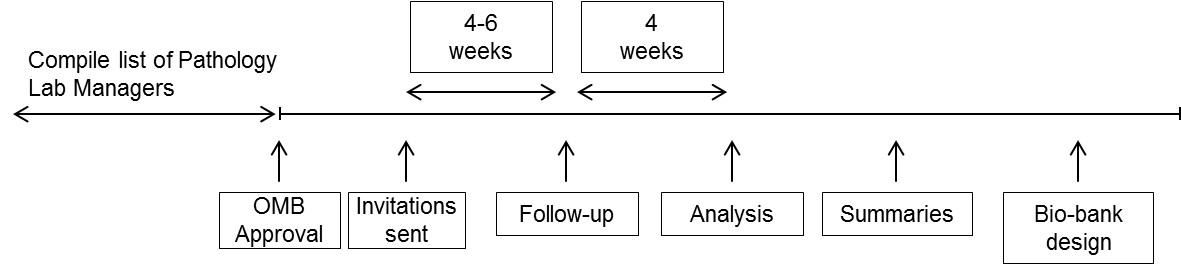
The surveys will be distributed once OMB approval is granted. Follow-up will occur 4-6 weeks following contact, by re-sending our original invitation (Attachment 1). Analysis and summaries will be conducted 4 weeks after follow-up. Our collaborators involved in the statistical analysis will provide a summary report of findings.
A.17 Reason(s) Display of OMB Expiration Date is Inappropriate
We are not requesting an exemption to the display of the OMB Expiration date.
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions
No exceptions are requested
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Subject | Supporting Statement A |
| Author | Lopez, Maria (NIH/NICHD) [E] |
| File Modified | 0000-00-00 |
| File Created | 2021-01-23 |
© 2026 OMB.report | Privacy Policy