Supporting Statement Part A
RMM SSA FINAL (7.2.2019).docx
CDC/ATSDR Formative Research and Tool Development
Supporting Statement Part A
OMB: 0920-1154
Information Collection Request
New
Reaching Minority Men Where They Are: Formative Research to Build Capacity for Enrollment in Diabetes Prevention and Management Programs
GENIC submitted under generic clearance 0920-1154
Supporting Statement: Part A
Program Official/Contact
Andrew Lanza, MPH, MSW, NCCDPHP
Division of Diabetes Translation
National Center for Chronic Disease Prevention and Health Promotion
Centers for Disease Control and Prevention
Atlanta, Georgia 30341
404-610-1294
FAX: 770-488-5939
Email: [email protected]
TABLE OF CONTENTS
Supporting Statement A
Justification – page 4
A.1 Circumstances Making the Collection of Information Necessary – page 6
A.2 Purpose and Use of Information Collection – page 7
A.3 Use of Improved Information Technology and Burden Reduction -page 8
A.4 Efforts to Identify Duplication and Use of Similar Information – page 8
A.5 Impact on Small Businesses or Other Small Entities – page 9
A.6 Consequences of Collecting the Information Less Frequently – page 9
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 – page 9
A.8 Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency – page 9
A.9 Explanation of any payment or gift to respondents – page 12
A.10 Protection of the privacy and Confidentiality of Information Provided by Respondents – page 13
A.11 Institutional Review Board (IRB) and justification for sensitive questions – page 14
A.12 Estimates of Annualized Burden Hours and Costs – page 15
A.13 Estimates of Other total Annual Cost Burden to Respondents or Record Keepers – page 17
A.14 Annualized Cost to the Government – page 17
A.15 Explanation for Program Changes or Adjustments – page 18
A.16 Plans for tabulation and publication and project time schedule – page 17
A.17 Reason Display of OMB Expiration Date is Inappropriate – page 19
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions – page 20
List of Attachments
Attachment 1 - Public Health Service Act, Section 301
(42 U.S.C.241)
Attachment 2 – Focus Group Guide – At Risk for
Diabetes
Attachment 3 – Focus Group Guide - Diabetes
Attachment 4 – Focus Group Screener
Attachment 5 – The Questionnaire (Survey)
Attachment 6 – IRB Approval Letter
Attachment 7 – Survey Consent Form
Attachment 8 – Focus Group Consent Form
Attachment 9 – Data Table Examples
Attachment 10 – Online Survey Intro Screenshot
Exhibits
Exhibit 1 – Project Workstreams
Exhibit 2 – External Consultants
Exhibit 3 – Burden Estimates
Exhibit 4 – Cost Burden Associated with Information Collection
Exhibit 5 – Cost Burden
|
Justification
This statement supports a request to conduct formative research to identify and understand effective strategies that will increase the enrollment of minority men in the National Diabetes Prevention Program (National DPP) lifestyle change program (LCP) and diabetes self-management, education, and support (DSMES). Since men in minority racial/ethnic groups are at higher risk for developing type 2 diabetes, some communities have developed innovative and novel strategies to reach, enroll, and retain men in chronic disease prevention and management programs. These novel approaches are not widely known. The purpose of this project is to:
Identify key elements and principles of novel approaches to incorporate into interventions to increase enrollment of minority males in the National DPP LCP and DSMES.
Increase partners’ ability to tailor current programs to target minority males with or at risk for diabetes for enrollment in DSMES programs or National DPP LCPs.
The National Diabetes Prevention Program – or National DPP – is a partnership of public and private organizations working to prevent or delay type 2 diabetes. The partners work to make it easier for people with prediabetes to participate in an evidence-based, affordable, and high-quality lifestyle change program to reduce their risk of type 2 diabetes and improve their overall health. A key part of the National DPP is the lifestyle change program (LCP) to prevent or delay type 2 diabetes. Hundreds of lifestyle change program organizations nationwide teach participants to make lasting lifestyle changes, like eating healthier, adding physical activity into their daily routine, and improving coping skills. The National DPP landmark randomized trial demonstrated that participants with prediabetes could reduce their risk for type 2 diabetes by 58% (71% if > 60 years old) if they achieved 5%–7% weight loss through healthy eating and increasing physical activity. While data show that the program is effective in diverse populations, enrollment among men from low-income and minority communities remains suboptimal.
Overall, the goal of this research is to identify key principles and novel approaches, initially described in the landscape analysis, to include in the National DPP LCP or DSMES programs targeting minority men. This is a request for approval to implement a total of six focus groups and a survey targeting 2,000 minority men to determine reactions to and perception of identified key elements and principles from the landscape analysis. The Focus Group Guide (Attachment 2 and 3), Focus Group Screener (Attachment 4), and Survey (Attachment 5) are included in this document for review.
A.1 Circumstances Making the Collection of Information Necessary
This information collection request is authorized by the Public Health Service Act, Section 301 (42 U.S.C.241) (Attachment 1). The term for this new request is to expire on September 30, 2020.
According to the Centers for Disease Control and Prevention (CDC) diabetes was the 7th leading cause of death in the United States in 2015, with 79,535 death certificates listing it as the underlying cause of death, and a total of 252,806 death certificates listing diabetes as an underlying or contributing cause of death. The 2017 CDC National Diabetes Statistics Report states that an estimated 33.9% of U.S. adults aged 18 years or older (84.1 million people) have prediabetes in 2015, based on their fasting glucose or A1C level. Age-adjusted data for 2011–2014 indicated that more men (36.6%) than women (29.3%) had prediabetes. Research led by the National Institutes of Health (NIH) and CDC showed that people, across various racial and ethnic groups, with prediabetes who take part in a structured lifestyle change program can cut their risk of developing type 2 diabetes by 58% (71% of > 60 years old). However, men of color are less likely to participate in the National DPP LCP or DSMES program opportunities. Therefore, there is a need to collect additional information regarding minority men’s attitudes and perceptions about prediabetes, diabetes, the National DPP LCP and DSMES programs. More research is needed to better understand why minority men experience poorer health outcomes (Jack Jr & Griffith, 2013). Significant work remains in increasing the uptake of minority men enrollment and participation in both the National DPP LCP and DSMES programs.
CDC is seeking approval for a new generic information collection request for the Reaching Minority Men Where They Are project. This information collection involves formative research to assess perceptions of identified novel strategies for increasing the participation of minority men in the National DPP LCP and DSMES programs. We will employ a mixed-methods approach to the information collection.
A.2 Purpose and Use of Information Collection
An estimated 33.9% of U.S. adults aged 18 years or older (84.1 million people) had prediabetes in 2015 and more men than women have prediabetes. The Reaching Minority Men Where They Are project seeks to capture reactions of minority men to identified key elements and principles through a national survey and focus groups. This data collection is formative as the project will culminate in a demonstration of the strategies and elements that are found to be most promising in increasing minority men enrollment and completion of National DPP LCP and DSMES programs. For this project, the data will be used only once, to inform the development of a future demonstration project in which we will apply findings from our formative research.
The Reaching Minority Men Where They Are Project
The project will identify, assess, and support the implementation and evaluation of key elements and principles identified from novel approaches that will increase the enrollment of eligible minority men in evidence-based diabetes prevention and management interventions. Subsequent to identification of innovative approaches, primary data collection using focus groups, and a national survey will be completed to understand the reaction of minority men to the identified key principles and elements and therefore leading to a refinement of these key principles and elements. Finally, community partners will be engaged and supported in testing and evaluating the innovative elements and principles among cohorts of minority men at risk for and managing diabetes. The lessons learned from the demonstration sites will inform a white paper and will be placed in a database of novel approaches to engaging minority men. Additionally, a webinar will be developed to share findings of the demonstration program. This project is divided into three work streams that build on each other.
Exhibit 1
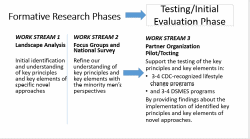
A.3 Use of Improved Information Technology and Burden Reduction
All (100%) respondents to the survey will respond electronically through an online opt-in panel. The survey platform that will be used allows for computer-generated skip patterns to reduce the number of questions displayed to the respondent. Respondents will only see and respond to those questions that are relevant to them, thus lessening the burden on each respondent.
The in-person focus groups will be recorded (with permission from participants) with audio and video to ensure the participant responses are captured accurately. The video recordings allow us to observe and analyze non-verbal communication in addition to verbal communication. Video recordings will also help with transcription and is a standard practice. The audio and video recordings will be secured on a password protected computer.
A.4 Efforts to Identify Duplication and Use of Similar Information
Through our landscape analysis which included a comprehensive review of academic and grey literature, we have determined that to our knowledge, this project will be the first to conduct formative research about novel enrollment interventions targeting minority men to encourage participation in the National DPP LCP and DSMES. Although CDC has evaluated the effectiveness of National DPP LCP and DSMES, there has been no previous work to date that examines strategies designed to increase the perceived urgency and importance of enrolling in National DPP LCP and DSMES for minority men. As this is unique research targeting minority men, the formative research and database of collected research to identify unique and novel approaches for minority men does not exist.
A.5 Impact on Small Businesses or Other Small Entities
This data collection will not involve small businesses. Upon confirmation of partnering minority organizations participation, we will confirm that there will be no impact on small businesses or entities.
A.6 Consequences of Collecting the Information Less Frequently
This is a one-time information request.
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This information collection request is in connection with a statistical survey (Attachment 5) that is not designed to produce valid and reliable results that can be generalized to the universe of the study. Rather, the data are designed to be used to inform a small demonstration project that implements key principles of novel approaches to reach minority men.
A.8 Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
CDC published a Federal Register notice on the scope of this generic clearance on July 18, 2016, (Vol. 81, No. 137, page. 46680). No public comments were received. Additional Federal Register notices are not required for project-specific submissions under the generic clearance.
CDC has been working with cooperative agreement awardee (the National Association of Chronic Disease Directors (NACDD)) and their subcontractor (Leavitt Partners) on the identification, assessment and potential support of novel approaches to increase the enrollment of minority men in evidence-based diabetes prevention and control programs.
In preparation for this work, in 2018 and 2019, CDC discussed the parameters of this effort with its cooperative agreement awardee partners and received their understanding and proposal on the availability of data, frequency of collection, clarity of instructions for record keeping, disclosure, reporting format and data elements to be recorded, disclosed and reported. Please refer to Exhibit 2 for the names and contact information of those consulted.
Below is a table that describes all required information as it pertains to the organizations, we have consulted with outside of the agency to obtain their views on this project.
Exhibit 2 External Consultants
Individuals Consulted Outside of the Agency
Name |
Organization |
Contact Information |
Robyn Taylor MBA |
National Association of Chronic Disease Directors (NACDD) |
Email: [email protected] Phone: (614) 327-1441 |
Marti Maachi, MEd, MPH |
NACDD |
Email: [email protected] Phone: (404) 924-8302 |
Kerstin Edwards |
Leavitt Partners |
Email: [email protected] Phone: (801) 326-3576 |
Chris Wilks |
Leavitt Partners |
Email: [email protected] Phone: (917) 538-0765 |
Erik Krisle |
Leavitt Partners |
Email: [email protected] Phone: (801) 326-3584 |
Bo Nemelka |
Leavitt Partners |
Email: [email protected] Phone: (801) 808-7089 |
Carlie Rhiness |
Leavitt Partners |
Email: [email protected] Phone: (517) 420-7621 |
Thomas Fisher, MD, MPH |
Headwaters Consulting, LLC |
Email: [email protected] Phone: (312) 876-1707 |
Jeannie Belinda Concha, PhD, MPH |
University of Texas at El Paso Diabetes Program |
Email: [email protected] Phone: (915) 747-8308 |
Mildred Hunter, MPH |
US Department of Health and Human Services, Office of Minority Health |
Email: [email protected] Phone:(312)-353-1386 |
Charles Modlin, MD, MBA |
Minority Men’s Health Center, The Cleveland Clinic |
Email: [email protected] Phone: (216) 312-3253 |
Devero Yellow Earring |
Great Plains Tribal Chairman’s Health Board |
Email: [email protected] Phone: (605) 786 8271 |
Summary of Comments Received:
In addition to the helpful comments we received from our cooperative agreement partners, NACDD and Leavitt Partners, we also received favorable comments and suggestions from other subject matter experts outside of the Federal government as well as those who represent other DHHS components.
In summary, these comments clearly encouraged us to move this important work forward. For example, outside subject matter experts were very supportive of the initial plan and methodology and offered concrete suggestions to improve the plan, including offers to serve as hosts for planned focus groups. These external (to CDC) experts also counseled us to keep in mind the tradeoffs between the depth and the breadth of the project, suggesting that we focus our work on what we can reasonably accomplish given our time and resources.
A.9 Explanation of any payment or gift to respondents
Survey response rates have fallen over the years but there is evidence that providing monetary incentives to respondents will improve response rates particularly for groups that are less likely to participate in survey research projects.1 The literature shows that African Americans, Hispanics, and other minority men groups are less likely to participate in survey research projects and are thus a prime group to whom to offer an incentive for participation.2,3 With this in mind, Dynata (formerly known as ResearchNow SSI) will offer a point-based incentive, valued between $2 and $4, per survey respondent which can be redeemed for other items. This will allow us to quickly and efficiently recruit participants to the survey in time to inform the design and implementation of a novel strategies to improve the uptake of the National DPP lifestyle change program and DSMES.
CDC proposes using an established online opt-in panel for its survey. The chosen vendor for this project, Dynata offers a variety of incentives aimed at increasing the diversity of the sampling frame. These rewards include sweepstakes, points, charity donations, points for gift cards, music downloads, and loyalty points such as airline miles. Dynata’s point system allows respondents to claim their points as gifts or cash via PayPal. The number of points offered for a survey is commensurate with the level of effort required and the size of the target population in the sampling frame compared to the required sample size. That is, Dynata offers a higher number of points for longer or more complex surveys and for surveys with smaller, or hard-to-reach populations. Dynata establishes an incentive amount that they think will be needed for each survey based on their experience fielding thousands of surveys every year.
This study will also use an incentive to recruit participants for its focus groups. As is true for survey research, minority men with or at risk for type 2 diabetes represent a relatively small proportion of the overall population and are less likely to seek out opportunities to participate in health-related research projects, making this a hard-to-reach population. Additionally, focus group participants will be expected to travel to the focus group facility and spend 90-minutes participating in the group discussion. For these reasons, the CDC proposes offering an incentive of a gift card valued at $75 per participant. This incentive level was chosen based on consultation with focus group vendors who suggested this incentive level based on their experience recruiting people for hundreds of focus groups each year.
A.10 Protection of the Privacy and Confidentiality of Information Provided by Respondents
The National Center for Chronic Disease Prevention and Health Promotion’s Information Systems Security Officer reviewed this submission and determined that the Privacy Act does not apply to this information collection request. CDC and its cooperative agreement partners will not collect information in identifiable form (IIF) and will not retrieve the data by IIF elements such as name or ID number.
The process we will use for notice or consent for the focus groups will be in a document form that will clearly and succinctly describe the purpose of the project and the need for and use of the requested information for the formative research components of this project (Attachment 8). Opt-in survey respondents provide consent to the opt-in panel when they sign up (Attachment 7).
We will keep all information collected through these formative research activities secure and private. CDC’s cooperative agreement awardees will keep interview notes and other related information locked in cabinets in the awardees’ offices. CDC awardees will also store audio and video files of focus group interviews and electronic copies of documents on a secure shared drive and password protected computers. CDC awardees will enter data from the focus groups and national surveys into an electronic database that will be stored on a password protected computer.
In summary, CDC and its cooperative agreement awardees will treat all data and information in a secure manner and will not disclose any data information, unless otherwise compelled by law. Activities do not involve the collection of individually identifiable information. The PIA process has been completed and has determined that we are not collecting personally identifiable information.
A.11 Institutional Review Board (IRB) and justification for sensitive questions
Institutional Review Board Approval
To ensure the protection of the human subjects participating in this formative research, the data collection protocol and instruments have been reviewed and approved through a commercial institutional review board, Sterling IRB, which the CDC awardee (NACDD) and the sub-awardee (Leavitt Partners) have engaged for this purpose. Sterling IRB is fully accredited by the Association for the Accreditation of Human Research Protection Programs, Inc. The IRB approval letter is attached in Attachment 6 and the methodology statement submitted to the IRB is included as an attachment with supporting statement B.
Justification for Sensitive Questions
The survey and focus group screening questionnaire will ask potentially sensitive questions such as those about ethnicity, race, diabetes and hypertension disease status, and height and weight. Each of these questions are required to ensure survey respondents are a part of the target population for this study. Race and ethnicity data are required to ensure the participant is part of a minority racial or ethnic group. Diabetes status is required to identify men who have been diagnosed with either diabetes or prediabetes. Height and weight will be used to calculate Body Mass Index (BMI). Individuals with a BMI greater than 25, 23 for Asian Americans, 45-years of age or older, having a hypertension diagnosis, having a family member (parent or sibling with type 2 diabetes), and/or physically inactive are considered at risk for type 2 diabetes for the purpose of this study. In addition to the need for these data for screening purposes, these data will be used to segment the resulting survey and focus group data into meaningful groups. For example, it will be important to look at differences in responses between men that have been diagnosed with diabetes and those that are at risk for type 2 diabetes.
Survey participants sign a consent form through the opt-in panel vendor, Dynata, (See attachment 7). Focus groups attendees will also sign a consent form (See attachment 8) and will be informed that they are not required to answer any question they do not wish to answer.
A.12 Estimates of Annualized Burden Hours and Costs
Exhibit 3 delineates the burden estimates of the average burden per focus group participant and response for average survey participant.
This project will engender different burdens and costs on different groups of participants. Focus groups for example, account for the time each participant must spend in the focus group. We estimate this will result in an annualized burden of 447 hours for all 4,048 unique respondents.
Exhibit 3 Annualized Burden
Respondents |
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Average Burden Per Response (in hours) |
Total Burden (in hours) |
Opt-in Panel |
Screener |
3,000 |
1 |
.5/60 |
25 |
Reaching Minority Men Where They Are Survey Questionnaire |
1,500 |
1 |
10/60 |
250 |
Community-based Minority Health Partners |
Screener |
1,000 |
1 |
.5/60 |
9 |
Reaching Minority Men Where They Are Survey Questionnaire |
500 |
1 |
10/60 |
84 |
|
Focus Group Participants |
Demographic/Background Questionnaire for Qualified Participants (completes) |
48 |
1 |
3/60 |
3 |
Demographic/Background Questionnaire for Qualified Participants (incomplete/screened out) |
200 |
1 |
1/60 |
4 |
|
Focus Group Questions – Men at risk for type 2 diabetes |
24 |
1 |
1.5 |
36 |
|
Focus Group Questions – Men with type 2 diabetes |
24 |
1 |
1.5 |
36 |
|
Total |
|
6,296 |
|
|
447 |
Exhibit 4 below describes the cost burden associated with these information collections. For focus group attendees and the survey respondents, costs were calculated based on the hourly wage rates for “all occupations” from the Bureau of Labor Statistics May 2017 National Occupational Employment and Wage Estimates (BLS, 2017) and from the U.S. Department of Labor Federal Minimum Wage Standards.
Exhibit 4 Cost Burden
Respondents |
Form Name |
Total Burden (in hours) |
Hourly Wage Rate |
Total Cost Burden |
Opt-in Panel |
Screener |
25 |
$24.34 |
$608 |
Reaching Minority Men Where They Are Survey Questionnaire |
250 |
$24.34 |
$6,085 |
|
Community-based Minority Health Partners |
Screener |
9 |
$24.34 |
$223 |
Reaching Minority Men Where They Are Survey Questionnaire |
84 |
$24.34 |
$2,045 |
|
Focus Group Participants |
Demographic/Background Questionnaire for Qualified Participants (completes) |
3 |
$24.34 |
$73 |
Demographic/Background Questionnaire for Qualified Participants (incomplete/screened out) |
4 |
$24.34 |
$97 |
|
Focus Group Questions – Men at risk for type 2 diabetes |
36 |
$24.34 |
$876 |
|
Focus Group Questions – Men with type 2 diabetes |
36 |
$24.34 |
$876 |
|
Total |
|
447 |
|
$10,883 |
A.13 Estimates of Other total Annual Cost Burden to Respondents or Record Keepers
There are none.
A.14 Annualized Cost to the Government
The total annualized cost to the government is $32,500. The breakdown of how that estimate was reached is below.
Governmental costs for this project include personnel costs for federal staff involved in managing the project. This includes overseeing the data collection methods and design, reviewing data collection tools, and overseeing demonstrations of the findings from the data collection. Federal staff will also manage an internal workgroup of subject matter experts who will provide their expertise about the project.
This level of effort includes approximately 25% percent of a GS-14 Public Health Advisor’s time at the annual rate of $130,000, ($32,500). There are no equipment or overhead costs; however, a contractor is being used to support the development of the instruments, data collection, and data analysis.
The data collection and analysis will be conducted by contractors, which amounts to $99,570. This fee includes the development of data collection tools and methods, revisions of data collection tools and methods, data collection, data analysis, and conducting focus groups. The grand total for the project, including federal employee and contractor cost, is $132,070.
A.15 Explanation for Program Changes or Adjustments
This is a new information collection.
A.16 Plans for tabulation and publication and project time schedule
Data from the survey and focus groups will be collected and analyzed and recommendations for the design of a diabetes prevention program demonstration study will be developed.
A high-level project schedule is included in the table below.
Exhibit 5
Project Time Schedule |
|
Activity |
Time Schedule |
Data collection |
2-6 weeks after OMB approval |
Data analysis |
6-10 weeks after OMB approval |
Survey Analysis Plan
The survey data collected from the opt-in online panel will be analyzed separately from the survey data that is collected from the community partner organizations.
For the panel survey, the data will be post-weighted so that the overall estimates are comprised of data that is representative of the minority male population based on race and ethnicity. Next, we will produce data tables displaying the tabulated results for each survey question and a stub and banner report that will include each survey question cross-tabulated by key independent variables including race/ethnicity classification, disease status, age, and income (see attachment 9 for an example). Statistically significant differences between population segments will be detected using the Second Order Rao-Scott Test of Independence of a Contingency Table using a 90% confidence level. This test is a design-adjusted version of the Pearson chi-square test that looks for differences in expected and observed frequencies in weighted survey data. Keeping in mind that this study is exploratory in nature, reviewing the analysis described above will narrow the field of viable potential interventions to include in a future diabetes prevention program demonstration and will introduce further research questions. Additional analysis will be conducted to answer these questions and may include exploratory factor analysis, cluster analysis, or other appropriate statistical analyses that can provide evidence to answer the research questions that emerge.
The same analysis will be conducted on the data collected through the community partners survey with the exception that the data will be not be post-weighted due to the lack of a well-defined population to use as a reference point.
Focus Group Analysis Plan
The focus group recordings will be transcribed, and responses will be coded and categorized. Researchers will aim to analyze data using content analysis as well as elements of the phenomenological approach. This approach will focus on gaining insights into the motivations and perspectives of the individual men involved using their own voices and lived experiences as well as providing insight into perspectives by sub-group. Researchers will use the coded data to look for patterns and connections, taking note of both verbal and non-verbal communication and differences in response from participants representing different segments of the population. Specifically, researchers will also look for variation in phenomenon, with the aim of understanding similarities and differences in program enrollment and retention based on the different circumstances groups of minority men navigate. Using content analyses, researchers will identify the key themes and learnings related to participant reaction to the innovative elements and principles identified in the landscape assessment. This information will be summarized in a narrative report, including key quotes, and used to inform design of a future demonstration study.
Data from the survey and focus groups will be collected and analyzed and recommendations for the design of a diabetes prevention program demonstration study will be developed. Findings from the survey and focus groups will not be shared publicly.
A.17 Reason Display of OMB Expiration Date is Inappropriate
The display of OMB expiration date is not inappropriate.
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
1 https://link.springer.com/article/10.1023/A:1025023600517, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5406995/
2 https://www.ncbi.nlm.nih.gov/pubmed/29738190
3 https://www.ncbi.nlm.nih.gov/pubmed/24328648/
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Robyn Taylor |
| File Modified | 0000-00-00 |
| File Created | 2021-01-15 |
© 2026 OMB.report | Privacy Policy