Justification for Change
Combined Nonsubs 071416.docx
National Health and Nutrition Examination Survey
Justification for Change
OMB: 0920-0950
National Health and Nutrition Examination Survey
OMB No. 0920-0950
(Expires December 31, 2017)
Nonsubstantive Change to conduct
NHANES Special Studies
Contact Information
David Woodwell, MPH
Planning Branch Chief
National Health and Nutrition Examination Survey
National Center for Health Statistics/CDC
3311 Toledo Road
Hyattsville, MD 20782
Telephone: 301-458-4327
FAX: 301-458-4028
E-mail: [email protected]
July 14, 2016
This is a request for nonsubstantive changes to the National Health and Nutrition Examination Survey (NHANES) (OMB No. 0920-0950, exp. December 31, 2017), conducted by the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC). The proposed changes would not alter the currently approved burden hours.
The projects planned include the following:
Ambulatory Blood Pressure Monitoring (ABPM) Feasibility Study
Currently, 67 million US adults have hypertension, and nearly all were diagnosed using clinic blood pressure measurements per national US guidelines (JNC VII). However, casual, periodic clinic-based blood pressure measurements cannot capture the significant variability in blood pressure that can occur throughout a 24 hour period. The ABPM Feasibility Study aims to test the methods that would be used to evaluate systolic and diastolic blood pressure variation over a 24 hour period.
Multi-Mode Screening Feasibility Study
Currently NHANES only conducts in-person screenings of potential participants. For several reasons, NHANES would like to test, and if successful, implement a multi-mode (online and in-person) screening approach. The primary reason would be to identify and more quickly screen out households which do not contain persons eligible to be in NHANES. There would be no need to send an interviewer to such households which self-screened out. Interviewers who spend less time screening could instead spend more time with individuals who screened into the survey, thereby potentially saving time and money. The language of the screener would not change.
NHANES Consent Form Revisions
The National Center for Health Statistics (NCHS) Ethics Review Board (ERB) has requested small edits to the following NHANES consent forms.
2016 Home Interview Consent
2016 Mobile Exam Center (MEC) Assent
Consent/Assent and Parental Permission for Specimen Storage and Continuing Studies
The request for these changes came after these forms had received OMB approval. NHANES has made these changes and is including the revised forms with this submission.
A. Justification
Circumstances Making the Collection of Information
Necessary.
NHANES is conducted annually. It includes a household interview, done in participants’ homes and physical measures and additional interviews done in the NHANES MECs. There may also be follow-up interviews or components (such as a 2nd dietary interview) that take place after the MEC exam. A major advantage of continuous NHANES data collection is the ability to address emerging public health issues and provide objective data on more health conditions and issues by changing/modifying survey content. Though collected annually, NHANES data are released in two year cycles. Some survey content stays the same across multiple cycles of NHANES. But new survey content may be added, existing content may be modified or some content may be dropped at the beginning of each two-year survey cycle.
There is great value in testing new methodologies before they are implemented in the main survey. Testing allows NHANES staff to determine how long the protocol will take and how well received the procedure will be among our participants. The results of such testing also allow the NHANES program to make changes or adjustments to improve the methodology without affecting the results from the main study. Finally, it also provides hands on training opportunities for NHANES survey staff responsible for collecting the data. Testing is a vital step in making sure NHANES is effective and efficient in its use of resources. Such measures promote improved data quality once the data is collected in the actual survey. Since data collection is continuous, methodology studies must be conducted during ongoing NHANES data collection.
Purpose and Use of the Information Collection
The purposes and uses of each proposed study are detailed below. Tests will include NHANES participants or remunerated volunteers (in circumstances such as when there aren’t enough NHANES participants in the pilot’s target group or when the pilot cannot be conducted in the NHANES setting, etc.). Participation is voluntary. Tests will be conducted as soon as clearance is received.
Ambulatory Blood Pressure Monitoring (ABPM) Feasibility Study
The recent U.S. Preventive Services Task Force report1 recommends ambulatory blood pressure monitoring (ABPM) as the reference standard for confirming the diagnosis of hypertension. The accurate diagnosis and management of high blood pressure (HBP) is a critical element to reduce cardiovascular morbidity and mortality. Currently in the U.S. healthcare system, clinic-based blood pressure is used as the primary method to diagnose and manage patients with HBP. However, it may significantly over- and under-estimate true blood pressure as experienced in everyday life (i.e., 24 hours a day) leading to missed opportunities in accurately identifying and managing patients by both over and under-treatment of HBP. There are currently no national estimates of blood pressure using ABPM standards, and estimates used for diagnosis and management are derived from populations with limited representativeness.
This proposal is to test the feasibility of conducting 24-hour ambulatory blood pressure monitoring (ABPM) (Attachment 1a). The objectives of this feasibility study can be summarized as follows:
To assess the feasibility and test all procedures related to the 24-hour ABPM including the instructions, participant preparation, and completion of questionnaire.
To test response rates
To evaluate and select one 24-hour ABPM device among three validated 24-hour ABPM devices (Spacelabs 90217, SunTech Oscar 2, and Welch Allyn Mobil-O-Graph)
To evaluate completeness of valid blood pressure measurements during the day and at night
To examine how participants respond to wearing a particular device for 24-hours.
To understand participants’ perception of monitor comfort, ease of use, and degree of inconvenience.
Three hundred and sixty volunteers would participate in the study and would be randomized into 3 groups of 120 individuals per device (see Figure 1 below). All three devices have been validated and cleared to be used for 24-hour ABPM.2,3,4 The devices are being tested to identify which device is the most comfortable, easiest to use and remove, has the least sleep disturbance and the least amount of pain for participants. One of the objectives of the feasibility study is to select one 24-hour ABPM monitor that will be used in the NHANES program.
In order to ensure usability of the device and facilitate utilizing a device with the lowest level of burden for the NHANES program, information about the technology and the participant’s experience need to be evaluated. The evaluation and the selection of a device will include: performance, convenience, comfort, safety measure on the device and simplicity in use. Key performance indicators that will be measured will include: product instructions, how quickly it takes to set up the device, reliable operation, did the device take measurements at the pre-specified time interval, percentage of measurement error, percentage of repeat measurements and data completion (the proportion of successful/valid blood pressure measurements during the day, at night, and 24-hour period and the proportion of ABPM recordings that satisfy pre-specified criteria for a satisfactory recording defined has having at least 70% valid BP readings during the 24 hour study period with at least 1 BP reading recorded per hour; with at least 14 readings for the awake period or at least 5 readings for the nighttime period)5. Device burden will be assessed from the tolerability questionnaire. The impact of the devices on sleep quality and sleep quality after 24-hour BP monitoring will be compared across the three devices. If all three devices compare favorably to each other, the determining factor will be cost. All three devices provide the same measurement; however, there is a substantial price difference among the devices.
Figure 1.
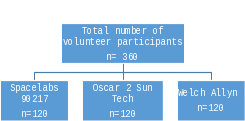
Volunteer participants will be screened for eligibility (Attachment 1b). Eligible participants who agree to participate will provide written informed consent (Attachment 1c). After giving consent, participants will have three resting blood pressure measurements taken to assess eligibility. If eligible, participants will then be asked to wear an ambulatory blood pressure monitor programmed to take blood pressure measurements every 30 minutes for the duration of the study time. Prior to wearing the monitor, participants would be asked questions about demographics and sleep quality. These questions (Attachment 1d) consist of seven demographic questions covering race/ethnicity, education, marital status, chronic condition and general health; the Pittsburgh Sleep Quality Index (PSQI)6 and the Richards-Campbell Sleep Questionnaire (RCSQ)7, both are validated survey instruments for measuring sleep quality.
Participants would also be given an information sheet describing the ambulatory blood pressure monitor (Attachment 1e), an accelerometer (the same devise used in previous NHANES) to wear on their wrists and a Study Diary to evaluate sleep/wake times, impact of eating, impact of anti-hypertensive use while wearing the monitor (Attachment 1f). When participants make the second visit at the end of the study, they would also be given post-ABPM questionnaires focusing on sleep quality within the 24-hour period and the potential inconveniences of wearing the blood pressure monitors8 (Attachment 1g). This post ambulatory BP monitoring questionnaire has been used in several studies assessing tolerability when wearing a specific ambulatory BP monitor. In addition, all participants will receive a report of findings on their blood pressure readings whether they are eligible to participate in the study or not (Attachment 1h).
If this testing yields a response rate of at least 80% and the protocol proves satisfactory, the eventual goal would be to add this exam procedure to the full NHANES. The addition of this component to NHANES would allow us to obtain estimates of the US prevalence of high blood pressure based on ambulatory blood pressure monitoring.
More details about the Ambulatory Blood Pressure Monitoring ABPM) Feasibility Study are provided in Attachments 1a through 1h.
Multi-Mode Screening Feasibility Study
NHANES is a multi-stage probability sample that requires rostering (creating a list of everyone living in a home) of households for its sample selection. The process of rostering is also known as “screening”. Screening to find people eligible to participate in NHANES is difficult. The steps involved include identifying sampled household addresses and sending field interviewers in-person to these addresses, often more than once, to ask screening questions to find out the make-up of the household.
Usually, the screener interview is conducted on the doorstep. Based on the answers obtained from the screener interview, a randomly assigned, computer-generated message tells the interviewer which individuals, if any, are eligible to participate based on their demographic characteristics. The characteristics used are gender, age, race/ethnicity and income. The field interviewer does not know in advance if anyone in the household will be selected as eligible to participate.
Currently NHANES only conducts screening of potential participants in-person. It is a time consuming process. There are several reasons for wanting to implement a multi-mode (online and in-person) screening approach in NHANES. A primary reason for adding online screening to NHANES would be to identify and screen out households which do not have anyone eligible to be in NHANES.
There would be no need to send an in-person interviewer to households which screened out online. Not needing to send an in-person interviewer to homes to determine that no one in the home is eligible to be in NHANES would save both money and time for NHANES and its staff. There would be a savings due to reduced travel and operational costs, because interviewers would be making fewer in-person screening trips. And interviewers would not have to spend any travel time going to those homes.
Not needing to send an in-person interviewer to homes would also help reduce the burden of the survey on the public in general. For example, individuals in the NHANES sample who filled out the online screener could do so at whatever time worked best for them. And they would not have to coordinate this time with NHANES interview staff. If these individuals were determined not to be eligible to participate in NHANES because of the information they provided, there would be no need to have anyone come to their homes in-person. And their names could be removed from mailing lists for announcements, reminders or other mailings regarding NHANES participation. So these households would be contacted less frequently.
Another reason to use this multi-mode screening approach is that it would allow NHANES to more efficiently identify and interview eligible sample participants. Because of the increasing challenges of obtaining cooperation from the public to participate in surveys, more of the field interviewers’ time is currently devoted to screening activities in order to reach acceptable response rates. When interviewers spend more of their time conducting screeners, they have less time to conduct actual NHANES interviews for people who do agree to participate in NHANES. This dynamic contributes to an overall lower response rate for the survey. If interviewers are saving time by not going to households that screen out online, then that will give interviewers more time to make contact with and conduct interviews with people actually eligible to participate in NHANES. This change is expected to help improve NHANES response rates.
Having both an online and in-person approach to screening may also allow for improved non-response bias analysis. A main goal of the study is to determine whether NHANES can successfully screen out households using an internet-based screening tool.
The feasibility study (Attachment 2a) will be conducted in six NHANES locations. The in-person screening procedures used in NHANES are not changing during this feasibility study. During this project, a randomly selected subset of households, in the selected locations, will also complete the web based screener (Attachment 2b). These households will first be contacted via an advance letter (Attachment 2c). If needed a postcard reminder (Attachment 2d) and a second letter (Attachment 2e) will be sent. On-line screening results will be validated in-person by an NHANES field interviewer.
More details about the Multi-Mode Screening are provided in Attachment 2a and 2b. Screenshots for the screener are in Attachment 2f.
NHANES Consent Form Revisions
To comply with requests from the NCHS ERB, minor changes have been made to NHANES consent forms. No text was deleted from the original language. A few short explanatory phrases have been added to the consent language. Both the original language and revised text are provided in Attachment 3a. The full revised consent document is in Attachment 3b.
9. Explanation of any payment or gift to respondents.
Participants in the Ambulatory Blood Pressure Monitoring (ABPM) Feasibility Study will be remunerated $100.
Participants in the Multi-Mode Screening Feasibility Study will not receive any additional remuneration, since they will be remunerated as part of the regular NHANES.
12. Estimates of Annualized Burden Hours and Cost
The Ambulatory Blood Pressure Monitoring Feasibility Study is budgeted for 25 hours. The maximum number of respondents would be 360 adults. The maximum burden is 9000 hours (360 respondents*25 hours = 9000 hours).
The Multi-Mode Screening project is budgeted for 20 minutes. The maximum number of respondents would be 2,100 and the maximum burden 700 hours (2,100 respondents*20/60 hour = 700 hours).
The total burden for all projects combined is 9,700 hours. This time was already budgeted and approved in line 2 (Special Studies) of the original submission. No additional burden is sought.
TABLE 3 – ANNUALIZED BURDEN HOURS AND COSTS
Type of Respondent |
Form |
Number of Respondents |
Number of Responses per respondent |
Average Burden per Response (in hours) |
Total Burden Hours |
Ambulatory Blood Pressure Monitoring Feasibility Study Participants |
Ambulatory Blood Pressure Monitoring Feasibility Study Screener |
360 |
1 |
10/60 |
60 |
Ambulatory Blood Pressure Monitoring Feasibility Study Participants |
Ambulatory Blood Pressure Monitoring Feasibility Study Pre ABPM Form |
360 |
1 |
30/60 |
180 |
Ambulatory Blood Pressure Monitoring Feasibility Study Participants |
Ambulatory Blood Pressure Monitoring Feasibility Study & Diary Form |
360 |
1 |
24 |
8,640 |
Ambulatory Blood Pressure Monitoring Feasibility Study Participants |
Ambulatory Blood Pressure Monitoring Feasibility Study Post ABPM Form |
360 |
1 |
20/60 |
120 |
Multi-Mode Screening Feasibility Study Participants |
Multi-Mode Screening Feasibility Study Form |
2,100 |
1 |
20/60 |
700 |
Total |
|
|
|
|
9,700 |
15. Explanation for Program Changes and Adjustments. The two special studies projects described in this submission do not change the burden hours from the previously approved clearance. The burden hours in this submission are captured in the “special studies” line of the burden table currently approved for this clearance.
List of attachments:
1a. ABPM Description
1b. ABPM Screener
1c. ABPM Consent Form
1d. ABPM Pre ABPM Form
1e. ABPM Info Sheet
1f. ABPM Study & Diary Form
1g. ABPM Post ABPM Form
1h. ABPM Report of Findings
2a. Multi-Mode Screening Description
2b. Multi-Mode Screening Form
2c. Advance Letter
2d. Postcard Reminder
2e. Second Letter
2f. Screener Screenshots
3a. NHANES Consent Form Changes
3b. NHANES Revised Consent Forms
Reference
Screening for High Blood Pressure in Adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:I-32. doi:10.7326/P15-9036
Baumgart P, Kamp J. Accuracy of the Spacelabs Medical 90217 ambulatory blood pressure monitor. Blood Press Monit 1998;3:303-307
Jones, SC, Bilous M, Winship S, Finn P, Goodwin J. Validation of the OSCAR 2 oscillometric 24-hour ambulatory blood pressure monitor according to the International Protocol for the validation of blood pressure measuring devices. Blood Press Monit 2004;9:219-223
Franssen, Pascal ML.; Imholz, Ben PM. Evaluation of the Mobil-O-Graph new generation ABPM device using the ESH criteria. Blood Pressure Monitoring: August 2010 - Volume 15 - Issue 4 - pp 229-231 doi: 10.1097/MBP.0b013e328339be38
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-21
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193-213.
Richards K. Techniques for measurement of sleep in critical care. Focus Crit Care. 1987; 14(4):34–
Viera AJ, Lingley K, and Hinderliter, AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol. 2011; 11: 59. PMCID: PMC3097008
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | National Health and Nutrition Examination Survey |
| Author | vlb2 |
| File Modified | 0000-00-00 |
| File Created | 2021-01-23 |
© 2026 OMB.report | Privacy Policy