Developmental Projects and Special Studies
National Health and Nutrition Examination Survey
Att 2b Blood - Infants Form 083117
Developmental Projects and Special Studies
OMB: 0920-0950
Attachment 2b
National Health and Nutrition Examination Survey (NHANES)
Blood Draw – Infants (0 to less than 12 months) Pilot Study
Form Approved
OMB No. 0920-0950
Assurance of Confidentiality - We take your privacy very seriously.
All information that relates to or describes identifiable
characteristics of individuals, a practice, or an establishment will
be used only for statistical purposes. NCHS staff, contractors, and
agents will not disclose or release responses in identifiable form
without the consent of the individual or establishment in accordance
with section 308(d) of the Public Health Service Act (42 U.S.C.
242m(d)) and the Confidential Information Protection and Statistical
Efficiency Act of 2002 (CIPSEA, Title 5 of Public Law 107-347). In
accordance with CIPSEA, every NCHS employee, contractor, and agent
has taken an oath and is subject to a jail term of up to five years,
a fine of up to $250,000, or both if he or she willfully discloses
ANY identifiable information about you. In addition, NCHS complies
with the Federal Cybersecurity Enhancement Act of 2015 (6 U.S.C. §§
151 & 151 note). This law requires the federal government to
protect federal computer networks by using computer security
programs to identify cybersecurity risks like hacking, internet
attacks, and other security weaknesses. If information sent through
government networks triggers a cyber-threat indicator, the
information may be intercepted and reviewed for cyber threats by
computer network experts working for, or on behalf of, the
government. Public reporting
burden of this collection of information is estimated to average 8
minutes per response, including the time for reviewing instructions,
searching existing data/information sources, gathering and
maintaining the data/information needed, and completing and
reviewing the collection of information. An agency may not conduct
or sponsor, and a person is not required to respond to a collection
of information unless it displays a currently valid OMB control
number. Send comments regarding this burden estimate or any other
aspect of this collection of information, including suggestions for
reducing this burden to CDC/ATSDR Information Collection review
Office, 1600 Clifton Road NE, MS D-74, Atlanta, GA 30333. ATTN: PRA
(0920-0950).
This attachment represents the burden for the heel stick (0 to less than 6 months) and for the finger stick
(6 months to less than 12 months). Depending on age, the infants will have either a heel stick or finger stick,
not both.
Blood Draw – Infants (0 to less than 11 months) Pilot Study
Heel Stick Blood Collection into Microtainer®
The heel stick is performed on the lateral or medial portions of the foot (left and right); never the central area of the foot, the arch, or the back of the heel.
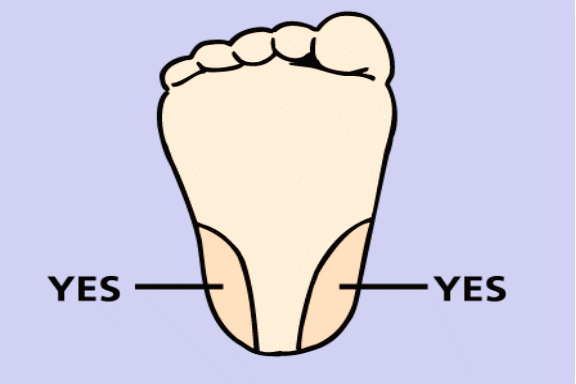
Use of trade names and commercial sources is for identification only and does not imply an endorsement by the U.S. Department of Health and Human Services (DHHS) and the Centers for Disease Control and Prevention (CDC).
Finger Stick Blood Collection into Microtainer®
The finger stick will be on the patient’s middle finger or ring finger.
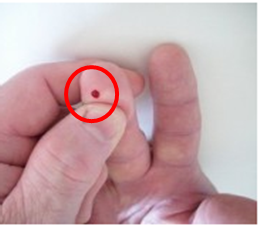
Use of trade names and commercial sources is for identification only and does not imply an endorsement by the U.S. Department of Health and Human Services (DHHS) and the Centers for Disease Control and Prevention (CDC).
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Attachment A |
| Author | vlb2 |
| File Modified | 0000-00-00 |
| File Created | 2021-01-22 |
© 2026 OMB.report | Privacy Policy