SSB_0920-1140_2017_Revision
SSB_0920-1140_2017_Revision.docx
Zika virus persistence in body fluids of patients with Zika virus infection in Puerto Rico (ZIPER Study)
OMB: 0920-1140
Zika virus persistence in body fluids of patients with Zika virus infection in Puerto Rico (ZIPER Study)
Request for OMB approval of a Revision ICR
0920-1140
Expiration Date: 10/31/2017
Supporting Statement B
May 5, 2017
Contact:
Lee Samuel
National Center for Emerging and Zoonotic Infectious Diseases
Centers for Disease Control and Prevention
1600 Clifton Road, NE
Atlanta, Georgia 30333
Phone: (404) 718-1616
Email: [email protected]
Table of Contents
1. Respondent Universe and Sampling Methods 2
2. Procedures for the Collection of Information 3
3. Methods to Maximize Response Rates and Deal with No Response 3
4. Tests of Procedures or Methods to be Undertaken 11
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data 11
Participants will include patients with ZIKV infection of all ages identified through the Sentinel Enhanced Dengue Surveillance System (SEDSS) that was established in 2012 by the CDC Dengue Branch at Saint Luke’s Episcopal Hospital and more recently at the outpatient clinic, Center for Emergencies and Integral Medicine, El Tuque, both in Ponce Puerto Rico. Participants will also include household contacts of persons with RT-PCR-positive ZIKV infection. These contacts will be recruited into the study with the use of recruitment coupons (Attachment H) that will be provided to the ZIKV RT-PCR-positive cases. The patient coverage area for this hospital consists of residents from 20 municipalities (county equivalent) who seek care at these facilities. These 20 municipalities have a combined population of 853,389 residents, and have a demographic profile similar to that of Puerto Rico at large which consists of 78 municipalities. Saint Luke’s Episcopal Hospital in Ponce is a tertiary care, teaching hospital that has 425 inpatient beds and 2,367 full-time employees. It is the largest hospital in southern Puerto Rico and it is the fifth largest hospital in Puerto Rico. Each year, approximately 54,000 patients seek care in the Emergency Department (ED) and 20% of them are admitted. It has primary medical specialties including cardiology, urology, obstetrics/gynecology, and neurology services. In addition, Saint Luke’s Episcopal Hospital in Ponce is the main affiliated teaching hospital of the Ponce School of Medicine. The Center for Emergency Medicine and Integral Medicine (CEMI), El Tuque is an outpatient clinic that had 1,500 visits per month. El Tuque is a sector of the Canas Neighborhood of Ponce with a population of 50,000. CEMI, the urgent clinic that serves this population has 1,500 visits per month and refers all patients requiring secondary and tertiary services to Saint Luke’s Episcopal Hospital in Ponce.
From December 2015 to April 20, 2016 there have been 626 (http://www.salud.gov.pr/Estadisticas-Registros-y-Publicaciones/Informes%20Arbovirales/Reporte%20ArboV%20semana%2014-2016.pdf) confirmed Zika cases in Puerto Rico. Of these, 71 cases are from the Ponce health region and 39 have been identified through SEDSS. We will expand recruitment of RT-PCR Zika virus positive cases into the ZIPER study by recruiting cases reported through passive surveillance in selected municipalities in Puerto Rico, such as San Juan, Guayama and Caguas.
Participant inclusion criteria
Residents of Puerto Rico of all ages with RT-PCR-positive ZIKV infection in any body fluid or,
Household contacts of RT-PCR-positive ZIKV cases with a valid coupon.
Participant exclusion criteria
Having previously participated in the ZIKV persistence study,
We aim to recruit a total of 350 participants with RT-PCR-positive ZIKV infection for the cohort study. Participants with RT-PCR-positive ZIKV infection can refer up to 5 household contacts for a total of 1,750 contacts. We estimate that not all coupons will be used and that we will get on average 3 recruits per participant for a total of 1,050 contacts. An estimated 20% will be ineligible or decline to participate for a total of 840 household contacts. Based on surveys among Chikungunya contacts, we expect 5% of contacts to have ZIKV RNA (n=42) in body fluids. We present the following scenarios on the point prevalence of shedding among contacts and the confidence interval around the estimate based on different sample sizes (Table 1). We used the formula n=Z2P(1-P)/d2, where Z is the Z statistic for a level of confidence (Z=1.96), P is the estimated prevalence (P ranged from 1%-10%) and d is the precision (d ranged from 0.0005 – 0.003).
Table 1. Estimated sample size for different prevalence and margin of error scenarios.
Sample size to estimate confidence
interval |
||||
Margin of Error (d) |
Prevalence (P) |
LCI* |
UCI** |
Sample Size |
0.50% |
1.00% |
0.50% |
1.50% |
1521 |
1.00% |
1.00% |
0.00% |
2.00% |
380 |
1.00% |
5.00% |
4.00% |
6.00% |
1825 |
2.00% |
5.00% |
3.00% |
7.00% |
456 |
2.00% |
10.00% |
8.00% |
12.00% |
864 |
3.00% |
10.00% |
7.00% |
13.00% |
384 |
This is a prospective, descriptive cohort study. The prospective nature of the proposed cohort study allows for determining the persistence of shedding ZIKV in bodily fluids through repetitive specimen data collection among individuals with RT-PCR-positive ZIKV. The study will also have a cross-sectional component to assess the prevalence of anti-ZIKV IgM and IgG in serum and RNA in body fluids among household contacts of RT-PCR-positive ZIKV cases.
Case definition
Because of the unknown duration of anti-ZIKV IgM among infected individuals and the possibility of those presenting with an illness caused by another infection still being positive for anti-ZIKV IgM antibodies, we will recruit ZIKV reverse transcription-polymerase chain reaction (RT-PCR) positive cases into the study and their household contacts.
A RT-PCR-positive case of ZIKV infection is defined as ZIKV RNA detected in any body fluid by RT-PCR.
Household contacts are defined as persons of all ages who live in the same residence as an RT-PCR-positive case of ZIKV infection.
Identification of RT-PCR-positive participants
Participants will be identified through routine procedures for SEDSS (Figure 1). SEDSS procedures have been described extensively in the CDC approved protocol # 6214. In brief, SEDSS is a nurse-initiated system with potential participants identified by triage nurses who take vital signs including body temperature in the triage area. The triage nurse identifies all patients with an AFI defined by presence of fever at time of triage (≥ 38.0°C or 100.5F) or complaint of having fever lasting seven days or less. Every patient who meets the inclusion criteria for symptomatic participants (i.e., fever or history of fever for less than or equal to 7 days) is invited to participate in SEDSS by the Emergency Department nurse, resident or study staff. It has been reported that only a subset of patients with confirmed ZIKV infection present with fever. In order to capture potential ZIKV cases during the duration of the ZIKV persistence study, the eligibility criteria for SEDSS will be expanded to include rash, conjunctivitis and arthralgia. Patients for SEDSS may first seek services at the emergency room, affiliated outpatient clinic, or they may be a direct admission to the inpatient service. All age group patients are included in SEDSS, however, enrollment is limited to infants after they are discharged from the hospital after birth. This means that infants are not enrolled right after birth during their birth hospitalization. If an infant goes home and is re-admitted 1 week after birth, this infant is eligible for enrollment. Infants weighting less than 10 pounds will not be enrolled in the study, due to the limit of blood collection per kg (see below under Specimen Collection). Based on Puerto Rico laws, adults are defined as age 21 years or older. Patients 14 to 20 years old who fulfill one or more of the following criteria are considered emancipated and will provide independent consent to participate in the study as adults and parental permission will not be sought: 1) legally emancipated, 2) support themselves financially, 3) live independent of their parents, 4) are pregnant, or 5) have children.
As part of SEDSS, physicians order blood, nasopharyngeal swabs and urine to be collected for all participants. Specimens are sent for testing at the Dengue Branch Laboratory in San Juan where testing is done for dengue, Zika and other infectious diseases (Leptospira, Burkholderia pseudomallei, enterovirus, and chikungunya). In SEDSS, Zika testing is performed in blood (serology and RT-PCR) and urine (RT-PCR). Any RT-PCR-positive cases of ZIKV will be contacted over the phone by study staff and offered enrollment in the ZIKV persistence study.
In SEDSS, all orders for laboratory testing are given a special code upon entry into the hospital computer system so that specimens can be tracked via the hospital electronic Meditech system. In the ZIKV persistence study a new participant study ID will be assigned; however, the code from SEDSS will also be retained in order to link SEDSS laboratory and clinical results to data collected in the ZIKV persistence study.
In addition to SEDSS, we will recruit RT-PCR Zika virus positive cases identified through passive surveillance in Puerto Rico. Patients presenting with Zika-like symptoms to private physicians and public health care facilities are offered blood-based RT-PCR testing for diagnosis. All samples are being tested either at the Salud Laboratory or at the CDC Dengue Branch Laboratory. On average, results are available within 7 days after specimen collection. For those with RT-PCR ZIKV positive results (ZIKV+), staff from the Puerto Rico Health Department contact health care providers to inform them of their patients ZIKV positive test results.
CDC will ask permission from the Puerto Rico Department of Health to contact the physicians of patients with laboratory-confirmed ZIKV+ infection. We will analyze passive surveillance data to target this strategy areas that have higher numbers of Zika cases reported. Once identified, we will contact physicians/hospitals with high numbers of ZIKV+ individuals who are located in areas of Puerto Rico that are logistically feasible for our study, for example, the municipalities of Ponce, San Juan and Caguas. We will briefly explain to the physician or physician’s surrogate the ZIPER study, objectives, eligibility criteria, and requirements of participation in the study.
Enrollment
As part of SEDSS procedures, patients are requested to attend a 7-day convalescent follow-up visit or receive a phone call from study staff to collect follow-up information. RT-PCR-positive ZIKV cases will be invited at that time to participate in the ZIKV persistence study. Upon contact with the patient, the study objectives and methods will be explained and eligibility assessed (Attachment C). The person will be asked whether they would like to participate in the study. Study staff will offer consent (Attachment D) among those interested in participating. At this same visit, participants will be enrolled in the study which will be participants’ week 1 visit.
For those recruited over the phone (Attachment F), the participant’s contact information will be verified (Attachment G) and an appointment set for a home visit or clinic visit. During the visit, consent will be administered, and procedures for the participant’s week one visit will be implemented.
For recruitment through passive surveillance, when an individual is identified as having a ZIKV+ result, the Puerto Rico Department of Health Laboratory or the CDC Laboratory will send the result to the physician or physician’s surrogate. We will also include information about the ZIPER study and request that this information be passed along to the ZIKV+ individual. We will ask the physician or physician’s surrogate to contact the ZIKV+ individual to report their results and explain the ZIPER study. Upon contact with the patient, they will inform the ZIKV+ individual of their test results and use a scripted introduction to explain the objectives, goals, and requirements of participation in the ZIPER study (Attachment K). The physician or physician’s surrogate will explain that they may be eligible and if they are interested in participating or finding out more information they can contact the ZIPER study directly at [email protected] or at the study coordinator phone number. The physician or physician’s surrogate will also ask the ZIKV+ individual to provide permission to be contacted by the ZIPER study staff. If the ZIKV+ person gives permission for ZIPER study staff to contact them, the physician or physician’s surrogate will confirm the ZIKV+ individual’s contact information (Attachment G) and then pass this information along to the ZIPER study coordinator. If the ZIKV+ individual does not give permission for the information to be shared with ZIPER study staff, then it will be up to the ZIKV+ individual to decide if they want to contact the ZIPER study. When potential participants contact the ZIPER study or the study staff calls the potential participant, the study coordinator will use the recruitment script (Attachment F). Participants will be able to ask any questions they may have for clarification and understanding the study procedures. If the individual is eligible and interested in participating, a date, time, and location will be scheduled for the study visit. At this point, other study procedures as described in the protocol will ensue.
A study ID will be assigned to each participant to link the forms and specimens. A base-line questionnaire (Attachment H) will be administered by interviewers in laptop computers or tablets that includes questions on socio-demographics, symptoms, and sexual activity (subjects age 21 and older, only).
All participants will provide blood, a saliva, and urine specimen. Adult participants (ages 21 years or older) and legally emancipated minors (support themselves financially, live independent of their parents, are pregnant, or have children) will be asked to self-collect a vaginal swab if female or provide a semen specimen if male. We will also collect breastmilk among lactating women. The study staff will label and mark all specimen containers before collection, with the study ID number. Procedures for specimen collection will be explained by study staff (Attachment I) and specimen collection will be completed in a secure, private space at the study site using appropriate infection control precautions.
Study kits will be prepared with all the materials necessary for specimen collection. Study kits will be contained in a large zip lock bag and will include: 1 tiger top blood collection tube, 1 sterile polyester swabs, 3 sterile collection containers, collection bag for children, one sterile transport media and a laboratory specimen collection biohazard bag. Study kits will be stored in the study office and distributed to designated area. The volume of blood collected will depend on the characteristics (i.e. symptomatic, healthy, pregnant, adults) of participants.
Blood collection among minors, pregnant women and participants with symptoms
At enrollment, pregnant women, minors and participants who report symptoms compatible with Zika or other major illness will have 7 ml of blood collected following standard procedures. If the participant weighs less than 37 pounds, the amount of blood drawn will depend on their weight, but will not be more than 3 ml/kg during any 8-week period, and the volume will not exceed 7 ml at one time. The volumes to be drawn for children weighting less than 37 pounds can be found in Table 2 below. These volumes were determined by first calculating the maximum 8-week volume by body weight (3 ml/kg), subtracting 7 ml (which are collected at the baseline screening for SEDSS), dividing by the maximum number of remaining visits in 8 weeks (5), and then rounding down to the nearest milliliter. Among participants weighing ≥ 31 pounds, the 7ml will be split into a tiger top tube (5ml) and a purple top tube (2ml). Among those weighing < 31 pounds, only a red top tube will be collected. These blood collection procedures will be repeated at each study visit. A laboratory form will be completed and will accompany the specimens to the laboratory (Attachment J).
Table 2. Blood volume to be drawn from children weighting less than 37 pounds.
Week |
0* |
V01 |
V02 |
V03 |
V04 |
V06 |
|
|
Weight (lbs) |
|
|
total ml |
|||||
≥ 31 |
7 |
7 |
7 |
7 |
7 |
7 |
|
42 |
28-30.9 |
7 |
6 |
6 |
6 |
6 |
6 |
|
37 |
24-27.9 |
7 |
5 |
5 |
5 |
5 |
5 |
|
32 |
20-23.9 |
7 |
4 |
4 |
4 |
4 |
4 |
|
27 |
17-19.9 |
7 |
3 |
3 |
3 |
3 |
3 |
|
22 |
13-16.9 |
7 |
2 |
2 |
2 |
2 |
2 |
|
17 |
9-12.9 |
7 |
1 |
1 |
1 |
1 |
1 |
|
12 |
*Day 0 blood draw occurs as part of the SEDSS protocol.
Blood collection among healthy non-pregnant adults and emancipated minors
At enrollment, healthy non-pregnant adults will have 20 ml of blood collected following standard procedures. Two tiger top tubes of 8.5 ml and one 3ml purple top tubes will be collected. These procedures will be repeated at each follow-up visit, see below. This amount is under the 500ml per 8 weeks maximum among healthy participants. A laboratory form will be completed and will accompany the specimens to the laboratory (Attachment J).
Other Specimens
All participants will receive instructions on how to collect saliva and urine specimens in sterile cups. Among children 4 years of age and younger saliva specimen will be collected with a swab. Among infants and non-toilet trained children urine specimens will be collected with a sterile urine collection bag. The study staff will instruct the participant to collect the vaginal swab and semen sample for adult participants and emancipated minors. Vaginal swabs will be placed in viral transport media. Women who are lactating will be asked to produce 1ml of breast milk with a pump or manually. Semen will be collected into a sterile container. Women will be offered pregnancy testing at the baseline visit (week 1), and if positive they will be referred to antenatal care (ANC) for services.
Follow-up
After the initial visit, participants will have to attend follow-up visits as described in Figure 1. They will be provided the option to: 1) come to the study site to complete study procedures; or 2) have a study team (phlebotomist and an interviewer) visit their home at a date and time previously agreed upon. If a participant chooses to come to the study site to do the interview and provide specimens, they will still be provided the option to self-collect the semen or vaginal swab (adults and emancipated minors only), and urine specimens at home and bring them to the clinic within 2 hours after specimen collection. At each visit study staff will administer a follow-up questionnaire (Attachment H), review the participant’s health status since the previous specimen collection(s), report any available testing results, review the next specimen collection needs and provide counseling on how to prevent Zika and answer any questions the participant may have (Attachment K).
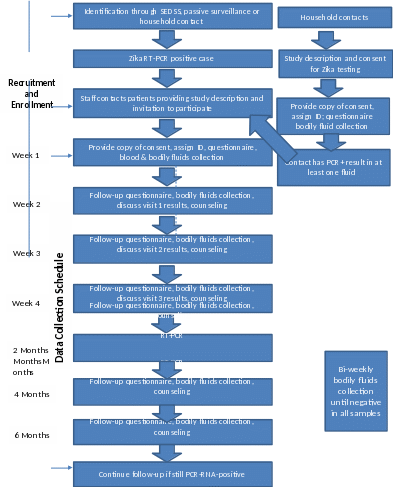
Figure
1. Study Flow Chart
For PCR in bodily fluids (blood, saliva, urine, breastmilk, and semen/vaginal secretions), participants will repeat specimen collection weekly for at least 4 weeks (Figure 1). Beginning at week 6, PCR testing for the presence of Zika virus will continue bi-weekly until all fluids have negative tests. In addition, every subject will have blood and other body fluids for serology and RT-PCR collected at 2, 4, and 6 months. If all fluids test negative at week 4, additional visits to collect specimens will be done at 2, 4, and 6 months. If at least one fluid remains positive after 4 weeks, bi-weekly visits will continue until all fluids have negative tests during a visit (Table 3). For subjects who are still undergoing biweekly visits at months 2, 4, and 6 months, only one set of specimens will be collected.
RT-PCR test results are estimated to be available within one week of specimen collection and will be shared with the study participant during follow-up visits or phone calls. Any specimen positive for ZIKV RNA by RT-PCR will be processed for culture/viral isolation at one of laboratory. Because we do not yet know how long Zika virus persists in body fluids, we do not have a set time frame or a maximum number of visits for the study. At present, the study is designed to obtain body fluids at 2, 4, and 6 months for all subjects, even if they no longer have detectable virus in their samples. The minimum number of visits will be 8, and there could be as many as 14 or more, if subjects still have detectable virus at 6 months.
A second study visit will be scheduled with household contact at 2 or 4 months, to detect new infections and estimate incidence. Because the original study consent forms do not include this visit, household contacts will be contacted by study staff and will be consented again using the same consent form.
Table 3. Collection of specimens by study week, Zika Persistence Study, 2016.
Specimen |
Week |
||||||||
0 |
1 |
2 |
3 |
4 |
6 |
8 |
16 |
24
|
|
Blood |
X |
X |
Y |
Y |
Y |
* |
Y |
Y |
Y
|
Saliva |
X |
Y |
Y |
Y |
Y |
* |
Y |
Y |
Y
|
Urine |
X |
Y |
Y |
Y |
Y |
* |
Y |
Y |
Y
|
Semen/vaginal swab |
|
Y |
Y |
Y |
Y |
* |
Y |
Y |
Y
|
Breastmilk |
|
Y |
Y |
Y |
Y |
* |
Y |
Y |
Y
|
X = specimen collected as part of SEDSS
Y = always collected
* = will continue every 2 weeks until has all samples have negative tests
Contact information including names, addresses, phone numbers and email, will be collected and entered into an access file that will allow to schedule appointments, manage staffing work hours and send text and email remainders to participants (Attachment G). This system will also allow to log and monitor the number of times participants have been contacted. Follow-up study site visits or home visits will be scheduled and agreed upon at each preceding communication. Contact information will be reviewed during each contact or visit and reminder calls will be made if applicable. Study participants will be supported to fulfill their participation in the study by counseling offered during telephone contact, clinic visit or home visit together with assistance with referrals to clinical care as needed. Carefully timed reminder calls will be made to participants who have missed their scheduled calls or appointments. Flexibility will be provided in terms of appointment bookings and any needs for additional counseling or information. Participants who fail to attend a follow-up visit will be contacted by phone or home visits. After 5 failed attempts to contact them, participants will be considered lost to follow-up. Women will be offered pregnancy testing at the week 1 visit by study staff, and if positive they will be referred to antenatal care. Care for such patients will be recommended in line with national guidelines.
Identification of household contacts
Every RT-PCR-positive ZIKV case identified through SEDSS will receive five study coupons (Attachment C) to invite up to 5 household contacts to participate in the study. Recruitment coupons have been used successfully in other fields such as in surveillance among populations at high risk for HIV. RT-PCR-positive ZIKV participants will be instructed to provide a coupon to any member of their household irrespective of age or sex. If they have less than 5 household contacts they will receive an appropriate number of coupons based on the number of household contacts. They will be instructed that when recruiting: “participation should be completely voluntary and no one should be influenced or pressured into participating.” We will not provide a recruitment incentive for participants referring household contacts. If referred household contacts are eligible (Attachment D) and consent to participate, the number on the coupon will be entered into a database to keep track of ZIKV RT-PCR-positive participants and their household contacts. Upon contact with the potential participant, an introduction to the study will be given and the requirements of participation in the study will be explained. If agreeable to participation, study staff will seek consent (Attachment E) for the collection of blood, saliva, urine and among adults and emancipated minors, semen/vaginal secretions specimens and breastmilk if appropriate. Household contacts who have a positive PCR result in any body fluid will be invited to participate in the cohort study. Results for household contacts will be provided by phone and study staff will answer questions participants may have (Attachment K). When providing the results over the phone to RT-PCR-positive household contacts they will be asked to return to the study site to invite them to participate in the cohort study, explain study procedures and administer consent (Attachment E). Participants will also be offered the option to have the study staff conduct a home visit. Household contacts who are Zika RT-PCR or IgM negative at V01 will be contacted and consented for a follow-up visit at month 2 or 4 after V01 to determine if they have been infected with the Zika virus and estimate incidence among household contacts.
Semen sub-study
To better understand the effect of Zika virus infection on sperm in men who have Zika virus RNA detected in their semen, 8-14 semen ejaculates from 10-20 men participating in the ZIPER study will be used to determine the presence and/or detection of the Zika virus in different fractions of the semen ejaculate (i.e., seminal plasma, cellular debris, including White Blood Cells [WBCs] and spermatozoa) (Attachment N). PSA, testosterone and inhibin will be measured in the men participating in this sub-study to investigate if there is an association between Zika RNA levels in semen and hormone levels (ng/ml). Ejaculates from 10-20 male ZIPER participants not infected with Zika will also be evaluated to compare sperm volume, sperm count and other parameters as described below. Participants will receive $50 dollars in addition to the reimbursement for the standard ZIPER visit in order to compensate them for lost time and effort. Different ejaculate fractions will be tested for flavivirus by culturing in Vero cells. Subsequent passages of cellular fractions, and supernatants from such passages will be tested for Zika virus RNA by RT-PCR. All individuals providing semen samples will be given an informed consent and other procedures will be as described for the ZIPER study.
Methods to Maximize Response Rates and Deal with No Response
Participants will be provided a stipend to compensate them for their time. The stipend will reimburse for the time off work to attend the study visit or complete procedures, transportation, snacks, and any other needs. In the past, SEDSS participants received an incentive of $20 to return for a convalescent study visit; however, only 30% returned for the follow-up visit. Informal conversations with participants suggested that increasing incentive could help with attrition. Thus, participants will receive $50 dollars for each study visit. For participants under 7 years of age the parent will receive the full incentive. Participants will receive compensation for attending the study visit even if there is incomplete specimen collection.
Tests of Procedures or Methods to be Undertaken
No pilot testing will be done for either project.
No individuals were consulted on statistical aspects of these projects.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Samuel, Lee (CDC/OID/NCEZID) |
| File Modified | 0000-00-00 |
| File Created | 2021-01-22 |
© 2026 OMB.report | Privacy Policy