Improving FDA Health Communications with Older Women Regarding FDA-Regulated Products
Data To Support Social and Behavioral Research as Used by the Food and Drug Administration
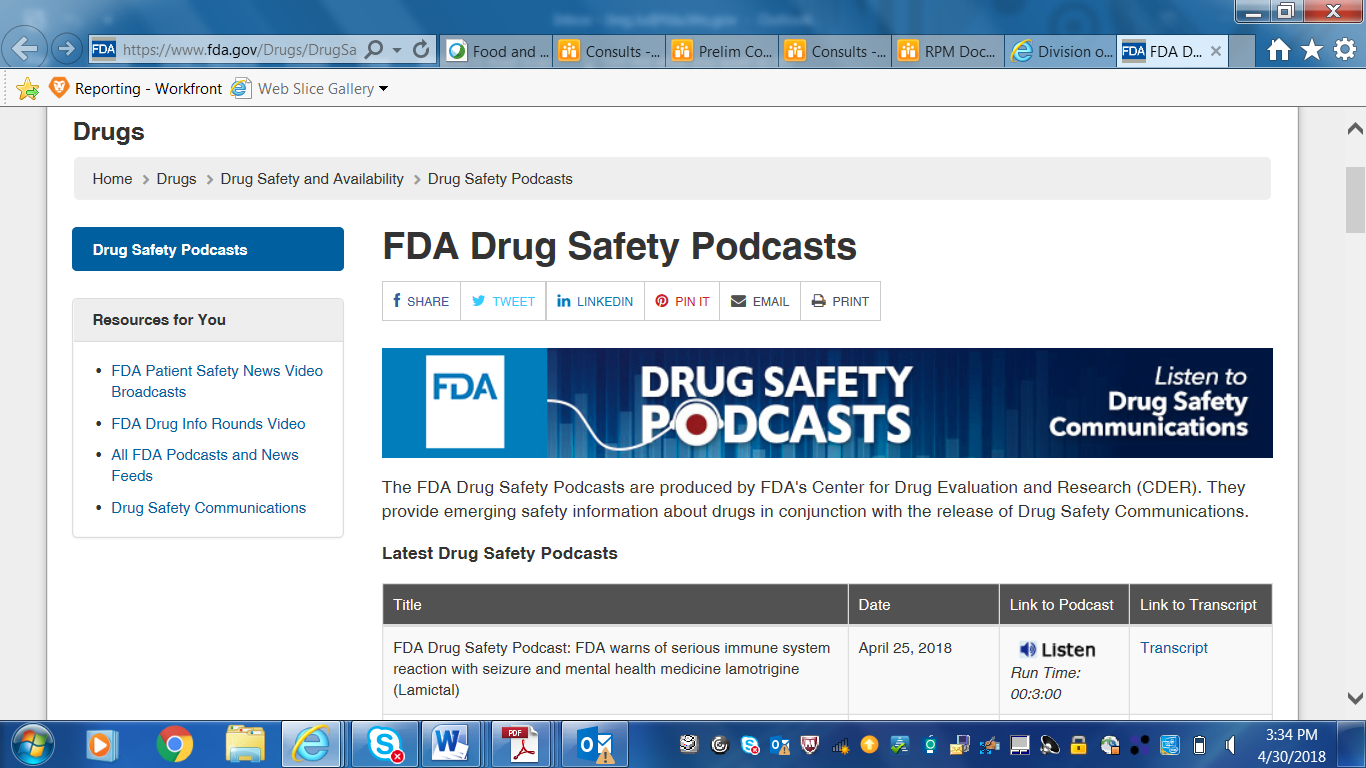
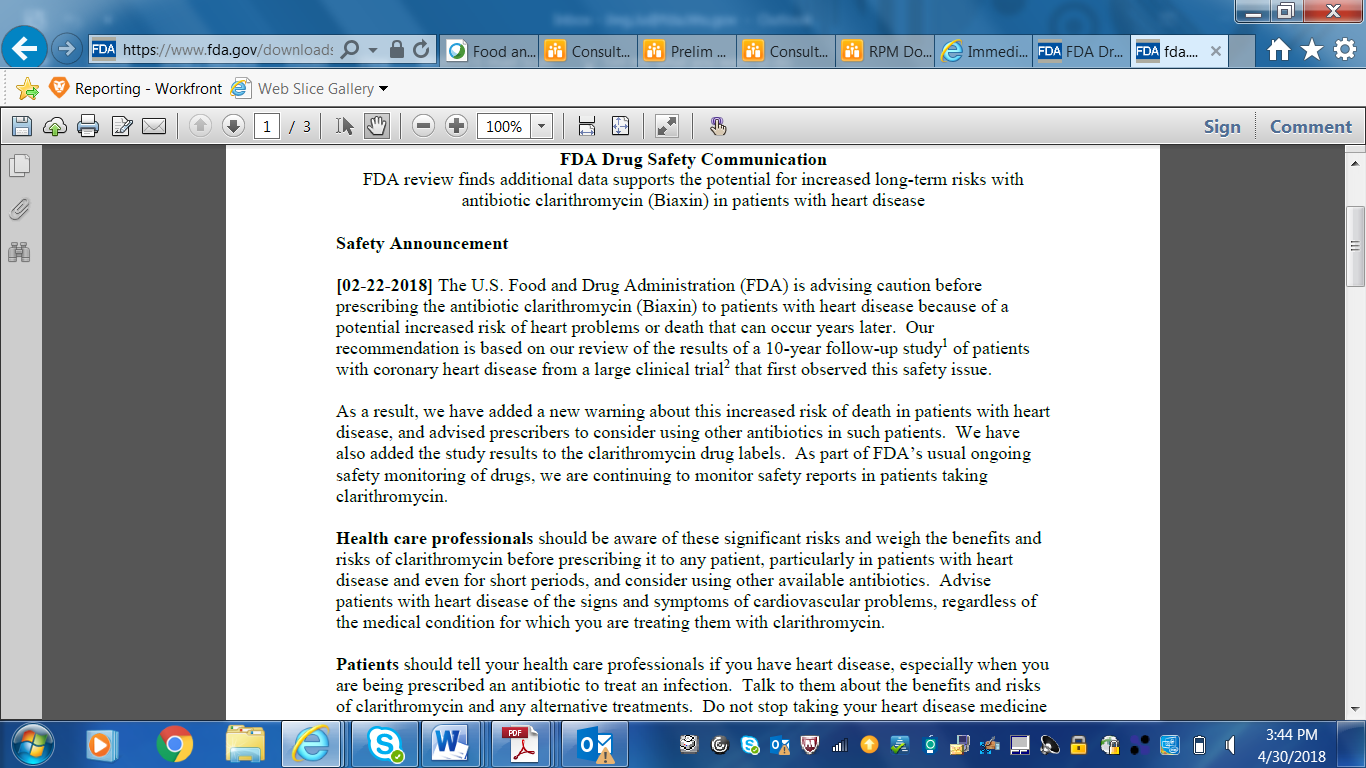
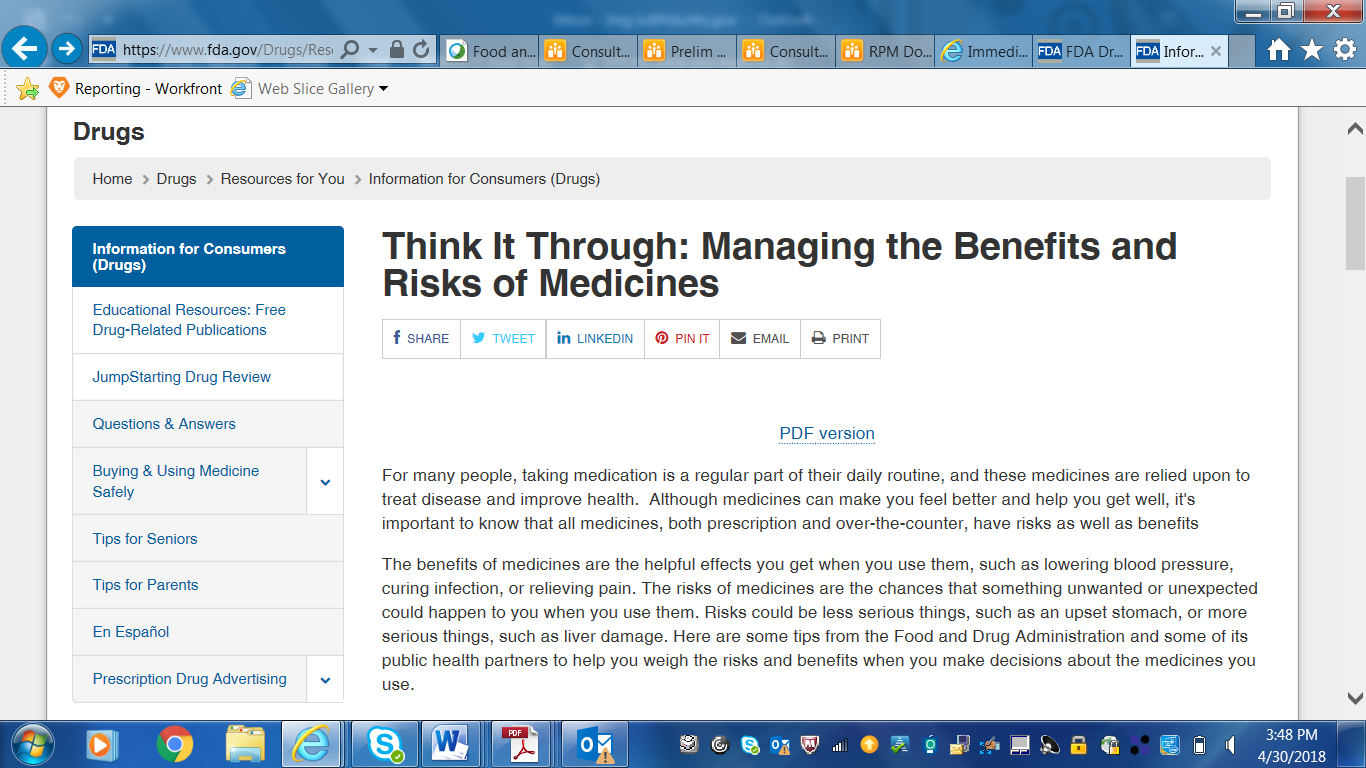
Screenshots of CDER Communications to Patients
Improving FDA Health Communications with Older Women Regarding FDA-Regulated Products
OMB: 0910-0847
⚠️ Notice: This form may be outdated. More recent filings and information on OMB 0910-0847 can be found here:
© 2026 OMB.report | Privacy Policy