2014 Ss 0317 Rev
2014 SS 0317 REV.docx
Citrus Canker; Interstate Movement of Regulated Nursery Stock and Fruit from Quarantined Areas
OMB: 0579-0317
` June 2017
SUPPORTING STATEMENT
Citrus Canker; Interstate Movement of Regulated Nursery Stock
And Fruit From Quarantined Areas
OMB No. 0579-0317
A. Justification
1. Explain the circumstances that make the collection of information necessary. Identify any legal or administrative requirements that necessitate the collection.
The United States Department of Agriculture, Animal and Plant Health Inspection Service (APHIS), is responsible for preventing plant diseases or insect pests from entering the
United States, preventing the spread of pests and noxious weeds not widely distributed in the United States, and eradicating those imported pests when eradication is feasible.
Under the Plant Protection Act (7 U.S.C. 7701 et seq.), the Secretary of Agriculture, either independently or in cooperation with the States, is authorized to carry out operations or measures to detect, eradicate, suppress, control, prevent, or retard the spread of plant pests (such as citrus canker) new to or not widely distributed throughout the United States. APHIS’ Domestic Quarantines (7 CFR Part 301) are issued under this authority.
APHIS has regulations in place to prevent the interstate spread of citrus canker. These regulations, contained in 7 CFR 301.75, restrict the interstate movement of regulated articles from and through areas quarantined because of citrus canker. APHIS’ citrus canker quarantine regulations also prohibit the interstate movement of regulated nursery stock from a quarantined area. The interstate movement of nursery stock from an area quarantined for citrus canker poses an extremely high risk of spreading citrus canker outside the quarantined area. APHIS is also continuing to allow the interstate movement of regulated nursery stock for immediate export, under certain conditions. These regulations are necessary to address the risk associated with the interstate movement of citrus nursery stock and other regulated articles from areas quarantined for citrus canker.
APHIS is asking OMB to approve this information collection activity, for an additional 3 years, to prevent the interstate movement of citrus canker into noninfested areas of the United States.
2. Indicate how, by whom, and for what purpose the information is to be used. Except for a new collection, indicate the actual use the agency has made of the information received from the current collection.
APHIS uses the following information activities to address the risk associated with the interstate movement of citrus nursery stock and other regulated articles from areas quarantined for citrus canker.
7CFR 301.75.13(a) and 301.75-7 (a)(4) - Compliance Agreements (PPQ Form 519) (business) - Any person engaged in the business of growing or handling regulated articles for interstate movement may enter into a compliance agreement with the Animal and Plant Health Inspection Service to facilitate the interstate movement of regulated articles in accordance with this subpart.
If the fruit is repackaged after being packed in a commercial packinghouse and before it is moved interstate from the quarantined area, the person that repackages the fruit must enter into a compliance agreement with APHIS in accordance with §301.75-13 and issue and attach a certificate for the interstate movement of the fruit in accordance with §301.75-12.
7CFR301.75-6(d), 301.75-7(b), and 301.75-12 - Limited Permit (PPQ Form 530)
business) - Limited permits may be issued for the interstate movement of regulated articles only by an inspector or by persons operating under a compliance agreement. Limited permits are used to authorize movement of regulated articles that are not certifiable to specified destinations for processing, treatment, or utilization.
7 CFR 301.75.13(b) - Written Appeal for Cancellation of Compliance Agreement
(business) - Any compliance agreement may be cancelled orally or in writing by an inspector if the inspector finds that the person who entered into the compliance agreement has failed to comply with this subpart, or any term or condition of the compliance agreement itself. If the person is given notice of cancellation orally, written confirmation of the decision and the reasons for it must be provided as promptly as circumstances allow. Any person whose compliance agreement is cancelled may appeal the decision, in writing, to the Administrator within 10 days after receiving the written notification. The appeal must state all of the facts and reasons upon which the person relies to show that the compliance agreement was wrongfully cancelled. The Administrator must grant or deny the appeal, in writing, stating the reasons for the decision as promptly as circumstances allow. If there is a conflict as to any material fact, a hearing will be held to resolve the conflict. Rules of practice concerning the hearing will be adopted by the Administrator.
7 CFR 301.75-12(a)(2) - Written Appeal for Cancellation of Limited Permit (business) - A certificate or limited permit may be withdrawn by an inspector if the inspector determines that any of the applicable requirements of this subpart have not been met. The decision of the inspector and the reason for the withdrawal must be confirmed in writing as promptly as circumstances allow. Any person whose certificate or limited permit is withdrawn may appeal the decision, in writing, to the Administrator within 10 days after receiving the written notification. The appeal must state all of the facts and reasons upon which the person relies to show that the certificate or limited permit was wrongfully withdrawn. The Administrator must grant or deny the appeal, in writing, stating the reasons for the decision, as promptly as circumstances allow. If there is a conflict as to any material fact, a hearing will be held to resolve the conflict. Rules of practice concerning the hearing will be adopted by the Administrator.
7 CFR 301.75.7(a) - Federal Certificate (PPQ Form 540) (business) - Regulated fruit produced in a quarantined area or moved into a quarantined area for packing may be moved interstate with a certificate issued and attached in accordance with §301.75-12 if all conditions are met.
3. Describe whether, and to what extent, the collection of information involves the use of automated, electronic, mechanical, or other forms of information technology, e.g., permitting electronic submission of responses, and the basis for the decision for adopting this means of collection. Also describe any consideration of using information technology to reduce burden.
Compliance Agreements (PPQ Form 519) are automated and posted at:
http://www.aphis.usda.gov/library/forms/pdf/ppq519.pdf.
The PPQ Form 530 and PPQ Form 540 are not automated for several reasons. These forms have a unique identifier (serial number) and they are accountable forms that must be issued by a PPQ employee. APHIS needs to have strict control over the issuance of these forms since they allow the movement of regulated products that are subject to restrictions. In addition, the forms must accompany the shipment throughout transport and until destinastion.
4. Describe efforts to identify duplication. Show specifically why any similar information already available cannot be used or modified for use for the purpose described in item 2 above.
The information APHIS collects is exclusive to its mission of preventing the incursion or interstate spread of plant pests and noxious weeds and is not available from any other source.
5. If the collection of information impacts small businesses or other small entities, describe any methods used to minimize burden.
The information APHIS collects associated with this program is the minimum needed to prevent the spread of citrus canker into noninfested areas of the United States. APHIS has determined that approximately all of the respondents are small entities.
6. Describe the consequences o Federal program or policy activities if the collection is not conducted or is conducted less frequently, as well as any technical or legal obstacles to reducing burden.
Failing to collect this information, or if this information was collected less frequently, could cause a severe economic loss to the citrus industry.
7. Explain any special circumstances that require the collection to be conducted in a manner inconsistent with the general information collection guidelines in 5 CFR 1320.5:
requiring respondents to report information to the agency more often than quarterly;
requiring respondents to submit more than an original and two copies of any document;
requiring respondents to retain records, other than health, medical, government contract, grant-in-aid, or tax records for more than three years;
in connection with a statistical survey, that is not designed to produce valid and reliable results that can be generalized to the universe of study;
requiring the use of a statistical data classification that has not been reviewed and approved by OMB;
that includes a pledge of confidentiality that is not supported by authority established in statute or regulation, that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or
requiring respondents to submit proprietary trade secret, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information's confidentiality to the extent permitted by law.
No special circumstances exist that would require this collection to be conducted in a manner inconsistent with the general information collection guidelines in 5 CFR 1320.5.
8. Describe efforts to consult with persons outside the agency to obtain their views on the availability of data, frequency of collection, the clarity of instructions and recordkeeping, disclosure, or reporting form, and on the data elements to be recorded, disclosed, or reported. If applicable, provide a copy and identify the date and page number of publication in the Federal Register of the agency’s notice, soliciting comments on the information collection prior to submission to OMB.
APHIS held productive consultations with the following concerning this program:
Drew Love, Director of State Legislative Affairs
Florida Citrus Mutual
411 E. Orange Street
Lakeland, FL 33802
863-682-1111
Suzy Peckett, President
Peckett’s Inc.
5643 Round Tree Lake Rd.
Apopka, FL 32712
407-886-5901
Lisa Lockridge, Director of Public Relations
Florida Fruits and Vegetables Association
P.O. Box 948153
Maitland, FL 32794
321-214-5206
On Friday, April 21, 2017, APHIS published in the Federal Register, a 60-day notice seeking public comments on its plan to request a 3-year renewal of this information collection. No comments from the public were received.
9. Explain any decision to provide any payment or gift to respondents, other than reenumeration of contractors or grantees.
This information collection activity involves no payments (other than appropriate, program-related payments) or gifts to respondents.
10. Describe any assurance of confidentiality provided to respondents and the basis for the assurance in status, regulation, or agency policy.
No additional assurance of confidentiality is provided with this information collection. Any and all information obtained in this collection shall not be disclosed except in accordance with
5 U.S.C. 552a.
11. Provide additional justification for any questions of a sensitive nature, such as sexual behavior and attitudes, religious beliefs, and others that are considered private. This justification should include the reasons why the agency considers the questions necessary, the specific uses to be made of the information, the explanation to be given to persons from whom the information is requested, and any steps to be taken to obtain their consent.
This information collection activity asks no questions of a personal or sensitive nature.
12. Provide estimates of the hour burden of the collection of information. Indicate the number of respondents, frequency of response, annual hour burden, and an explanation of how the burden was estimated.
• Indicate the number of respondents, frequency of response, annual hour burden, and explanation of how the burden was estimated. If this request for approval covers more than one form, provide separate hour burden estimates for each form and aggregate the hour burdens in Item 13 of OMB Form 83-I.
See APHIS Form 71 for hour burden estimates.
• Provide estimates of annualized cost to respondents for the hour burdens for collections of information, identifying and using appropriate wage rate categories.
APHIS arrived at the estimated annualize cost to respondents by multiplying the total burden hours (2,742) by the estimated average hourly wage of respondents ($32.12).
2,742 X $32.12 = $88,073.04. The hourly rate was taken from http://www.salaryexpert.com.
13. Provide estimates of the total annual cost burden to respondents or recordkeepers resulting from the collection of information, (do not include the cost of any hour burden shown in items 12 and 14). The cost estimate should be split into two components: (a) a total capital and start-up cost component annualized over its expected useful life; and (b) a total operation and maintenance and purchase of services component.
There is zero annual burden associated with capital and start-up costs, maintenance costs, and purchase of services in connection with this program.
14. Provide estimates of annualized cost to the Federal government. Provide a description of the method used to estimate cost and any other expense that would not have been incurred without this collection of information.
See APHIS Form 79 for the annualized cost to the Federal Government which is estimated to be $110,487.
15. Explain the reasons for any program changes or adjustments reported in Items 13 or 14 of the OMB Form 83-1.
ICR Summary of Burden:

|
Requested |
Program Change Due to New Statute |
Program Change Due to Agency Discretion |
Change Due to Adjustment in Agency Estimate |
Change Due to Potential Violation of the PRA |
Previously Approved |
Annual Number of Responses |
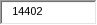
14,402 |
0 |
4,002 |
758 |
0 |
9,642 |
Annual Time Burden (Hr) |
2,742 |
0 |
642 |
157 |
0 |
1,943 |
There are increases in the burden for this information collection because APHIS reported more businesses are now participating in the program. The number of respondents increased from 371 to 400, burden hours increased from 1,943 to 2,742, and annual responses increased from 9,642 to 14,402. Adjustments out of APHIS’ control totaled an increase of 157 burden hours and 758 annual responses.
In addition, APHIS added burden for: (1) written appeals for the cancellation of compliance agreements, (2) written appeals for withdrawn limited permits; and (3) Federal Certificates because this burden was erroneously omitted from the previous collection. The three additional burden items that were added in this information collection were program changes totaling 642 burden hours and 4,002 annual responses
16. For collections of information whose results are planned to be published, outline plans for tabulations and publication.
APHIS has no plans to tabulate or publish the information collected.
17. If seeking approval to not display the expiration date for OMB approval of the information collection, explain the reasons that display would be inappropriate.
PPQ Form 519, PPQ Form 530, and PPQ Form 540 are all used in multiple APHIS information collections; therefore, it is not practical to include an OMB expiration date because of the various expiration dates for each information collection. APHIS is seeking approval to not display the OMB expiration date on these forms.
18. Explain each exception to the certification statement identified in the “Certification for Paperwork Reduction Act.”
APHIS is able to certify compliance with all the provisions under the Act.
B. Collections of Information Employing Statistical Methods
Statistical methods are not used in this information collection.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | SUPPORTING STATEMENT |
| Author | Government User |
| File Modified | 0000-00-00 |
| File Created | 2021-01-22 |
© 2026 OMB.report | Privacy Policy