Form CMS-10629 Waiver Application for Providers and Suppliers Subject t
Waiver Application for Providers and Suppliers Subject to an Enrollment Moratorium (CMS-10629)
CMS-10629 - Waiver Application Final - wpas
Waiver Application
OMB: 0938-1313

OMB Control Number: 0938-1313 Expiration Date: XX/20XX
MEDICARE
Waiver Application for Providers and Suppliers Subject to an Enrollment Moratorium
CMS-10629 PROSPECTIVE PROVIDERS AND SUPPLIERS SHOULD COMPLETE THIS APPLICATION IF:
|
SECTION 1: INSTRUCTIONS
The information collected in this application will be used by CMS, in addition to a comprehensive access to care statistical analysis, to determine whether the applicant will be recommended for submission of a Medicare enrollment application. This determination will be made based on the following information:
Beneficiary access to care issue(s) in the applicant’s intended service area
Comprehensive background investigation of the applicant
If you are a provider or supplier type that is affected by a current moratorium and would like to provide service in a county/s where beneficiaries have limited access to care, please complete:
The waiver application for providers and suppliers subject to an enrollment moratorium, and
The appropriate Form CMS-855 for Medicare enrollment
Payment of the enrollment application fee at https://pecos.cms.hhs.gov/pecos feePaymentWelcome.do.
If CMS determines that a beneficiary access to care issue exists in the intended service area and the applicant passes the comprehensive background check, CMS will recommend that the Medicare Administrative Contractor (MAC) process the Medicare enrollment application. If an application is recommended for processing, eligibility for Medicare enrollment will be determined, based on current policy, by the Medicare contractor.
The waiver application for providers and suppliers subject to an enrollment moratorium, and form CMS-855 Medicare enrollment application should be submitted, by email, to the provider enrollment waiver mailbox [email protected], with the subject heading “PE Waiver Request”.
If you have a question regarding the application process, please submit it to [email protected] .
If you have a question regarding completion or submission of the Form CMS-855 enrollment application process, please contact your MAC. Contact information for your Medicare contractor is located at
https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/MedicareProviderSupEnroll/Downloads/ contact_list.pdf.
If this application is denied, the provider or supplier may submit an appeal to CMS within 15 days of denial. The appeal must specifically address the reason(s) for denial and detail the action(s) taken to resolve any deficiency. Any person or entity completing this application waives the right to further appeal, including to an administrative law judge or the Departmental Appeals Board.
SECTION 2: ACCESS TO CARE EVALUATION
CMS will perform a statistical analysis to determine whether additional providers/suppliers are required in the proposed service area in order to address an existing beneficiary access to care deficiency. This evaluation will be utilized in conjunction with all other information that is collected in the waiver application for providers and suppliers subject to an enrollment moratorium to determine whether a provider or supplier will be recommended for enrollment.
Please provide information that demonstrates that beneficiaries have limited access to care in the area of question, including any information that may not be identified through statistical analysis. This information may include, but is not restricted to:
Information that demonstrates lack of providers or suppliers in your intended service area, or
Socio-economic, cultural, geographical or other barriers that prevent existing providers or suppliers from servicing the beneficiaries
These factors alone may not determine whether the applicant will be recommended for approval. They are merely intended to assist the reviewer in making a comprehensive access to care determination.
Please copy and attach up to two (2) additional pages if necessary.
SECTION 3: GENERAL INFORMATION
PROVIDER/SUPPLIER INFORMATION
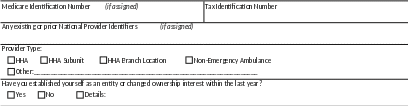
CONTACT INFORMATION

Contact Person for questions regarding this application
Address Line 1 (Street Name and Number)
Address Line 1 (Street Name and Number)
City/Town |
State |
Zip Code + 4 |
Telephone Number |
Fax Number (if applicable) |
E-mail Address |
PROPOSED SERVICE AREA

State/s
Zip Codes*
County/s*
*List all counties where you intend to provide service and have determined that an access to care issue exists
SECTION 4: FINGERPRINTING
All individuals with a 5 percent or greater ownership interest (including a 5 percent or greater general or limited partnership interest) in a provider or supplier, and any managing employee, as defined in 42 C.F.R. § 424.502 must undergo a fingerprint-based criminal background check as part of the waiver application for providers and suppliers subject to an enrollment moratorium. You may, but are not required to, submit your fingerprints prior to submitting your application. We will not process your waiver application without prints and we will approve it only after a satisfactory fingerprint based criminal background check. This application will be rejected and the provider or supplier notified thereof if the provider or supplier has not submitted fingerprint results within 30 days.
Providers and suppliers should contact the organization listed below for information regarding being fingerprinted for the Medicare program:
Accurate Biometrics
CMS Processing
866-361-9944
Monday – Friday , 9:00 AM – 7:00 PM, EST http://www.cmsfingerprinting.com/
Accurate Biometrics will provide a list of possible fingerprint locations that are convenient based on your location and will send the fingerprint-based criminal background check to the Centers for Medicare & Medicaid Services (CMS). Accurate Biometrics must be contacted prior to fingerprinting to receive all required information and to ensure that the fingerprint-based criminal background check is processed correctly.
You may also check the status of the fingerprint process with Accurate Biometrics at http://www.cmsfingerprinting.com/
SECTION 5: DISCLOSURE

A. Affiliations

Section
1866(j) of the Social Security Act (42 USC 1395cc(j)) requires that a
provider of medical or other items or services or supplier who
submits an application for enrollment or revalidation of enrollment
in the program under this title, title XIX, or title XXI must
disclose any current or previous affiliation (directly or indirectly)
with a provider of medical or other items or services or supplier
that has uncollected debt, has been or is subject to a payment
suspension under a Federal health care program, has been excluded
from participation in Medicare, Medicaid or CHIP, or has had its
billing privileges denied or revoked.
5.1 Have you had any current or previous affiliation with an individual or entity as described above?
 Yes
No
Yes
No
5.2 Are you currently affiliated with an individual or entity as described above?
 Yes
No
Yes
No
If answering yes to either question above, list any provider of medical or other items or services or supplier as described above.
5.3 Provider or Supplier Affiliations


B. Unpaid Federal Debt
This section captures information about all unpaid debt to any Federal Government entity.
Examples of federal debt include Medicare overpayments, delinquent taxes and/or liens, audit disallowances, FHA loans and other miscellaneous debts.
5.4 Does the applicant, under any current or former name or business identity, have unpaid debt to the Federal Government?
 Yes
– Continue below
Yes
– Continue below
 No
– Skip to Section 6
No
– Skip to Section 6
If yes, report each debt below, when it was accrued, the federal agency to which you are indebted and the terms of payoff. Please include information regarding adherence to payoff terms and any other relevant information about the debt in the additional information section. Copy and complete additional pages as necessary. Provide documentation for all Federal debt
5.5 Applicant Debt
Amount of Debt
Name of Debtor (individual or entity owing debt)
Name of Debtee (federal government entity name)
Payoff Terms (if applicable)
Additional information (please include any other information that may be relevant, including circumstances of debt, age of debt, adherence to payoff terms, etc…)
SECTION 6: PENALTIES FOR FALSIFYING INFORMATION

This section explains the penalties for deliberately falsifying information in this application to gain or maintain enrollment in the Medicare program.
Failure to fully or truthfully disclose all information required for this application will result in revocation of Medicare billing privileges. Pub. 100-08, Chapter 15 Section 27(a)(4)
18 U.S.C. § 1001 authorizes criminal penalties against an individual who, in any matter within the jurisdiction of any department or agency of the United States, knowingly and willfully falsifies, conceals or covers up by any trick, scheme or device a material fact, or makes any false, fictitious or fraudulent statements or representations, or makes any false writing or document knowing the same to contain any false, fictitious or fraudulent statement or entry.
Individual offenders are subject to fines of up to $250,000 and imprisonment for up to five years. Offenders that are organizations are subject to fines of up to $500,000 (18 U.S.C. § 3571). Section 3571(d) also authorizes fines of up to twice the gross gain derived by the offender if it is greater than the amount specifically authorized by the sentencing statute.
Section 1128B(a)(1) of the Social Security Act authorizes criminal penalties against any individual who,
“knowingly and willfully,” makes or causes to be made any false statement or representation of a material fact in any application for any benefit or payment under a Federal health care program. The offender is subject to fines of up to $25,000 and/or imprisonment for up to five years.
The Civil False Claims Act, 31 U.S.C. § 3729, imposes civil liability, in part, on any person who:
knowingly presents, or causes to be presented, to an officer or any employee of the United States
Government a false or fraudulent claim for payment or approval;
knowingly makes, uses, or causes to be made or used, a false record or statement to get a false or fraudulent claim paid or approved by the Government; or
conspires to defraud the Government by getting a false or fraudulent claim allowed or paid.
The Act imposes a civil penalty of $5,000 to $10,000 per violation, plus three times the amount of damages sustained by the Government
Section 1128A(a)(1) of the Social Security Act imposes civil liability, in part, on any person (including an organization, agency or other entity) that knowingly presents or causes to be presented to an officer, employee, or agent of the United States, or of any department or agency thereof, or of any State agency…a claim…that the Secretary determines is for a medical or other item or service that the person knows or should know:
was not provided as claimed; and/or
the claim is false or fraudulent.
This provision authorizes a civil monetary penalty of up to $10,000 for each item or service, an assessment of up to three times the amount claimed, and exclusion from participation in the Medicare program and State health care programs.
18 U.S.C. 1035 authorizes criminal penalties against individuals in any matter involving a health care benefit program who knowingly and willfully falsifies, conceals or covers up by any trick, scheme, or device a material fact; or makes any materially false, fictitious, or fraudulent statements or representations, or makes or uses any materially false fictitious, or fraudulent statement or entry, in connection with the delivery of or payment for health care benefits, items or services. The individual shall be fined or imprisoned up to 5 years or both.
SECTION 6: PENALTIES FOR FALSIFYING INFORMATION, continued

18 U.S.C. 1347 authorizes criminal penalties against individuals who knowing and willfully execute, or attempt, to executive a scheme or artifice to defraud any health care benefit program, or to obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by or under the control of any, health care benefit program in connection with the delivery of or payment for health care benefits, items, or services. Individuals shall be fined or imprisoned up to 10 years or both. If the violation results in serious bodily injury, an individual will be fined or imprisoned up to 20 years, or both. If the violation results in death, the individual shall be fined or imprisoned for any term of years or for life, or both.
The government may assert common law claims such as “common law fraud,” “money paid by mistake,” and
“unjust enrichment.”
Remedies include compensatory and punitive damages, restitution, and recovery of the amount of the unjust profit.
SECTION 7: CERTIFICATION STATEMENT

An Authorized Official means an appointed official (for example, chief executive officer, chief financial officer, general partner, chairman of the board, or direct owner) to whom the organization has granted the legal authority to enroll it in the Medicare program, to make changes or updates to the organization’s status in the Medicare program, and to commit the organization to fully abide by the statutes, regulations, and program instructions of the Medicare program.
NOTE:
By his/her signature(s), an authorized official binds the supplier to all of the requirements listed in the Certification Statement and acknowledges that the supplier may be denied entry to or revoked from the Medicare program if any requirements are not met. All signatures must be original and in ink. Faxed, photocopied, or stamped signatures will not be accepted.
Only an authorized official has the authority to sign a waiver application for providers and suppliers subject to an enrollment moratorium on behalf of the supplier. A delegated official does not have this authority.
First Name |
MI |
Last Name |
|
|
Suffix |
Telephone Number |
|
|
Title/Position |
|
|
Social Security Number (required) |
|
|
E-mail Address |
|
|
Authorized Official Signature |
Date Signed (mm/dd/yyyy) |
||||
By signing this application, an authorized official agrees to immediately notify the Medicare fee-for-service contractor if any information furnished on the application is not true, correct, or complete. In addition, an authorized official, by his/her signature, agrees to notify CMS of any future changes to the information contained in this form, within ten days
The provider/supplier may have as many authorized officials as it wants. If the supplier has more than two authorized officials, it should copy and complete this section for each authorized official.
Each Authorized Official must have and disclose his/her social security number.
AUTHORIZED OFFICIAL SIGNATURE
I have read the contents of this application. My signature legally and financially binds this provider or supplier to the laws, regulations, and program instructions of the Medicare program. By my signature, I certify that the information contained herein is true, correct, and complete and I authorize CMS to verify this information. If I become aware that any information in this application is not true, correct, or complete, I agree to notify CMS of this fact within ten days.
 I
authorize CMS or its contractors to perform a credit check for all 5%
or greater owners
I
authorize CMS or its contractors to perform a credit check for all 5%
or greater owners
SECTION 8: SUPPORTING DOCUMENTS

This section lists the documents that, if applicable, must be submitted with the waiver application for providers and suppliers subject to an enrollment moratorium.
Mandatory for all Provider/supplier types
Access to care determination and supporting documentation.
Documentation of all Federal debt (if applicable)
Fingerprint Submission to Accurate Biometrics (if completed)
Application Fee
PRIVACY ACT STATEMENT
The Authority for maintenance of the system is given under provisions of sections 1102(a) (Title 42
U.S.C. 1302(a)), 1128 (42 U.S.C. 1320a–7), 1814(a)) (42 U.S.C. 1395f (a)(1), 1815(a) (42 U.S.C. 1395g(a)), 1833(e)
(42 U.S.C. 1395I(3)),1871 (42 U.S.C. 1395hh), and 1886(d)(5)(F), (42 U.S.C. 1395ww(d)(5)(F) of the Social Security Act; 1842(r) (42 U.S.C.1395u(r)); section 1124(a)(1) (42 U.S.C. 1320a–3(a)(1), and 1124A (42 U.S.C. 1320a–3a), section 4313, as amended, of the BBA of 1997; and section 31001(i) (31 U.S.C. 7701) of the DCIA (Pub. L. 04– 134), as amended.
The information collected here will be entered into the Provider Enrollment, Chain and Ownership System (PECOS).
PECOS will collect information provided by an applicant related to identity, qualifications, practice locations, ownership, billing agency information, reassignment of benefits, electronic funds transfer, the NPI and related organizations. PECOS will also maintain information on business owners, chain home offices and provider/chain associations, managing/ directing employees, partners, authorized and delegated officials, supervising physicians of the supplier, ambulance vehicle information, and/or interpreting physicians and related technicians. This system of records will contain the names, social security numbers (SSN), date of birth (DOB), and employer identification numbers (EIN) and NPI’s for each disclosing entity, owners with 5 percent or more ownership or control interest, as well as managing/directing employees. Managing/directing employees include general manager, business managers, administrators, directors, and other individuals who exercise operational or managerial control over the provider/ supplier. The system will also contain Medicare identification numbers (i.e., CCN, PTAN and the NPI), demographic data, professional data, past and present history as well as information regarding any adverse legal actions such as exclusions, sanctions, and felonious behavior.
The Privacy Act permits CMS to disclose information without an individual’s consent if the information is to be used for a purpose that is compatible with the purpose(s) for which the information was collected. Any such disclosure of data is known as a “routine use.” The CMS will only release PECOS information that can be associated with an individual as provided for under Section III “Proposed Routine Use Disclosures of Data in the System.” Both identifiable and non-identifiable data may be disclosed under a routine use. CMS will only collect the minimum personal data necessary to achieve the purpose of PECOS. Below is an abbreviated summary of the six routine uses. To view the routine uses in their entirety go to: https://www.cms.gov/ Research-Statistics-Data-and-Systems/ Computer-Data-and-Systems/Privacy/Downloads/0532-PECOS.pdf.

To support CMS contractors, consultants, or grantees, who have been engaged by CMS to assist in the performance of a service related to this collection and who need to have access to the records in order to perform the activity.To assist another Federal or state agency, agency of a state government or its fiscal agent to:
Contribute to the accuracy of CMS’s proper payment of Medicare benefits,
Enable such agency to administer a Federal health benefits program that implements a health benefits program funded in whole or in part with federal funds, and/or
Evaluate and monitor the quality of home health care and contribute to the accuracy of health insurance operations.
To assist an individual or organization for research, evaluation or epidemiological projects related to the prevention of disease or disability, or the restoration or maintenance of health, and for payment related projects.
To support the Department of Justice (DOJ), court or adjudicatory body when:
The agency or any component thereof, or
Any employee of the agency in his or her official capacity, or
Any employee of the agency in his or her individual capacity where the DOJ has agreed to represent the employee, or
The United States Government, is a party to litigation and that the use of such records by the DOJ, court or adjudicatory body is compatible with the purpose for which CMS collected the records.
To assist a CMS contractor that assists in the administration of a CMS administered health benefits program, or to combat fraud, waste, or abuse in such program.
To assist another Federal agency to investigate potential fraud, waste, or abuse in, a health benefits program funded in whole or in part by Federal funds.
The applicant should be aware that the Computer Matching and Privacy Protection Act of 1988 (P.L. 100-503) amended the Privacy Act, 5 U.S.C. section 552a, to permit the government to verify information through computer matching.

According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0938-1313. The time required to complete this information collection is estimated to 6 hours per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have any comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Baltimore, Maryland 21244-1850.
Do Not Mail Applications To This Address. Mailing your application to this address will significantly delay application processing.
Form CMS-10629 (02/28/17) 12
Form
CMS-10629
(02/28/17)
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | CMS waiver |
| Author | JAMAA HILL |
| File Modified | 0000-00-00 |
| File Created | 2021-01-22 |
© 2026 OMB.report | Privacy Policy


