Supporting Statement B template_Backyard Integrated Tick Management WCSU project_06282017
Supporting Statement B template_Backyard Integrated Tick Management WCSU project_06282017.docx
Backyard Integrated Tick Management Project
OMB: 0920-1203
Backyard Integrated Tick Management Project
Request for OMB approval of an Existing Collection in Use without an OMB Control Number
July 20, 2017
Supporting Statement B
Contact:
Lee Samuel
National Center for Emerging and Zoonotic Infectious Diseases
Centers for Disease Control and Prevention
1600 Clifton Road, NE
Atlanta, Georgia 30333
Phone: (404) 718-1616
Email: [email protected]
Table of Contents
1. Respondent Universe and Sampling Methods 2
2. Procedures for the Collection of Information 3
3. Methods to maximize Response Rates and Deal with No Response 4
4. Tests of Procedures or Methods to be Undertaken 4
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data 5
The collection of information is conducted by Western Connecticut State University (WCSU), and its subcontractor, the University of Rhode Island (URI), as part of a Cooperative Agreement with the Centers for Disease Control and Prevention (CDC) (1U01CK0004912-01). The Cooperative Agreement was established based on WCSU competing successfully for CDC RFA-CK-16-002 (Spatially Scalable Integrated Tick Vector/Rodent Reservoir Management to Reduce Human Risk of Exposure to Ixodes scapularis Ticks Infected with Lyme Disease Spirochetes). The collection involves statistical methods. Study results may be generalizable to some extent but this cannot be ascertained without follow-up studies in other ecological and environmental settings.
Respondent Universe and Sampling Methods
The primary objective of the study is to compare the effectiveness of an integrated tick management (ITM) approach at single-treated residential properties vs. contiguously-treated residential properties to reduce the abundance of host-seeking ticks infected with Lyme disease spirochetes and human tick bites. The secondary objective of this study is to increase understanding of where people encounter ticks, both near their homes and in other outdoor settings. The study will utilize a single-blinded, placebo-controlled design to evaluate the effectiveness of ITM on single properties vs. ITM on contiguous properties. The study timespan that will involve participants is from 2017 ̶ 2020.
The respondent universe will consist of all persons (adults and children) living in a freestanding home with tick habitat (i.e., brushy or wooded area on property) in the study catchment area towns of CT and RI. Geographic Information System (GIS) technology will be used to identify and target recruitment at residential cul-de-sacs or dead-end streets that 1) contain 3–8 properties each, 2) border upon deciduous or mixed deciduous forest, 3) are greater than 30 m from water features such as rivers, streams or ponds (because of the potential for aquatic toxicity of pyrethroid pesticides), and 4) have a parcel size of 0.5–3 acres. Approximately 115 properties will be recruited from each of CT and RI that will comprise the study population through the four years of the study. The 230 properties should represent at least 690 people at risk of tick bites and acquisition of tick-borne diseases (at least three people per household).
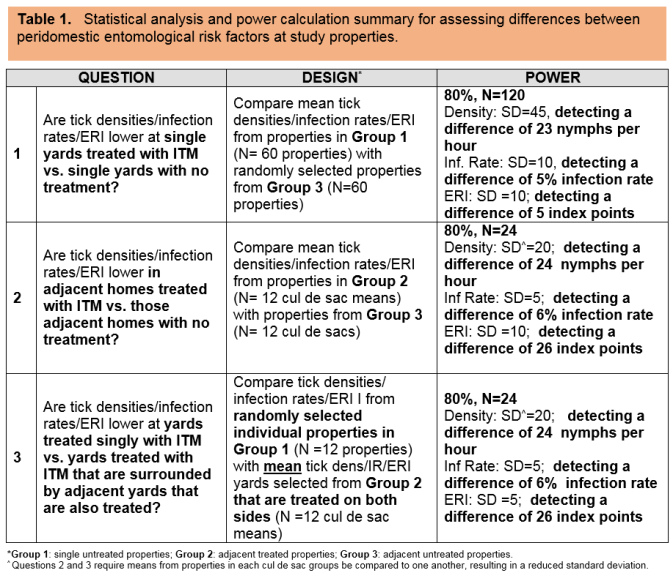
The sampling scheme will allow us to compare mean entomologic risk factors (density, infection rates, and ERI) each year at treatment versus placebo groups using a two-tailed t-test for each comparison (Table 1). Entomological data will be transformed if needed to address non-normality or unequal variances. Using previous entomologic data from studies of peridomestic risk in Connecticut and Rhode Island that estimated reasonable tick densities, infection rates, ERI, and standard deviations, we determined that enrolling 180 households would yield 80% Power with an alpha-level of 0.05 for two-tailed t-tests that detect significant differences between mean entomologic factors (Table 1). Previous TickNET prevention studies had attrition rates of 7% and 5%. Estimating a 10% attrition rate, we will seek to enroll at least 200 total properties at the beginning of the study. The stated number of respondents for this information collection has been set at 230 to ensure that loss of study participants does not unduly impact the power of the study over its planned 3-year course.
Households will be recruited from the study towns by using GIS-assisted targeted mailings (Attachment M) or door hangers (Attachments N and O). Based on previous CDC-funded tick-borne disease prevention studies conducted in CT, approximately 10,000 mailings and door hangers will be required to enroll eligible households from the study towns. Interested recipients may complete an online eligibility survey via www.surveymonkey.com, or may call WCSU/URI research study staff to complete the eligibility questionnaire by telephone. Final selection of study respondents for the three arms of the study (single-treated residential properties; contiguously-treated residential properties; single control residential properties) will be achieved based on information provided in the eligibility survey (Attachment C).
Procedures for the Collection of Information
Information is collected, under protocols approved by the IRBs at WCSU and URI, from inhabitants of residential properties to (i) compare the effectiveness of an integrated tick management approach at single-treated residential properties vs. contiguously-treated residential properties to reduce human tick bites and (ii) increase the understanding of where people encounter ticks, both near their homes and in other outdoor settings.
Initial surveys will include an eligibility survey (Attachment C) and the consent form (Attachment D) to recruit study participants. Consented participants will complete one introductory survey by telephone, projected to last no more than 15 minutes (Attachment E). In May–August of Years 1–4, participants will also complete an emailed monthly tick encounter survey about the number of ticks found on each member of the household and each household member’s tick-borne disease status, projected to take no more than 10 minutes per month to complete (Attachment F). An end-of-season survey will also be administered in March/April each year, projected to take no more than 10 minutes to complete (Attachment H). In addition, participants will be asked to record location of daily activity on behalf of themselves and household members each day over the first week of June in a single year via emailed daily surveys, projected to take 70 minutes over the week of participation (Attachment G). Lastly, an end-of-study survey will be administered in September 2020, projected to take no more than 15 minutes (Attachment I).
Surveys will, with the exception of the introductory telephone survey, be administered electronically. Surveys will be administered by the WCSU PI, Dr. Neeta Connally, or the URI PI, Dr. Thomas Mather; or individuals directly trained by the PIs in survey administration.
Individuals will be able to visit a study website (housed on the WCSU web server) that will contain a frequently asked questions (FAQs) section, contact information, and general study information for potential participants and others interested in learning about the study. For all persons who indicate interest in study participation by mail, phone, email, or web, and who meet the eligibility requirements, investigators will schedule a time to speak with the individual over the phone to discuss the study and request permission to mail study forms, including consent forms, to their home for their review. Potential participants will be re-contacted as needed to maximize response and participation rates. One adult in each home (≥18 years of age, and with the authority to allow synthetic pyrethroid application and rodent bait box placement on the property) will be asked to provide consent to participate and to respond to study surveys. Based on previous studies with similar study designs but examining different tick control methods, low rates (<10%) of attrition are expected over the full study period.
Tests of Procedures or Methods to be undertaken
Information collection instruments (Attachments E-I) represent refinements of instruments used successfully in previous studies with similar study designs but examining different tick control methods.
The statistical aspects of the study and the appropriateness of the key individuals collecting and/or analyzing data were evaluated during the process of external subject matter expert reviews of the project proposal that competed successfully under CDC RFA-CK-16-002 (Spatially Scalable Integrated Tick Vector/Rodent Reservoir Management to Reduce Human Risk of Exposure to Ixodes scapularis Ticks Infected with Lyme Disease Spirochetes), was funded under CDC Cooperative Agreement 1U01CK0004912-01 to WCSU, and is referred to here as the Backyard Integrated Tick Management Project.
Individuals collecting or analyzing data will include:
Name |
Title/Affiliation |
|
Dr. Neeta Connally |
PI/WCSU |
|
Dr. Rayda Krell |
Study Coordinator/WCSU |
|
Dr. Daniel Barrett |
Senior Contributor/WCSU |
|
Dr. Thomas Mather |
PI/URI |
|
Kim Downes |
Study Coordinator/URI |
|
Dr. Howard Ginsberg |
Senior Contributor/URI |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Samuel, Lee (CDC/OID/NCEZID) |
| File Modified | 0000-00-00 |
| File Created | 2021-01-22 |
© 2026 OMB.report | Privacy Policy