Phc Ssb Clean Ch 12.15.17 Finalx
PHC SSB CLEAN CH 12.15.17 FINAL.DOCX
Positive Health Check Evaluation Trial
OMB: 0920-1211
Positive Health Check Evaluation Trial
Supporting Statement B
OMB No. 0920-New
December 15, 2017
Contact:
Camilla Harshbarger
Behavioral Scientist, Prevention Research Branch
Division of HIV/AIDS Prevention
Centers for Disease Control & Prevention
1600 Clifton Rd, NE, MS E-46
Phone (404) 639-4267
Fax (404) 639-1950
Table of Contents
B. Collection of Information Employing Statistical Methods
1. Respondent Universe and Sampling Methods
2. Procedures for the Collection of Information
3. Methods to Maximize Response Rates and Minimize Nonresponse
4. Tests of Procedures or Methods to be Undertaken
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
B. Collection of Information Employing Statistical Methods
1. Respondent Universe and Sampling Method
Aim 1 of the study is to implement a randomized trial to test the effectiveness of the Positive Health Check PHC intervention for improving clinical health outcomes, specifically viral load and retention in care. The respondent population for the Positive Health Check trial will be HIV-positive persons attending one of four clinics with most recent viral load lab result of ≥200 copies/mL, who are newly diagnosed patients, or are considered out of HIV care (last attended appointment at the clinic was more than 12 months ago).
PHC is designed to improve antiretroviral therapy (ART) initiation, ART adherence and retention in care as well as reduce unprotected sex. The primary outcome on which the trial is powered is viral suppression. The four clinics were selected for this study because of the high numbers of patients with elevated viral load (≥200 copies/mL). Table B1 displays the number of patients within each clinic not virally suppressed, the estimated eligible patients within each clinic, and the projected sample size per clinic.
Sample Size
The primary research question for this trial (Aim 1) will be whether patients in the intervention arm are more likely to achieve viral suppression within 12 months after enrollment compared to persons in the control arm. The proportions of persons in the control and intervention arms who achieve HIV-1 viral load suppression will be compared using Fisher's Exact Test with a two-sided significance level of 5%. Assuming that the proportion of success in the control and intervention arms are 50% and 62%, respectively, an absolute difference of 12 percentage points, and allowing for an annual attrition rate of 25%, a minimum sample size of 1,010 will be required to have 80% power to reject the null hypothesis that the true proportion of success is the same in both arms.
Table B1. Projected sample size for each clinic
Clinic |
Number not virally suppressed |
Number of new patients/year |
Total potential N |
Projected N based on previous meetings |
Florida Department of Health, Hillsborough County Specialty Care Clinic |
856 |
371 |
1,227 |
235 |
Rutgers Infectious Disease Practice |
333 |
150 |
483 |
235 |
Atlanta VA |
300 |
82 |
382 |
305 |
New Orleans Crescent Care |
267 |
500 |
762 |
235 |
Total |
|
|
2,854 |
1,010 |
Respondent Population
Based on estimates provided by the clinics, the potential respondent population is 2,854 for the PHC intervention trial (see Table B1). It is estimated that 30% to 35% of the total respondent sample will participate in the trial.
Eligible patients must be:
18 years of age or older
Diagnosed with HIV
English-speaking
Attending one of the four HIV Primary Care clinics
Meet at least one of the following:
Most recent viral load lab result of ≥200 copies/mL
Attended an initial HIV appointment with a provider at one of the four clinics within the past 12 months
Out of care (last attended appointment at the clinic was more than 12 months ago)
The PHC Project Coordinator will confirm each patient’s eligibility using the clinic electronic medical records (EMR). The Project Coordinator will also determine whether the patient is sober and cognitively able to complete the tool. This will be done via their best judgment. If the patient does not meet one or more of the eligibility criteria, they will be recorded as ineligible.
A Project Coordinator at each site will identify eligible patients with upcoming scheduled appointments through each clinic’s EMR system. Additionally, clinical staff will conduct systematic PHC telephone outreach to engage patients back into care for patients who meet the study eligibility criteria but who are out of care (defined as patients whose last attended appointment at the clinic was more than 12 months ago) or have missed appointments. Patients who are successfully reengaged in care will be eligible to participate in the study.
Aim 2 of the study is to conduct a qualitative feasibility assessment to determine strategies to facilitate implementation and integration of PHC into the workflow of HIV primary care clinics. For this Aim, three to five staff at each clinic will be selected to participate in interviews and an online survey. The staff will include PHC implementation staff (such as the Project Coordinator, outreach coordinator, and data manager) and clinic providers.
Aim 3 of the study is to collect and document data on the cost of PHC intervention implementation (PHC research and non-research costs). The Project Coordinator will collect all the required data for the cost questionnaires from other staff members, will submit it to RTI, and will serve as our main contact for the cost study data collection.
Aim 4 of the study is to document the standard of care at each participating clinic. The clinic’s medical director will provide all data for the questionnaire by phone or email to document descriptive data on clinics’ standard of care. The medical director will serve as the main contact for clinics’ standard of care data collection.
2. Procedures for the Collection of Information
Collection of Information
During the PHC telephone outreach process, the outreach coordinator will document barriers preventing the patient from returning to care, whether the patient is receiving care at another clinic, number of attempts to reach the patients, date, method, and person being contacted during each attempt, and whether outreach was successful. If PHC outreach is successful, the PHC outreach coordinator will work with clinic staff to have the patient schedule an appointment. The procedures are consistent with the clinic’s standard outreach protocol that is used to keep patients in care.
If an eligible participant expresses an interest in participating in the PHC intervention trial, they will be consented and randomized to the intervention arm or the control arm. At the time of enrollment, they will be asked their date of diagnosis. During the study period, patients in the intervention arm will be expected to log on and complete the PHC intervention before each regularly scheduled clinic visit. Each patient enrolled in the trial will complete the intervention up to three times in a 12-month period, with anticipated scheduled clinic visits at least 2 months apart. Patients assigned to the intervention arm will log in and use Positive Health Check before seeing their clinician. At their first visit, patients will be assigned a study ID that they will use to log in to the tool and a default password. For visits 2 and 3, patients may be approached at their HIV primary care visits as well as ancillary services. Examples of ancillary services include case management, pharmacy pick-up, mental health services, sexual health services, well-visits, financial services, social services, OBGYN appointments, dental appointments, and AIDS Drug Assistance Programs (ADAP). Appointments will only be counted towards the retention in care outcome if the patient attends an HIV primary care visit with a provider. At the time of consent into the study, the project coordinator will ask participants if they would be willing to be approached to complete subsequent PHC visits during ancillary services they receive at the clinic.
Intervention arm patients will experience PHC and they will answer the PHC tailoring questions (Attachment 7). In the intervention patients will view educational videos that are tailored to each patient’s answers to the tailoring questions, a patient handout tailored to each patient’s selected behavioral tips to practice before the next clinic visit. At the end of the tool, patients can click a link to the ‘Extra Info’ web page where they can find CDC-approved links to resources addressing a variety of topics. If a handout is generated by the tool based on participants’ responses, the Project Coordinator will deliver it to the patient before they are called back to see their provider. Participants that complete PHC at ancillary services provided by the clinics will also have the option to email the handout by entering their email address into the tool. The exact procedures for handling the printed handouts for participants that complete PHC during these ancillary services will be tailored for each of the clinics. Patients can also complete part of the intervention by receiving a link via email after their clinic visit. If an email address is entered into the intervention database a link is automatically generated and sent to the address. Then the email address is automatically deleted. This email is not saved in any of the study databases or at CDC after the email link is sent. Thus no identifying information is retained.
Patients randomized to the control arm will not use the intervention. Following signing informed consent, these patients will return to the waiting room and receive the standard of care at their clinic. These patients will only consent to have their de-identified clinical values be made available via passive data collection via the EMR. Both the control and intervention participants will be asked for the date of diagnosis at the time of enrollment- “When were you diagnosed with HIV? If you cannot remember the exact date, can you estimate the month and year?” (Attachment 6).
The table above (Table B1) provides information on potentially eligible patients at each of the four study sites. These numbers reflect only those with a viral load ≥200. The actual numbers will be larger given those who have fallen out of care will also be eligible.
Table B2. illustrates the timeline for PHC implementation and data collection efforts.
Table B2. Timeline for PHC Implementation and Data Collection
C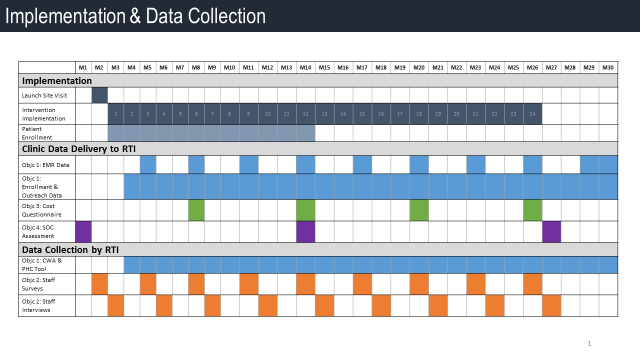 DC
will collect user metrics from the PHC tool that are not linked to
participants’ personally identifiable information (PII) also
referred to as backend data. These data, captured via the PHC tool,
include responses to the PHC Tailoring Questions (Attachment
7)
related to ART use, clinic attendance, and behaviors that may
increase risk of HIV transmission. Monthly, CDC will send RTI an
Excel file with these data through a secure FTP connection. RTI staff
will access the FTP site to download the data and then enter into the
master database. These PHC data include patients’ answers to
tailoring questions on ART, adherence, clinic attendance, sex risk,
pregnancy planning, and injection drug use (IDU). Backend data do
not contain identifiable information (Study ID only) and will be
stored in CDC servers behind a firewall.
DC
will collect user metrics from the PHC tool that are not linked to
participants’ personally identifiable information (PII) also
referred to as backend data. These data, captured via the PHC tool,
include responses to the PHC Tailoring Questions (Attachment
7)
related to ART use, clinic attendance, and behaviors that may
increase risk of HIV transmission. Monthly, CDC will send RTI an
Excel file with these data through a secure FTP connection. RTI staff
will access the FTP site to download the data and then enter into the
master database. These PHC data include patients’ answers to
tailoring questions on ART, adherence, clinic attendance, sex risk,
pregnancy planning, and injection drug use (IDU). Backend data do
not contain identifiable information (Study ID only) and will be
stored in CDC servers behind a firewall.
For all participants, EMR data will be collected every three months. Depending on each clinic’s system, some data may also be collected from other electronic systems. For example, data pertaining to patients’ attendance at primary care visits may be extracted from clinics’ electronic scheduling systems and provided to RTI. Dates of scheduled clinic appointments are considered identifiable information so we will work with each clinic’s Institutional Review Board (IRB) to ensure privacy and other regulatory rules are followed. The data collected from the EMR and/or other electronic systems includes laboratory results, ART prescriptions, appointment attendance, STD test results, and demographic information (Attachment 8). The sites will collect historical EMR data for 24 months prior to the date of randomization. The consent form informs participants that we will collect EMR data for the 24 months prior to randomization and up to 18 months after randomization. We will use Python or another program to harmonize data before entering it into the master database. If an enrolled patient moves out of the area during the study period and they have no further data in the clinic’s EMR, we will only use data that are available. Data after the move will be recorded as missing. We will not be contacting other clinics to obtain patient information. If any plans to obtain data from other clinics is proposed by sites we will prepare an amendment.
For Aim 2, three to five staff at each clinic (a total of 12 to 20) will be selected to participate in interviews and an online survey. Staff who agree to participate will complete a 15-minute clinic staff survey (Attachment 10) at the beginning of the study and then at every 3 months of the 2-year implementation period. The qualitative interviews will last approximately 40 minutes and will be conducted at the beginning and then at every 3 months during the 3-year study period in the month following the quantitative survey. RTI will contact clinic staff by phone or email to ask staff to participate and to schedule individual or small group interviews.
The clinic staff survey has closed-ended questions asking information such as (1) participant background information (e.g., age, race, gender, education level), and (2) Likert-scale ratings for several questions regarding the implementation context (e.g., implementation readiness and implementation climate) and perceived fit of the intervention (e.g., appropriateness, acceptability, compatibility) (Attachment 10). The clinic staff qualitative interviews include collecting data on staff feedback on open-ended questions about the implementation of PHC, including their perceptions of the intervention’s impact on their patients and staff, how they perceive the effectiveness of the intervention, to what extent they perceive clinic leadership support for the implementation of the intervention, the implementation climate, readiness at their clinic, and the likelihood of intervention adoption in other clinics (Attachment 11).
For Aim 3, clinic staff who participate in the PHC intervention will complete the non-research labor cost questionnaire (Attachment 12), the PHC labor cost questionnaire (Attachment 13), and the PHC non-labor cost questionnaire (Attachment 17). We received a waiver of informed consent for Aim 3. We will not be collecting personal or sensitive data for Aim 3. Data will be collected using the clinics’ systems and is part of understanding the costs of implementation to the clinics. Staff will submit labor cost questionnaires (Attachments 12 and 13) to RTI three times: (1) after the first month of PHC intervention implementation; (2) after the 6th month of PHC intervention implementation; and (3) after the 12th month of PHC intervention implementation. The PHC non-labor cost questionnaire (Attachment 17) will be completed and submitted to RTI on a monthly basis during PHC intervention implementation. Cost data includes seven program activity categories: (1) staff training and preparation; (2) patient identification and recruitment; (3) intervention delivery; (4) mobile device management; (5) patient outreach; (6) report generation; and (7) administration/general oversight.
For Aim 4 the medical director will be the point of contact on this data collection and will gather information to complete the Standard of Care Questionnaire (Attachment 14). The questionnaire will document descriptive data on clinics’ standard of care. These data will be used to determine to what extent if any patients’ health outcomes can be attributed to variation in clinics’ medical standard of care provided to HIV patients. Fewer than 9 persons will be involved in the data collection for this aim. We will not be collecting personal or sensitive data for Aim 4. The medical director will submit standard of care data to RTI three times: (1) during the first month of PHC implementation; (2) during month 14 of implementation; and (3) during month 27 of the study.
Quality Control Measures
The study has several quality control measures to protect the quality of the data collected as well as to protect the privacy of participants. For the intervention trial, study ID numbers will be generated in advance, and processed through a random number generator system (e.g., Sealed Envelope, an online study ID randomization program where a list of study IDs is entered into the program and they are randomized into either the control or intervention arm). These numbers will be preprogrammed into each clinic’s Access database and will be blinded to clinic staff. After eligibility is confirmed and patients are informed about the study and agree to participate, the coordinator will click a button in the Access database that will reveal the next unassigned ID and intervention arm. The date of generation will be recorded in the database so that RTI can confirm that IDs were assigned sequentially to the appropriate patient (date of generation should match with the patient’s appointment). This approach protects against any bias where a Project Coordinator would independently assign patients into either intervention arm.
At their first visits, patients will be assigned a study ID that they will use to log in to the tool and a default password. The participant will not need to remember their study ID. They will be provided this ID by the project coordinator who will enroll them into the study and onboard them into the intervention. The Access database will include study patients’ names. The consent form documents that any patient’s personal information obtained through the study will be password protected, stored on a secure site and only be used by a limited number of assigned clinic staffers working on the project team, not RTI or CDC. To protect their privacy, patients are required to generate their own password after logging in with the default password. The password must be at least eight characters long and have at least one uppercase letter, one lowercase letter, a symbol such as the pound sign, and a number. Patients will be asked to write down and secure their password. If they forget their password at subsequent visits, the Project Coordinator can reset the password through the PHC Clinic Web Application (CWA).
Eligible patients who use the tool will be given a tablet with privacy screen, a set of headphones, and a unique login ID and will be prompted by the tool to create their own private password that clinic staff cannot access. RTI will provide Android or iOS tablets with the Positive Health Check app installed. The devices are encrypted and password-locked. The project coordinator will maintain the devices and they will be stored in a locked cabinet in a secure area of the clinic when not in use. Since patient information is not being stored on the device, clinic staff will not need to erase any information between patients. RTI will set the clinics up with either “Find my iPhone” or “Android Device Manager” in order to manage the security of the devices. This allows the devices to be located if lost/stolen and allows them to be erased remotely.
The Project Coordinator will review the written PHC intervention trial consent form (Attachment 9) with patients. Each clinic will retain the patient consent forms so that RTI and CDC do not receive names of participants. The Project Coordinator will record the date and time at which the participant consented and record it in the clinic’s Access database. The key file linking names to Study IDs will be stored in the password-protected Access database. The Project Coordinator, Data Manager and Outreach Coordinator will have access to the Access database where this key file is stored. The database will be stored on the clinic’s servers or device, depending on their security. The clinics will be sending de-identified data to RTI in the form of Excel spreadsheets or some other data management tool that is to be determined. The Access database will not be sent to RTI or CDC and will be destroyed from the server after the study is completed.
For the staff interviews (Aim 2), two RTI staff will conduct each interview, one to lead the interview and one to take notes. All interviews will be digitally recorded. Interview notes will be typed in Microsoft Word and saved directly to the secure project share drives. The interviews will also be audio-recorded on RTI devices, transcribed, and entered into NVivo for analysis. Identifiable study data will not be given to the clinics about clinic staff that participate in the interviews or surveys as part of Aim 2. In addition, data will not be shared across clinic sites. Data will be reported in aggregate and quotes of any responses will be anonymous. Anonymous identifiers will be used instead of respondents’ names on all transcriptions of qualitative interviews. Notes and audio recordings of all individual and small group interviews will be created on RTI devices and stored on the secure project share drive. Clinic Staff Survey data will be collected through Qualtrics, a secure survey hosting website, and the data will be stored on a project share drive only accessible to project staff.
The clinical site principal investigators and co-investigators at their respective clinics will oversee all aspects of project activities. The sites will implement all components of the intervention, conduct all data collection activities, and transmit data to RTI electronically using a secure FTP site or whatever secure method is approved by each site’s IRB. RTI will provide training for data transmission procedures and will provide all necessary data management guidelines to the sites.
RTI will receive data transmittals from all sites and conduct data management. All data submitted to RTI will only be identified by a study participant ID number. There will be no patient identifying information such as names, EMR IDs, or email addresses. The study sites will maintain a study database using Access that will contain the link between the patient name, EMR ID, and the study ID. It will be password protected and only accessible by selected study staff at each clinic. CDC will format and transfer anonymous backend data from the PHC tool to RTI, including process data on PHC use and answers to tailoring questions presented in the tool. RTI and CDC will not have access to any personal identifying information (PII). RTI and CDC will take collaborative responsibility for statistical analysis of the data, although sites may also participate in data analysis activities. CDC will run quality assurance checks as another way to ensure that data quality is maintained throughout the life of the project.
3. Methods to Maximize Response Rates and Minimize Nonresponse
The investigators involved in this project have extensive experience recruiting and retaining clinic patients for projects involving the collection of behavioral and clinical data. PHC project staff will approach clinic patients identified through the routinely collected clinic data as eligible for the study. PHC project staff will also conduct systematic outreach to patients who meet the study criteria but who are out of care. Patients who are successfully re-engaged in care will be eligible to participate in the study. If the patient agrees, the Project Coordinator will bring the patient into a private space for the informed consent process after which they will be randomized to either the treatment or control arm.
Achieving sufficient enrollment in the study is critical to the success of the PHC Evaluation Trial and we propose to offer two tokens of appreciation to all enrolled participants. Participants will be informed of both tokens of appreciation during the consenting process. Tokens of appreciation will be provided to each study participant at enrollment upon consent and at the 12-month data collection end point. Each of the two tokens of appreciation will be a $50 gift card. (Please see Statement A, Section A9 for a detailed description of the rationale and how the PHC study will administer tokens of appreciation.)
This intervention trial is being conducted within each clinic, fully integrated as part of patients’ clinic scheduling. Therefore, as long as patients are returning for their clinical appointments, they should also be retained in the study. The study will rely on each clinic’s standard of care for making appointment reminder calls and emails. Intervention participants who have no scheduled appointments at the 12-month end point are contacted through PHC outreach to schedule their 12-month visit. During this call, participants will be reminded about the second token of appreciation. Outreach coordinators that are contacting participants who have not returned to the clinic and have no scheduled appointments for the 12-month data collection endpoint will be trained to follow specific procedures on notifying participants of the second $50 gift card (Attachment 15).
Recruitment and retention will be monitored through ongoing data reports generated weekly and monthly and submitted by RTI to CDC. The project area staff and CDC will use the data in these reports to identify problems with recruitment or retention. When a problem with recruitment or retention arises during data collection, research staff will be instructed to consult with clinic staff to identify solutions to the problem.
Non-response bias will be assessed using data from the EMR. Study participants who completed the PHC intervention 3 times will be compared to those who enrolled but did not complete PHC at least 3 times in a 12-month period.
For Aim 2, clinic staff will be assured that their responses to the online survey and qualitative interviews will be kept private. They will also provide informed consent to participate in the study.
4. Tests of Procedures or Methods to be Undertaken
The data collection elements were developed by CDC and RTI. Clinic staff are being provided with an orientation to project tools and processes prior to beginning work at their clinic.
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
CDC Consultant on Statistical Aspects
Craig Borkowf, PhD
Biostatistician
Division of HIV/AIDS Prevention
U.S. Centers for Disease Control and Prevention
(404) 639-5235
Cooperative Agreement Awardee
CDC awarded a cooperative agreement to RTI in 2016 to conduct the Positive Health Check Intervention trial. The RTI staff is involved with all aspects of designing and implementing the study. The study data will be collected by CDC and RTI staff and merged using a unique study ID. No identifiers will be included in datasets prepared for CDC. RTI will deliver the de-identified but sensitive information from the study using an encrypted File Transfer Protocol.
Megan Lewis, PhD |
Principal Investigator (PI): Responsible for overall scientific integrity for the project, across all aims; fiscal management; primary contact for CDC |
Jen Uhrig, PhD |
Co-investigator: Consulting on design and implementation |
Carla Bann, PhD |
Co-investigator and Lead Statistician: Responsible for conducting study analysis and overseeing data management |
Catherine Slota, PhD |
Co-investigator: Responsible for assisting the Principal Investigator with overseeing data management flows, including clinical electronic medical record (EMR) data, EMR data reporting, and interaction with site personnel; helping with regulatory approvals |
Ryan Paquin, PhD |
Assistant Analyst: Responsible for working with Carla Bann and Shawn Karns to process and analyze study data |
Bryan Garner, PhD |
Co-investigator: Responsible for leading Aim 2 quantitative data collection and analysis |
Barry Blumenfeld, MD |
Co-investigator: Responsible for leading design of EMR harmonization and procedures for EMR data collection |
Olga Khavjou, MA |
Co-investigator: Responsible for cost analysis data collection for Aim 3 |
Olivia Taylor, MPH |
Project Manager: Responsible for project management; leading qualitative data collection and analysis for Aim 2; leading data collection for Aim 4 assessing standard of care at each clinical site; assisting PI with cooperative agreement management, site subcontracts, consultant agreements; supervising other study research associates |
Alexa Ortiz, MSN |
Research Analyst: Responsible for outreach protocol and assisting the PI and Associate Project Director with site management |
Shawn Karns, BA |
Data Manager: Responsible for managing data for all sites; assisting Dr. Bann with data analysis and reporting |
Brittany Zulkiewicz, BS |
Research Associate: Responsible for intervention implementation protocols across sites; coordinating data management with Dr. Bann and Ms. Karns |
Kate Ferriola-Brukenstein, BA |
Research Associate: Responsible for assisting Ms. Taylor with site administration and project organization |
CDC Project Staff
The CDC staff members who are involved with the various aspects of designing and implementing the study are listed below. CDC staff will not be in contact with study participants. CDC will receive only study data with no information in identifiable form. The data collected will be analyzed by CDC staff. All CDC project staff can be reached at the following address and phone number:
Prevention Research Branch
Division of HIV/AIDS Prevention
Centers for Disease Control and Prevention
1600 Clifton Rd, NE MS E-37
Atlanta, GA 30333
Phone: (404) 639-1900
Camilla Harshbarger, PhD, Project Officer
Prevention Research Branch
Division of HIV/AIDS Prevention
Ann O’Leary, PhD, Consultant
Prevention Research Branch
Division of HIV/AIDS Prevention
Carla Galindo, MPH, Project Officer
Prevention Research Branch
Division of HIV/AIDS Prevention
Arin Freeman, MPH, Project Coordinator
Prevention Research Branch
Division of HIV/AIDS Prevention
Gary Marks, PhD, Consultant
Epidemiology Branch
Division of HIV/AIDS Prevention
Cari Courtenay-Quirk, PhD, Consultant
Prevention Research Branch
Division of HIV/AIDS Prevention
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | ziy6 |
| File Modified | 0000-00-00 |
| File Created | 2021-01-22 |
© 2026 OMB.report | Privacy Policy